Rapid Whole Genome Sequencing Uncovers a Triple Diagnosis: X-Linked Chondrodysplasia Punctata, MECP2-Related Disorder, and Mosaic Jacobs Syndrome
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Rapid Whole Genome Sequencing (rWGS) is increasingly being used in neonatal intensive care units, as there is growing evidence that rare singe gene disorders present in the neonatal period and early identification can change management. While the diagnostic utility is increased with this broad testing, the possibility of unexpected findings also increases significantly. Here, we present a patient found to have three distinct genetic conditions through rWGS testing, with significant psychosocial and health consequences.
Methods and Results
This case report describes a patient who was identified with a form of chondrodysplasia punctata, as well as incidental findings of MECP2-related disorder and Jacobs' syndrome. To our knowledge, this is one of the first documented cases of triple genetic diagnoses in the literature, underscoring the expanding clinical utility of rWGS.
Conclusion
Our patient represents a unique example of the utility of rWGS in the NICU setting. As two of the three conditions were unexpected results, his case is an important reminder of the possibility of unexpected findings for both providers and families. His case demonstrates the importance of pretest counseling and consenting processes, particularly in an acute setting. It also will add to our understanding of MECP2 variant presentations in males in the future.
1 Case Presentation
The patient was born full-term to non-consanguineous parents via cesarean-section. The pregnancy, conceived using in vitro fertilization following a 7-year history of infertility, was complicated by polyhydramnios. Prenatal imaging, preimplantation genetic testing for aneuploidy, and carrier screening were reportedly normal. After delivery, the patient was transferred to the neonatal intensive care unit (NICU) for transient tachypnea of the newborn. He was noted to have a right club foot, dysmorphic features, and hypotonia. Imaging demonstrated rib and vertebral anomalies as well as an atrial septal defect (ASD). The patient was transferred to our hospital at 6-days-of-life for higher level of care. A skeletal survey showed excessive stippling in the spine and multiple epiphyses with normal length long bones, concerning for non-rhizomelic chondrodysplasia punctata. Genetics was consulted at that time and rapid whole genome sequencing (rWGS) was ordered due to crucial prognostic differences depending on the underlying cause of chondrodysplasia punctata. The rWGS was ordered clinically through a CLIA-certified lab. Based on the results, the patient was given a diagnosis of X-linked chondrodysplasia punctata due to a maternally inherited variant in ARSL, associated with a good prognosis. However, two additional genetic disorders were detected as well: MECP2-related disorder from a maternally inherited pathogenic variant in MECP2, and de novo mosaic 47, XYY or Jacobs syndrome. Specifically, he had a c.185G > T, p.R62M variant (NM_000047.3) in ARSL that was classified as a variant of uncertain significance, but fit his phenotype of non-rhizomelic chondrodysplasia punctata, inherited from his mother. He had a c.1155_1200del, p.L386Afs*8 variant (NM_004992.3) in MECP2 classified as pathogenic also inherited from his mother. Additionally, he had mosaic Jacobs Syndrome (XXY) present at 70%. The results of the genetic testing helped direct his medical care during his 38-day stay in the NICU as well as his subsequent outpatient surveillance and management.
Since discharge from the NICU, the patient has followed with orthopedics for his right club foot and has been undergoing bracing with Ponseti shoes and Dobbs bar. At 1 year of age, a repeat X-Ray of the entire spine showed appropriate development with a decrease in stippling and progressive ossification as compared to prior study. The imaging showed no spinal curvature and the patient's hips were well reduced.
Additionally, the patient follows with neurosurgery following a multilevel thoracic laminectomy and posterior spinal fusion due to significant upper thoracic spinal stenosis while admitted to the NICU. Repeat brain MRIs at 1-year-of-age showed significant ventriculomegaly as well as an increased frontal-occipital head circumference (FOC). A VP shunt was placed at that time. His parents have not noted any abnormal movements, staring spells, or other episodes concerning for seizure activity.
The patient was evaluated by otolaryngology (ENT) in the NICU due to stridor. A flexible fiberoptic laryngoscopy exam at that time showed a narrow nasal vault but no other significant anatomic abnormalities. He continues to follow ENT in the outpatient setting for his small airway, small ear canals, noisy breathing at baseline, and bilateral mixed conductive and sensorineural hearing loss. A CT of the internal auditory canals demonstrated bilateral absence of the osseous external auditory canals and potential fusion between the malformed malleus and incus.
Additionally, he continues to be followed by genetics. At his 5-month-old follow-up visit, he was noted to have motor delay and hypotonia. He was unable to fully support his head or roll over but was appropriately engaged socially. At 11 months of age, he continues to have global delays, most notably with gross motor skills. His lower extremities are more hypotonic than his upper extremities. He does not have any words yet, though he has learned how to clap and is reportedly working on learning body parts. He has not experienced any developmental regression. Currently, he is receiving occupational therapy, speech therapy, physical therapy, and hearing therapy. While in the NICU, he received a gastrotomy-tube due to difficult feeding. At his 5 month follow up visit with genetics, he was receiving adequate caloric intake by mouth, and his G-tube had been removed. His weight is in the 10th–25th percentile, but he has poor linear growth with length < 3rd percentile (50th percentile for a 6 months), and 90th percentile for FOC. Accurate diagnosis paved the way for proper multidisciplinary care and surveillance based on the standard of care for each of the genetic conditions diagnosed in our patient.
2 Discussion
This report outlines a patient who underwent rWGS for prognostic information and received a triple diagnosis that was surprising and unanticipated, but still helpful in determining prognosis and for directing his patient care. While he was found to have a form of chondrodysplasia punctata with a good prognosis, he was also found to have MECP2-related disorder, associated with variable and serious prognostic implications. He was also found to have Jacobs syndrome, which can also be variable in its presentation and symptom onset. To the best of our knowledge, this is one of the first reported cases of triple genetic diagnoses in the literature (Guzel Dirim et al. 2024; Matis et al. 2020; Pasquetti et al. 2023), and points to further utility of rWGS.
At the time that the rWGS was ordered for our patient, his phenotype best fit with the maternally inherited variant in ARSL (c.185G > T, p.R62M), which is associated with X-Linked Chondrodysplasia Punctata (CDPX1). CDPX1 has been associated with pathogenic variants in ARSL (Braverman et al. 1993). Fewer than 50 individuals have been reported with CDPX1, which is characterized by chondrodysplasia punctata, brachytelephalangy, and nasomaxillary hypoplasia. The bone findings associated with this condition often improve by childhood, although adults with this condition can have short stature. Individuals with CDPX1 are at increased risk for respiratory problems, hearing loss, cervical spine abnormalities, and developmental delays/intellectual disability. Unlike some other chondrodysplasia punctatas, CDXP1 is associated with a normal life span. Our patient had a variant of uncertain significance in ARSL; however, due to the phenotypic overlap of epiphyseal stippling, short stature with relative macrocephaly, hearing loss, cervical spinal stenosis, and nasomaxillary hypoplasia the variant was the most likely explanation for his presentation, and he was given a diagnosis of CDPX1.
While this variant was a very good fit for the features that first prompted genetics' involvement, it was not the only significant finding. Pathogenic variants in MECP2 were originally associated with classic Rett syndrome in females and early or embryonic lethality in males. However, with the expansion of genetic testing, a spectrum of other phenotypes have emerged in males and females. The most common is severe neonatal encephalopathy, but syndromic or non-syndromic intellectual disability, and pyramidal signs, parkinsonism and macroorchidism, X-Linked (PPM-X syndrome) have also been described (Kaur and Christodoulou 1993). There are some genotype–phenotype correlations for MECP2 including null variants being associated with classic Rett syndrome in females and lethality in most males. Genotypes that maintain some protein function are found to cause the milder, recently described expansion of phenotypes (Inuzuka et al. 2021). This patient's rWGS identified a maternally inherited pathogenic variant in MECP2 (c.1155_1200del, p.L386Afs*8). While the variant leads to a premature stop, it is close to the 3′ terminus of the gene and thus may still result in protein production that is not rapidly degraded, missing only the last XX amino acids. There have been no reports of males with this specific MECP2 variant, but females with C-terminal deletions have been reported with somewhat milder presentations when compared to individuals with other MECP2 variants, specifically having better growth parameters and ambulation (Bebbington et al. 2010; Ronen, Brady, and Tarnopolsky 2017). Additionally, there are reports of two boys with MECP2 variants near our patient's (PMID: 19914908 and PMID: 28089766) who inherited these changes from unaffected mothers. Both had developmental delays followed by regression and the eventual development of seizures (Dayer et al. 2007; Zhang et al. 2017). While not clinically indicated, measurement of plasmalogen levels in the future might further define the physiological impact of the mutation. While predicting the exact impact this variant will have on our patient in the future is challenging, significant developmental delays and intellectual disability are expected.
Finally, mosaic Jacobs syndrome is a common condition, present in about 1 in 1000 males, that goes undiagnosed in up to 85% of affected individuals. It is caused by an additional copy of the Y chromosome. Symptoms are generally very mild and can include an increased risk for asthma, seizure disorders, learning disabilities, autism, and behavior problems (Sood and Clemente Fuentes 2024). The proband was mosaic for Jacobs syndrome, present at 70%. Although the symptoms of this diagnosis did not contribute to the reason for testing, if this diagnosis does impact our patient's clinical picture, it will be difficult to parse out the effects from this diagnosis versus one of his other two diagnoses, as behavior problems, learning disabilities and seizures can also be attributable to his variant in MECP2 and/or ARSL.
We found only one other report in the literature of a patient that has undergone genetic testing that came back positive for three distinct genetic diagnoses (Pasquetti et al. 2023). The patient was a 44-year-old-man with a genetic workup positive for autosomal dominant polycystic kidney disease (ADPKD) due to a pathogenic variant, c.2152C > T–p.(Gln718Ter), in the PKD1 gene, phenylketonuria due to the presence of two missense variants, c.842C > T–p.(Pro281Leu) and c.143 T > C-p.(Leu48Ser) in the PAH gene, and a 915 Kb duplication on chromosome 15. While very few triple diagnoses have been reported in the literature, Kurolap et al. published a thorough literature review in 2016 that describes the natural history of 14 separate cases identified to have double genetic diagnoses (Kurolap et al. 2016). Recognizing multiple diagnoses in a patient based solely on clinical evaluation can be complicated by the different degrees of penetrance as well as variable expressivity seen in many genetic syndromes. Kurolap describes factors they found in their research that increase the risk of a second genetic diagnosis, including consanguinity, founder mutation, reproductive isolate, having at least one relatively common condition, and a family history of learning disabilities, mental illness, or a specific disorder.
With advancements in technology and genetic testing, the occurrence of multiple diagnoses will become increasingly common (Capra et al. 2023). This evolution necessitates meticulous clinical judgment, as overlapping symptoms can obscure the individual contributions of each genetic condition. The variable expressivity and penetrance of genetic disorders further complicates accurate diagnosis. Given these complexities, advanced multidisciplinary care is essential. This approach ensures that patients receive comprehensive evaluations and treatments from a range of specialists, each bringing their expertise to address different aspects of the patient's health. Such coordinated care can significantly enhance the quality of life for patients with multiple genetic conditions. Moreover, the process of genetic testing must include careful pretest counseling and informed consent. Patients and their families need to understand the potential outcomes, implications, and limitations of genetic testing. Pretest counseling provides a platform for discussing the possibility of identifying multiple genetic conditions, the potential impact on the patient's health and well-being, and the subsequent steps that may be required for management and treatment.
While early identification of certain rare diseases can be highly beneficial, especially when effective treatments or surveillance guidelines exist, identifying many other rare diseases may not influence clinical management due to a lack of treatment options or regulatory guidance. In such cases, the diagnosis may inadvertently cause unnecessary stress and health-related anxiety for patients and their families. We recommend exercising caution in these scenarios by engaging families in discussions about the possibility of identifying conditions without specific treatments that alter the long-term outcomes of disease or other conditions which may not present until later in life. This approach respects their preferences and ensures alignment with their values and priorities. Additionally, the potential psychological and emotional impacts on siblings should be carefully considered as part of this process. Comprehensive informed consent should be obtained before testing, emphasizing the implications of discovering conditions without actionable medical interventions.
Informed consent ensures that patients and their families are fully aware and agreeable to the testing process, respecting their autonomy and rights. As genetic testing technology continues to advance, it is also important to consider the ethical and psychosocial aspects of identifying multiple genetic conditions in a single patient. The emotional and psychological impact on patients and their families can be profound, necessitating ongoing support and counseling Figure 1.
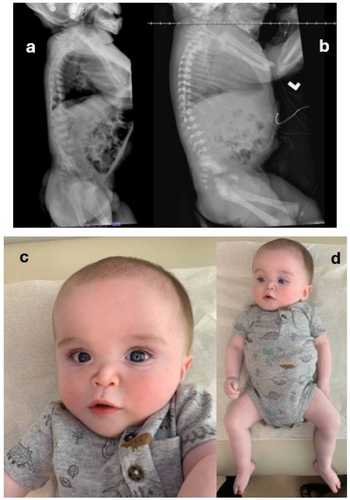
3 Conclusion
The rise of multiple genetic diagnoses due to advanced testing technologies underscores the importance of careful clinical judgment, multidisciplinary care, and thorough pretest counseling and informed consent. This holistic approach helps manage the complex interplay of symptoms from multiple conditions and supports patients and their families through the diagnostic and treatment journey. Our patient represents a unique example of a patient identified to have three distinct genetic conditions through rWGS in the NICU setting. As two of the three conditions were unexpected results, his case is an important reminder of the possibility of unexpected findings for both providers and families. His case demonstrates the importance of pre-test counseling and consenting processes, particularly in an acute setting. It also will add to our understanding of MECP2 variant presentations in males in the future.
Author Contributions
M.S. reviewed the literature and wrote original draft. K.S., P.H., and L.F. clinically evaluated the patient and revised manuscript for final version.
Acknowledgements
We wish to thank the family reported for allowing their story to be shared.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




