Dietary supplements and human health: For better or for worse?
Abstract
Encouraged by the potential health benefits of higher dietary intake of substances with beneficial properties, the use of supplements containing these compounds has increased steadily over recent years. The effects of several of these, many of which are antioxidants, have been supported by data obtained in vitro, in animal models, and often by human studies as well. However, as carefully controlled human supplementation trials have been conducted, questions about the efficacy and safety of these supplements have emerged. In this Educational Paper, three different supplements were selected for consideration of the benefits and risks currently associated with their intake. The selected supplements include β-carotene, selenium, and genistein. The use of each is discussed in the context of preclinical and clinical data that provide evidence for both their use in reducing disease incidence and the possible liabilities that accompany their enhanced consumption. Variables that may influence their impact, such as lifestyle habits, baseline nutritional levels, and genetic makeup are considered and the application of these issues to broader classes of supplements is discussed.
1 Introduction
Foods have been used to prevent or alleviate illness for centuries: ancient Egyptians consumed liver (high in vitamin A) to treat night blindness; citrus foods (rich in vitamin C) were found to prevent scurvy in the 18th century; and William Fletcher demonstrated that unpolished rice (rich in vitamin B1) prevents Beriberi in 1905 1-3. In the 1930s, British and Polish chemists artificially synthesized vitamin C, and it was the first vitamin to be produced and marketed on a commercial scale 4. At that time, vitamin C supplements were advocated for the treatment of many viral infections, including polio 5. In the latter half of the 20th century and beyond, diseases due to overt nutrient deficiencies (including some of the examples mentioned above) have virtually disappeared in developed countries, and dietary supplements are now discussed primarily in terms of “optimal health” and chronic disease prevention.
Antioxidant nutrients are a diverse group of compounds characterized by their ability to quench reactive oxygen and/or nitrogen species—free radicals that cause significant damage to DNA, lipids, and proteins 6. Common antioxidants include vitamins E and C, carotenoids (including β-carotene), selenium, and polyphenols (including genistein); many are concentrated in foods of plant origin, particularly fruits and vegetables. Since excessive amounts of free radicals contribute to the pathogenesis of numerous chronic diseases, including cancer, cardiovascular disease, and diabetes 7, it has long been believed that antioxidant supplementation might offer a promising approach to the prevention of these devastating diseases.
Vitamin supplement use in the United States is very prevalent and has been increasing over time (Fig. 1). The National Health Interview Survey (NHIS) – a nationwide survey designed to assess health information in noninstitutionalized civilians – assessed the frequency of intake of multivitamins and single nutrient supplements at three time points; in 2000, daily use of any vitamin supplement, multivitamins, vitamin E, vitamin C, and vitamin A was reported by 33.9, 27.9, 11.4, 10.8, and 1.2% of the sampled population, respectively, which represents a substantial increase over percentages reported in 1987 8. Females, non-Hispanic Whites, moderate alcohol drinkers, and former smokers were more likely to report consuming antioxidant nutrient supplements in the NHIS 2000 survey, as were individuals who were older, more educated, nonobese, and lived in the Western and Northeastern regions of the United States 8. Annual sales of vitamin supplements currently exceed 25 billion dollars 9, which reflects the aging population and people's growing desire to achieve good health and prevent chronic disease.
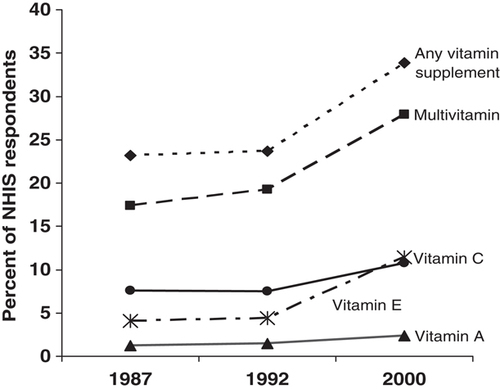
Trends in daily supplement use in the United States. Data were derived from the 1987, 1992, and 2000 National Health Interview Surveys (NHIS), as reported in 8.
Despite a plethora of in vitro, animal, and observational epidemiological data indicating that high antioxidant intake from foods is protective against disease, many clinical trials have failed to demonstrate any beneficial effects of antioxidant supplementation. Furthermore, some trials have shown adverse effects in specific population subgroups. In this Educational Paper, we discuss the potential benefits and risks of antioxidant consumption – including dietary intake and supplement use – in human health, and use of three specific examples – β-carotene and lung cancer; selenium, cancer, and diabetes; and genistein and breast cancer— to illustrate the ongoing controversy surrounding this important issue. This discussion is timely, given the continuing rise in antioxidant supplement use, the potential hazards associated with consumption of pharmacologic doses of these nutrients, and reports that many consumers are not well-informed about these risks 10.
1.1 β-Carotene and lung cancer
The unexpected adverse effects of β-carotene supplementation on lung cancer risk in heavier smokers and drinkers have been discussed in several excellent reviews 11-13. Nevertheless, the story merits revisiting because it is one of the first and most striking examples of how physiological amounts of a specific antioxidant nutrient obtained through the diet can be beneficial for disease risk, whereas high-dose supplements of that same nutrient can actually cause harm in certain subgroups of people.
1.1.1 The rise of β-carotene as a chemopreventive agent
Carotenoids are a family of yellow, orange, and red compounds that are synthesized by all photosynthetic organisms. In plants and certain forms of bacteria, carotenoids function as accessory pigments in photosynthesis and also inactivate damaging-free radicals that are generated during this process. Humans ingest only a small fraction of the more than 600 carotenoids identified thus far in nature; of these, β-carotene is one of the most abundant forms found in our blood and tissues 14. β-Carotene exhibits a wide range of potentially anticarcinogenic functions: it is a powerful quencher of singlet oxygen – a highly reactive species that can cause significant damage to cellular macromolecules, including DNA and lipids; it is a precursor of retinoic acid, which is a critical regulator of epithelial cell differentiation; and it has been shown to facilitate intercellular communication and enhance immune function 14.
In 1981, Sir Richard Peto and colleagues published a landmark article in which they suggested that β-carotene may reduce the risk of cancer, particularly in the lung 15. This was based on data from more than 20 observational epidemiological studies showing a consistently lower incidence of lung cancer among individuals with higher self-reported fruit and vegetable intake, higher estimated consumption of β-carotene from these foods, and higher blood levels of β-carotene. These associations were noted in men and women and for nonsmokers, former smokers, and current smokers. In addition to provocative findings obtained from epidemiologic studies, results from in vitro and animal experiments also tended to support the chemopreventive potential of β-carotene. For example, many studies conducted prior to the initiation of human trials consistently showed that β-carotene was an effective chemopreventive agent in mouse skin tumor models; later studies also demonstrated efficacy against buccal pouch carcinogenesis in hamsters 16. Notably, however, β-carotene was not effective in studies of respiratory-tract carcinogenesis in hamsters nor against lung carcinogenesis in mice, although these results were not available until well after human trials had already been initiated 16. Based on the overwhelmingly positive body of evidence available at the time, randomized trials of β-carotene supplementation were initiated in the early 1980s.
1.1.2 Unexpected adverse effects of supplemental β-carotene
Three large, primary prevention trials tested whether β-carotene supplementation reduced the incidence of lung cancer, and all three produced results that were strikingly discordant with those observed in previous epidemiologic studies. The α-Tocopherol, β-Carotene Cancer Prevention (ATBC) Study was a randomized, double-blind, placebo-controlled trial that tested whether daily supplementation with β-carotene (20 mg) and/or α-tocopherol (50 mg) for 5–8 years reduced the risk of lung and other cancers in 29 133 male cigarette smokers in Finland. Unexpectedly, the trial demonstrated statistically significant increases in lung cancer incidence (16%) and overall mortality (8%) among men randomized to receive β-carotene supplements versus placebo 17, 18. Notably, the observed increase in lung cancer risk was only evident among men who reported smoking 20 or more cigarettes per day (for β-carotene versus no β-carotene, relative risk (RR)=1.25, 95% confidence interval (CI): 1.07–1.46) or in men consuming at least 11 g of ethanol (equivalent to one standard drink) daily (RR=1.35, 95% CI: 1.01–1.81), with no adverse supplementation effects noted in lighter smokers or lighter drinkers 18. Similar findings were obtained in the β-Carotene and Retinol Efficacy Trial (CARET), which randomized 18 314 men and women at high risk of lung cancer due to cigarette smoking and asbestos exposure to daily supplementation with a combination of β-carotene (30 mg) and retinyl palmitate (25 000 IU) or placebo. After an average of 4 years of intervention, there was a 28% increase in lung cancer risk and a 17% increase in overall mortality among participants who received the active intervention compared with the placebo 19. As in the ATBC study, these adverse effects were apparent only among participants who were current smokers (for active intervention versus placebo, RR=1.42, 95% CI: 1.07–1.87) or in the highest category of alcohol consumption (RR=1.99, 95% CI: 1.28–3.09) at randomization 20. In contrast to ATBC and CARET, the Physicians Health Study (PHS) of 22 071 male, predominantly nonsmoking physicians in the United States showed no beneficial or adverse effects of 12 years of supplementation with 50 mg β-carotene on alternate days on lung cancer incidence 21. There were also no differences in risk associated with supplementation in any specific subgroup defined by smoking status or alcohol consumption 22. Several other randomized trials that tested β-carotene, either alone or in combination with other micronutrients, have also demonstrated no effect on lung cancer incidence or mortality 23-26. However, lung cancer was not the primary endpoint in these latter studies, with small numbers of cases accruing during the intervention period; in addition, low numbers of smokers were enrolled in most of these trials.
1.1.3 Explanations for adverse effects and discrepancies with observational studies
Experimental studies were initiated after the completion of the aforementioned trials in order to better understand the adverse interaction between cigarette smoking and β-carotene supplementation on lung tumorigenesis. A series of studies conducted in ferrets – an animal that absorbs, accumulates, and metabolizes β-carotene similarly to humans – provided strong mechanistic leads. In these studies, animals simultaneously fed pharmacologic doses of β-carotene (equivalent to 30 mg/day in humans) and exposed to cigarette smoke for 6 months had increased expression of several phase I carcinogen metabolizing enzymes, reduced retinoid signaling due to altered retinoic acid concentrations and retinoic acid receptor-β expression, and increased indicators of cellular proliferation in comparison to control animals that were not exposed to β-carotene or cigarette smoke 27-29. Unfortunately, these results have not been corroborated by recent investigations of supplementation effects on similar molecular markers in lung tissues procured from ATBC study 30 or PHS 31 participants.
The sharp contrast in findings obtained from β-carotene supplementation trials and previous observational epidemiological studies of dietary intake and circulating levels of β-carotene led to a reconsideration of the utility and safety of this nutrient as a chemopreventive agent. It was noted that the ATBC study and CARET utilized pharmacological doses of β-carotene (20–30 mg/day), resulting in serum concentrations that were far higher than those achieved through dietary intake alone (300, 210, and 5–50 μg/dL in the ATBC study, CARET, and normal US population, respectively); in contrast, the PHS achieved lower serum β-carotene levels during supplementation (120 μg/dL) than either ATBC or CARET 32. Trials were also conducted over a relatively short period of time, whereas observational studies evaluated habitual intake that is assumed to be reflective of lifelong dietary habits. Also of note is that the trials examined a single nutrient, whereas epidemiologic studies estimated intake of β-carotene from foods that are rich in many other potentially anticarcinogenic substances. Finally, it is well known that dietary habits correlate with other lifestyle factors, most notably smoking, that influence cancer risk 33; randomized trials are essentially immune to such confounding effects, whereas observational studies are much more susceptible to them.
1.1.4 Lessons learned from β-carotene
It is important to recognize that epidemiologic studies carried out in the 1990s and beyond have continued to support protective associations between carotenoid-rich food consumption, estimated β-carotene intake from these foods, circulating concentrations of β-carotene, and lung cancer risk 34. Although the magnitude of these associations is modest and point estimates are sometimes nonsignificant, the consistency of findings across studies continues to be impressive. Hence, what can we conclude about the risks and benefits of β-carotene with respect to lung cancer? A particularly informative study carried out in 59 910 French women may have provided an answer. This study prospectively evaluated the relationship of β-carotene intake from diet and supplements with the incidence of tobacco-related cancers (a combined endpoint that included colorectal, thyroid, ovarian, cervical, and lung cancers, in addition to rarer tumors) in ever smokers and nonsmokers 35. Among nonsmokers, increasing β-carotene intake from diet and supplements was associated with a decreased risk of tobacco-related cancers (for β-carotene supplement users versus women in the lowest tertile of dietary β-carotene intake, RR=0.44, 95% CI: 0.18–1.07), whereas high β-carotene intake (from supplements) increased cancer risk among ever smokers (RR=2.14, 95% CI: 1.16–3.97). For the first time, results from an observational epidemiologic study have corroborated findings obtained from the previous supplementation trials. It therefore seems advisable that persons at high risk for lung cancer by virtue of cigarette smoking or other high-risk exposures should avoid β-carotene supplements, based on the observed interactions in randomized trials and the aforementioned epidemiologic study. The evidence for a beneficial dietary and biochemical association for β-carotene, however, remains convincing; because there is currently no Dietary Reference Intake for β-carotene, all individuals should aim to consume the recommended daily amounts of fruits and vegetables – the primary sources of this micronutrient in our diets.
1.2 Selenium
1.2.1 Interest in selenium as a dietary supplement
Selenium is an essential trace element that is commonly taken as a dietary supplement, both alone and as part of a multivitamin complex. The potential benefits of selenium stem from the pioneering work of Klaus Schwarz who, in 1957, reported that an unknown factor, referred to as “Factor 3” and later discovered to be selenium, protected rats from liver necrosis resulting from being fed a purified casein diet 36. In subsequent years, selenium deficiency in livestock was associated with a range of disease in several organ systems, including muscle and heart, and also contributed to infertility. In humans, selenium deficiency has been associated with several diseases including a pediatric cardiomyopathy endemic to China called Keshan's disease, a disorder of skeletal growth associated with abnormal neurological development and hypothyroidism referred to as Myxedematous cretinism that occurs most often in Central Africa, and an osteoarthropathy endemic in low selenium and iodine areas of China 37, 38. Etiological factors in addition to selenium deficiency are likely to contribute to disease development in each of these cases 39, 40.
Although the examples presented above, both in animals and people, represent circumstances of extreme selenium deficiency, human epidemiology has indicated that selenium intake even at the lower end of the “normal” nutritional range of most countries might be associated with increased risk of disease 41, 42. Although this has been shown for heart disease, perhaps the most dramatic evidence for a role for selenium levels in affecting human health is in the prevention of cancer 43. An impressive history of animal studies has established that nontoxic, low-level supplementation of the diets of experimental animals with selenium is effective in reducing cancer incidence, does so in most tissues types, and that selenium is protective against a wide variety of carcinogens in these models 44. The number of animal studies that support the chemopreventive action of selenium has now been published in more than 200 independent manuscripts. Consistent with these animal experiments, an inverse association between selenium levels and the risk of several cancers has been reported in humans 45. Among the cancer types investigated, the data are most consistent for both colon and prostate cancer 46. Based on the collective data, human selenium supplementation trials have been initiated and the results of some of these will be discussed below.
1.2.2 Historical concerns over selenium-associated toxicity
The interest in the possible benefits of selenium supplementation follows a long history in which selenium was more known for its associated toxicity. Thousands of animals were killed by selenium over-dose and experienced adverse events associated with high-level selenium intake, referred to as selenosis. This has also been reported in humans, most often in regions of the world where there is very high levels of selenium in the soil and consequently, in the plants grown in that soil 47. Symptoms of selenosis include hair loss, brittle and discolored nails, dermatitis if exposure was topical, peripheral neuropathy, nausea, diarrhea, fatigue, and irritability 48. Selenosis can occur due to exposure to selenium from industrial sources, and several cases of individuals over-dosing on selenium appear in the literature. Perhaps the most striking example of selenium toxicity in recent years occurred in 2008 when an outbreak of selenosis affecting 201 individuals in nine different states in the United States was reported 49. Each was taking a multivitamin supplement that was labeled to contain 200 μg of selenium, approximately four times higher than the Dietary Reference Intake of 55 μg/day 50, per ounce of supplement, the recommended dose. Subsequent analysis of the product indicated that it in fact contained 40 800 μg/ounce, approximately 200 times which was indicated on the label.
The exact causes for the symptoms associated with selenium toxicity are likely to be multifactorial. There exists a considerable literature indicating both cytotoxic and genotoxic effects of selenium when cells in culture or animals are provided high doses, and consequences include enhanced mutagenesis, induction of chromosomal abnormalities such as micronuclei formation, as well as induction of cell-cycle arrest and apoptosis 51. The reasons for these effects are likely to include the erroneous substitution of selenium for sulfur in the sulfur-containing amino acids cysteine and methionine, as well as induction of selenium-associated oxidative stress. Although examples of selenosis continue to be reported, they are rare and perhaps of greater concern are adverse consequences of selenium supplementation resulting from taking doses only a few fold higher than the recommended doses, concerns that emerged following selenium supplementation clinical trials.
1.2.3 Risks and benefits of selenium supplementation: evidence from clinical studies
Encouraged by the preclinical animal data indicating a chemopreventive role for selenium and human epidemiology indicating an inverse association for selenium intake and risk of certain cancer types, the Nutritional Prevention of Cancer (NPC) Trial was initiated in 1993, designed to investigate the efficacy of 200 μg/day selenium in the form of selenized yeast, for the prevention of secondary nonmelanoma skin cancer 52. With 1312 participants in the study and 10 years of followup, the results failed to indicate any benefit for the selenium arm in the prevention of the primary endpoint. However, examination of secondary endpoints indicated that selenium was protective against total cancer and mortality, and in particular, prostate cancer in men with the lowest baseline levels of selenium in their serum 53. The positive results were met with enthusiasm for the wider use of selenium in cancer prevention. Although the limitations of the study have been discussed in detail, such as small sample size and prostate cancer not being the primary endpoint, these results stimulated the development of larger selenium supplementation trials.
Although the results of the NPC trial were encouraging for the development of selenium's use in prostate cancer prevention, data from the beginning of the study through 1996 indicated a statistically significant increase of basal cell (RR=1.17, CI: 1.02–1.35), squamous cell (RR=1.32, CI: 1.09–1.60), and total nonmelanoma skin cancer (RR=1.27, CI: 1.11–1.45) in the supplementation arm 54. The increased risk of a new skin cancer was most pronounced among participants with the highest baseline concentrations of plasma selenium, with those in the highest tertile experiencing a 60% increase in risk associated with selenium supplementation. In addition to the potential increased risk of nonmelanoma skin cancer, a secondary analysis from NPC was conducted to assess whether selenium supplementation would reduce the incidence of type 2 diabetes 55. Surprisingly, the results of this analysis indicated a higher incidence of type 2 diabetes in the selenium-supplemented group (RR=1.55, 95% CI: 1.03–2.33). Additional examination of the data indicated a statistically significant dose–response gradient with the greatest risk for type 2 diabetes occurring in the supplemented group within the highest tertile of baseline selenium levels (RR=2.7, CI: 1.30–5.61). As indicated by the authors of the study, the association between selenium supplementation and diabetes had significant limitations, including the use of self-reported data, diabetes not being the primary endpoint, the absence of significant data on diabetes-associated risk factors, and sampling from a subset population that were relatively older and from a low selenium region.
Encouraged by the collective data indicating a possible protective effect of selenium on prostate cancer incidence, the Selenium and Vitamin E Cancer Prevention Trial, a double blind placebo-controlled supplementation study involving over 35 000 men from the United States, Canada, and Puerto Rico randomized to selenium, vitamin E, both or placebo, began in 2001 56. Unlike the NPC trial, men were provided selenium in the form of selenomethionine. The Selenium and Vitamin E Cancer Prevention Trial was terminated ahead of schedule in 2008 for an apparent lack of efficacy of the supplements. It is worth noting that an analysis of the collected data indicated a nonsignificant increase in the risk of diabetes in the group receiving selenium (RR=1.07, 99% CI: 0.94–1.22; p=0.16) 57. Thus, the possibility that selenium might increase the risk of diabetes emerged as a result of two independent clinical supplementation trials, conducted in two different populations and with different forms of selenium, and has raised concerns over the possible risks of selenium supplementation.
The data obtained from the two supplementation trials discussed above indicated a potential benefit (or at least no harm) to increasing selenium levels; however, data from both studies also indicated that there may be a threshold above which higher selenium levels are deleterious. With regard to the selenium-associated risk of type 2 diabetes, two studies of National Health and Nutrition Examination Survey (NHANES) data on US populations have demonstrated an increased prevalence of type 2 diabetes among those with the highest (≥147 μg/L in NHANES 2003–2004 and ≥137.66 ng/mL in NHANES III) versus lowest (<124 μg/L and <111.62 ng/mL, respectively) levels of serum selenium 58, 59, as well as positive associations to adverse serum lipid profiles and arterial disease 60, the latter being consistent with the report of a similar analysis of British citizens 61. Reconciliation among conflicting reports about the risks and benefits of selenium supplementation can be found in the report of Bleys et al. 62 who investigated the relationship between serum selenium levels and causes of death, also using NHANES data. They reported a nonlinear association between serum selenium levels and deaths by all causes, and specifically those due to cancer. Below a baseline serum concentration of 130 ng/mL, increased selenium levels were associated with a reduced risk of all-cause mortality and cancer-associated deaths. On the contrary, selenium had no effect between 130 and 150 ng/mL, and above this level, there was evidence for increased risk of death from all-causes, including cancer.
1.2.4 To supplement or not to supplement?
The accumulating data indicate that supplementation of the diet with selenium should be considered carefully with regard to potential risks and benefits. Relatively low levels of selenium are associated with increased risk of cardiovascular disease and certain cancers; furthermore, results from both epidemiology and supplementation trials raise caution that some could increase their risk of diabetes and cancer if they were to supplement their diets with selenium. How selenium could impact human health remains unclear; however, many of the biological consequences of selenium intake appear to revolve around its role as a constituent of selenium-containing proteins, or selenoproteins. In humans, 25 selenoproteins have been identified 63 and while their functions remain under investigation, many are involved in redox reactions and serve roles in maintaining cellular homeostasis 64. In this regard, it is interesting that the first and best-studied selenoprotein, the cytoplasmic glutathione peroxidase (GPx-1), is an anti-oxidant enzyme 65 shown to protect cells from genotoxic damage 66, 67 and is regulated by selenium availability at multiple levels 68. Although the benefits of GPx-1 expression can be inferred from its biochemistry, it is also plausible that its enhanced expression could have less desirable consequences. Although reduction in oxidative damage could minimize the levels of potentially carcinogenic mutations and other forms of DNA damage, overexpression of GPx-1 can also inhibit apoptosis 69 and therefore potentially attenuate the clearance of premalignant lesions. It is also intriguing that overexpression of GPx-1 in transgenic mice results in glucose intolerance 70, raising the possibility that GPx-1 can contribute to the risk of diabetes that might be associated with elevated selenium status.
The picture that is emerging is that baseline selenium status might be a significant determinant in deciding who might benefit from supplementation. This is supported by the data obtained from the discussed supplementation trials, indicating that the risk of developing nonmelanoma skin cancer and diabetes was most apparent among study participants with the highest baseline selenium levels. This could have regional impact in making recommendations for taking selenium supplements as certain European regions and other parts of the world have populations that would be considered deficient compared with those levels seen in the United States. Furthermore, selenium adequacy might be a more complicated issue that combines intake with genetic identity. For example, polymorphisms in the human GPx-1 gene are associated with increased risk of cancer 71 and may impose a requirement of higher selenium concentrations being needed to obtain maximal activity as compared with individuals that express the alternative allele 72. Understanding the basic biology of selenium and selenoprotein function is likely to help clarify these issues.
1.3 Genistein: a bioactive isoflavone component of soy
1.3.1 Benefits of a diet rich in soy
Soybean – an excellent source of protein and oil – is a legume whose popular use originated in East Asia. Interest in soy as a possible chemopreventive agent stemmed from ecological studies showing that countries with high soy consumption (Japan) had relatively low rates of breast and prostate cancers, whereas countries with low soy consumption (United States) had higher rates of these malignancies 73-75. Notably, Asians who migrate to Western countries have prostate and breast cancer incidence rates that are similar to those of the host country, indicating that environmental factors such as lifestyle and diet are likely to influence the risk of developing these diseases 76.
Results obtained from observational epidemiological studies also provide support for a chemopreventive role for soy. One of the first such studies was published by Lee et al. in 1991, and showed that premenopausal Singapore Chinese women in the highest tertile of soy product intake had a significant 66% reduction in the risk of breast cancer compared with participants in the lowest intake category 77. Numerous investigations of this association followed, and a recent meta-analysis of 18 studies including over 9000 breast cancer cases concluded that there is a modest yet significant inverse association between soy intake and breast cancer risk (for highest versus lowest intake category, odds ratio=0.86; 95% CI: 0.75–0.99) 78. Consistent with the earlier findings from Lee et al., these associations were most evident in premenopausal women. Strikingly, the magnitude of the protective association was similar in both Asian and Western populations, despite their markedly different levels of intake. Aside from its chemopreventive potential, higher soy consumption has also been associated with reductions in cholesterol levels and menopausal symptoms, as well as improvements in bone mineral density 79-84.
1.3.2 Genistein – a soy isoflavone
Individual constituents of soy have been examined in order to determine which ones are responsible for the aforementioned chemopreventive potential. Soybeans are a rich source of isoflavones – polyphenolic compounds found primarily in legumes. Like their major food sources, isoflavone intake varies widely among populations (25–50 mg of isoflavones per day in Japanese and Chinese adults versus ∼3 mg/day in Europeans and Americans) and appears to inversely coincide with rates of breast and prostate cancer 73, 85. There has been considerable interest in one of the most prominent isoflavones contained in soy – genistein. Genistein exhibits several potentially anticarcinogenic functions: it has the highest antioxidant activity of any soy isoflavone 86, 87, it can inhibit protein tyrosine kinases that are often overexpressed in cancers 88, and it is a phytoestrogen, as indicated by its ability to bind and compete for estrogen receptors (ER). ER-α and ER-β are nuclear hormone receptors that undergo a conformational change on hormone binding, triggering a translocation of the receptor from the cytoplasm to the nucleus. The dimerized receptor binds to hormone-response elements and activates or represses hormone-responsive genes, depending on whether the DNA-receptor complex recruits coactivator or corepressor proteins 89-91. Although 17β-estradiol – produced by the ovary and the major estrogen in humans – binds ER-α and -β with equal affinity, genistein preferentially binds ER-β, albeit less strongly than estrogen 92. The relative estrogenic potency of genistein at ER-β is ∼30-fold higher compared with that at ER-α 92. Although genistein appears to exert anti-estrogenic effects at ER-β, high concentrations of genistein can actually exert weak estrogenic effects at ER-α, at least in vitro 93-95. Notably, a recent study demonstrated that genistein exposure induced different gene and protein expression patterns in the T47D human breast cancer cell line, depending on the ER phenotype and the ratio between the ER subtypes in the cells 96.
Plasma genistein levels have been evaluated in relation to breast cancer risk in a small number of epidemiological studies. Provocative findings were obtained from a nested case–control study of Japanese women in which higher concentrations of plasma genistein (>354 ng/mL), but not daidzein (the other major soy isoflavone), were linked with significant 66% reductions in breast cancer risk 97. This protective effect was also observed among women in Western countries, where plasma genistein concentrations are typically much lower 98. Results obtained from a randomized, double-blinded, placebo-controlled study of 389 osteopenic, postmenopausal women supplemented with 54 mg/day of genistein for 2–3 years also support the chemopreventive potential of genistein. In this trial, women receiving the genistein supplement had stable levels of tumor suppressor proteins BRCA1 and BRCA2, as well as fewer chromosomal breaks and recombination events (assessed using sister chromatid exchanges), than the placebo group after 3 years of active intervention 99. Nevertheless, several studies have found no association between blood or urine genistein concentrations and breast cancer risk 100-103.
In addition to the human studies presented above, genistein also has anti-tumorigenic efficacy as determined using animal models of cancer 104-109, can be selectively cytotoxic to neoplastic and preneoplastic cells grown in culture 110-112, and may inhibit metastasis as evidenced by its ability to attenuate the adhesion and migration of prostate, breast, lung, and pancreatic cancer cell lines in vitro 113-118. In addition to functioning as an anti-oxidant and exhibiting anti-estrogenic and anti-tyrosine kinase properties as described above, genistein may be protective by inducing the cyclin-dependent kinase inhibitor p21, resulting in cell-cycle arrest, inhibiting DNA topoisomerase II affecting cell-cycle progression, and by inducing expression of tumor suppressor proteins 88, 119-122. Although considered for its possible utility in reducing cancer risk, genistein supplementation may also have additional benefits, including the reduction of cardiovascular disease 123, relieving unwanted symptoms associated with menopause 124, and improving the health of those suffering from central nervous system disorders 125.
1.3.3 Possible risks associated with isoflavones and genistein
Although the benefits of increased isoflavone and genistein intake have received considerable attention, there are also data indicating the need for caution before supplementation is generally recommended. One of the concerns revolves around genotoxicity associated with higher levels of genistein exposure that are typically not achievable through dietary intake, with most of these data obtained from in vitro experiments using cells in culture. For example, genistein exposure of L5178Y mouse lymphoma cells at concentrations less than 100 nM induced micronuclei formation and mutagenesis at the thymidine kinase locus 126 and chronic exposure (3 months) of human MCF-10A cells induced genomic instability 127. Others have reported that genistein at higher concentrations could also result in DNA damage detectable by either the comet assay or the chromosomal aberrations in both cells in culture 128, 129 and in human sperm and lymphocytes obtained from donors 130, 131. These genotoxic effects are likely to be due, at least in part, to the known role of genistein in inhibiting topoisomerase II 132.
The weak estrogenic properties of genistein (due to its weak affinity for ER-α (see above)) have also stimulated interest in its possible harmful effects on reproductive health. A wide range of reproductive biological and behavioral defects were observed in male rats fed a genistein supplemented diet 133 and recent data have demonstrated a direct effect of genistein on Leydig cells 134, 135. Providing physiologically relevant doses of genistein (i.e. those achievable through dietary intake) neonatally resulted in a wide range of defects in the developing female reproductive system and this has been extensively reviewed by Jefferson et al. 136. Additional concerns about possible teratogenic effects of genistein have been raised in the studies using zebrafish 137, 138.
There is a particular concern as to whether genistein supplementation may have an adverse effect on cancer survival. An extensive and comprehensive review of the preclinical and clinical literature on both the benefits and the possible risks of genistein has been published 92. Although several reports have indicated that genistein could stimulate the growth of the ER+ human breast cancer cell line MCF-7, it was shown early on that genistein concentrations as low as 10–100 nM can be growth stimulatory with higher concentrations of 20 μM being inhibitory, presumably due to toxicity 139. Consistent with this observation, genistein supplementation enhanced mammary gland growth and tumor development of estrogen-dependent MCF7 cells in athymic mice in a dose-dependent manner 140 and when ovariectomized mice were treated with the chemical carcinogen 1-methyl-1-nitrosourea to induce mammary tumorigenesis 141.
Supplementation studies conducted in humans have raised the possibility that soy may promote breast cancer development. For example, in a trial that randomized women with benign or malignant breast disease to soy supplementation (60 g of soy containing 45 mg of isoflavones) or their normal diet daily for 2 wk, women receiving the soy supplements had increased serum genistein levels in comparison to women on a standard diet, and their histologically normal breast tissue exhibited enhanced breast epithelial cell proliferation and significantly increased progesterone receptor levels 142. Some caution is warranted, however, as this study was conducted in a small number of women and the intervention effects may be attributable to multiple components in soy. Overall, the data suggest that short-term exposure to soy caused a weak estrogenic effect. An additional concern about the potential harm that might be done by genistein stems from data that it can stimulate proliferation of tamoxifen-sensitive cells 143-145 and attenuate the anti-tumor activities of both tamoxifen 146 and the aromatase inhibitor letrozole 143 in mouse models of breast cancer.
Despite apparent beneficial effects of genistein on bone health, menopausal symptoms, and some cancer endpoints, there remains some concern regarding its possible genotoxic, teratogenic, and proestrogenic activity at high concentrations, and therefore its long-term safety as a dietary supplement. Genistein may have very different effects on healthy breast tissue as compared with breast cancers, and this may be further complicated by the ER status of the tumor. There is also a possibility of an interaction between genistein and common approaches to manage breast cancer (tamoxifen and letrozole) that reduce the efficacy of these drugs and this needs to be examined further. In addition, it is important to consider attainable physiological doses of genistein in humans when assessing its safety. As pointed out by Klein and King 147, serum levels of genistein from soy consumption or supplementation are typically below 5 μM, with many of the reports of growth stimulation of cells in culture involving concentrations ranging from 0.01 to 10 μM and the growth inhibitory and genotoxic effects occur at higher concentrations. Baseline levels of genistein and genetic differences that contribute to inter-individual variation in response to genistein exposure may ultimately influence the consequences of higher genistein intake.
2 Concluding remarks
Antioxidant supplement use is widespread in the US general population. Most people consume these supplements in order to attain optimum health and prevent chronic disease, but do so with little consideration of their potential benefits and risks. Based on the available evidence, including the examples discussed above, antioxidant nutrient supplementation is unlikely to yield any benefits for chronic disease prevention in healthy, well-nourished populations, except possibly in subgroups of individuals that harbor specific genetic variants (e.g. GPx polymorphisms). Of concern is that antioxidant supplements can cause significant harm, particularly in individuals at higher risk of disease due to lifestyle behaviors, genetic makeup, and/or pre-existing (and possibly undiagnosed) conditions. Adverse supplementation effects are not limited to these groups, however. For example, apparently healthy French women randomized to receive a multivitamin containing β-carotene, selenium, vitamins E and C, and zinc at nutritional doses for an average of 7.5 years had a significant elevation in melanoma skin cancer risk compared with the placebo group 148. In addition, an observational epidemiological study of 300 000 American Association of Retired Persons members showed that excessive use of multivitamins (more than seven times per week) was associated with increased risks of advanced and fatal prostate cancer, particularly when coupled with use of individual β-carotene, selenium, or zinc supplements 149. Adverse effects have also been observed for other chronic diseases; for example, high-dose vitamin E supplementation was found to slightly increase the risk of all-cause mortality in a meta-analysis of 19 randomized controlled trials 150, and was also linked to a higher risk of heart failure in individuals with existing disease 151. In addition, high-dose vitamin C and E supplements not only failed to decrease pre-eclampsia in at-risk pregnant women, but resulted in increased numbers of adverse events such as low birth weight and hypertension 152.
It is important to recognize that populations with low dietary intakes of antioxidant nutrients may benefit from multiple vitamin/mineral supplements at nutritional doses. This was observed in the General Population Trial conducted in Linxian, China – a region with the highest rate of esophageal cancer in the world. Adult residents of Linxian – most of whom exhibited multiple borderline nutritional deficiencies – were randomized to one of the four vitamin combinations at nutritional doses for over 5 years. At the end of the trial, the group that received the β-carotene-vitamin E-selenium combination experienced significant reductions in gastric cancer incidence and mortality, as well as overall cancer mortality 153. In the French Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) trial described above, men who received the multivitamin intervention had significantly lower total cancer incidence and overall mortality compared with the placebo arm 154. These benefits were not observed in female trial participants, and this discrepancy has been ascribed in part to their higher baseline micronutrient status.
It is well established that antioxidant intake through foods, particularly fruits and vegetables, is beneficial for overall health, and maintenance of this type of diet throughout life appears to offer the most protection against the development of chronic disease. If individuals are contemplating (or are already using) antioxidant supplements, it is imperative that they discuss the potential benefits and risks of such a regimen with their physician, and that physicians carefully consider their patient's medical history, lifestyle habits, and possibly genetic makeup before delivering a recommendation. Caution over antioxidant supplement use should also extend to cancer patients and survivors, over 65% of whom report taking some kind of vitamin or mineral supplement, often without consulting their physician 155. This may be a serious cause for concern, given that antioxidant supplements may interfere with radiotherapy and chemotherapy 156 or promote the growth of residual disease in these patients.
Acknowledgements
The authors have declared no conflict of interest.




