The Functions and Implications of MicroRNAs in Premature Ovarian Insufficiency
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Premature ovarian insufficiency (POI) is a condition in women characterized by the premature decline of ovarian function before age 40, evident through menstrual irregularities such as amenorrhea or oligomenorrhea, elevated FSH levels exceeding 25 U/L, and a progressive decrease in estrogen levels. Despite considerable research, the exact pathogenic mechanisms underlying POI remain unclear. This study focuses on the role of microRNAs (miRNAs) in the development of POI. As critical regulators of gene expression, miRNAs may play significant roles in diagnosis and the development of innovative therapeutic approaches for POI.
Methods
A comprehensive literature search was conducted on PubMed for this review. We included studies published in English up to September 2024. Our search utilized a combination of the following keywords: microRNA, premature ovarian insufficiency (POI), and premature ovarian failure (POF). Articles were filtered by title and subsequently through full-text review, selecting only those pertinent to our topics of interest and their related areas.
Results
miRNAs have emerged as critical regulators in POI, mediating a range of biological processes that contribute to the disease's progression. Their involvement offers promising insights for early diagnosis, prognostic assessments, and therapeutic interventions, highlighting their potential as biomarkers and therapeutic targets.
Conclusion
Elucidating miRNAs' roles in POI opens new avenues for managing the disease. By understanding how miRNAs influence the molecular mechanisms of POI, innovative strategies can be developed for diagnosis and treatment, potentially improving patient outcomes.
1 Introduction
Premature ovarian insufficiency (POI) is a reproductive disorder that occurs in women under the age of 40 and is characterized by a decline in ovarian function (Nelson 2009). Clinical manifestations of POI include a hypogonadal state, amenorrhea, anovulation, early depletion of the ovarian reserve, signs of estrogen deficiency, and elevated follicle-stimulating hormone (FSH) levels. The incidence of POI is age-related and varies between 0.9%–1.2% (Nippita and Baber 2007). About 5%–10% of patients with POI can conceive and have healthy children with the appropriate treatment (Nelson 2009). The diagnosis of POI is confirmed by elevated FSH levels (> 25 IU/L) on two occasions 1 month apart, along with low estradiol (E2) levels (< 50 pg/mL) and amenorrhea for at least 4 months in women under 40 (De Vos et al. 2010). Anti-Müllerian hormone (AMH) levels and antral follicle count (AFC) are other indicators used to assess ovarian reserve. Recent studies have suggested that cortisol, a biomarker typically associated with stress responses, may also play a role in the assessment of POI, potentially reflecting the impact of stress-related physiological pathways on ovarian function (Kloster et al. 2024; Moysés-Oliveira et al. 2023). In addition to genetic abnormalities, autoimmune disorders, and exposure to radiotherapy, chemotherapy, or surgery, alternative biomarkers like cortisol provide a broader understanding of the etiological spectrum affecting POI (Ulin et al. 2021). However, many cases of POI have idiopathic causes. POI has adverse outcomes related to estrogen deficiency, including an increased risk of cardiovascular diseases, impaired sexual function, and low bone density (Wesevich et al. 2020). The precise etiology and molecular mechanisms underlying POI are still unclear.
MicroRNAs (miRNAs) are short, highly conserved noncoding RNAs, ranging from 19 to 25 nucleotides, that explicitly regulate gene expression. They facilitate the recruitment of a ribonucleoprotein (RNP) complex to complementary RNAs, culminating in the cleavage and degradation of mRNAs or other noncoding RNAs, thereby diminishing the expression of target molecules. Remarkably, a single miRNA is capable of targeting hundreds of mRNAs, significantly influencing the expression of numerous genes frequently engaged in functionally interacting pathways (Lu and Rothenberg 2018). We hypothesize that the precise control of gene networks by microRNAs may be crucial in the tissue development and disease pathogenesis of POI.
Understanding miRNAs in these contexts is crucial for several reasons. Firstly, their regulatory capacity allows insight into the complex gene expressions underlying these diseases. Secondly, miRNAs can serve as biomarkers for early diagnosis due to their specific expression patterns in different tissues and conditions. Finally, targeting miRNAs could lead to innovative therapies that adjust pathological gene expressions without altering the genetic code. Thus, research into miRNAs expands our understanding of POI and opens new avenues for tailored and effective treatments.
2 Methods
In conducting this review, we initiated an extensive literature search targeting both animal and clinical studies. The search, conducted through PubMed and Web of Science databases, covered publications from the inception of these databases up to September 2024. We employed a set of keywords including ‘microRNA,’ ‘premature ovarian insufficiency (POI),’ and ‘premature ovarian failure (POF).’ We included only studies published in English that were pertinent to our research topics. Our inclusion criteria were strictly studies that explored the role of microRNAs in the pathogenesis, diagnosis, or treatment of POI and POF. We excluded commentaries, editorials, experience introductions, conference articles, reviews, and graduation theses. Duplicate publications, studies with inaccessible original data, and articles lacking a clear outcome index were also excluded. The selection process commenced with title screening for relevance, followed by a full-text review to include only those studies providing significant insights into the relationship between microRNAs and premature ovarian insufficiency or failure while omitting those with only cursory mentions of these terms unrelated to our focus areas. This methodical approach ensured a comprehensive and focused review of the literature relevant to the role of microRNAs in POI and POF from the earliest available records.
3 Innovative Therapies for POI
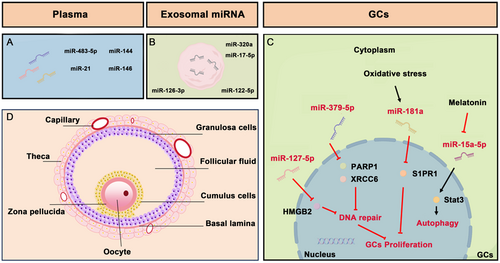
Due to the limited understanding of the processes and complex etiology of POI, there is currently no effective treatment available. Hormone replacement therapy (HRT), which involves the physiological replacement of estrogens (+ progestin), is commonly used for POI treatment. However, it fails to restore ovarian function fully and even increases the risk of reproductive cancer (Mohamed et al. 2018). To address this challenge, scientists have developed several innovative therapeutic approaches. These include stem cell-based therapy (Mohamed Rasheed et al. 2023), in vitro activation (Kawamura et al. 2013), mitochondrial activation (Kasapoğlu and Seli 2020), biomaterial strategies (Ding et al. 2018), and intra-ovarian infusion of platelet-rich plasma (Sfakianoudis et al. 2020). Notably, recent advances highlight the role of miRNAs in deciphering the molecular underpinnings of POI, offering new insights into both disease biomarkers and therapeutic targets (Figure 1 and Table 1). With miRNAs influencing key biological processes like apoptosis and cell proliferation, their manipulation could pave the way for novel treatments to restore ovarian function and mitigate long-term health sequelae associated with POI. This emerging field promises significant clinical applications, potentially revolutionizing the management of POI by targeting specific miRNA molecules.
| miRNAs | Species | Target | Biological role | Expression | Source | Ref. |
|---|---|---|---|---|---|---|
| miR-4516 | Human | Diagnostic marker of POI | Up | POI patients | Umair et al. (2022) | |
| miR-376a | Mouse | PCNA | Regulated primordial follicle assembly | Zhang et al. (2014) | ||
| miR-144-3p | Mouse | MAP3K9 | Prevented premature primordial follicle depletion | Down | Cisplatin-induced POI mouse model | Liu et al. (2023) |
| miR-379-5p | Human | PARP1 and CRCC6 | Inhibited proliferation and impairment of DNA repair | Up | Biochemical POI | Dang et al. (2018) |
| miR-127-5p | Human | HMGB2 | Inhibited GCs proliferation and impaired DNA damage repair capacity | Up | Biochemical POI | Zhang et al. |
| miR-181a | Human | S1PR1 | Mediated oxidative stress-induced GC apoptosis | Up | Zhang et al. (2019) | |
| miR-15a-5p | Human | Stat3 | Involved in serum starvation-induced autophagy of GCs | Up | Wu et al. (2022) | |
| miR-483-5p | Human and Mouse | FKBP4 | Increased ovarian sensitivity to cisplatin and cause severe ovarian dysfunction | Up | Zhao et al. (2021) | |
| miR-21 | Human and Mouse | Peli1 | Associated with the pathogenesis of autoimmune POI | Down | Autoimmune POI | Li et al. (2020) |
| miR-144 | Human and Rat | Interfered with the effect of VCD on autophagy in KGN cells | Down | Zhou et al. (2023) | ||
| miRNA-146 | Mouse | IL-6 and TNF-a | Inhibited the TLR4/NF- κB signaling pathway | Down | LPS-induced POI mouse model | He et al. 2022 |
| miRNA-145 | Rat | VEGF-B and RGC32 | Protective effect against cyclophosphamide-induced ovarian toxicity | Cyclophosphamide induced POI Rat Model | Abogresha et al. (2021) | |
| miR-320a | Human | SIRT4 | Promise of exosome-based therapy for POI treatment | CTX-induced POI model mice | Ding, Qian, et al. (2020) | |
| miR-21-5p | Human | MSX1 | Promoted IFN-γ-induced KGN cell proliferation and hormone synthesis, and inhibited apoptosis | Mouse model of autoimmune POI | Yang et al. (2024) | |
| miR-17-5P | Human | SIRT7 | Promise of exosome-based therapy for POI treatment | CTX-induced POI model mice | Ding, Zhu, et al. (2020) | |
| miR-126-3p | Human | PIK3R2 | Promoted ovarian angiogenesis and inhabited apoptosis | Cisplatin-induced POF rat model | Qu et al. (2022) | |
| miR-122-5p | Mouse | BCL9 | Promoted GCs apoptosis | CTX-induced POI model mice | Zhang et al. (2022) |
- Abbreviations: BCL9, B-cell CLL/lymphoma 9 protein; CRCC6, Chinese hamster cells 6; FKBP4, FKBP Prolyl Isomerase 4; GCs, granulosa cells; HMGB2, high mobility group box 2; IL-6, Interleukin-6; KGN, granulosa-like tumor cells; LPS, lipopolysaccharide; MAP3K9, mitogen-activated protein kinase kinase kinase 9; MSX1, Msh homeobox 1; PARP1, poly ADP-ribose polymerase1; PCNA, proliferating cell nuclear antigen; Peli1, Pellino-1; PIK3R2, Phosphoinositide-3-Kinase Regulatory Subunit 2; RGC32, regulator of cell cycle; SIRT4, Sirtuin 4; SIRT7, Sirtuin 7Stat3, transcription 3; S1PR1, sphingosine-1-phosphate receptor 1; TLR4, toll-like receptor 4; TNF-a, tumor necrosis factor-a; VCD, 4-vinylcyclohexene diepoxide; VEGF-B, vascular endothelial growth factor B.
4 The Role of miRNAs in the Pathophysiology of POI
Current research on miRNAs in POI encompasses three main aspects: identifying key genes and mutation sites through bioinformatics for diagnosis; elucidating the molecular mechanisms of miRNA regulation during POI's onset and progression; and exploring related treatments, including cellular exosomal miRNA (Table 1). miR-4516 was found to be upregulated in human patients with POI and in cyclophosphamide (CTX) and a busulfan-induced mouse model of POI, alongside a downregulation of STAT3 protein levels, suggesting its diagnostic and pathogenic relevance (Umair et al. 2022). Additionally, identifying the rs10061133 A>G single nucleotide polymorphism (SNP) in the mature region of miR-449b represents another significant finding. The AA genotype is notably associated with an increased risk of POI. In the ovarian context, elevated miR-449b expression could inhibit granulosa cell proliferation and promote apoptosis. The expression changes associated with this SNP might decrease the levels of prosurvival genes such as CDK6, HDAC1, CCND1, and MET, critical factors that could contribute to diminished ovarian function (Pan et al. 2016).
4.1 miRNAs and Oocytes
Primordial follicle assembly is crucial for female reproduction as it determines the pool of available oocytes. Disruptions in this process can result in ovarian disorders, including POI (Sullivan and Castrillon 2011). Folliculogenesis, the development of follicles from the primordial to the antral stage, is tightly regulated by the hypothalamic–pituitary-ovarian axis. For instance, gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the anterior pituitary to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are crucial for follicular recruitment, activation, and maturation (Mikhael et al. 2019). The transition from primordial to primary follicles involves intricate mechanisms. Disruptions in these mechanisms can cause premature activation and depletion of the follicular reserve, characteristic of POI. miR-376a plays a pivotal role in regulating the formation of primordial follicles in fetal and neonatal mouse ovaries by controlling the expression of proliferating cell nuclear antigen (Pcna), a crucial regulator of oocyte apoptosis. miR-376a inversely affects Pcna mRNA levels by binding to its 3′ untranslated region, inhibiting its expression and thus impacting protein and mRNA levels. This regulatory effect has been demonstrated in mouse ovary transfection studies, where miR-376a not only increases the number of primordial follicles but also significantly reduces oocyte apoptosis (H. Zhang et al. 2014). Furthermore, miR-144-3p specifically targets MAP3K9, leading to decreased activation of the PI3K/AKT pathway and thereby preventing premature depletion of primordial follicles in cisplatin-induced POI models in mice (Liu et al. 2023).
Despite these insights, the broader understanding of microRNA functions in primordial follicle recruitment, activation, and maturation remains limited. Several microRNAs have been identified as regulators in these processes. For instance, miR-143 plays a vital role in the formation of primordial follicles (Zhang et al. 2013), whereas miR-125b negatively affects primordial follicle assembly (Wang et al. 2016). Additionally, miR-92b-3p has emerged as a novel regulator in follicle assembly (Li et al. 2019), and miR-378-3p contributes to maintaining the mouse primordial follicle pool size by regulating cell autophagy and inhibiting apoptosis (Sun et al. 2020). However, the relationships of these microRNAs with POI have yet to be established. Further research could uncover novel biomarkers for assessing ovarian reserve, potentially improving both the understanding and management of POI. This advancement could expand the diagnostic and therapeutic options available for this condition.
4.2 miRNAs and Granulosa Cells
Granulosa cells (GCs), crucial for follicular health and oocyte maturation, produce hormones and growth factors such as E2, inhibin, and insulin-like growth factor (IGF)-1 (Almeida et al. 2018). In idiopathic POI cases, GC dysfunction often manifests as accelerated apoptosis, disrupting follicular maturation and leading to follicle depletion. This dysfunction can be attributed to chemotherapy, environmental toxins, or genetic predispositions, which are mediated by disruptions in noncoding RNA pathways that regulate cell survival and apoptosis (Williams and Erickson 2000). Such damage correlates with reduced estrogen and AMH production, which in turn leads to elevated FSH levels, a diagnostic marker for POI (Hikabe et al. 2016). It is imperative to understand the health of GCs and the molecular pathways that influence their survival, with a particular focus on miRNAs.
Research progress has revealed significant insights into the role of miRNAs in GCs and their relationship with POI (Figure 1C). Specifically, miR-379-5p and miR-127-5p exhibit aberrant expression in GCs from women with biochemical premature ovarian insufficiency (bPOI). This condition is characterized by elevated FSH levels, indicating transitional ovarian failure. This stage is part of a continuum in ovarian function decline that includes occult (incipient ovarian insufficiency) and overt phases (De Vos et al. 2010; Tucker et al. 2016; Welt 2008). The identification of pivotal miRNAs in POI has progressed slowly due to challenges in sample collection. Unlike overt POI, follicle depletion is not complete at the bPOI stage, enabling the collection of GCs from individuals at this early phase of POI. miR-379-5p suppresses GC proliferation and diminishes DNA repair efficiency by targeting PARP1 and XRCC6, thereby playing a pathogenic role in POI through the disruption of DNA repair mechanisms (Dang et al. 2018). MiR-127-5p negatively affects GC proliferation and DNA damage repair (DDR) by downregulating HMGB2, potentially serving as a biomarker for ovarian reserve and highlighting the importance of DNA repair disruptions in POI pathogenesis. (Zhang et al. 2020). Furthermore, the dysfunction of GCs due to accumulated DNA damage and defective DDR mechanisms induces cellular senescence, which is now recognized as a key pathogenic process in POI. Recent studies have also spotlighted the abnormal expression of DDR-associated genes in GCs (Ma et al. 2024). To substantiate the direct linkage between DDR abnormalities in GCs and POI pathogenesis, detailed pathological assessments of DDR levels and induced senescence in GCs are necessary during the progression of POI.
Research progress has revealed significant insights into the role of miRNAs in GCs and their relationship with POI (Figure 1C). Specifically, miR-379-5p and miR-127-5p exhibit aberrant expression in GCs from women with biochemical premature ovarian insufficiency (bPOI). This condition is characterized by elevated FSH levels, indicating transitional ovarian failure. This stage is part of a continuum in ovarian function decline that includes occult (incipient ovarian insufficiency) and overt phases (De Vos et al. 2010; Tucker et al. 2016; Welt 2008). The identification of pivotal miRNAs in POI has progressed slowly due to challenges in sample collection. Unlike overt POI, follicle depletion is not complete at the bPOI stage, enabling the collection of GCs from individuals at this early phase of POI. miR-379-5p suppresses GC proliferation and diminishes DNA repair efficiency by targeting PARP1 and XRCC6, thereby playing a pathogenic role in POI through the disruption of DNA repair mechanisms (Dang et al. 2018). MiR-127-5p negatively affects GC proliferation and DNA damage repair (DDR) by downregulating HMGB2, potentially serving as a biomarker for ovarian reserve and highlighting the importance of DNA repair disruptions in POI pathogenesis. (Zhang et al. 2020). Furthermore, the dysfunction of GCs due to accumulated DNA damage and defective DDR mechanisms induces cellular senescence, which is now recognized as a key pathogenic process in POI. Recent studies have also spotlighted the abnormal expression of DDR-associated genes in GCs (Ma et al. 2024). To substantiate the direct linkage between DDR abnormalities in GCs and POI pathogenesis, detailed pathological assessments of DDR levels and induced senescence in GCs are necessary during the progression of POI.
Studies on oxidative stress, known to induce GC apoptosis leading to follicular atresia, have shown that miR-181a, upregulated in response to oxidative stressors, promotes apoptosis in GCs by suppressing sphingosine-1-phosphate receptor 1 (S1PR1), a critical mediator in this pathway (Zhang et al. 2019). Additionally, the role of melatonin in modulating GC autophagy in POI, focusing on the miR-15a-5p/Stat3/PI3K-Akt–mTOR pathway, suggests melatonin's protective effects on the ovary may involve suppression of miR-15a-5p and activation of autophagy-related pathways (Wu et al. 2022). This antioxidative action or limitation of oxidative stress-induced autophagy shields oocytes and GCs from oxidative damage, potentially preventing ovarian aging. Since GC apoptosis adversely impacts pregnancy outcomes in in vitro fertilization (IVF) treatments, safeguarding GCs from increased apoptosis could significantly enhance therapeutic strategies for managing ovarian dysfunction and improving IVF outcomes.
4.3 miRNAs and the Circulatory System
Several miRNAs identified in the serum of peripheral blood of patients with POI or in POI mouse models are crucial in the disease's onset and progression (Figure 1A). For example, in cisplatin-induced POI, miR-483-5p interacts with FKBP4 protein, unveiling a critical pathway for understanding the disease's etiology. This interaction suggests that miR-483-5p may increase ovarian sensitivity to cisplatin, leading to ovarian dysfunction. Additionally, serum levels of miR-483-5p could serve as a predictive biomarker for POI development and progression (Zhao et al. 2021). Similarly, miR-21, which is vital for ovarian folliculogenesis in autoimmune POI, interacts with several genes, including Pellino-1 (Peli1). Autoimmune POI in the study was characterized by an onset before age 40, FSH levels above 25 mIU/mL on two separate occasions (≥ 4 weeks apart), and the presence of autoimmune disorders and/or autoimmune antibodies. Individuals with autoimmune POI, like their counterparts in the control group, excluded women who received hormone or immunosuppressive therapy (≤ 3 months prior), had undergone oophorectomy, pelvic irradiation, or previous chemotherapy. Also excluded were women with thromboembolic processes, severe hypertension, severe obesity, hepatic or renal insufficiency, ovarian abnormalities, or known karyotype abnormalities. Reduced expression levels of miR-21 and Peli1 have been documented in both mouse models and patients with autoimmune POI, indicating its role in the autoimmune mechanisms contributing to POI (Li et al. 2020). Furthermore, miR-144, studied in the context of 4-vinyl cyclohexene diepoxide (VCD)-induced POI, exhibits down-regulation in serum and ovarian tissues, correlating with increased autophagy and hormonal alterations. Thus, enhancing miR-144 expression might mitigate VCD-induced autophagy and restore ovarian function (Zhou et al. 2023). The up-regulation of miRNA-146 in response to lipopolysaccharide (LPS)-induced ovarian dysfunction suggests its protective role. MiRNA-146 can alleviate ovarian damage by inhibiting the TLR4/NF-κB signaling pathway, reducing pro-inflammatory cytokines IL-6 and TNF-α expression, thereby enhancing cell viability and reducing apoptosis in ovarian GCs (He et al. 2022). These circulating miRNAs offer non-invasive biomarkers for earlier POI detection, presenting new therapeutic targets to modulate gene expression involved in POI and suggesting a potential for clinical intervention before the onset of overt symptoms. However, the specificity and sensitivity of these miRNAs as biomarkers for POI require rigorous validation to realize their full diagnostic and therapeutic potential.
4.4 miRNAs and Other Noncoding RNAs
The intricate roles of miRNAs in POI are underscored by extensive studies utilizing high-throughput data and network analyses. For instance, Liu et al. examined miRNA expression profiles in GCs from patients with bPOI, constructing a detailed lncRNA-miRNA-mRNA network from the GEO database. This study highlighted significant variations in 131 mRNAs, 191 lncRNAs, and 28 miRNAs, identifying key miRNAs like hsa-miR-27a-3p and hsa-miR-17-5p as central regulators. These miRNAs are crucial for orchestrating gene expression that affects ovarian function, highlighting their potential regulatory impact on reproductive pathophysiology (Liu et al. 2024). Another study focused on miRNA regulatory element (MRE) analysis in 30 patients with POI, identifying several miRNAs such as hsa-miR-548c-3p, hsa-miR-924, and hsa-miR-4677-5p, among others, as targets of specific circular RNAs. These circular RNAs act as “molecular sponges,” modulating the signaling pathways controlled by miRNAs, thus influencing the progression of POI (Z. Wang et al. 2023). These findings demonstrate the profound influence of miRNAs in the molecular mechanisms governing POI, offering insights into potential therapeutic targets and biomarkers for this condition.
5 The Role of miRNAs in Potential Treatment of POI
Recent investigations into miRNAs have illuminated potential therapeutic strategies for POI. For instance, the naturally occurring flavonoid diosmin has demonstrated efficacy in mitigating CTX-induced POI in Rat models. These protective effects are further supported by the upregulation of miRNA-145 and its target genes, such as vascular endothelial growth factor B (VEGF-B) and regulator of the cell cycle (RGC32), which play crucial roles in maintaining cellular integrity and promoting angiogenesis within ovarian tissues (Abogresha et al. 2021).
The therapeutic potential of mesenchymal stem cell (MSC) transplantation in treating POI has been well-documented across various studies. Specifically, bone marrow-derived MSCs (BM-MSCs), along with their conditioned media, significantly express various lncRNAs and miRNAs, such as Neat-1, Hotair1, mir-21-5p, mir-144-5p, and mir-664-5p. When transplanted into CTX-induced POI rat models, BM-MSCs enhance ovarian and hypothalamic function, improve IGF-1 and kisspeptin signaling within the hypothalamic–pituitary-gonadal (HPG) axis, reduce ovarian granulosa cell apoptosis, and support steroidogenesis, angiogenesis, energy balance, and reduce oxidative stress (Ahmed et al. 2024). Similarly, miR-21 improves the ovarian function recovery of zona pellucida 3 (ZP3)-induced autoimmune POI mice with UCMSCs transplantation, and the mechanisms may be through suppressing the PTEN/AKT/FOXO3a signal pathway and up-regulating the circulating of the CD8+ CD28−T cells (Yin et al. 2024).
Moreover, exosomal miRNAs, derived from various sources of MSCs including human amniotic mesenchymal stem cells (hAMSCs), and human umbilical cord MSCs (hucMSCs), have shown significant therapeutic potential in POI models (Figure 1B). For example, in a study conducted on POI mouse models, exosomes from hAMSCs were found to enhance ovarian function and reduce apoptosis in ovarian cells by delivering miR-320a. This microRNA targets the expression of Sirtuin 4 (SIRT4), which is associated with decreased levels of reactive oxygen species and improved cellular proliferation in mouse ovaries and human granulosa cells (hGCs) (Ding, Qian, et al. 2020). Exosomes derived from BM-MSCs that carry miR-21-5p can regulate the Msh homeobox 1 (MSX1)-mediated Notch signaling pathway. This interaction inhibits apoptosis in GCs and enhances hormone synthesis, providing therapeutic benefits in models of ZP3-induced autoimmune POI mice (Yang et al. 2024). Additionally, exosomes from hUMSCs carrying miR-17-5P have been shown to restore ovarian function by inhibiting SIRT7, a key regulator of DNA repair and cellular stress responses. This effect was observed both in CTX-damaged hGCs and in the ovaries of CTX-induced POI model mice, suggesting a broad regulatory impact that extends beyond apoptosis to enhance DNA repair capabilities in ovarian cells (Ding, Zhu, et al. 2020). Furthermore, in studies conducted on cisplatin-induced POF rat models, miR-126-3p-modified exosomes from hucMSCs were found to promote angiogenesis and reduce apoptosis in ovarian granulosa cells (OGCs), emphasizing the angiogenic and reparative roles of specific miRNAs in ovarian rejuvenation (Qu et al. 2022). Exosomal miRNAs derived from ovarian tissues also exhibit therapeutic potential. Utilizing a POI mouse model induced by CTX and busulfan, a study highlighted the role of exosomal miR-122-5p in modulating GC apoptosis, revealing significant therapeutic implications (Zhang et al. 2022). Exosomal miRNAs represent a targeted approach to modulate specific cellular pathways that are dysregulated in POI. Their capacity to regulate apoptosis, oxidative stress, and angiogenesis addresses the multifactorial nature of ovarian decline. Additionally, the non-invasive delivery mechanism of exosomes presents a substantial advantage over traditional therapies, which often suffer from systemic side effects and limited targeting capabilities.
6 Conclusions and Future Perspective
MiRNAs are pivotal in regulating a variety of cellular functions across different cell types, including oocytes, and GCs in POI. They regulate multiple genes, influencing a broad spectrum of cellular processes through the expression and activity of numerous proteins. The extensive impact of miRNAs suggests that their regulation and dysregulation play crucial roles in the development and progression of POI. miRNAs are involved in various critical pathophysiological processes such as the proliferation or apoptosis of oocytes and GCs, steroidogenesis, folliculogenesis, autophagy, and oxidative stress, which are essential in the onset and progression of POI. Due to variations in the expression patterns of many miRNAs in the circulatory system at different disease stages, they could serve as valuable biomarkers for POI patients. Utilizing miRNAs as biomarkers could greatly enhance early detection and lead to improved clinical outcomes through earlier therapeutic intervention. Moreover, these studies also suggest that manipulating certain miRNAs could lead to therapeutic advancements in treating POI.
While considerable progress has been made in understanding the roles of miRNAs in the pathogenesis of POI, there are still many unknowns about the precise mechanisms, and the limitations of current experimental methods should not be overlooked. For instance, a significant limitation in the study of microRNA mechanisms in vitro is the disparity between overexpression systems and actual physiological conditions. The excessive abundance of hypothetical miRNAs in these systems far exceeds natural levels, potentially overstating the function of miRNAs. As a result, the phenomena observed in the lab may not accurately reflect what occurs under natural conditions. Another significant challenge is the diversity of miRNA targeting, which complicates the identification of specific miRNA-gene interactions and their physiological impacts on POI. The combined effects of multiple miRNAs can significantly alter gene expression and cellular functions, crucial in POI contexts. Moreover, the focus on a limited set of well-known miRNAs might neglect less studied miRNAs that could play critical roles in POI. Furthermore, methodological choices can introduce biases in miRNA expression, potentially skewing our understanding of their role in POI pathophysiology. It is essential to validate miRNA expression through in situ, histological examinations. Additionally, clinical studies on POI patients often suffer from small sample sizes, compromising the statistical strength and generalizability of the results. The lack of extensive, diverse patient cohorts limits our ability to draw reliable conclusions about the impact and therapeutic potential of miRNAs in managing POI. Future research should expand to multi-center, multi-sample, and multi-ethnic studies to enhance our comprehension and treatment of POI.
This article emphasizes the influence of microRNA on regulating POI progression. Understanding the specific roles of these miRNAs is crucial for identifying new biomarkers and developing effective treatment options for POI, ultimately enhancing patient prognosis and outcomes.
Author Contributions
Study conception and design: Hua Hua and Hui Zhu; data collection: Xi-Xia Cao; draft manuscript preparation: Hui Gao; review and editing: Hui Gao and Xi-Xia Cao; project administration: Hua Hua and Hui Zhu. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
Thanks to Dr. Chaowen Shi for the assistance in reviewing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
This review is based on the literature searched in the NCBI database and individual knowledge of the authors on the topic. It does not include any unpublished data or code.




