A novel intronic variant causing aberrant splicing identified in two deaf Chinese siblings with enlarged vestibular aqueducts
Suyang Wang and Yi-Ming Zhu contributed to the work equally and should be regarded as co-first authors.
Yufen Guo and Xiaowen Liu contributed to the work equally and should be regarded as co-corresponding authors.
Abstract
Objective
We aimed to evaluate the genotype–phenotype relationship in two Chinese family members with enlarged vestibular aqueduct (EVA).
Methods
We collected blood samples and clinical data from each pedigree family member. Genomic DNA was isolated from peripheral leukocytes using standard methods. Targeted next-generation sequencing and Sanger sequencing were performed to find the pathogenic mutation in this family. Minigene assays were used to verify whether the novel intronic mutation SLC26A4c.765+4A>G influenced mRNA splicing.
Results
Hearing loss in the patients with EVA was diagnosed using auditory tests and imaging examinations. Two pathogenic mutations, c.765+4A>G and c.919-2A>G were detected in SLC26A4. In vitro minigene analysis confirmed that c.765+4A>G variant could cause aberrant splicing, resulting in skipping over exon 6.
Conclusions
The SLC26A4c.765+4A>G mutation is the causative variant in the Chinese family with EVA. Particular attention should be paid to intronic variants.
1 INTRODUCTION
Enlarged vestibular aqueduct (EVA), a common type of autosomal recessive hearing loss, is the most common inner ear imaging abnormality in children and adolescents with sensorineural hearing loss. Most patients with EVA present with non-syndromic hearing loss (NSHL), which is typically fluctuating and sometimes progressive, accompanied by speech delay, tinnitus, vertigo, vomiting and other symptoms, while a small number of them are complicated by goiter, called Pendred syndrome (PDS). Imaging examination is the golden standard for diagnosing EVA at present, and temporal bone high-resolution computed tomography (HRCT) is the first choice in the clinic. There are two main diagnostic criteria: (1) Valvassori standard, i.e., the median point of the semicircular canal angle to the VA outer diameter is more than 1.5 mm (Valvassori & Clemis, 1978) and (2) the Cincinnati standard, i.e., the diameter of the midpoint is greater than 0.9 mm or the outer diameter of VA is more than or equal to 1.9 mm (Vijayasekaran et al., 2007). At present, the main treatment methods for EVA include drugs, wearing hearing aids and cochlear implantation. Cochlear implants have been widely used in the clinical treatment of patients with severe NSHL. As an important means of treating EVA, this technology can not only fully compensate for the hearing loss of patients, but also can effectively maintain the hearing of patients at the level of normal communication, which is enough to return pre-lingual deaf children to mainstream society.
Genes that have been associated with non-syndromic EVA include SLC26A4, GJB2, FOXI1, KCNJ10, EPHA2, TMC1 and POU3F4 (Roesch et al., 2021). Variants of SLC26A4 are among the most common causes of EVA worldwide. In different regions of China, biallelic pathogenic variants in SLC26A4 have been found in 79%–100% of EVA patients (Qing et al., 2015; Tian et al., 2021; Wu et al., 2019). In our previous study, we found biallelic pathogenic variants in SLC26A4 in 47 of 53 EVA patients (88.68%) (Wang et al., 2019).
The human SLC26A4 gene is located on chromosome 7q31 and consists of 21 exons, the first of which is non-coding. SLC26A4 gene encodes a highly hydrophobic membrane protein named pendrin, which is composed of a transmembrane (TM) domain and an intracellular sulfate transporter–antisigma factor agonist (STAS) domain. It mediates the transport of anions like Cl−, OH−, HCO3−, and I−, as well as nitrate, formate, and thiocyanate. Reduction in pendrin functionality causes endolymph acidification and is thought to be responsible for Ca2+ re-absorption inhibition, yielding auditive sensory transduction defects (Wangemann et al., 2007). Pendrin is mainly expressed in the inner ear, thyroid and kidney, so pathogenic variants in the SLC26A4 gene are involved in syndromic deafness (PDS, OMIM 274600), characterized by congenital sensorineural hearing loss, goiter, and congenital deafness (DFNB4; OMIM 600791), both of which are associated with EVA. To date, 8647 mutations have been reported in SLC26A4, of which 487 are pathogenic (https://deafnessvariationdatabase.org/gene/SLC26A4).
In the present study, we identified two compound heterozygous mutations of SLC26A4: c.919-2A>G/c.765+4A>G in a family with non-syndromic hearing loss. Although some intron variants, splice site variants and synonymous variants may be more destructive than coding variants, research on intronic variants is relatively limited (Kallel-Bouattour et al., 2017).
To determine whether c.765+4A>G variant is a pathogenic site for the affected patients, a pathogenicity study of the SLC26A4 gene c.765+4A>G variant was performed. This will extend the scope of knowledge of the spectrum of SLC26A4 mutations in the Chinese population and will allow us to further explore the relationship between mutations and phenotypes.
2 MATERIALS AND METHODS
2.1 Subjects
One 2-generation Chinese family members with NSHL were introduced to the Department of Otolaryngology and Head and Neck Surgery at Lanzhou University Second Hospital (Lanzhou, China) in July 2021. The proband (II-1) and her little brother (II-2) were diagnosed with EVA based on a physical examination. Their parents had normal hearing.
2.2 Audiological, imaging evaluation and ultrasonic inspection
The pedigree members underwent a full medical history and comprehensive audiological evaluation, which were completed both by otorhinolaryngology and a clinical geneticist, including otoscopic examination, tympanometry, pure-tone audiometry (PTA) or behavioral observation audiometry (BOA), distortion product evoked otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABRs). HRCT, Magnetic resonance imaging (MRI), the thyroid function tests include thyroid ultrasound and measurements of hormone levels were also performed to verify whether the family members had other complications disorders.
2.3 Mutation screening
After informed consents were obtained, blood samples (2 mL) were obtained from the core pedigree members, 500 normal control individuals (263 males and 237 females, aged from 18 to 25 years old) and 122 unrelated Chinese families. The common deafness genes (GJB2, GJB3, SLC26A4, and MT-RNR1) had been screened on these family members by direct sequencing, common deafness genes GJB2, GJB3, SLC26A4 and MT-RNR1 by a custom-bydesign 48-Plex SNPscan TM Kit (Genesky Biotechnologies Inc., Shanghai, China), which was developed using patented SNP genotyping technology by double ligation and multiplex fluorescence PCR (Roesch et al., 2021). The methods are the same as Du, et al. (2014). To further clarify the molecular etiology, targeted next-generation sequencing technology was applied to identify the causative gene in this family. The specific experimental methods are the same as Zhu, Li, Gao, et al. (2021). Confirmation of the identified variants and analysis of their co-segregation with phenotype were evaluated by Sanger sequencing in the family members. Primer sequences are shown in Table 1.
| Primer | ||
|---|---|---|
| chr7-107315558 | F | TTTAGAGTGGTGGAGGAAGGG |
| R | TCAAGCAATCTGCCCACC | |
| chr7-107323898 | F | CCATTGTCGTCTGTATGGCA |
| R | AGAGGAACACCACACTCACC | |
2.4 In silico analysis
The bioinformatics splicing tool Human Splicing Finder (HSF) version 3.1(http://www.umd.be/HSF3/HSF.shtml), SpliceAI (https://spliceailookup.broadinstitute.org/), and Varseak (https://varseak.bio/index.php) software were used to predict the possible influence of this mutation in the intron.
2.5 Minigene vector construction strategy
Using the patient's genomic DNA (gDNA) containing the AC variant and wild-type human DNA as templates, partial intron 5 (343 bp)–exon 6 (165 bp)–partial intron 6 (612 bp) was inserted into the pcMINI vector. pcMINI was used as the carrier backbone to construct pcMINI-WT/mut. Intron B and exon B are highly recognized sequences, which were selected from human gDNA, and are independent of the inserted minigene sequences. The selected sequence was used as part of a tool vector to detect whether the mutated minigene vector affects the splicing of the associated exon.
Two pairs of nested primers, 12027-A-F/14981-A-R and 12216-A-F/14528-A-R, were designed to perform nested PCR using patient gDNA as a template. The second PCR was performed using products from the first round of nested PCR as a template, with pcMINI-A-KpnI-F/pcMINI-A-EcoRI-R as primers, for 30 cycles; a fragment of 1120 bp of pcMINI was amplified. The third PCR was performed using PCR products from the second round as a template, with pcMINI-A-KpnI-F/pcMINI-A-EcoRI-R as primers, for 30 cycles; a fragment of 1685 bp of pcMINI was obtained. gDNA, gene names, and primers are shown in Table 2. The standard PCR program was as follows: 94°C for 2 min, followed by 30 cycles of 94°C for 10 s, 57°C for 30 s, and 68°C for 20 s, with a final extension step at 72°C for 7 min.
| Primer | Sequence (5′–3′) |
|---|---|
| 12027-SLC26A4-F | CCTACGTGTCTCTCTCGCCT |
| 12216-SLC26A4-F | TAGCTGGGACCATAGGGACG |
| 14528-SLC26A4-R | GGCATGGTGATTGGCTCACA |
| 14981-SLC26A4-R | ATGGTATCATTCCCCCCAAG |
| pcMINI-SLC26A4-KpnI-F | GGTAGGTACCAGAGAAGGGAAATATACGGG |
| pcMINI-SLC26A4-EcoRI-R | TGCAGAATTCGGCGCCTGTAATCCCAGCTA |
| pcMINI-N-SLC26A4-KpnI-F | GGTAGGTACCGGACCTTTTCCAGTGGTGAGT |
| pcMINI-N-SLC26A4-EcoRI-R | TGCAGAATTCTGTAATCCCAGCACTTTGGG |
Finally, the PCR products were purified by electrophoresis and gel recovery. Both pcMINI-A-wt (wild-type) and pcMINI-Amut (c.765+4A>G) contained the entire sequence of exon 6 and part of the upstream and downstream introns.
2.6 Transfection of eukaryotic cells
The recombinant vectors (pcMINI-SLC26A4-wt and pcMINI-SLC26A4-mut) were transiently transfected into cervical cancer cells (HeLa) and human embryonic kidney cells (HEK-293T) according to the manufacturer's instructions (Lipo2000), and then, four samples were obtained. The transfected cells were cultured for 48 h and then collected for analysis.
2.7 Reverse transcription-polymerase chain reaction
Total RNA was extracted from HeLa and 293T cells using the Trizol method (RNAiso PLUS, TaKaRa). The concentrations and purity of RNA were determined by UV spectrophotometry, and cDNA was synthesized by transcription kit (HifairTM 1st Strand cDNA Synthesis SuperMix for qPCR, YEASEN). The PCR products were identified by 2% agarose gel electrophoresis and verified by sequencing.
3 RESULTS
3.1 Family investigation and clinical evaluation
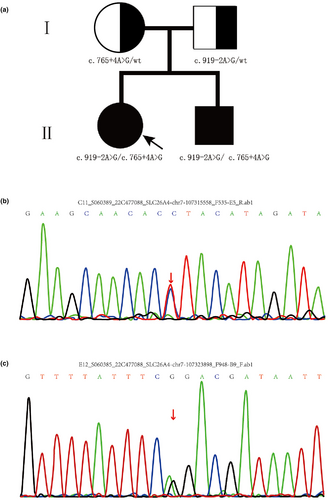
Here, we identified a Chinese Hui family with four members (Figure 1a). All family members had no systemic and thyroid diseases, and the results of physical examinations and otoscopy were normal. None of them had a history of ototoxic drug exposure.
The proband (II-1) had passed the newborn hearing screening. However, when she was 2, BOA, ABR and ASSR indicated severe sensorineural deafness in her right ear and moderate hearing loss in her left ear after a bad cold. In particular, the ABR suggested an air-bone gap (ABG) of about 25 dB. Her parents fitted her with bilateral hearing aids for better speech and language development. The results presented in Figure 2a show a progressive aggravation of hearing loss at 2, 3, and 4 years of age. Based on the tympanogram, the proband was classed as type A (this is normal); HRCT scans of the inner ear showed bilateral EVA without cochlea malformations (Figure 2c); and MRI showed lymphatic enlargement and no vestibular endolymphatic hydrops (Figure 2d). Thyroid goiter was excluded based on thyroid function tests and ultrasonography. Overall, the proband (II-1) was diagnosed with non-syndromic hearing loss with EVA. When she was 4 years old, she received a cochlear implant in her right ear (Figure 2e), and the procedure went smoothly without blowouts, dizziness, and other vestibular symptoms. At present, her hearing has recovered well and she can communicate with people normally.
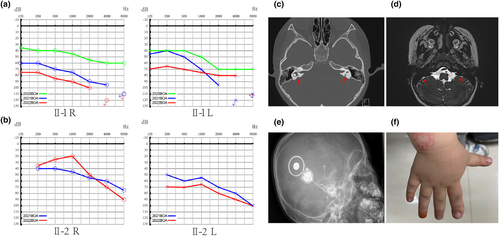
Another member of this family, the younger brother of the proband (II-2), visited the hospital due to delayed language development at the age of 2. He also had EVA, and behavioral hearing assessment revealed moderate hearing loss (Figure 2b). Interestingly, his right index finger is thicker than the other fingers (Figure 2f), but the relationship with the deafness phenotype could not be defined.
3.2 Genetic analysis
After the screening of common deafness genes 13 pathogenic variants (GJB2: 35delC; 176_191del116; 235delC; 299_300delAT; 155delTCTG. GJB3: 538C>T. SLC26A4: 2168A>G; 919-2A>G; 1229C>T. MT-12SrRNA: 1494C>T; 1555A>G; 7445A>G; 12201T>G) in GJB2, GJB3, SLC26A4 and MT-DNA in the family members, the results showed that the siblings (II-1 and II-2) and their father carried the heterozygous SLC26A4 c.919-2A>G mutation, but no other mutation in SLC26A4 was detected in the mother at any mutation site. The results cannot explain their molecular etiology, so targeted next-generation sequencing (about 139 known genes associated with deafness, as detailed in Table S1) was used to identify the pathogenic genes in this family. It was demonstrated that the proband and the little brother had compound heterozygous variants of c.765+4A>G in intron 6 and c.919-2A>G (IVS7-2A>G) in intron 7. In addition, the father and the mother were heterozygous carriers of the c.919-2A>G variant and c.765+4A>G variant, respectively. The identified mutation and its co-segregation are shown in Figure 1. The c.919-2A>G variant is a known pathogenic variant, while c.765+4A>G is novel. The SLC26A4c.765+4A>G mutation site was not analyzed by Sanger sequencing in 500 normal control individuals (263 males and 237 females, aged 18 _ 25 years old) and 122 unrelated Chinese families. GJB2: 35delC; 176_191del116; 235delC; 299_300delAT; 155delTCTG. GJB3: 538C>T. SLC26A4: 2168A>G; 919-2A>G; 1229C>T. MT-12SrRNA: 1494C>T; 1555A>G; 7445A>G; 12201T>G.
3.3 Splicing analysis
Splice AI showed that the original donor splicing site confidence score was reduced by 0.56 in the variant, suggesting that the AC variant affects splicing (https://spliceailookup.broadinstitute.org/). HSF predicted that the c.765+4A>G alters the wild-type donor splicing site and is likely to affect the splicing of mRNA (http://www.umd.be/HSF3/HSF.shtml). Varseak showed that splicing of the donor splicing site is significantly decreased after mutation, suggesting that AC affects mRNA splicing (https://varseak.bio/index.php) (Figure S1).
3.4 SLC26A4 mRNA expression in cells transfected with recombinant plasmids
Since the patient's RNA was not available, minigene splicing assays were performed to verify whether this variant affected the splicing products. A total of four samples were collected 48 h after transfection. A schematic diagram of the minigene construction and RT-PCR results are shown in Figure 3.
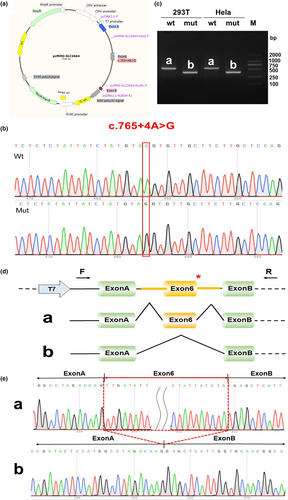
The minigene in vitro assay showed that the mutation c.765+4A>G could affect the normal splicing of mRNA, which led to the skipping of exon 6 (c.601_765del, p.Leu201_Tyr255del). The overall deletion of exon6 did not cause subsequent changes in the reading frame, but only resulted in an internal deletion of 55 amino acids(aa) (from Leu201 to Tyr255). To verify the reliability of the experiment, we designed a parallel experiment, and the same results were obtained (Figure S2).
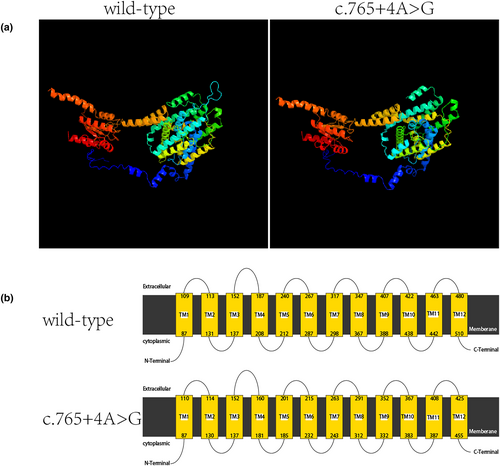
Phyer2 was used to predict the influence of the loss of these 55 aa, which is located in the TM domain (Figure 4). The internal deletion did not reduce the number of TM segments but significantly affected the length of TM segments (especially TM3–TM5) and extracellular loops of the TM domain.
4 DISCUSSION
It is well known that EVA may be accompanied by congenital malformations of the cochlear and semicircular canals. In recent years, EVA as the only inner ear abnormality for which imaging is the diagnostic golden standard, has been recognized as a distinct pattern of congenital inner ear abnormality (Qing et al., 2015; Tian et al., 2021; Wu et al., 2019). The diagnostic tool of choice for VA is HRCT. However, indirect visualization of lymphatic vessels by MRI is also feasible and commonly used, showing enlargement of endolymphatic vessels and endolymphatic sacs. The original Valvassori standard of VA midpoint diameter >1.5 mm has been largely replaced by the Cincinnati standard of VA midpoint diameter ≥0.9 mm or opercular widths ≥1.9 mm (Honda & Griffith, 2022). The VAs with greater widths may reasonably be considered enlarged. In the present study, patients in the family all showed radiographic abnormalities, and the VA midpoint diameter of proband was 2.9 mm. As hearing loss in EVA patients may fluctuate, and the degree of hearing loss at the time of diagnosis may range from normal to profound, diagnosis of EVA is often delayed. The mechanism of hearing loss and vertigo caused by EVA has not been determined yet.
It is noteworthy that the results of PTA in some EVA patients may have obvious ABG differences at low frequencies. In a recent study (Pang et al., 2021), ABGs were observed in 17 ears, accounting for 53.1% of all affected ears. In particular, all the 17 ears showed ABGs of at least 10 dB at 250 Hz and 500 Hz, and only 13 of them showed ABGs at 1000 Hz. As a result, the average gap became narrower by 4–5 dB at each increment from 250 to 1000 Hz compared to that at 250–500 Hz. Currently, the ABG in patients with EVA is regarded as a “third-window” effect of EVA, because the power of sound transmission within the labyrinth is shunted away from the EVA cochlea (Honda & Griffith, 2022; Zhou et al., 2008). In the present study, the ABR of the proband suggested an ABG of about 25 dB, which is consistent with previous studies.
To clarify the molecular etiology, we conducted deafness gene screening (GJB2, SLC26A4 and MT-RNR1) for the proband, who was a heterozygous carrier of the c.919-2A>G mutation. It has been reported that the presence of monoallelic mutations of SLC26A4 is etiologically associated with EVA. Furthermore, monoallelic variants have indeed been found, but in compound heterozygosity with a typical haplotype, suggesting that a monoallelic variant in SLC26A4 by itself is not pathogenic (Pang et al., 2015). The father carrying the SLC26A4c.919-2A>G mutation had normal hearing in this family, so the results cannot explain their molecular etiology. Targeted next-generation sequencing was used to identify the pathogenic genes in this family. Finally, two mutations of the SLC26A4 gene in this study were detected: one in intron 7 (c.919-2A>G) from the father and one in intron 6 (c.765+4A>G) from the mother.
The SLC26A4 c.919-2A>G mutation, situated at the 3′ splice site of exon 8, has previously been reported to be pathogenic. It has been identified that transcripts of the SLC26A4 gene carrying the mutation skip exon 8 completely, resulting in a new connection between exon 7 and exon 9 (Wang et al., 2007). In China, large screenings of SLC26A4 mutations in non-syndromic deafness have been extensively conducted. c.919-2A>G variant was the most prevalent mutation, followed by c.2168A>G. For the Chinese mutation spectrum of SLC26A4 gene, the c.919-2A>G variant was the most common form, accounting for 51.9% (Pang et al., 2015) 58% (Wang et al., 2007) of all the mutant alleles. A recent study of 32,512 neonates with hearing combined with genetic screening found that among 142 children with hearing loss, 31 cases were positive for common deafness gene screening (15 pathogenic variants in GJB2, GJB3, SLC26A4 and MT-RNR1), including three cases (3/31, 9.7%) with homozygous SLC26A4 c.919-2A>G mutations. In the normal hearing population, 425 cases were heterozygous carriers of A mutations, including 328 cases (328/425, 77.2%) of the c.919-2A>G variant (Zhu, Li, Zhuang, et al., 2021).
To verify the pathogenicity of intron mutation c.765+4A>G, we constructed minigene vectors for reverse transcription-PCR splicing validation. The results showed that the intron c.765+4A>G mutation caused aberrant splicing, resulting in the skipping of exon 6, leading to an internal deletion of 55 aa (Figure 3D,). Although the mutation did not cause a frame shift, the large deletion still would consequently result in an altered protein structure. As predicted by Phyer2 (Figure 4), the change affects only the TM domain, especially the partial core domain (Bassot et al., 2017). Site mutations within the lost 55 aa have been reported to cause EVA or NSHL (Table 3).
| Nucleotide change | Residue change | Protein location | Pathogenic effect | Predicted molecular effect | References |
|---|---|---|---|---|---|
| c.611G>T | p.Gly204Val | TM4 | EVA | Disruption of the Gly interaction between TM4 and TM10 | Wang et al. (2007) |
| c.626G>A | p.Gly209Glu | Amphipatic helix | EVA | Clashes with TM11 and TM12 | Wang et al. (2007) |
| c.644T>C | p.Leu215Ser | Intracellular loop | EVA | Formation of Hbond with Ala131 | Chai et al. (2013) |
| c.664G>A | p.Gly222Ser | TM5 | NSHL | Formation of hydrogen bond withThr485 or Cys486 | Yao et al. (2013) |
| c.665G>T | P.Gly222Ala | TM5 | EVA | Insertion of a side chain at the interaction interface of TM13 | Chai et al. (2013) |
| c.668T>C | p.Phe223Ser | TM5 | NSHL | Insertion of a polar residue in a hydrophobic pocket | Han et al. (2007) |
| c.679G>C | p.Ala227Pro | TM5 | NSHL | Insertion of a proline in the α helix | Jiang et al. (2010) |
| c.691G>A | p.Val231Met | TM5 | NSHL | Clashes in the protein core | Anwar et al. (2009) |
| c.707T>C | p.Val239Asp | TM5 | NSHL | Disruption of hydrophobic interaction between TM5 and TM6 | Lazarin et al. (2013) |
| c.754T>C | p.Ser252Pro | External loop | NSHL | Disruption of hydrophobic interaction between TM5 and TM6 | Park et al. (2003) |
The SLC26A4 c.765+4A>G variant is also an intronic region mutation that has not been reported before, but the genotypic variant and phenotypic characterization near this locus have been widely reported (Table 4). To the best of our knowledge, there is no report about this variant in any databases, including the Single Nucleotide Polymorphism Database, The Human Gene Mutation Database, the 1000 Genomes Project, ClinVar, and Exome Sequencing Project v. 6500. However, SLC26A4: NM_000441.2:c.765+4A>C has been reported to be likely pathogenic in the Deafness Variation Database. As suggested by the ACMG/AMP guidelines, a pathogenicity analysis was performed: (a) By minigene vector validation of the in vitro experiment was found that intron SLC26A4 c.765+4A>G variant resulted in aberrant splicing. However, the protein domain and function after splicing are not very clear. (pathogenic evidence PS3). (b) The novel variant SLC26A4 c.765+4A>G was not identified in control groups, the frequency in the normal population database is “-” when compared to GnomAD (moderate pathogenic evidence PM2). (c) EVAS is a recessive genetic disorder. The new mutation was present in trans with another pathogenic mutation (compound heterozygous with another pathogenic mutation); therefore, the SLC26A4 c.765+4A>G variant is considered evidence of pathogenicity (moderate evidence PM3). (d) The deaf siblings' hearing loss and EVA are highly characteristic of the SLC26A4 gene (supporting pathogenic evidence, PP4). and (e) The affected siblings were found to carry the SLC26A4 c.919-2A>G and c.765+4A>G compound heterozygous mutations. The normal-hearing individuals in this family only had monoallelic mutations in the SLC26A4 gene (the father carried the SLC26A4 c.919-2A>G mutation, and the mother carried the SLC26A4 c.765+4A>G mutation), confirming that the phenotype of hearing loss and EVA was co-segregated with genotype in this family (supportive pathogenic evidence, PP1). Taken together, the evidence for the c.765+4A>G mutation is “PS3 + PM2 + PM3 + PP4 + PP1” and is judged to be a pathogenic mutation (very strong pathogenic evidence).
| Genotype | Phenotype |
|---|---|
| c.765_765+3del | Pendred syndrome |
| c.765+1G>A | Hearing loss with dilation of vestibular aqueduct |
| c.765+2T>C | Deafness/pendred syndrome |
| c.765+3A>C | Hearing loss |
| c.765+3A>T | Deafness |
| c.765+4A>C | Hypothyroidism |
5 CONCLUSIONS
To sum up, we report the presence of a novel intronic variant c.765+4A>G in the SLC26A4 gene with c.919-2A>G leading to compound heterozygosity as the cause of EVA. Our findings expand the SLC26A4 gene mutation spectrum and provide additional information for diagnosis and genetic counseling that is associated with EVA-induced hearing loss. Particular attention should be paid to intronic variants that are usually overlooked. We propose to combine predictive bioinformatics with functional analysis to reveal the genetic etiology of undiagnosed genetic diseases.
AUTHOR CONTRIBUTIONS
Yufen Guo, Xiaowen Liu, Suyang Wang, and Yi-Ming Zhu designed the project. Suyang Wang, Wenjuan Ding, and Hui Jia performed patient workup. Xiaowen Liu, Baicheng Xu, Suyang Wang, ChenYang Xu and Yi-Ming Zhu involved genetic analysis. Suyang Wang, Yi-Ming Zhu, Panpan Bian and Xiaowen Liu drafted the manuscript. Yufen Guo, Xiaowen Liu, and Baicheng Xu approved the final version to be published and agreement to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
We thank the families for their invaluable cooperation and participation. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
FUNDING INFORMATION
The authors acknowledge the support from all recruited families for their invaluable cooperation and participation and the financial support from the National Natural Science Foundation of China (Nos. 82160214, 81960192), the Natural Science Foundation of Gansu Province (20JR5RA334, 21JR1RA039, 21JR11RA119, 22JR5RA652), the Cuiying Scientifc and Technological Innovation Program of Lanzhou University Second Hospital (Nos. CY2017-MS18, CY2022-MS-A16), Innovation Fund for colleges and Universities in Gansu Province (2020B-043), the Research Fund for Doctoral Tutor of Lanzhou University Second Hospital (No. bdkyjj-02) and Gansu Provincial Hospital Intra-hospital Research Fund Project-Master Tutor Cultivation Plan (22GSSYB-1).
CONFLICT OF INTEREST STATEMENT
We declare that we have no conflict of interests.
CONSENT FOR PUBLICATION
Any form of data contained in this study involving an individual was consented to all the participants or the next of kin on the behalf of the minors/children participants who were involved in this study.
ETHICS APPROVAL AND CONSENT FOR PARTICIPATION
This study was approved by the Committee of Medical Ethics of Lanzhou University Second Hospital. Written informed consent was obtained from all the participants or the next of kin on the behalf of the minors/children participants who were involved in this study.
Open Research
DATA AVAILABILITY STATEMENT
Please contact author for data requests.




