Assessing exposure to phthalates – The human biomonitoring approach
Abstract
Some phthalates are developmental and reproductive toxicants in animals. Exposure to phthalates is considered to be potentially harmful to human health as well. Based on a comprehensive literature research, we present an overview of the sources of human phthalate exposure and results of exposure assessments with special focus on human biomonitoring data. Among the general population, there is widespread exposure to a number of phthalates. Foodstuff is the major source of phthalate exposure, particularly for the long-chain phthalates such as di(2-ethylhexyl) phthalate. For short-chain phthalates such as di-n-butyl-phthalate, additional pathways are of relevance. In general, children are exposed to higher phthalate doses than adults. Especially, high exposures can occur through some medications or medical devices. By comparing exposure data with existing limit values, one can also assess the risks associated with exposure to phthalates. Within the general population, some individuals exceed tolerable daily intake values for one or more phthalates. In high exposure groups, (intensive medical care, medications) tolerable daily intake transgressions can be substantial. Recent findings from animal studies suggest that a cumulative risk assessment for phthalates is warranted, and a cumulative exposure assessment to phthalates via human biomonitoring is a major step into this direction.
1 Introduction
Phthalates (dialkyl or alkyl aryl esters of o-phthalic acid) have been used in a large variety of industrial and consumer applications for more than 50 years. They are the most commonly used plasticizers worldwide, primarily in soft polyvinyl chloride (PVC), where they can account for up to 40% within the final product. The annual production volume of phthalates in Western Europe alone is currently around one million tons 1-6.
Depending on the alcohol that makes up the alkyl chain (from methanol up to tridecanol, either straight chain or branched), phthalates have a wide range of different properties for diverse applications. The long-chain phthalates di(2-ethylhexyl) phthalate (DEHP), di-iso-nonyl phthalate (DiNP), di-iso-decyl phthalate (DiDP) and di(2-propylheptyl) phthalate (DPHP) are primarily used in PVC polymer and plastisol applications. They can be found in building and construction materials, cables and wires, floorings, clothing, furnishings, car interiors and car underbody coatings, toys and also food contact materials. DEHP, which had been the most common phthalate for many years, has been substituted by DiNP and DiDP/DPHP for the most part. Today, DiNP and DiDP – both are complex mixtures of variously branched alkyl chain isomers – account for about 60% of the total plasticizer market in Europe 1. Short-chain phthalates, such as dimethyl phthalate (DMP), diethyl phthalate (DEP), butyl benzyl phthalate (BBzP), di-n-butyl phthalate (DnBP) and di-iso-butyl phthalate (DiBP), are often used also in non-PVC applications such as personal-care products, paints, adhesives or enteric-coated tablets 1-15.
Phthalates are constantly released into the environment by direct release, migration, evaporation, leaching and abrasion of and from the products they are used in. As a result, the general population is widely and continuously exposed to phthalates 16-19. This has raised scientific and public concern about possible detrimental health effects 2-14. Some phthalates, such as DnBP 20, 21, DiBP 22-24, DEHP 25-28, BBzP 29, 30 and DiNP 27, 31, are developmental and reproductive toxicants. They modulate the endogenous production of (foetal) testicular testosterone and influence insulin-like factor 3 and follicle-stimulating hormone production 32. Critical effects are related to functional and structural impairment of male reproduction and development 33-35 and manifest in malformations of the epididymis and the external genitalia (hypospadias), undescended testicles (cryptorchidism), impaired spermatogenesis and a general reduction of male fertility 20. Phthalates also cause signs of feminization (retention of nipples/areolae in male rodents) and a reduced anogenital distance as a first indication of general demasculinization 21, 30. This group of symptoms in animals is called the “phthalate syndrome” 36, 37.
Exposure to phthalates is suspected to be at least partially responsible for increasingly common developmental disorders in humans like an increasing prevalence of cryptochordism, hypospadias and testicular cancer as well as an impairment of sperm quality. This so-called human testicular dysgenesis syndrome in many ways resembles the phthalate syndrome in rats 38-44. However, data on phthalate exposure related to human health effects are still limited and sometimes contradictory. Some recent epidemiologic studies suggest that internal exposure to a number of phthalates at environmental levels may be associated with alterations in semen parameters 45-47, DNA damage in sperm 48, 49, reduced reproductive hormone levels in adult men 50, decreased anogenital distance in male infants 44, 51, abdominal obesity and insulin resistance 52-54, conduct or attention-deficit hyperactivity disorders 55 or a less male-typical behavior in young boys 56. However, all the above human studies lack an adequate subject number and a robust exposure verification in the relevant window of susceptibility to count as an unequivocal proof of these effects in humans to be related to phthalates. Current discussions on phthalates also focus on the cumulative toxicity of the various phthalates among each other and in combination with other endocrine disruptors. Mixtures of phthalates among each other and mixtures of phthalates with other chemicals that alter the androgen signalling pathway (via diverse mechanisms) can disrupt male rat reproductive tract differentiation and induce malformations in a cumulative, dose-additive manner 57-62.
2 Different approaches to assess human exposure to phthalates
Exposure to chemicals like phthalates can occur through a variety of sources, such as foodstuff, water, air, dust and the use of consumer and personal-care products. From all these sources, phthalates end up in the human body via ingestion, inhalation or dermal absorption.
One way to perform exposure assessments relies on measuring chemicals in environmental media, foodstuff and consumer products. Based on these data, together with survey/questionnaire data on personal lifestyle, product use, and food consumption, scenarios representing realistic exposure situations are generated to calculate the range in daily exposure through these pathways. Combining these external exposure estimates with organ- and situation-specific uptake rates, the daily internal exposure in (μg/kg bodyweight (bw)/day) can be calculated. The main aim of these models, however, is to estimate possible contributions of different pathways to the total exposure and not to reliably estimate the overall or average extent of exposure of the general population 63-67. Furthermore, in the case of phthalates, the ubiquitous presence of these chemicals in the environment poses an analytical challenge known as the phthalate blank problem. Phthalates are detected even in the cleanest laboratory chemicals, sampling equipment and analytical apparatus. These circumstances hamper the reliable quantification of phthalates in real-life scenarios. As a result, all ambient monitoring data and all data in general related to measurements of low levels of phthalate diesters have to be interpreted with utmost caution because of possible external contamination 68-70.
Another way to perform an exposure assessment is by human biomonitoring. Human biomonitoring determines internal exposure (i.e. body burden) by measuring the chemicals, their metabolites or specific reaction products in human specimens (e.g. urine or blood). Progress in human biomonitoring has opened up new possibilities in assessing phthalate exposures, because most of the biomarkers used in modern phthalate biomonitoring are specific metabolites generated in the human body (secondary, oxidized metabolites) which are not prone to external phthalate contamination. Furthermore, as biomonitoring represents an integral measure of exposure from multiple sources and routes, biomonitoring data permit a new approach to exposure assessment even when the quantity and quality of external exposures are unknown or ambiguous. Biomonitoring data can also be used to compare exposures of the general population with special subpopulations. This way, although biomonitoring is an integral measure from all sources, special routes or sources of exposure, contributions of exposure routes (e.g. foodstuff) or exposures caused by individual life style can be identified in combination with survey/questionnaire data and/or a selective study design 71-78. In the following sections, we will focus on the recent advances in phthalate exposure assessment by means of human biomonitoring.
3 Sources of phthalate exposure
3.1 Sources of ubiquitous nature
Food is generally regarded as a major source of phthalate exposure in the general population. Contamination of food can occur during processing, handling, transportation, packaging and storage. For instance, relatively high phthalate levels have been found in some fatty food due to direct contact to gaskets of twist-off lids 79. There is considerable variability in the degree of phthalate contamination of foods depending e.g. on packaging and processing practices and the lipid content. Nevertheless, phthalates are found in all kinds of food 80-87. Breast milk is an additional source of phthalates for infants 88-90.
Testing the hypothesis that a major part of phthalate exposure is food-borne, Koch et al. 91 investigated the influence of fasting on the body burden to phthalates. Three volunteers (two males and one female, between 27 and 32 years) drank mineral water only, for 48 h. They collected their consecutive urine samples before, during and after the fasting period. In these samples, the excretion of specific urinary metabolites of DEHP, DiNP, DnBp, DiBP and BBzP was quantified over time (Fig. 1). For DEHP (Fig. 1A) and DiNP (Supporting Information Fig. 1), urinary metabolite levels sharply decreased to very low levels within the first 24 h of fasting. These very low metabolite levels persisted throughout the second day of fasting. Furthermore, elimination characteristics of the DEHP an DiNP metabolites were similar to elimination after a single oral dose in human metabolism studies 92-94. Figure 1B shows the excretion of the short-chain phthalates DnBP, DiBP and BBzP over the period of fasting. In contrast to the long-chain phthalates, foodstuff clearly is not the only source of exposure to these phthalates. Some excretion peaks during the period of fasting e.g. after 36 and 48 h have to be related to other sources than foodstuff. Furthermore, exposure sources to these three phthalates seemed to be the same, since internal exposure to all three metabolites followed the same pattern, sharing all peak exposures.
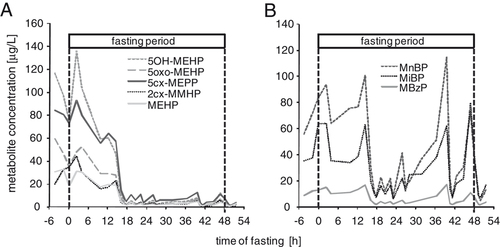
The influence of fasting on the renal excretion of metabolites of the long-chain phthalate DEHP (A) and the short-chain phthalates DnBP, DiBP and BBzP (B) presented as individual metabolite data from one volunteer.
Taking the data for all three volunteers together (Fig. 2 and Supporting Information Fig. 2), one can see that for the long-chain phthalates, median metabolite levels were approximately 7.5 times lower on the second day of fasting than initial median levels (t-test, p<0.01) and the median levels of a local reference population (n=102). This indicates that during the fasting no significant DEHP and DiNP exposure occurred. The authors concluded that for DEHP and DINP food seems to be the dominant exposure pathway for adults. For the short-chain phthalates median internal exposures were lower (approx. factors 2–3) on day 2 of the fasting compared with the prephase and the first 12 h of the fasting, however, with no statistical significance (t-test, p>0.05). Clearly, for DnBP, DiBP and BBzP the effect of fasting on metabolite excretion was less pronounced and other sources next to foodstuff have to be of equal importance.
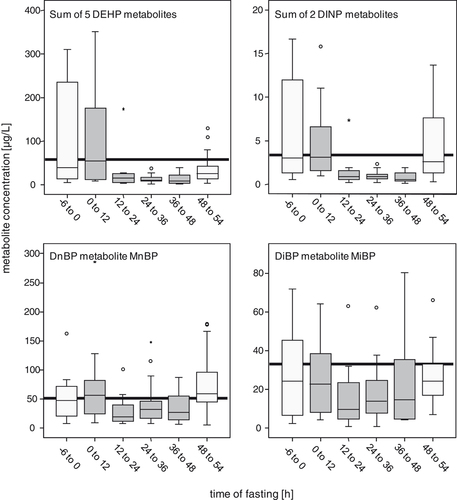
The influence of fasting (48 h) on the internal exposure to phthalates. Combined metabolite excretion data of three volunteers over time increments of 12 h (plus pre and postphase) for each of the phthalates investigated. In total, 75 urine samples, 10 (prephase), 14 (0–12), 12 (12–24), 15 (24–36), 10 (36–48) and 14 (postphase). The boxes depict the inter-quartile range (P25–P75), the horizontal line in the boxes the median (P50). 95P and 5P are depicted by T and ⟂, respectively. The reference median (bold horizontal line) has been derived from 102 volunteers sampled around the same time (64 μg/L for DEHP, 3.4 μg/L for DiNP, 51 μg/L for DnBP and 36 μg/L for DiBP). For DEHP and DiNP, five (MEHP, 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP and 2cx-MMHP), respectively, and two (OH-MiNP, oxo-MiNP) metabolites were summed up for each sample.
Fromme et al. 95 compared phthalate intakes they calculated from urinary metabolite levels in 50 German adults with intakes they calculated from duplicate diet samples analyzed for phthalate content. Duplicate diet and urine samples were collected over seven consecutive days, cumulating to 350 single-diet samples and 249 urine samples. For DEHP, both approaches yielded quite similar intake values, suggesting that food was the dominant source for DEHP exposure. In addition, significant correlations between food and urine data were observed with r-values ranging from 0.69 to 0.80 (p<0.001), depending on the different DEHP metabolites. DiNP was found only in 1% of the diet samples above the LOD (not specified), which did not allow a comparison of diet with body burden data in that study. For DnBP and DiBP, Fromme et al. calculated higher intakes (median increased by factors of 3 and 6, respectively) in the biomonitoring approach compared with the food data, indicating that there are other significant sources of DnBP and DiBP exposure next to food. A weak but significant correlation was found for DiBP contamination in foodstuff and mono-iso-butyl phthalate (MiBP) excretion in urine (r=0.25; p<0.001). They found no correlation for DnBP. BBzP was detected only in 10% of the foodstuff.
Itoh et al. 96, 97 calculated daily phthalate intakes from urinary metabolite concentrations in 36 Japanese volunteers and compared them with estimated daily intakes via air and diet derived from point-of-contact measurements and scenario evaluations in previous exposure assessments in Japan. For DEHP and BBzP, they found similar values for the back-calculated exposure values and the external aggregate exposures estimates. On the other hand, the exposure estimates for DMP, DEP and DnBP via diet and air were lower (half or less) than the exposure estimates based on the urinary metabolite excretion. The authors concluded that dietary intake is responsible for most exposure to DEHP and BBzP in Japan, but suggested further exposure pathways (e.g. personal-care products) for the short-chain phthalates.
Several other studies also identified additional sources of phthalate exposure in the general population to be mainly related to the use of personal-care and cosmetic products, and other consumer products (clothing, vinyl gloves, etc.) 98-104.
Duty et al. 102 investigated the association between the use of personal-care products and exposure to several phthalates (DMP, DEP, DnBP, BBzP and DEHP), indicated by phthalate metabolite levels measured in urine. A frequent use of cologne and aftershave was significantly associated with higher urinary levels of the monoester of DEP. Additionally, a relationship was found between the number of different types of personal-care products used and the urinary DEP metabolite concentrations.
Adibi et al. 105 found statistically significant correlations between personal air concentrations of DEP, DnBP and BBzP and urinary levels of the respective monoester metabolites in 25 pregnant women from New York. No such correlation was found for DEHP. Becker et al. 106 investigated the association between urinary DEHP metabolite levels in 239 German children and DEHP concentrations in house dust samples from their homes. Although the house dust analyses revealed high contamination levels with phthalates, there was no significant association with the body burden determined via monitoring urinary phthalate metabolites. Therefore, the data from Koch et al. 91, Fromme et al. 95 and the other studies strongly indicate that the major source of exposure to long-chain phthalates such as DEHP and DiNP is foodstuff, whereas for the shorter chain phthalates such as DnBP, DiBP and BBzP other sources next to foodstuff are of the same or even higher importance.
In addition, in several scenario-based exposure models food was identified as a major source of human phthalate exposure 80, 81, 86, 87. In infants and toddlers, mouthing of toys and other items made of phthalate plasticized PVC may lead to additional oral intake of some phthalates 2, 6, 9, 11, 107, 108. In a recent study, Wormuth et al. 81 assessed the average contribution of various exposure sources in Europeans. In all age groups, ingestion of food was assessed to be the most dominant pathway for exposure to DEHP (>90% of total DEHP exposure in children, teenagers and adults; 50% in infants and toddlers), DnBP (between 40% in female teens and 90% in male adults) and DiBP (60% in infants and toddlers; >90% in the other age groups). The relative high shares for food in total exposure to the dibutyl phthalates (DnBP and DiBP) are in some contrast to the above biomonitoring studies. For the two other short-chain phthalates, DMP and DEP, the contribution of food to total exposure was assessed to be low in all age groups. For the long-chain phthalates, DiNP and DiDP, Wormuth et al. derived exposure pathways differing from those for DEHP. This is somewhat surprising, because these phthalates are one-to-one substitution products for DEHP. However, Wormuth et al. state that due to the substitution process, it is probable that the exposure patterns of DINP and DEHP will become similar in the near future and food might become a major source of exposure to DINP. Most probably, as biomonitoring data shows, this substitution has already taken place.
3.2 Sources of specific nature
A specific source of high DEP or DnBP exposure is the intake of enteric-coated tablets that can contain several milligrams of these phthalates. After intake of such medications, metabolite levels in urine are several-fold above those of the general population 109-112. In medical patients, high exposure to DEHP is possible through PVC medical devices such as blood bags and tubings 113-115. In infants undergoing intensive care 116-118, exposures of up to several milligrams DEHP/kg/day have been estimated. Weuve et al. 116 found a monotonic association between urinary levels of DEHP metabolites and the intensity of DEHP-containing product use in neonatal intensive-care unit infants. In dialysis patients and blood donors, medical procedures are a source of DEHP exposure 119-121. The measurement of urinary DEHP metabolites in athletes as screening measure for illicit blood doping has recently been suggested by Monfort et al. 122 because elevated urinary DEHP metabolites can indicate to a recent blood transfusion which is difficult to detect otherwise.
3.3 EU legislation
In the EU, the use of phthalates in materials and articles intended to come into contact with food is restricted and standard migration limits apply, e.g. 0.3 mg DBP/kg food simulant or 1.5 mg DEHP/kg food simulant 123. DEHP, DnBP and BBzP are banned from toys and in childcare articles. Di-n-octyl phthalate (DnOP), DiNP and DiDP are approved only for such toys that cannot be placed in the mouth of children 124. All substances classified as toxic to reproduction categories 1 and 2 (which applies to DEHP, DnBP, DiBP and BBzP) are banned in cosmetic products and restricted in preparations such as paints and varnishes for end-consumers 125-128.
4 Exposure assessment by human biomonitoring
In the last years, phthalate exposure assessment has increasingly focused on human biomonitoring. A great advantage of human biomonitoring compared with exposure assessment based on external levels is that the individual and the actual internal exposure can be assessed covering all routes and sources of exposure independent of their relevance and magnitude.
4.1 Human metabolism – biomarkers of exposure
One precondition for valid human biomonitoring is a profound knowledge of the metabolism of the respective substances in order to interpret internal concentrations of these substances (or their metabolites) in terms of doses taken up and time point of exposure(s). Phthalates, once incorporated, are rapidly metabolized by hydrolysis and subsequent oxidation reactions. Phthalate metabolites – not the parent phthalates – are almost completely excreted via urine, partly as glucuronides. The content of phthalate metabolites in human urine represents a measure of the exposure to the respective parent phthalate that occurred within the last 24 h 129.
Human metabolism studies have shown that the simple monoesters are the major urinary metabolites of the short-chain phthalates such as DnBP, DiBP or BBzP. Their urinary excretion represents approx. 70% of the oral dose 130 (Table 1). In the case of the long-chain phthalates such as DEHP, DiNP, DiDP and DPHP, the major share of the simple monoester is further metabolized to produce a number of oxidative metabolites (alcohols, ketones and carboxylic acids). Only between 2 and 7% of the dose is excreted as the simple monoester for these long-chain phthalates. The secondary, oxidized metabolites that are particularly formed by ω-, ω-1- and ß-oxidation (Table 1) are the main metabolites excreted in human urine 92-94, 131-135.
| Phthalate | Metabolite | UEF (%) | Reference | ||
|---|---|---|---|---|---|
| DnBP | MnBP | 69 | Anderson et al. 130 | ||
| BBzP | MBzP | 73 | Anderson et al. 130 | ||
| DEHP | MEHP | 5.9 | Sum: 62.7 | Koch et al. 92, 93 | |
| 5OH-MEHP | 23.3 | ||||
| 5oxo-MEHP | 15.0 | ||||
| 5cx-MEPP | 18.5 | ||||
| 2cx-MMHP | 4.2 | ||||
| DiNP | cx-MiNP | 9.1 | Sum: 39.6 | Koch and Angerer 94 | |
| OH-MiNP | 18.4 | ||||
| oxo-MiNP | 10.0 | ||||
| MiNP | 2.1 | ||||
| DiDP/DPHP | cx-MiDP | n.a. | Sum: 34 | Wittassek and Angerer 135 | |
| OH-MiDP | n.a. | ||||
| oxo-MiDP | n.a. | ||||
In the first biomonitoring approaches, only the simple monoesters of the phthalates were measured in urine samples to assess phthalate exposure in the general population 18. When comparing simple monoester data of different phthalates, one has to be aware that the same urinary monoester levels of a short- and a long-chain phthalate do not mean that exposures to these phthalates have also been the same. Taking DEHP and DnBP as an example, the same urinary levels (in μg/L) of mono(2-ethylhexyl) phthalate (MEHP) and mono-n-butyl phthalate (MnBP) can easily mean that the exposure to the parent long-chain phthalate (DEHP) has been ten times higher than exposure to the parent short-chain phthalate (DnBP). Furthermore, special attention has to be paid to the analyses of the simple monoesters: these metabolites are prone to external contamination, as they can relatively easily be generated out of the omnipresent phthalate diesters before or during the analytical procedure. This is a particular problem in biological matrices containing lipase activity such as breast milk, blood or amniotic fluid 89, 136-139. By contrast, the secondary, oxidized phthalate metabolites are not susceptible to external contamination.
Moreover, these oxidized metabolites possess longer half-lives of elimination than the simple monoesters and therefore are more suitable to capture the average background exposure 93, 94. For all these reasons, in most of the subsequent biomonitoring studies, oxidative phthalate metabolites of DEHP and DiNP have been implemented in the parameter spectrum 16, 19, 106, 140-142. The two oxidative DEHP metabolites mono(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (5oxo-MEHP) are excreted in at least three times higher concentrations than MEHP in the general population, proving these oxidative metabolites as much more sensitive biomarkers of DEHP exposure than MEHP. Basing exposure assessment solely on MEHP might therefore underestimate the real extent of DEHP exposure, which has been illustrated impressively by the data of Kato et al. 142, who analyzed urine samples of 127 US Americans. Looking only at the median exposure for MEHP, which is below the LOD (35%>LOD), might suggest that the study population is negligibly exposed to DEHP, whereas only the secondary metabolites (95% of 5OH-MEHP and 91% of 5oxo-MEHP>LOD) prove the omnipresent internal exposure to DEHP. The most prominent oxidative DEHP metabolite in urine is mono(2-ethyl-5-carboxypentyl) phthalate (5cx-MEPP) and has been first used in biomonitoring studies in 2005 93, 133. 5cx-MEPP can be detected in literally all urine samples from the general population at the highest concentration of all DEHP metabolites and furthermore exhibits the longest half-live of elimination (>15h) 129, 143. Regarding DiNP and DiDP, the secondary, oxidized metabolites are of even greater value as biomarkers of exposure because the simple monoesters make up only 2% or less of the dose excreted in urine. In urine samples of the general population oxidative products of the DiNP and DiDP monoesters can be detected in nearly 100% of the samples proving the widespread exposure to these phthalates as well, whereas the simple monoesters are detectable only at trace levels (with a high chance of external contamination) 129, 131, 144. Interestingly, the concentrations of the secondary metabolites of DEHP and DiNP, respectively, strongly correlate among each other 131, 132, 145, 146. This demonstrates the consistency of oxidative phthalate metabolism among individuals and underlines the diagnostic validity of these biomarkers. Correlations between the simple monoester MEHP and the oxidative DEHP metabolites are weaker. Reasons for this are the different half-lives of elimination of the metabolites but possibly also external contamination of MEHP. Individual variations in (oxidative) metabolism have been discussed as a third possibility for a weaker correlation between MEHP and oxidized metabolites than within the oxidized metabolites 129, 147. However, Lorber et al. 147 have shown in their PBPK-model of DEHP that, due to the elimination kinetics of the different DEHP metabolites, depending on the time point of exposure, and the time point of sample collection (morning or afternoon sample), the ratio between MEHP and the oxidized metabolite 5OH-MEHP can vary between 2.89 and 5.4, whereas the ratio between the oxidized metabolites 5OH-MEHP and 5oxo-MEHP varies within a close margin of 1.50–1.78. Therefore, most of the weaker correlation of MEHP with the oxidized metabolites can be explained by differences in elimination kinetics between these metabolites and not inter-individual differences in phthalate metabolism. For the shorter chain phthalates such as DnBP and especially DiBP, the importance of oxidized metabolites has only recently been pointed out as valuable supplements to the simple monoesters. Two promising metabolites are 3-hydroxy-MnBP (3OH-MnBP) and 2-hydroxy-MiBP (2OH-MiBP) 129, 148, and although these urinary metabolites represent only between 5 and 20% of the dose, they are not prone to contamination and they have considerably longer half-lives of elimination compared with the monoester metabolites MnBP and MiBP.
4.2 Quantifying internal phthalate exposure by urinary metabolite levels
Since the turn of the millennium, there is a sharp increase in studies that investigated the internal phthalate exposure in the general 16-18, 129, 140 and specific populations 45-50, 105, 118, 149-154 by measuring urinary concentrations of phthalate metabolites (Tables 2 and 3). All these studies have revealed the widespread exposure to a number of phthalates. Metabolites of DMP, DEP, DiBP, DnBP, BBzP, DEHP, DiNP and DiDP have been detected in a high percentage of the study populations or could even be found in each urine sample analyzed.
| Reference | Sampling year | n (age) | DEHP | DiNP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5cx-MEPP | 5oH-MEHP | 5oxo-MEHP | 2cx-MMHP | MEHP | cx-MiNP | OH-MiNP | oxo-MiNP | |||
| USA | ||||||||||
| Blount et al. 18 | 1988–1994 | 298 (20–60) | – | – | – | – | 2.7 (21.5) | – | – | – |
| Brock et al. 154 | 2000 | 19 (1–3) | – | – | – | – | 4.6a) | – | – | – |
| Barr et al. 141 | n.s. | 62 (n.s.) | – | 35.9 | 28.3 | – | 4.5 | – | – | – |
| Silva et al. 17 | 1999/2000 | 328 (6–11) | – | – | – | – | 4.9 (34.5) | – | – | – |
| 752 (12–19) | – | – | – | – | 3.7 (22.8) | – | – | – | ||
| 1461 (≥20) | – | – | – | – | 3.0 (22.4) | – | – | – | ||
| 2541 (>6) | – | – | – | – | 3.2 (23.8) | – | – | – | ||
| Kato et al. 142 | 2001 | 127 (n.s.) | – | 17.4 (220) | 15.6 (243) | – | <LOD (20.4) | – | – | – |
| CDC 140 | 2001/2002 | 393 (6–11) | – | 32.9 (210) | 22.6 (142) | – | 4.4 (29.9) | – | – | – |
| 742 (12–19) | – | 25.2 (202) | 18.5 (118) | 4.5 (42.5) | – | – | – | |||
| 1647 (>20) | – | 17.7 (175) | 12.2 (115) | – | 4.1 (39.5) | – | – | – | ||
| 2541 (>6) | – | 20.1 (192) | 14.0 (120) | 4.1 (38.9) | – | – | – | |||
| Marsee et al. 153 | 1999–2002 | 214 pregnant women | – | 10.8 (76.4) | 9.8 (65.0) | – | 4.3 (38.6) | – | – | – |
| Duty et al. 50 | 1999–2003 | 295 men (18–54) | – | – | – | – | 5.0 (131) | – | – | – |
| Duty et al. 102 | 2000–2003 | 406 menb) (20–54) | – | – | – | – | 5.2 (135) | – | – | – |
| Silva et al. 132, 133 | 2003/2004 | 129 adults | 15.6 (159.3) | 15.3 (120.8) | 7.1 (62.4) | 5.9 (20.7) | 3.1 (17.0) | 8.4 (46.2) | 13.2 (43.7) | 1.2 (6.6) |
| Adibi et al. 202 | 2000–2004 | 283 pregnant women | – | 11.2 (99.4) | 9.9 (68.4) | – | 3.5 (40.2) | – | – | – |
| Adibi et al. 203 | 1999–2005 | 246 pregnant women | 37.1 (232.2) | 19.9 (149.6) | 17.5 (107.6) | – | 4.8 (46.8) | – | – | – |
| Germany | ||||||||||
| Wittassek et al. 19c) | 1988/1989 | 120 (21–29) | 34.9 (88.0) | 28.2 (76.6) | 21.3 (58.9) | 11.7 (37.5) | 9.7 (32.2) | – | 1.7 (11.4) | 0.66 (3.5) |
| Becker et al. 106 | 2001/2002 | 254 (3–14) | – | 52.1 (188) | 41.4 (139) | – | 7.2 (29.7) | – | – | – |
| Koch et al. 16 | 2002 | 85 (7–63) | – | 46.8 (224) | 36.5 (156) | – | 10.3 (37.9) | – | – | – |
| Wittassek et al. 19c) | 2001/2003 | 120 (20–29) | 19.5 (68.6) | 14.6 (58.6) | 13.4 (42.3) | 5.8 (25.0) | 5.0 (28.6) | – | 2.2 (13.5) | 1.3 (5.7) |
| Koch et al. 150 | 2003 | 19 (2–6) | – | 49.6 (107) | 33.8 (71.0) | – | 9.0 (29.0) | – | – | – |
| 36 (20–59) | – | 32.1 (64.0) | 19.6 (36.7) | – | 6.6 (14.6) | – | – | – | ||
| Fromme et al. 143 | 2005 | 50 (14–60) | – | 5.7 (11.5)e) | 3.1 (8.1)e) | |||||
| 399 samplesd) | 26.2 (93–6) | 19.2 (81.8) | 14.7 (56.0) | 8.3 (41.3) | 4.9 (21.7) | – | 5.5 (18.7)f) | 3.0 (9.3)f) | ||
| Becker et al. 146 | 2003–2006 | 599 (3–14) | 61.4 (209) | 46.0 (164) | 36.3 (123) | 20.4 (76.7) | 6.7 (25.1) | 12.7 (195) | 11.0 (198) | 5.4 (86.7) |
| Koch and Calafat 129 | 2007 | 45 adults | 13.9 (42.9) | 11.5 (35.0) | 8.2 (21.5) | – | 1.8 (8.5) | 5.3 (15.5) | 4.7 (16.8) | 1.7 (6.7) |
| Israel | ||||||||||
| Berman et al. 204 | 2006 | 19 pregnant women | 26.7 | 21.5 | 17.5 | – | 6.8 | 3.0 | – | – |
| Netherland | ||||||||||
| Ye et al. 205 | 2004–2006 | 99 pregnant women | 18.4 (31.5) | 14.0 (30.0) | 14.5 (27.4) | 6.2 (11.1) | 6.9 (82.8) | – | 2.5 (38.3) | 2.2 (30.0) |
| Japan | ||||||||||
| Itoh et al. 96 | 2004 | 36 (4–70) | – | – | – | – | 5.1 | – | – | – |
| Suzuki et al. 171 | 2005–2006 | 50 pregnant women | – | 10.6 | 11.0 | – | 3.96 | – | – | – |
| Taiwan | ||||||||||
| Huang et al. 206 | 2005–2006 | 76 pregnant women | – | – | – | – | 20.6 (273) | – | – | – |
| Sweden | ||||||||||
| Jönsson et al. 207 | 2000 | 234 men (18–21) | – | – | – | – | <LODg) (54) | – | – | – |
- a n.s.: not specified.
- a) a) Mean value.
- b) b) Partners of subfertile couples.
- c) c) In total, urine samples of 634 students, which were collected in nine years between 1988 and 2003, were analyzed.
- d) d) Urine samples of 8 consecutive days for each person were collected.
- e) e) Value for the 27 women measured. The overall median and 95th percentile are not available for the DINP metabolites. No significant differences were seen regarding gender.
- f) f) Value for the 23 men measured.
- g) g) LOD: 15 μg/L.
| Reference | Sampling year | n (age) | DMP | DEP | DnBP | DiBP | BBzP | DnOP | DiDP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monomethyl phthalate | MEP | MnBP | MiBP | MBzP | MnOP | 3cx-MPPa) | cx-MiDP | OH-MiDP | oxo-MiDP | |||
| USA | ||||||||||||
| Blount et al. 18 | 1988–1994 | 298 (20–60) | – | 305 (3750) | 41.0 (294)b) | – | 21.2 (137) | <LODc) (2.3) | – | – | – | – |
| Brock et al. 154 | 2000 | 19 (1–3) | – | 184.1d) | 22.0d) (203e)) | – | 20.2d) (118e)) | – | – | – | – | – |
| Silva et al. 17 | 1999/2000 | 328 (6–11) | – | 74.7 (756) | 40.0 (163)b) | – | 40.3 (214) | <LODc) (3.2) | – | – | – | – |
| – | 752 (12–19) | – | 193 (3260) | 36.1 (16)b) | 28.3 (125) | <LODc) (2.8) | – | – | – | – | ||
| 1461 (≥20) | – | 180 (3480) | 23.0 (142)b) | – | 13.8 (86.3) | <LODc) (2.9) | – | – | – | – | ||
| 2541 (>6) | 164 (2840) | 26.0 (149)b) | 17.0 (103) | <LODc) (2.9) | – | – | – | – | ||||
| CDC 140 | 2001/2002 | 393 (6–11) | 1.8 (11.6) | 71.9 (808) | 32.4 (157) | 4.4 (23.4) | 37.0 (226) | <LODf) (<LOD) | 6.6 (24.7) | – | – | – |
| 742 (12–19) | 2.1 (12.7) | 184 (2060) | 26.4 (147) | 3.8 (22.2) | 24.7 (169) | <LODf) (<LOD) | 4.0 (13.9) | |||||
| 1647 (>20) | 1.4 (9.1) | 181 (2720) | 19.1 (95.4) | 2.4 (16.3) | 13.8 (99.7) | <LODc) (<LOD) | 2.6 (12.0) | |||||
| 2782 (>6) | 1.5 (9.8) | 169 (2500) | 20.4 (108) | 2.6 (17.9) | 15.7 (122) | <LODc) (<LOD) | 3.0 (14.6) | |||||
| Marsee et al. 153 | 1999–2002 | 214 pregnant women | – | 117 (3199) | 16.2 (64.5) | 2.5 (13.1) | 9.3 (57.8) | – | – | – | – | – |
| Duty et al. 50 | 1999–2003 | 295 men | 4.6 (32.1) | 149 (1953) | 14.3 (75.4) | – | 6.9 (37.1) | – | – | – | – | – |
| (18–54) | ||||||||||||
| Duty et al. 102 | 2000–2003 | 406 meng) | 4.5 (31.3) | 145 (1953) | 14.5 (75.1) | – | 6.8 (41.3) | – | – | – | – | – |
| (20–54) | ||||||||||||
| Silva et al. 131 | 2003/2004 | 129 adults | – | – | – | – | – | – | – | 4.4 (104.4) | 4.9 (70.6) | 1.2 (15.0) |
| Adibi et al. 203 | 1999–2005 | 246 pregnant women | – | 202 (2753) | 35.3 (174.9) | 10.2 (36.1) | 17.2 (146.8) | – | 2.0 (8.0) | – | – | – |
| Germany | ||||||||||||
| Wittassek et al. 19h) | 1988/1989 | 120 (21–29) | – | – | 184 (700) | 25.5 (117) | 8.1(34.4) | – | – | – | – | – |
| Koch et al. 151 | 2001/2002 | 254 (3–14) | – | – | 166 (624) | – | 18.7 (123) | – | – | – | – | – |
| Wittassek et al. 19h) | 2001/2003 | 120 (20–29) | – | – | 57.4 (338) | 31.9 (132) | 5.6 (25.0) | – | – | – | – | – |
| Koch et al. 16 | 2002 | 85 (7–63) | – | 90.2 (560) | 181 (248) | – | 21 (146) | <LOQi) | – | – | – | – |
| Fromme et al. 143 | 2005 | 50 (14–60) | – | – | 49.6 (171.5) | 44.9 (183) | 7.2 (45.6) | – | – | – | – | – |
| 399 samplesj) | ||||||||||||
| Becker et al. 146 | 2003–2006 | 599 (3–14) | – | – | 93.4 (310) | 88.1 (308) | 18.1 (76.2) | – | – | – | – | – |
| Koch and Calafat 129 | 2007 | 45 adults | <LOD (17.2) | 77.5 (396) | 12.6 (43.5) | 13.8 (62.4) | 2.5 (8.4) | <LOQ | 0.7 (3.1) | 0.7 (2.6) | 1.0 (4.0) | 0.2 (1.1) |
| Israel | ||||||||||||
| Berman et al. 204 | 2006 | 19 pregnant women | – | 165 | 30.8 | 15.6 | 5.3 | – | 1.3 | 1.5 | – | – |
| The Netherland | ||||||||||||
| Ye et al. 205 | 2004–2006 | 99 pregnant women | <LOQ (20.1) | 117 (1150) | 42.7 (197) | 42.1 (249) | 7.5 (95.8) | <LOD (<LOD) | 1.0 (3.5) | – | – | – |
| Japan | ||||||||||||
| Itoh et al. 96 | 2004 | 36 (4–70) | – | – | 43 | – | – | – | – | – | – | – |
| Suzuki et al. 171 | 2005–2006 | 50 pregnant women | 6.61 | 7.83 | 57.9 | – | 3.74 | <LOQ | – | – | – | – |
| Taiwan | ||||||||||||
| Huang et al. 206 | 2005–2006 | 76 pregnant women | 4.3 (87.7) | 27.7 (2346) | 81.1 (368) | 0.9 (33.4) | – | – | – | – | – | |
| Sweden | ||||||||||||
| Jonsson et al. 207 | 2000 | 234 men | – | 240 (4400) | 78 (330) | – | 16 (74) | – | – | – | – | – |
| (18–21) | ||||||||||||
- a Abbreviations: n.s.: not specified.
- a) a) 3-cx-MPP is a main metabolite of DnOP and a minor metabolite of DiNP and DnBP.
- b) b) No differentiation between MnBP and MiBP.
- c) c) LOD 0.9 μg/L.
- d) d) Mean value.
- e) e) According to Koch et al.
- f) f) LOD 1.0 μg/L.
- g) g) Partners of subfertile couples.
- h) h) In total, urine samples of 634 students, which were collected in nine years between 1988 and 2003, were analyzed.
- i) i) LOQ: 0.5 μg/L.
- j) j) Urine samples of 8 consecutive days for each person were collected.
The urinary concentrations of the individual phthalate metabolites vary widely both within and between the subjects. In general, the highest metabolite levels have been measured for monoethyl phthalate (MEP), MnBP and the oxidative DEHP metabolites (Tables 2 and 3). However, as we have pointed out above, identical exposure to DnBP and DEHP at the same time may lead to a 5- to 20-fold higher urinary excreted amount of MnBP compared with MEHP 92, 94, 130, 155. Thus, the relative urinary level of a single (monoester) metabolite does not necessarily reflect the relative exposure level to the parent phthalate.
Most biomonitoring data on phthalate exposure have been collected for the German and the US general population. In general, the data from both countries are in good accordance. However, in consideration of the respective sampling years, the urinary levels of MnBP and particularly of MiBP were higher in Germany, whereas the concentrations of MEP and monobenzyl phthalate (MBzP) were higher in the USA 16, 17, 140, 156. In recent general population studies from Germany 129, 143, the median levels of MnBP were approximately two to three times higher and the median levels of MiBP were approximately ten times higher than in the US National Health and Nutrition Examination Survey (NHANES) from 2001 to 2002, whereas the medians for MBzP were two to three times lower. Possibly different patterns of use in Germany and the United States, for instance a preferred usage of DEP compared with DnBP in personal-care products in the USA, may have led to different exposure levels of the butyl phthalates and BBzP 16, 19. In the case of DiNP, Silva et al. 132 measured in 129 US American adults two to six times higher median concentrations for mono(carboxyisononyl) phthalate (cx-MiNP) and mono(hydroxyisononyl) phthalate (OH-MiNP) compared with levels measured in study populations from Germany 19, 129, 143, which indicates a higher exposure to DiNP in the USA. All current biomonitoring approaches, however, cannot distinguish between exposures to the two different DiNP isoforms in commercial use (“DiNP 1” and “DiNP 2”) and it is possible that one or the other isoform is predominately used in the USA or Europe 19.
The data of NHANES 1999–2000 and NHANES 2001–2002 were very similar for all metabolites. By contrast, the median values of MEP, MBP (sum of MnBP and MiBP) and MBzP determined in NHANES III (1988–1994) were approximately twice as high. Although the investigated subpopulation of NHANES III was not representative and therefore not directly comparable with the two later NHANESs, this might indicate a decrease in exposure to DEP, DBP and BBzP in the USA over the years. The MEHP concentrations remained rather stable over the years. Whether this exposure pattern over the years reflects the market use of phthalates in the USA cannot be verified, because no reliable market data are available for phthalates. Results from a retrospective human biomonitoring study based on biobanked urine samples of German students suggest that the general population in Germany has been exposed to at least five phthalates throughout the last two decades (1988–2003) 19. However, for MnBP and the DEHP metabolites, continuously decreasing urinary levels were found over the years, whereas the values for MiBP and the DiNP metabolites were slightly increasing. These observations are in accordance with a shift within the European phthalate market, which has taken place during the last years 1, 157. Fromme et al. 143, who investigated 399 urines samples from 50 German subjects taken on eight consecutive days in 2005, measured very similar values for all metabolites compared with the subset of German students from 2001 to 2003 19. Only for the DiNP metabolites, median levels were somewhat higher, again, hinting to a shift in market use.
Urinary phthalate metabolite data from other countries are scarce but emerging over the last years. In 36 Japanese volunteers, urinary concentrations of MnBP and MEHP 96 were comparable to concentrations recently measured in Germany. In urine samples of 234 Swedish men of recruitment age, relatively high MEP levels were measured 152.
Some studies show that women have significantly higher levels of the monoester metabolites of the short-chain phthalates than men, in particular of the dibutyl phthalates 16-19. One hypothesis is that this may be related to a more frequent and everyday application of body care and cosmetic products containing such phthalates by women compared with men. In children, higher urinary phthalate metabolite levels are generally measured than in adolescents and adults 17, 106, 140, 150. Only for MEP, an age-related trend opposite the direction of the other phthalate metabolites has been found in NHANES 1999–2000 and NHANES 2001–2002 17, 140.
When interpreting exposure to phthalates based on metabolite levels in urine, one has to be aware of several pitfalls. First, the metabolite pattern is different for the short-chain phthalates compared with the long-chain phthalates. Monoester metabolites are the preferred metabolites of the short-chain phthalates such as DEP or DnBP/DiBP, whereas oxidized metabolites are the preferred metabolites for the long-chain phthalates such as DEHP or DiNP. Therefore, when only monoester metabolites such as MEP, MnBP or MEHP are interpreted in regard of exposure, similar MnBP and MEHP levels point to several fold higher DEHP exposures. Second, when comparing urinary metabolite concentrations measured in subjects of different age, one has to make allowance for the biometric, physiological and potential toxicokinetic differences. In relation to the bw, young children excrete generally higher urine volumes (approx. 30 mL/kg/day in children younger than five) than adults (approx. 20 mL/kg/day) 158. Therefore, the same metabolite concentrations in the urine of young children, older children and adults undoubtedly reflect a higher body burden to phthalates per kg bw in the young children compared with the older children and adults 150, 151, 159 (Table 4). Third, creatinine excretion – often used as a corrective for urine dilution – is age and gender dependent 151, 159-161. Fourth, oxidative metabolism was found to be age-dependent to a certain degree and slightly favoured in children compared with adults 14, 76, 106, 118, 140, 162. All of this has to be taken into consideration when comparing urinary metabolite levels in adults with children or other subpopulations.
| Reference | Sampling year | n (age) | DEP | DnBP | DiBP | BBzP | DEHP | DiNP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 95th P (max) | Median | 95th P (max) | Median | 95th P (max) | Median | 95th P (max) | Median | 95th P (max) | Median | 95th P (max) | |||
| USA | ||||||||||||||
| David 166 | 1988–1994 | 289 (20–60) | 12.3a) | 93.3 (243) | 1.6a), b) | 6.9b) (117) | – | – | 0.73a) | 3.3 (19.8) | 0.60a), c) | 3.1c) (38.5) | 0.21a), d) | 1.1d) (14.4) |
| Kohn et al. 165 | 1988–1994 | 289 (20–60) | 12 | 110 (320) | 1.5b) | 7.2b) (110) | – | 0.88 | 4.0 (29) | 0.71c) | 3.6 c) (46) | <LOD | 1.7d) (22) | |
| Calafat and McKee 156 | 1999–2000 | 2536 (6 to >20) | 5.4a) | 64.7 | – | – | – | – | – | – | 0.7a), c) | 4.0c) | – | – |
| Calafat and McKee 156 | 2001–2002 | 2772 (6 to >20) | 5.5a) | 61.7 | – | – | – | – | – | – | 0.9a), c) | 7.1c) | – | – |
| 2.1a), e) | 16.8e) | |||||||||||||
| 2.2a), f) | 15.6f) | |||||||||||||
| Marsee et al. 153 | 1999–2002 | 214 pregnant women | 6.6 | 112 (1263) | 0.84 | 2.3 (5.9) | 0.12 | 0.41 (2.9) | 0.5 | 2.5 (15.5) | 1.3g) | 9.3g) (41.1) | – | – |
| Germany | ||||||||||||||
| Wittassek et al. 19h) | 1988/1989 | 120 (21–29) | – | – | 7.5 | 21.7 (70.1) | 1.1 | 3.6 (12.9) | 0.28 | 0.78 (6.6) | 3.9i) | 9.9i) (39.8) | 0.21j) | 1.4j) (12.9) |
| Koch et al. 16 | 2002 | 85 (7–63) | 2.3 | 22.1 (69.3) | 5.2 | 16.2 (22.6) | – | – | 0.6 | 2.5 (4.5) | [13.8]k) 4.6g) | [52.1 (166)]k) 17.0g) (58.2) | – | – |
| Koch et al. 151 | 2001/2002 | 239 (2–14) | – | – | 4.1l) | 14.9l) (76.4) | – | – | 0.42l) | 2.57l) (13.9) | 4.3g), l) | 15.2 g), l) (140) | – | – |
| Wittassek et al. 159 | 7.6m) | 30.5m) (110) | 0.77m) | 4.48m) (31.3) | 7.8g), m) | 25.2g), m) (409) | ||||||||
| Wittassek et al. 19h) | 2001/2003 | 119 (20–29) | – | – | 2.2 | 7.3 (116) | 1.5 | 4.2 (12.6) | 0.22 | 0.75 (1.7) | 2.7i) | 6.4i) (20.1) | 0.37j) | 1.5j) (4.4) |
| Fromme et al. 95 | 2005 | 50 (14–60) | – | – | 1.7 | 4.2 | 1.7 | 5.2 | 0.2 | 1.2 | 2.2j) | 7.0j) | 0.7j) | 3.5j) |
| Japan | ||||||||||||||
| Itoh et al. 96 | 2004 | 35 (20–70) | – | – | 1.3 | (4.5) | – | – | – | – | 1.8n) | (7.3)n) | – | – |
| Suzuki et al. 171 | 2005–2006 | 50 pregnant women | 0.28 | (42.6) | 2.18 | (6.91) | – | – | 0.132 | (3.2) | 1.73o | (24.6)o | 0.06d) | (4.38)d) |
- a Except for DEHP and DiNP all values are based on the urinary monoester levels.
- a) a) Geometric mean.
- b) b) No differentiation between DnBP and DiBP.
- c) c) Based on UEF of MEHP determined by Anderson et al. 130.
- d) d) Based on urine levels of MiNP.
- e) e) Based on UEF of 5OH-MEHP determined by Koch et al. 92, 93.
- f) f) Based on UEF of 5oxo-MEHP determined by Koch et al. 92, 93.
- g) g) Based on UEFs for MEHP, 5OH-MEHP and 5oxo-MEHP determined by Koch et al. 92, 93.
- h) h) In total, phthalate exposure in 634 persons, whose urine samples were collected in nine years between 1988 and 2003, were calculated.
- i) i) Based on UEFs of five DEHP metabolites determined by Koch et al. 93.
- j) j) Based on urine levels of OH-MiNP, oxo-MiNP, and cx-MiNP.
- k) k) Based on UEFs for MEHP, 5OH-MEHP and 5oxo-MEHP determined by Schmid and Schlatter 208.
- l) l) Creatinine based calculation model.
- m) m) Volume based calculation model.
- n) n) Based on UEF of MEHP determined by Koch et al. 92, 93.
One additional important point has also to be taken into consideration. In population-based studies and also in clinical studies, participants are sometimes asked to fast before blood and urine donation, e.g. in NHANES, participants who have appointments in the morning are asked to fast 9.5 h (overnight). Participants with appointments in the afternoon and evening are asked to fast 6 h 163. For substances that are mainly food borne (as has been shown for the long-chain phthalates such as DEHP and DiNP) and for substances with a short half-time of elimination (as is the case for all phthalates) the time of fasting is of importance when interpreting urinary phthalate metabolite values in terms of exposure and when comparing one study with another. Studies with fasting intervals before urine donation do most likely underestimate exposure to (some) phthalates, especially if dose extrapolations based on biomonitoring data do not take into account the fasting interval. In the German GerES, no fasting time is required before donating blood or spot urine samples.
4.3 Calculation of the daily phthalate intake based on biomonitoring data
With the knowledge on human metabolism and elimination characteristics of the phthalates as a precondition, a translation from the urinary metabolite levels to the doses of the parent phthalates taken up becomes feasible. To do so, some approximations have to be made such as a steady-state regarding exposure and metabolic clearance. When extrapolating intake doses from urinary metabolite levels, the urinary excretion factors (UEF) play a crucial role. These factors describe the molar ratio of the phthalate dose taken up to the amount excreted in urine as one or several specific metabolites. For several phthalates UEFs have been determined in human metabolism studies by the administration of isotope-labeled phthalate diesters (Table 1) 92-94, 118, 164. Phthalates are rapidly metabolized and excreted in urine. Most of the metabolites are almost completely excreted within 24 h post-exposure. Some metabolites, however, have clearly been shown to have half-lives of elimination longer than 24 h, like mono(2-carboxymethyl-hexyl) phthalate (2cx-MMHP), an oxidized metabolite of DEHP 93. Such metabolites might be of interest when evaluating phthalate exposures that took place several days in the past, or when trying to capture cumulative exposures to one phthalate over several days. For some phthalates and their metabolites, no UEFs have been determined in humans so far, e.g. for DEP, DiBP or DiDP. In these cases, factors from animal studies or from a phthalate of similar structure have been used for the exposure calculation. It can be expected that there is a certain intra and interpersonal variation in human phthalate metabolism, which may result in a certain variation of the UEFs. For example, children have shown to have a somewhat more pronounced oxidative metabolism compared with adults 14, 106, 118, 129, 140. But, given the excellent correlations among the different metabolites of DnBP, DiBP, DEHP and DiNP, respectively, which were seen in a large number of individuals, variations seem to be small.
In general, spot urine samples are collected in population-based biomonitoring studies. In these cases, one has to extrapolate from a single measurement of the metabolite level to the amount excreted over 24 h. Such an approximation is possible by referring to reference values for the daily urinary volume or creatinine excretion, respectively. In the latter case, the creatinine adjusted urinary metabolite concentrations have to be determined. The reference values applied should reflect the physiological differences between men, women and children of different ages in urinary excretion 158, 160. As already mentioned, daily urine volume is, among other things, a function of age. Creatinine excretion is a function of muscle mass and activity, and therefore indirectly depends on gender and age as well 161. For the individual case, a spot urine sample may not be representative for the mean daily concentration, but can also be a peak or bottom value. However, these extreme values may be balanced out in a larger study population. Compared with spot samples, 24 h urine samples are more appropriate for the daily phthalate intake calculation 19. Via the 24 h urine volume, the absolute metabolite amounts excreted during a whole day are directly accessible and variations in urinary dilution are irrelevant. However, sampling of 24 h urine specimens is logistically difficult and in some cases, for instance for young children, not a realistic approach.
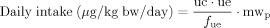
Kohn et al. 165 and David 166 were the first who performed such back-calculations with using creatinine-corrected phthalate metabolite levels in urine. Kohn et al. 165 applied a linear two-compartment model (fue=ku/kt, with ku=rate constant for elimination of urinary excreted monoester, kt=rate constant for total elimination), whereas David 166 used UEF values instead (fue=UEF). Both approaches have been shown to yield similar values with slightly lower values in the calculation model of David 153, 165, 166. In the vast majority of the phthalate exposure assessment studies based on biomonitoring data, creatinine-corrected urinary levels and UEF values have been used 95-97, 129, 155, 156, 159, 167.
Wittassek et al. 159 and Koch et al. 151 calculated daily intakes of German children by using the creatinine-based calculation model and compared the values with those obtained from the volume-based calculation model. Owing to the rapidly increasing creatinine excretion in developing children, body height and gender-based reference values for daily urinary creatinine excretion 160 were used in the creatinine calculation model. Corresponding detailed data for the daily urine volume excretion in children were not available 158. This might be one reason why the values were on average about two times higher with the volume-based model compared with the creatinine-based model.
4.4 Exposure assessment based on biomonitoring data
Table 4 summarizes daily phthalate intakes calculated from urinary metabolite levels measured in the general population of the USA, Germany and some other countries. The data suggest comparable exposure to DEHP in Germany and the USA, but higher exposure to the dibutyl phthalates and lower exposure to DEP and BBzP in Germany. Data from both countries indicate that total phthalate exposure has decreased to a certain extent during the last decades. Exposure to some phthalates, e.g. DiBP and DiNP is on the rise. In several studies, women were found to have significantly higher daily intake levels of the dibutyl phthalates (DnBP and DiBP) compared with men.
The NHANES data have been used to calculate daily phthalate exposure in the US general population 95, 129, 156, 165, 166 (Table 4). The median exposure levels calculated for the subjects from NHANES III (1988–1994) were, except for DEHP, about two times higher compared with the values for NHANES 1999–2000 and 2001–2002. Highest daily exposures were calculated for DEP with medians (95th P) between 5.4 and 12.3 μg/kg/day (approx. 100 μg/kg/day). For DBP (no differentiation between DnBP and DiBP), median (95th P) values of 0.7–1.6 (2.6–7.2 μg/kg/day) were calculated. Daily exposure to BBzP was calculated to be in median (95th P) 0.4–0.9 (1.9–4.0 μg/kg/day). In the case of DEHP, calculations became more and more reliable over the years, owing to an increasing number of (oxidized) metabolites next to the simple monoester MEHP and human-based UEFs for these metabolites. Solely based on MEHP and the UEF of MEHP determined by Anderson et al. 130, for NHANES III and NHANES 1999–2000 medians (95th P) of 0.6–0.7 (3.1–4.0 μg DEHP/kg/day) were calculated 156, 165, 166. By contrast, Fromme et al. 95 used a lower UEF for MEHP (6% instead of 13%) determined by Koch et al. 92, 93, resulting in approximately two times higher DEHP daily intake values for the NHANES 1999–2000 data with a median (95th P) of 1.4 (8.3 μg/kg/day). Based on the UEFs from Koch et al. 92, 93 and based on the three metabolites MEHP, 5OH-MEHP and 5oxo-MEHP, for NHANES 2001/2002 somewhat higher values have been calculated with a median (95th P) of approximately 2 (16 μg/kg/day) 95. For DnOP, DiNP and cyclohexyl phthalate, only marginal exposure levels have been estimated from the NHANES data. However, for both DnOP and DiNP, exposure may have been underestimated because only the monoesters MnOP and monoisononyl phthalate (MiNP) were measured. These metabolites are known in the meantime to be minor and unreliable metabolites 94, 129, 132, 144, 145, 168-170. Daily phthalate exposure estimated in 214 pregnant US women (1999–2002) was similar to the NHANES 2001–2002 data 153 (Table 4).
In German study populations, highest daily intake levels have been calculated for DnBP and DEHP, although continuously decreasing values have been observed for both phthalates between 1988 and 2001 in a retrospective human biomonitoring study among German students 19. In three recent studies 19, 95, 129, similar DEHP exposures have been estimated with medians (95th P) of 2.2–2.7 (6.4–7.0 μg DEHP/kg/day). Koch et al. 167 calculated clearly higher DEHP intakes for 85 German subjects sampled in 2002, however, when applying the reliable UEFs for the oxidized metabolites generated later by the same group 92, 93, intake values are in the same order of magnitude compared with other studies of the same sampling years. For DnBP and DiBP, comparable exposure levels have recently been estimated with medians (95th P) of 1.5–2.2 (4.2–5.2 μg/kg/day), respectively 19, 95, 129. Although for DnBP intake values seemed to be decreasing for German students over the last 20 years, for DiBP (next to DiNP) values are increasing 19. For DiNP and BBzP, the daily intakes are generally lower compared with DEHP and the dibutyl phthalates with medians (95th P) of 0.4–0.7 (1.5–3.5 μg/kg/day) and 0.2 (0.8–1.2 μg/kg/day), respectively 19, 95, 129. In the pilot study GerES IV of 2001/2002, daily phthalate intakes of DnBP, BBzP and DEHP were calculated for 239 German children (2–14 years) by using two calculation models (urinary creatinine and volume based) 151, 159. Comprising all children, the creatinine-based values were about twice as high as the values obtained in three recent studies 19, 95, 129 (Table 4). The volume-based values were about two times higher than the creatinine-based values, with medians (95th P) of 7.6 (30.5 μg DnBP/kg/day), 7.8 (25.2 μg DEHP/kg/day) and 0.77 (4.5 μg BBzP/kg/day). Daily phthalate exposure was found to increase with decreasing age of the children. For the youngest children (2–4 years), median exposure to all three phthalates was two to three times higher than in the oldest children (12–14 years), independently of the calculation model. The higher exposure levels may be due to a higher food consumption related to the bw, different mouthing behavior and/or ingesting dust by playing near the ground.
Itoh et al. 97 and Suzuki et al. 171 estimated daily intakes for Japan in the same range as in recent biomonitoring studies from the USA and Germany.
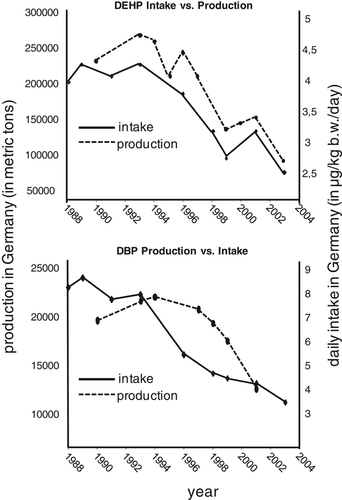
Recently, Helm 157 has shown that for DEHP daily intakes calculated from biomonitoring data 19 correlate very well with the industrial DEHP production in Germany. A similar effect can be observed for DBP (Fig. 3). Like for DEHP, a decrease in DBP production in Germany is accompanied by a decrease in daily DBP intake. However, both curves for DBP do not fit as impressively as for DEHP. One possible reason for this might be that exposure sources and routes of exposure to DBP are more complex than for DEHP. Also, because of the smaller production volume, the effects of the global market might have had a stronger influence on DBP production in Germany than on DEHP. Nevertheless, for both phthalates the decrease in production is clearly reflected in a decrease in internal exposure/daily intake.
5 Exposure assessment by biomonitoring data versus exposure media models
As already discussed above, human exposure assessment to environmental contaminants can also be performed by combining data on concentrations in different exposure media with assumptions on exposure scenario parameters.
Consumption rates of individual foods are generally taken from nutrition surveys using questionnaires and diaries. The scenarios for inhalation exposure have to take into account the time that consumers spend in various outdoor and indoor environments and activity-dependent inhalation volumes. Oral exposure due to children's mouthing of plastic objects or dermal exposure from skin contact with consumer products such as clothing (e.g. PVC gloves) or toys are based on estimations on the contact area, the time of the contact and the release rate per time unit. For the evaluation of the dermal exposure from personal-care products, the frequency of use, the amount of the product used per application and the fraction retained by the skin after use have to be estimated 66. A further difficulty is the conversion of the estimated external exposures resulting from oral, dermal and inhalation pathway into the internal body burden, i.e. the phthalate amount that is actually transferred into the human body. For this purpose, resorption rates of different organs (gastrointestinal tract, skin and respiratory tract) have to be considered, which, however, are not available in most cases or can only roughly be estimated from animal studies, respectively 66, 81. From contamination levels estimated or measured in different exposure media, point estimates and probabilistic calculations of the daily exposure to several phthalates in adults (Supporting Information Table 1a) and the younger general population (Supporting Information Table 1b), respectively, have been performed.
In the most recent exposure model Wormuth et al. 81 estimated age-specific ranges in daily consumer exposure to eight phthalates in Europeans by a probabilistic scenario-based approach. In this comprehensive study, 15 different oral, dermal and inhalation exposure pathways were considered. In contrast to most other studies, also organ- and situation-specific uptake rates were used for each pathway. In adults, the highest values were calculated for DEP (median 1.3 μg/kg/day), DnBP (3.6 μg/kg/day) and DEHP (2.7 μg/kg/day). The respective maximum estimates were 64.9, 38.6 and 16.3 μg/kg/day. These values are very similar to exposure levels recently determined by biomonitoring approaches. For DiBP, BBzP, DMP and particularly for DiNP and DiDP, the authors estimated clearly lower daily intakes with median exposure levels between 0.4 and <0.01 μg/kg/day. This is in contrast to calculations based on recent biomonitoring data 19, 95, 129, 143, which show considerably higher mean and upper intake levels for DiBP and DiNP. Biomonitoring studies also find a considerable increase in exposure over the last years for these two phthalates 19, 129, which can be explained by the substitution of DnBP with DiBP and DEHP with DiNP/DiDP. Changes in the exposure situation are directly reflected in biomonitoring-based exposure assessments, whereas external exposure model extrapolations always rely on the accuracy and up-to-dateness of the respective input data. Consequently, the correctness of external exposure models always needs verification by biomonitoring data. On the other hand, external exposure models would help to predict future exposure scenarios if substitution processes were correctly implemented in the models.
In children and adolescents (Supporting Information Table 1b), phthalate exposure levels were estimated to be generally higher compared with adults. These calculations are in accordance with findings from biomonitoring studies. Based on the maximum local exposure calculated with the European Union System for the Evaluation of Substances model very high maximum exposure levels of DnBP, DEHP, DiNP and DiDP were estimated for infants and children, reaching up to more than 400 μg/kg/day 2, 3, 86. In the cases of DEHP, DiNP and DiDP, mouthing of PVC toys was seen as a crucial pathway resulting in exposures of up to 200 μg/kg/day. Bosgra et al. 172 pointed out that these point estimates were based on the worst-case scenarios for each of the underlying parameters. Based on the same database, they estimated in a probabilistic approach much lower DEHP levels in children, caused by mouthing of PVC toys (95th P 0.88 μg/kg/day). The authors concluded that using the entire distribution may lead to more reasonable and likely scenarios than by selecting point estimates of all parameters in a deterministic approach. Nevertheless, biomonitoring data indicate that, indeed, extremely high exposures to the above phthalates can occur in children. Wittassek et al. 159 found a maximum daily DEHP intake of 409 μg/kg/day in 2- to 4-year-old children in Germany (urines collected between 2001 and 2002).
The former UK Ministry of Agriculture, Fisheries and Food (MAFF) analyzed infant formulae for several phthalates in 1996 and 1998 173, 174. The calculated exposure levels in 1998 were about half the exposures reported in the 1996 survey (Supporting Information Table 1b). It was suggested that this drop may have been due to the manufacturers' effort to eliminate potential sources of phthalate contamination. Just recently, exposure from fatty food being in contact with gaskets of twist-off caps was estimated to result in exposures of up to 110 μg DEHP/kg/day and 720 μg DiNP+DiDP/kg/day for children aged 4–6 years 79, 175, 176. Although these peak values were obtained in a worst-case approach, assuming that all considered foods (pasta sauces, dressings, vegetables and mushrooms preserved in oil, pestos) are packed in jars with respective lids and having the maximum phthalate concentrations of the respective food groups, relative high phthalate exposures from such foods have to be assumed. Again, based on the biomonitoring results from the German environmental survey on children, indeed, maximum intakes have been calculated to be in this range.
Although biomonitoring data portray a rather consistent picture of phthalate exposure in the general population (Table 4), there are higher variations in exposure assessments based on contamination levels, particularly in the upper exposure levels (Supporting Information Tables 1a and 1b), probably depending on the degree of worst-case assumptions. However, intake calculations based on biomonitoring have proven that assumptions based on external exposure assessments or scenario-based approaches are still rather conservative, i.e. underestimate the possible extent of exposure both in the mean and upper percentiles.
When assessing human exposure to environmental chemicals from (environmental) contamination levels, it is essential to account for all relevant routes and sources of exposure, which, however, is difficult in the case of the phthalates. On the one side, there certainly are a large number of potential sources and routes of exposure which have to be accounted for, on the other side probably a large number of still unknown sources of exposure exist. For example, in the previous external exposure assessments, phthalate exposure through food supplements, (prescription-free) medications or medical devices (like in blood donations) have not been taken into account, although these may be significant sources in some individuals as unveiled in biomonitoring studies 114, 115, 121, 177-179. Also, many models only evaluate the classical accumulation of a contaminant in the food chain which is more relevant for persistent chemicals. Contamination of food during manufacturing, processing, packaging, storage or inappropriate use of phthalate containing materials – pathways that are believed to be relevant for some phthalates – can only be revealed by actual measurements. This may be one reason why European Union System for the Evaluation of Substances 63, 64 apparently underestimates the phthalate contamination of foodstuff 86. That contaminated foodstuff, especially fatty foods (such as noodle sauces, foods in oil and pesto) can be a considerable source of phthalate exposure (both for children and adults) has recently been stated by the German Federal Institute for Risk Assessment (BfR) 79, 175, 176, 180.
Clearly, both approaches of exposure assessment have advantages and limitations. All in all, however, assessments based on biomonitoring data may provide a more accurate evaluation of the current and overall state of exposure. Urinary metabolite concentrations represent an integrative measure of exposure from all sources and routes and indicate not an hypothetical but actual internal exposure 73, 74, 129, 156, 181. Furthermore, biomonitoring can determine the current state of exposure to phthalates and therefore immediately detect any changes in the exposure situation of the population caused by e.g. substitution processes, new fields of application, critical applications or inappropriate usage. In scenario-based approaches, a considerable number of assumptions have to be made, which are associated with a high degree of uncertainty. On the other hand, contamination measurements in exposure media are indispensible for the identification, evaluation and finally the elimination of potential exposure sources.
6 Assessing risks associated with phthalate exposure
6.1 General population
For an assessment of the risk associated with phthalate exposure, the phthalate exposure estimates can be compared with exposure limit values established by authorities like the European Food Safety Authority (EFSA) and the US Environmental Protection Agency (US EPA) (Table 5). Based on the developmental and testicular toxicity in rats 21, 26, 30, the EFSA has allocated tolerable daily intake (TDI) values for DEHP (50 μg/kg/day), DnBP (10 μg/kg/day) and BBzP (500 μg/kg/day) 182-184 in 2005. The TDI values for DiNP (150 μg/kg/day) and DIDP (150 μg/kg/day) are based on liver effects 185, 186. However, also for DiNP detrimental reproductive effects have been observed in rats 27, 31, but no-observed-adverse-effect-level (NOAEL) has been determined for these effects so far. Between 1990 and 1993, the US EPA established reference doses (RfD) for DEP (800 μg/kg/day), DBP (100 μg/kg/day), BBzP (200 μg/kg/day) and DEHP (20 μg/kg/day) 187-190. These values, however, are based on other than reproductive toxic effects. For DEHP and DnBP increased liver weight and increased mortality, respectively, were the toxicological endpoints, which have been seen in animal studies already published in 1953 191, 192. In the cases of the RfD values, uncertainty factors of 1000 have been used, whereas the EFSA's Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) used a factor of 100 for the deduction of the TDI values.
| Phthalate | Abbreviation | EFSA TDI (μg/kg/day) | US EPA RfD (μg/kg/day) |
|---|---|---|---|
| Diethyl phthalate | DEP | – | 800 |
| Di-n-butyl phthalate | DnBP | 10 | 100 |
| Diisobutyl phthalate | DiBP | – | – |
| Butylbenzyl phthalate | BBzP | 500 | 200 |
| Di(2-ethylhexyl) phthalate | DEHP | 50 | 20 |
| Diisononyl phthalate | DiNP | 150 | – |
| Diisodecyl phthalate | DiDP | 150 | – |
The median daily intakes obtained from biomonitoring data are clearly below the EFSA TDI and EPA RfD values, respectively, of the individual phthalates. Also the 95th P and the maximum estimated exposure levels of DEP, BBzP, DiNP and DiDP were generally well below the limit values, although for NHANES 1999–2000 and 2001–2002 maximum daily intake estimates are not available. Marsee et al. 153 calculated in one woman an exposure of 1263 μg DEP/kg/day, which, however, was one order of magnitude higher than the 95th P of 112 μg DEP/kg/day of the study population. Regarding DEHP and DnBP, the respective 95th Ps were in many cases close to or above the RfD of 20 μg DEHP/kg/day and the TDI of 10 μg DnBP/kg/day 95, 156, 159, 167, respectively. For some individuals, values considerably above the limit values have been calculated. In GerES IV, three of the 239 investigated children (1%) had daily DEHP intakes above the TDI of 50 μg/kg/day, whereas – depending on the calculation model – 3% (max. 140 μg/kg/day) and 7.5% (max. 409 μg/kg/day), respectively, exceeded the RfD value of 20 μg/kg/day 159. For DnBP 11% (creatinine calculation model; max. 76.4 μg/kg/day) and 37.2% (volume calculation model; max. 110 μg/kg/day), respectively, of the children had exposures higher than the TDI value for DnBP (10 μg/kg/day) 151. But, also for some adult individuals, daily intakes of DnBP much higher than the TDI value (up to 230 μg/kg/day) have been estimated in population-based studies 19, 165-167. Application of medications containing DnBP has recently been shown to be able to cause even higher exposure levels 111, 178, 193. In many cases, these medications contain essential oils or herbal extracts, are obtainable without a prescription, and are recommended also for pregnant women, children and infants, which are all regarded as special risk groups for exposure to phthalates. Based on the urinary measurements after administration of a prescription-free drug for the treatment of bronchitis, Koch et al. 178, 193 calculated for a long-term application in women of reproductive age a daily dose of 266 μg DnBP/kg/day and for a child (16 kg) a dose of 1080 μg DnBP/kg/day, which equals a 100-fold overstepping of the TDI value and comes close to the lowest observed adverse effect level of 1.5–3.0 mg/kg/day seen in rats 21. In 2007, 132 medications containing DnBP were approved by the German Federal Institute for Drugs and Medical Devices (BfArM), with a significant DnBP content per unit up to 10.9 mg 194. The application of all of these medications (according to the instruction leaflet and disclosure of the BfArM) can result in considerable transgressions of the current EFSA TDI value.
In several worst-case scenarios phthalate exposure higher than the acceptable daily exposure levels have been estimated. For instance, mouthing of PVC items was regarded as an important pathway for exposure to DiNP and DiDP in infants, which might result in uptakes of up to 200 μg/kg/day 2, 3, 86. Consumption of fatty food being in contact with gaskets of twist-off lids was estimated to result in exposures of up to approx. 125 μg DEHP/kg/day in adults and children (4–6 years) and 320 and 720 μg DiNP+DiDP/kg/day, respectively 79, 175. Also, a more realistic exposure assessment approach, based on the food consumption surveys (95th P) and the empirical distribution of contamination concentrations, yielded significant DEHP exposure of up to 91 μg/kg/day. The German Federal Institute for Risk Assessment (BfR) concluded that the consumption of such foods can result in a pronounced transgression of the TDI values of the respective phthalates and their consumption might therefore result in adverse health effects. The BfR made the general recommendation to refrain from using critical phthalates like DEHP in materials in contact with food and to use less toxic substitutes 79, 175. Also in some edible oils, very high DEHP contamination levels have recently be found in Germany 176. Only 40 g (two tablespoons) of a highly DEHP contaminated oil could reach the TDI for DEHP. The BfR stated that effects on human health could not be excluded at long-term intake of such oils.
6.2 Cumulative exposure to phthalates
In general, toxicologically based exposure limit values refer to a single substance, deduced from adverse effects seen in laboratory animals that were exposed to the substance. Thus, when assessing potential risks to human health by comparing exposure levels with such limit values, possible dose-additive effects of different environmental chemicals acting in a similar way usually remain unconsidered. In the case of phthalates and other antiandrogens, recent research findings show that they induce reproductive malformations in rats in a cumulative, dose-additive manner 57-60, 195 and, more remarkably, independent of the mode of altering the androgen signalling pathway. Howdeshell et al. 59 found in rats during sexual differentiation that the coadministration of DnBP and DEHP, both acting by the same mechanism of action, increased the incidence of a number of reproductive malformations (e.g. epididymal agenesis, reduced androgen-dependent organ weights) in a cumulative, dose-additive manner. In total, 11 of the 16 considered hormone (androgen and/or insl3)-dependent end points were accurately predicted by dose addition. For hypospadias, gubernacular agenesis/hypoplasia and seminal vesicle malformation even synergistic interaction were observed. Based on these findings, it has to be presumed that exposures to several different phthalates at the same time might increase the risk for the development of endocrine disrupting/modulating effects also in humans. Therefore, for human risk assessment and for health prevention it may be more appropriate to account for the everyday and simultaneous exposure to a number of phthalates that act in a similar way. DnBP, DiBP, BBzP, DEHP and DiNP have already been proven to cause harmful effects on the developing male reproductive system through, at least in part, a common mode of action by the reduction of testosterone synthesis 21-26, 29-31, 35, 59, 196, 197. Therefore, current scientific attention in phthalate research is focussing on evaluating the cumulative effects of mixtures of phthalates in animal models and also the ubiquitously exposed general population 62. A recent approach in risk assessment to consider the cumulative effects of phthalates is the use of a cumulative TDI value for all endocrine active phthalates instead of individual TDIs 135. Moreover, phthalates have been shown to act in a dose-additive or synergistic manner with other anti-androgens/endocrine disrupters 37, 58, 60-62, 198, 199.
6.3 Groups at risk
Next to the widespread exposure to phthalates in the general population, there are additional specific population groups that are even much higher exposed. Long-term medication with tablets containing phthalates can result in very high and steady exposure to DEP and DnBP, respectively. Medical procedures using PVC medical devices can lead to DEHP exposure much higher than the background levels, albeit the extent of exposure largely depends upon type and the duration of the medical treatment 113-115. In adults, highest doses of DEHP may result by transfusions of blood components reaching up to several mg/kg/day. It has also been shown that apheresis procedures to donate blood products can cause significant exposure to DEHP 119-121, 200. Premature neonates in intensive care can receive even higher DEHP exposures relative to their bw compared with adults 116-118, 133. This is of greatest concern as neonates – next to the fetus – are regarded as most susceptible to the adverse reproductive and developmental effects of DEHP. For some treatments, the mg/kg bw/day range may easily be reached and for blood transfusion procedures peak values up to 22 mg/kg bw/day have been estimated 113, 201. These exposure estimates are in the same range as the doses initiating adverse reproductive effects in rats. Based on urinary measurements, Koch et al. 118 estimated for 45 premature neonates being in contact with PVC medical devices a median daily DEHP dose of 42 μg/kg/day and a 95th P of 1780 μg/kg/day. The large difference between the median and the 95th P indicate a great variability in DEHP exposure for newborns in intensive care. The maximum estimated daily DEHP intake was 2300 μg/kg/day, which is separated from the NOAEL (4.8 mg/kg/day) for testicular and developmental toxicity in rats 26 only by a factor of 2. Based on the biomonitoring data of Calafat et al. 117, even higher maximal DEHP exposures up to 6 mg/kg/day have been estimated for premature neonates in a neonatal intensive-care unit 14, which would be above the respective NOAEL in rats.
7 Concluding remarks
There is widespread exposure to a number of phthalates among the general population. Children have the highest exposure levels within the general population reaching levels near or above established exposure limit values for DnBP and DEHP. Food is the major source of phthalate exposure, in particular for DEHP and probably also the other long-chain phthalates. For the short-chain phthalates, indoor air and personal-care products (particularly DEP) seem to be relevant sources next to foodstuff. The mouthing behavior of infants and children can lead to additional intake of phthalates (DiNP, DiDP). High to very high phthalate exposure can occur from medication (DnBP) and medical devices (DEHP), especially in neonates undergoing intensive care. Further characterization and identification of the exposure pathways and the sources of food contamination is needed to reduce human phthalate exposure. Children and particularly the unborn life have to be regarded as special risk groups for the potential effects on reproduction and development of the phthalates. In pregnant women, a short-term exposure to high levels of antiandrogenic agents like phthalates during male sexual differentiation might have the potential to cause permanent effects on reproductive development of the fetus. Recent research findings from animal studies suggest that cumulative risk assessments for phthalates and other antiandrogens should be performed instead of considering risks for these substances one-by-one.
Acknowledgements
The authors have declared no conflict of interest.




