Fahr's disease linked to a novel mutation in MYORG variants manifesting as paroxysmal limb stiffness and dysarthria: Case report and literature review
Abstract
Background
Primary familial brain calcification (PFBC) is a rare hereditary neurodegenerative disorder associated with the MYORG gene; however, the clinical and radiological characteristics of MYORG-PFBC remain unclear.
Methods
We present relevant medical data obtained from a patient affected by PFBC with a novel MYORG variant and conducted a mutational analysis of MYORG in her family members. We reviewed all reported PFBC cases with biallelic MYORG mutations until April 1, 2023, and summarized the associated clinical and radiological features and mutation sites.
Results
The patient (22-year-old woman) exhibited paroxysmal limb stiffness and dysarthria for 3 years. Computed tomography revealed calcifications in the paraventricular white matter, basal ganglia, thalamus, and cerebellum. Whole-exome sequencing revealed a novel homozygous frameshift variant (c.743delG: p.G248Afs*32) in exon 2 of the MYORG gene (NM_020702.5). To date, 62 families and 64 mutation sites have been reported. Among the reported biallelic MYORG mutations, 57% were homozygous and 43% were compound heterozygous. Individuals with biallelic MYORG mutations experience more severe brain calcification with approximately 100% clinical penetrance. Ten single heterozygous mutation sites are associated with significant brain calcifications.
Conclusion
All patients with primary brain calcification, particularly younger patients without a family history of the disease, should be screened for MYORG mutations.
1 INTRODUCTION
Primary familial brain calcification (PFBC), also known as Fahr's disease, is a rare hereditary neurodegenerative disorder characterized by extensive and symmetric calcifications in the brain. PFBC has a wide range of clinical manifestations, including Parkinsonism, dysarthria, dystonia, seizures, headache, cognitive impairment, and psychosis (Balck et al., 2021). PFBC is considered an autosomal dominant genetic disease, and four pathogenic genes, namely—SLC20A2 (OMIM: 158378) (Wang et al., 2012), PDGFB (OMIM: 190040) (Keller et al., 2013), PDGFRB (OMIM: 173410) (Nicolas et al., 2013), and XPR1 (OMIM: 605237) (Legati et al., 2015)—have been identified since 2012. Approximately 36%–42% of patients with autosomal dominant PFBC (AD-PFBC) remain asymptomatic throughout their lifetime (Nicolas et al., 2015; Tadic et al., 2015; Xu et al., 2023). Recently, biallelic homozygous mutations in the MYORG (OMIM:618255) (Yao et al., 2018) or JAM2 (OMIM: 606870) (Schottlaender et al., 2020) genes have been identified as novel genetic causes of autosomal recessive PFBC.
The association between MYORG and PFBC was first reported in Chinese families in 2018 (Yao et al., 2018), and has since been confirmed in recessive PFBC cases in various ethnicities (Chelban et al., 2020; Grangeon et al., 2019; Kume et al., 2020; Malaquias et al., 2020; Ramos et al., 2019; Sadok et al., 2023; Tekin et al., 2021; Yao et al., 2018). MYORG mutations account for a large proportion of recessive PFBC cases. Compared with patients who possessed any of the four AD-PFBC gene types, individuals with the MYORG gene exhibited a higher prevalence of intracranial calcification lesions, which were more severe and extensive, primarily affecting the pons and cerebellum (Bauer et al., 2019). However, the clinical and radiological features of MYORG-PFBC have not been well understood.
We obtained detailed clinical, radiological, and genetic data from a patient with a novel MYORG variant of PFBC. Further mutational analysis of MYORG was performed on her family members.
2 MATERIALS AND METHODS
2.1 Ethical compliance
This study involving human participants received approval from the Ethics Committee of Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, China. The patient and her family provided written informed consent to participate in this study.
2.2 Genetic sequencing and data analysis
Total deoxyribonucleic acid (DNA) from peripheral blood leukocytes of the patient and her family members was extracted using the Genomic DNA Extraction Kit (Tiangen Biochemical Technology, Beijing, China). Nanodrop 2000 was used to qualitatively examine DNA. The genomic DNA was fragmented, end-repaired, adapter linked, purified, and amplified. The entire human exonic genome was captured using a target sequence capture probe (MyGenostics, GenCap, China). The captured DNA was enriched, eluted, amplified, and subjected to high-throughput sequencing on the Illumina HiSeq X10 sequencing platform (Illumina, San Diego, California, United States). The raw data were subjected to preliminary processing, including image recognition and sample differentiation. The filtered sequences were aligned to the NCBI database human genome reference sequence (GRCh37/hg19) using Burrows—Wheeler Aligner software (Li & Durbin, 2010), and related information on single nucleotide variation and indel mutations were obtained by analysis using the Genome Analysis Toolkit software(https://software.broadinstitute.org/gatk/). All single nucleotide polymorphisms and indels were annotated using the ANNOVAR (Yang & Wang, 2015). Mutation sites with a frequency less than 0.05 were screened for in human genetic databases, including the Thousand Genomes Project, Exome Variant Server, and Exome Aggregation Consortium. Missense mutations were predicted by Scale Invariant Feature Transform (http://sift.jcvi.org/), Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph2/), MutationTaster (http://www.mutationtaster.org/), and Genomic Evolutionary Rate Profiling (http://mendel.stanford.edu/SidowLab/downloads/gerp/index.html). The changes in splice sites were analyzed using SPIDEX (http://www.deepgenomics.com/spidex). Sanger sequencing was used to verify the candidate mutation sites obtained after analysis and screening. The Human Splicing Finder software was used to verify the function of abnormal gene mutations, and the pathogenicity of gene variants was analyzed according to the guidelines of the United States Society for Medical Genetics and Genomics (Richards et al., 2015).
3 RESULTS
3.1 Patient information
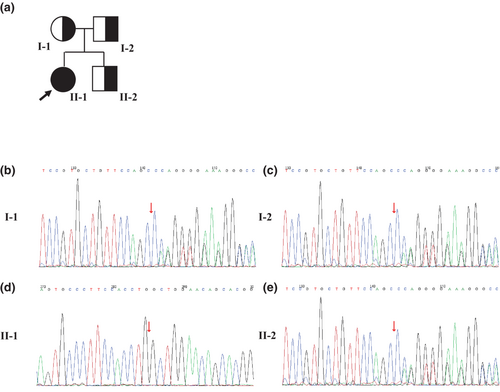
The index patient (Figure 1a, II-1) was a 22-year-old Chinese woman who presented to our department with a 3-year history of paroxysmal limb stiffness and dysarthria. Her symptoms occurred two to four times a week and lasted only a few seconds from 3 years ago. However, over the 6 months before the presentation, they had become more frequent and occurred almost daily for a few seconds, with irregular onset. She was previously in good health and had no family history of neurological disease. Her parents were healthy and not consanguineous (Figure 1a, I-1, I-2). Her younger brother was aged 13 years and healthy (Figure 1a, II-2). She denied experiencing dysphagia, cognitive impairment, syncope episodes, palpitations, constipation, gait disorder, trunk imbalance, or irregular tremors.
3.2 Clinical findings
On admission, the initial vital signs were as follows: blood pressure, 130/93 mmHg; pulse, 86 beats/min; respiration, 20 breaths/min; and body temperature, 37.3°C. The general physical examination results were normal. Neurological examination revealed normal cognition, attention, memory, affect, cranial nerve function, gait, posture, balance, coordination, motor function and sensory function interstitially. Brain computed tomography (CT) revealed symmetric calcifications in the paraventricular, basal ganglia, thalamus, and cerebellum (Figure 2a–c). Brain magnetic resonance imaging (MRI) revealed symmetric calcifications in the basal ganglia, dorsolateral nucleus of the thalamus, and dentate nucleus of the cerebellum (Figure 2d–f). The routine blood, urine, and stool tests showed normal results. Blood test results for alanine transaminase, aspartate aminotransferase, lactate dehydrogenase, creatinine, parathyroid hormone, total vitamin D, serum calcium, serum phosphate, thyroid hormones, albumin, blood glucose, uric acid, C-reactive protein, erythrocyte sedimentation rate, and glycosylated hemoglobin were normal. Blood lipid test showed values within normal limits, except for triglycerides, which were 5.78 mmol/L. Autoimmune tests, including antinuclear antibodies, SSA/SSB antibodies, and rheumatoid factor tests, showed negative results. Infectious test results for human immunodeficiency virus antibodies, syphilis antibodies, and hepatitis B and C antibodies were negative. Bone density, 24-hour electroencephalogram, and bilateral femur radiography showed normal findings. No obvious abnormality in the heart, liver, gallbladder, pancreas, spleen, kidneys, ureters, or bladder was observed by ultrasound. The Mini-mental State Examination and Montreal Cognitive Assessment scores were 30/30, respectively.

3.3 Diagnostic assessment and genetic screening
A diagnosis of PFBC was considered plausible after excluding secondary causes of brain calcification. Therefore, the patient's blood samples were subjected to whole-exome sequencing after obtaining informed consent. A novel homozygous frameshift variant (c.743delG: p.G248Afs*32) were identified in exon 2 of the MYORG gene (NM_020702.5), and validated using Sanger sequencing (Figure 1d). This mutation was absent from multiple population and disease databases, including the Human Gene Mutation Database, Clinvar, and The Genome Aggregation Database. According to the recommendations of the American College of Medical Genetics, the variant was categorized as a “pathogenic variant” based on its classification as PVS1, PM2, or PM3_Supporting(hom). Combined with the clinical manifestations, related imaging, and genetic studies, the patient was diagnosed with PFBC caused by MYORG mutation. Sanger sequencing was performed for the family members of the patients (Figures 1b,c,e). The patient's father (Figure 1c), mother (Figure 1b), and younger brother (Figure 1e) were carriers of heterozygous mutations. Notably, all the aforementioned family members were asymptomatic. Unfortunately, brain CT or MRI was not performed for the patient's parents and younger brother; hence, it was unknown whether they had intracranial symmetric calcifications.
3.4 Therapeutic intervention and follow-up
The patient was treated with oxcarbazepine to reduce neuronal excitability. Following treatment, the patient's symptoms improved remarkably, and the frequency of symptoms significantly decreased. However, 2 years later, the patient was re-examined by brain CT, and the results showed that the range of intracranial calcification had increased.
3.5 Literature review
We searched through PubMed until April 1, 2023 for relevant studies and scanned the reference lists in the identified articles. We reviewed all reported cases of PFBC with biallelic MYORG mutations and summarized all the mutation sites in Table 1 (Arkadir et al., 2019; Chelban et al., 2020; Chen, Lin, et al., 2020; Chen, Cen, et al., 2020; Chen et al., 2019; Fei et al., 2021; Ferreira & de Oliveira, 2019; Forouhideh et al., 2019; Gao et al., 2022; Grangeon et al., 2019; Kume et al., 2020; Li et al., 2022; Liu et al., 2021; Malaquias et al., 2020; Peng et al., 2018; Ramos et al., 2019; Sadok et al., 2023; Saranza et al., 2020; Taglia et al., 2019; Tekin et al., 2021; Tsai et al., 2022; Yang et al., 2022; Yao et al., 2018; Zeng et al., 2021). Data for 62 families have been reported, 51% of the patients were men and 49% were women. The ethnic groups included those from China, Japan, Israel, Italy, France, Turkey, Portugal, and Brazil. The median age of symptom onset was 46 years, and the affected age group ranged from 7.5 and 87 years. The diagnosis of MYORG-PFBC was for individuals aged between 12 and 87 years (average, 47 years).
| Reference | Country/region | Age at onset (y) | Clinical features | Location of calcification | cDNA alteration | Amino acid change |
|---|---|---|---|---|---|---|
| Yao et al. (2018) and Zeng et al. (2021) | China | 42 | Dysarthria, dysphagia, forced laughter or crying | Basal ganglia, thalamus, cerebellum, subcortical white matter | c.103A > G | p.M35V |
| Chen, Lin, et al. (2020) | Taiwan | 44 | Dysarthria, dystonia, bradykinesia, parkinsonism, ataxia | Basal ganglia, thalamus, caudate nucleus, red nucleus, subcortical white matter | c.104T>A | p.M35K |
| Chelban et al. (2020) | Caucasian | 39 | Parkinsonism, ataxia, dysarthria | Basal ganglia, cerebellum, subcortical white matter | c.176G>A | p.G59D |
| Grangeon et al. (2019) and Chen, Cen, et al. (2020) | France | 52–62 | Dysarthria, cognitive impairment, ataxia. akinetic hypertonic syndrome, psychotic symptoms, stroke | Basal ganglia, thalamus, cerebellar hemispheres, vermis, subcortical white matter, brain stem | c.191G>A | p.G64E |
| Yao et al. (2018) and Tsai et al. (2022) | Taiwan,South China | 65 | Dysarthria, dysphagia, ataxia, bradykinesia | Centrum semiovale, corona radiata, basal ganglia, thalamus, occipital lobe, central pons, cerebellum | c.225G>A | p.W75* |
| Malaquias et al. (2020) | Portugal | 51 | Depression, mild hemiparesis, dysarthria, akinetic-rigid parkinsonism, headache, urinary incontinence, cognitive impairment | Subcortical white matter, basal ganglia, choroid plexus, cerebellum | c.285_310delinsTTC | p.95fs |
| Chelban et al. (2020) | NA | 62 | Dysarthria, dysphagia | Basal ganglia, thalamus, cerebellar hemispheres, pons | c.325C>T | p.Q109* |
| Grangeon et al. (2019) | France | 45 | Dysarthria, ataxia, pyramidal signs, akinetic hypertonic syndrome with tremor, chorea, dystonia | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter, pons | c.338T>G | p.L113R |
| Yao et al. (2018), Grangeon et al. (2019), Li et al. (2022), Yang et al. (2022), Chen, Cen, et al. (2020) and Chen et al. (2019) | China,France | 14–51 | Parkinsonism, seizures, psychotic symptoms, dysarthria, hypomimia, bradykinesia, dyskinesia, akinetic hypertonic syndrome, headache, paroxysmal limb weakness and numbness, cognitive decline, urinary disturbance, stroke | Basal ganglia, thalamus, dentate nuclei, subcortical white matter, pons | c.348_349insCTGGCCTTCCGC | p.R116_S117insLAFR |
| Chelban et al. (2020) | NA | 56 | Parkinsonism, dysphagia, depression, ataxia, dysarthria | Basal ganglia, thalamus, cerebellar hemispheres, pons | c.349T>C | p.S117P |
| Taglia et al. (2019) | Italy | 61 | Depression, ataxia, bradykinesia, dysarthria, parkinsonism | Basal ganglia, cerebellum, thalamus, occipital lobe, subcortical white matter, brainstem | c.373_394delinsG | p.C125_L132delinsV |
| Chen, Cen, et al. (2020) and Chen et al. (2019) | East China | 52 | Dysarthria, gait disorder, parkinsonism, constipation, cognitive decline | Basal ganglia, dentate nuclei, thalamus, subcortex, brain stem | c.428_442delTGCACTTCTTCATCC | p.143_147delLHFFI |
| Zeng et al. (2021) | China | 66 | Dysarthria, parkinsonism, vertigo | Globus pallidus, thalamus, dentate nuclei, subcortical white matter | c.442C>T | p.Q148* |
| Malaquias et al. (2020) | Portugal | 51 | Depression, hemiparesis, dysarthria, akinetic-rigid parkinsonism, headache, urinary incontinence, cognitive impairment | Subcortical white matter, basal ganglia, choroid plexus, cerebellum | c.535_536insC | p.G179fs |
| Yao et al. (2018) | North China | NA | Dysarthria, ataxia | Basal ganglia, dentate nuclei, thalamus, subcortical white matter | c.607C>T | p.Q203* |
| Chen, Cen, et al. (2020) | China | 46 | Dysarthria | NA | c.679C>G | p.R227G |
| Li et al. (2022), Chen, Cen, et al. (2020) and Chen et al. (2019) | China,France | 40–41 | Dysarthria, hypomimia, dyskinesia, akinetic hypertonic syndrome, dystonia, parkinsonism, cognitive decline, urinary disturbance, stroke | Basal ganglia, thalamus, dentate nuclei, subcortical white matter, cerebellum, pons | c.687G>T | p.W229C |
| Yao et al. (2018) | West China | NA | Dysarthria, ataxia, chorea, cognitive impairment | Basal ganglia, dentate nuclei, thalamus, subcortical white matter | c.695C>T | p.S232L |
| Grangeon et al. (2019) | France | 53 | Dysarthria, akinetic hypertonic syndrome, pyramidal signs, depression | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.706_708dupGCC | p.A236dup |
| Our study | China | 19 | Body stiffness, dysarthria | Paraventricular, basal ganglia, thalamus, cerebellum | c.743delG | p.G248Afs*32 |
| Grangeon et al. (2019) | France | 51 | Dysarthria, ataxia, akinetic hypertonic syndrome, pyramidal signs, depression | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter, pons | c.747G>C | p.W249C |
| Yao et al. (2018), Zeng et al. (2021) and Chen, Cen, et al. (2020) | China | 45 | Vertigo, dysarthria, parkinsonism | Basal ganglia, dentate nuclei, thalamus, corona radiata, brain stem | c.782_783 GC>TT | p.R261L |
| Kume et al. (2020) | Japan | 41 | Dysarthria, headache, ataxia | Subcortical white matter, basal ganglia, cerebellum, brainstem | c.794C>T | p.T265M |
| Gao et al. (2022) | China | 28 | Dysarthria, stroke | Basal ganglia, thalamus, midbrain, pons, cerebellum | c.830delC | p.P277Qfs*3 |
| Chen, Lin, et al. (2020) | Taiwan | 3rd decade | Dystonia, bradykinesia | Basal ganglion, thalamus, caudate nucleus, subcortical white matter | c.850T>C | p.C284R |
| Ferreira and de Oliveira (2019) | Brazil | 41 | Dysarthria, dysphagia, ataxia | Pons, cerebellum, basal ganglia, choroid plexus, subcortical white matter | c.854_855dupTG | p.G286Wfs*149 |
| Tekin et al. (2021) | Turkey | 8.5 | Dizziness, headache, attention deficit, urinary urgency | Lentiform nuclei, cerebellar white matter | c.856G>A | p.G286S |
| Chen, Cen, et al. (2020) | China | 78 | Ataxia | Brain stem | c.893G>C | p.R298P |
| Sadok et al. (2023) | Brazil | 24 | Movement disturbances (chorea and dystonia), migraine, tinnitus, attention and memory deficit | Cerebellum, basal ganglia, pineal | c.912_914delGTC | p.S305del |
| Taglia et al. (2019) | Italy | 61 | Depression, ataxia, bradykinesia, dysarthria, parkinsonism | Basal ganglia, cerebellum, thalamus, occipital lobe, subcortical white matter, brainstem | c.940C>T | p.R314* |
| Zeng et al. (2021) | China | 42 | Dysarthria, dysphagia, forced laughter or crying | Basal ganglia, thalamus, dentate nuclei, subcortical white matter | c.972C>A | p.Y324* |
| Arkadir et al. (2019) | Middle Eastern | 21–57 | Dysarthria, hyperkinetic movement disorder, dystonia, parkinsonism, cognitive impairments, cerebellar signs, pyramidal signs, dysphagia | Cerebellum, basal ganglia, thalamus, brainstem, the deep midbrain nuclei, cortical areas, pons | c.1060_1062del GAC | p.D345del |
| Grangeon et al. (2019) | France | 57 | Dysarthria, pyramidal signs, akinetic hypertonic syndrome | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.1078delT | p.Y360fs |
| Yao et al. (2018) | South China | NA | Migraine | Basal ganglia, dentate nuclei, thalamus, subcortical white matter | c.1092_1097delCTTCGA | p.365_366delFD |
| Grangeon et al. (2019) | France | 52 | Depression, ataxia, dysarthria, akinetic hypertonic syndrome, pyramidal signs | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.1118C>A | p.A373D |
| Arkadir et al. (2019) | Middle Eastern | 21–57 | Dysarthria, hyperkinetic movement disorder, dystonia, parkinsonism, cognitive impairments, cerebellar signs, pyramidal signs, dysphagia | Cerebellum, basal ganglia, thalamus, brainstem, the deep midbrain nuclei, cortical areas, pons | c.1233delC | p.F411Lfs*23 |
| Fei et al. (2021) | China | 57 | Dysphagia and alalia | Basal ganglia, cerebellum, thalamus, periventricular white matter | c.1271_1272ins TGGTGCGC | NA |
| Grangeon et al. (2019) | France | 51 | Ataxia, dysarthria, bradykinesia, pyramidal signs, depression | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter, pons | c.1300G>C | p.D434H |
| Yao et al. (2018) | South China | NA | Migraine | Basal ganglia, dentate nuclei | c.1321C>G | p.R441G |
| Yao et al. (2018) | South China | NA | Dysarthria, ataxia, cognitive decline | Basal ganglia, dentate nuclei, thalamus, subcortical white matter | c.1328G>A | p.W443* |
| Grangeon et al. (2019), Yang et al. (2022) and Peng et al. (2018) | China | 40–51 | Headache, paroxysmal limb weakness and numbness, dysarthria, bradykinesia, central neuropathic pain, attention deficits, cognitive decline | Cerebellum, periventricular white matter, basal ganglia, thalamus, subcortical white matter, pons | c.1333C>T | p.Q445* |
| Chelban et al. (2020) | NA | 73 | Dysarthria | Basal ganglia, cerebellar hemispheres, subcortical white matter | c.1383C>G | p.P420R |
| Grangeon et al. (2019) | France | 57 | Dysarthria, pyramidal signs, akinetic hypertonic syndrome | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.1394dupG | p.E466fs |
| Chelban et al. (2020) | NA | 62 | Parkinsonism, dysarthria, dysphagia, depression | Basal ganglia, thalamus, cerebellar hemispheres, pons | c.1401_ 1402insCGCTGGTG | p.W426Cfs*11 |
| Grangeon et al. (2019) | France | 52 | Dysarthria. Stroke-like episode, akinetic hypertonic syndrome with freezing. ataxia | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.1427C>A | p.T476N |
| Chen, Cen, et al. (2020) and Chen et al. (2019) | East China | 43 | Dysarthria, ataxia, dysphagia, cognitive decline | Basal ganglia, dentate nuclei, thalamus, subcortical white matter, brainstem | c.1431C>A | p.Y477* |
| Fei et al. (2021) | China | 57 | Dysphagia and alalia | Basal ganglia, cerebellum, thalamus, periventricular white matter | c.1438T>G | NA |
| Chen, Cen, et al. (2020) | China | 46 | Dysarthria | Brain stem | c.1511G>C | p.R504P |
| Ramos et al. (2019) | Italian | 35 | Bradykinesia, dysarthria, ataxia | Basal ganglia, thalamus, pons, cerebellum, occipital lobe, frontal lobe | c.1530delG | p.N511Tfs*243 |
| Grangeon et al. (2019) | France | 52 | Depression, ataxia, dysarthria, akinetic hypertonic syndrome, pyramidal signs | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter | c.1538_1540delCCT | p.S513del |
| Chelban et al. (2020) | Caucasian | 87 | Parkinsonism, dysdiadochokinesia, wide-based gait | Basal ganglia, thalamus, cerebellar hemispheres | c.1598C>T | p.S533L |
| Chelban et al. (2020) | Middle East | 45–51 | Parkinsonism, headache, dizziness, ataxia, dysarthria | Basal ganglia, thalamus, and subcortical white matter | c.1611C>T | p.P496L |
| Chelban et al. (2020) | Caucasian | 87 | Parkinsonism, dysdiadochokinesia; wide-based gait | Basal ganglia, thalamus, cerebellar hemispheres | c.1634G>A | p.G545D |
| Tsai et al. (2022) | Taiwan | 65 | Dysarthria, dysphagia, ataxia, bradykinesia | Centrum semiovale, corona radiata, basal ganglia, thalamus, occipital lobe, pons, cerebellum | c.1727G>C | p.R576P |
| Grangeon et al. (2019) and Saranza et al. (2020) | China | 8 | Paroxysmal kinesigenic dyskinesia | Cerebellum, midbrain, thalamus, basal ganglia, frontal and occipital lobe, subcortical areas, pons | c.1831C>T | p.R611W |
| Chelban et al. (2020) | NA | 62 | Dysarthria and dysphagia | Basal ganglia, thalamus, cerebellar hemispheres, pons | c.1832G>T | p.R611L |
| Grangeon et al. (2019) | France | 45 | Dysarthria, ataxia, pyramidal signs, akinetic hypertonic syndrome with tremor, chorea, dystonia. | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter, pons | c.1865T>C | p.L622P |
| Forouhideh et al. (2019) | Turkey | 38–40 | Dysarthria, ataxia, dysphagia, cognitive deficit, depression, schizophrenia | Basal ganglia, red nucleus, thalamus, cerebellum, periventricular, brain stem, subcortical white matter | c.1964A>G | p.I655T |
| Grangeon et al. (2019), Chelban et al. (2020), Chen, Cen, et al. (2020) and Liu et al. (2021) | China, France | 30–62 | Dysarthria, ataxia, cognitive deficit, dysphagia, stroke-like episode, akinetic hypertonic syndrome, parkinsonism, depression | Basal ganglia, thalamus, cerebellum, pons | c.1967T>C | p.I656T |
| Zeng et al. (2021) | China | 45 | Vertigo | Basal ganglia, dentate nuclei, thalamus, corona radiata | c.1969G>C | p. G657R |
| Grangeon et al. (2019) | France | NA | Several fainting, normal neurological examination | Lenticular nuclei, thalamus, cerebellar hemispheres, vermis, subcortical white matter, pons | c.1979T>A | p.L660Q |
| Zeng et al. (2021) | China | 59 | Dysarthria, parkinsonism, ataxia | Basal ganglia, thalamus, dentate nuclei, subcortical white matter, cerebellar vermis, brainstem | c.2033C>G | p.P678R |
| Chelban et al. (2020) | NA | 73 | Dysarthria | Basal ganglia, cerebellar hemispheres, subcortical white matter | c.2162G>A | p.G680S |
| Chelban et al. (2020) | NA | 56 | Parkinsonism, ataxia, dysarthria, dysphagia, depression | Basal ganglia, thalamus, cerebellar hemispheres, pons | c.2211_2212del | p.L696Pfs*10 |
- Abbreviation: NA, not available.
4 DISCUSSION
Brain calcification is a medical condition characterized by the accumulation of calcium deposits in the brain tissue. It can be caused by various factors, including genetic disorders, tumors, vascular diseases, infections, metabolic disorders, and exposure to certain medications or toxins (Guedes et al., 2020). One genetic disorder associated with brain calcification is PFBC, a rare neurogenerative disorder that can be hereditary or sporadic. The onset of PFBC is often insidious, and its manifestations can differ widely among individuals. Recent research has focused on identifying the genetic mutations responsible for PFBC (Balck et al., 2021), which can help shed light on the underlying mechanisms of the disease and explain how different gene mutations might lead to similar neuroimaging findings but variable clinical outcomes.
PFBC is diagnosed based on clinical presentation, neuroimaging studies, and genetic testing. The diagnostic criteria include bilateral calcification in the basal ganglia and other brain regions, neurological or psychiatric symptoms, positive family history with an autosomal dominant inheritance pattern, or recessive mutations in known PFBC-associated genes (Mufaddel & Al-Hassani, 2014). The differential diagnosis of PFBC includes other disorders that can cause brain calcification, such as hypoparathyroidism, pseudohypoparathyroidism, tuberous sclerosis, aneurysm, oligodendroglioma, and infectious diseases such as tuberculosis, cysticercosis, and toxoplasmosis (Table S1). A comprehensive clinical evaluation, including medical history, physical examination, and laboratory tests, is necessary for an accurate diagnosis. Genetic testing can also be useful in confirming PFBC and excluding other conditions.
Of the four genes associated with AD-PFBC, SLC20A2 mutations are the most common, accounting for over 50% of cases. PDGFB and PDGFRB mutations account for approximately 10%–40% and 5%–10% of cases, respectively (Balck et al., 2021; Donzuso et al., 2019). Mutations in PDGFB and PDGFRB can lead to abnormal blood vessel development and disrupt the blood–brain barrier integrity (Xu et al., 2023). XPR1 mutations have also been identified in a few PFBC cases. Mutations in SLC20A2 and XPR1 can disrupt phosphate transport, leading to calcium deposition in the brain (Xu et al., 2023). Missense mutations were the most frequent and unique alterations in SLC20A2, PDGFB, PDGFRB, and XPR1 (Balck et al., 2021).
Autosomal recessive mutations that have been reported include MYORG and, more recently, JAM2. MYORG mutations are an infrequent cause of PFBC; however, with the widespread use of genetic testing, MYORG mutation account for 10%–20% of PBFC cases (Balck et al., 2021; Yao et al., 2018). The gene MYORG, also known as KIAA1161 or NET37, is located on chromosome 9p13.3, and encodes a 714-amino-acid(aa) protein. The MYORG protein is expressed in the skeletal muscles, colon, small intestine, liver, and brain. It plays a role in cell movement and organization. The MYORG protein is an α-galactosidase and has a conserved domain architecture consisting of a short N-terminal cytoplasmic domain (aa 1–58), single transmembrane fragment (aa 59–79), glycoside hydrolase family 31 domain (GH31) (aa 311–714), and domain of unknown function (aa 80–310) (Meek et al., 2022; Yao et al., 2018). The GH31 domain is a common glycosidase domain responsible for the decomposition of complex sugars. The unknown functional domain is a less well-understood domain present in various proteins, and is believed to be involved in protein–protein interactions.
Balck et al. reported that the age of onset and diagnosis of MYORG-PFBC are similar to those of SLC20A2, XPR1, and PDGFRB mutations, and significantly higher than those of PDGFB and JAM2 mutations (Balck et al., 2021). Most of the 64 reported mutation sites in MYORG located in the GH31 domain, followed by the unknown functional domain, transmembrane fragment, and short N-terminal cytoplasmic domain. Thirty-six mutation sites were found only in compound heterozygous mutations, 23 only in homozygous mutations, and five mutation sites, including c.225G>A, c.348_349insCTGGCCTTCCGC, c.1333C>T, c.1831C>T, and c.1967T>C, were found in both homozygous and compound heterozygous mutations. The most frequent mutation sites were c.348_349insCTGGCCTTCCGC, followed by c.1967T>C, c.1333C>T, c.687G>T, and c.782_783GC>TT, which were found in 10 families (Chinese and Frenchman; Chen, Cen, et al., 2020; Chen et al., 2019; Grangeon et al., 2019; Li et al., 2022; Yang et al., 2022; Yao et al., 2018), five families (Chinese and Frenchman; Chelban et al., 2020; Chen, Cen et al., 2020; Grangeon et al., 2019; Liu et al., 2021), three families (Chinese; Grangeon et al., 2019; Peng et al., 2018; Yang et al., 2022), three families (Chinese and Frenchman; Chen, Cen, et al., 2020; Chen et al., 2019; Li et al., 2022), and three families (Chinese; Chen, Cen, et al., 2020; Yao et al., 2018; Zeng et al., 2021), respectively. We opined that these mutation sites may be “hot spots” for Chinese and Frenchmen. Missense mutation was the most frequent type of unique alteration, followed by frameshift, nonsense, in-frame indels, and duplication. To the best of our knowledge, this report describes a newly discovered homozygous frameshift mutation, c.743delG in the MYORG gene. The mutation c.743delG (p.G248Afs*32) has not been previously reported and may have been pathogenic in this case. Our patient with this mutation had a relatively young age of onset.
Bilateral symmetric calcifications in the basal ganglia (approximately 100%) and other brain regions are characteristics of PFBC. CT and MRI are commonly used imaging techniques for diagnosing of PFBC. Calcification can also affect the cerebellum, thalamus, subcortical white matter, cerebral cortex, and brain stem. Compared with patients with AD-PFBC, abnormal calcium deposits in the brains of patients with MYORG-PFBC are more severe, widespread, and always contain three to four brain calcification regions (Balck et al., 2021; Grangeon et al., 2019). However, the severity of calcification in patients with MYORG-PFBC can vary among patients and does not necessarily correlate with the severity of the clinical symptoms. Unlike AD-PFBC, there appears to be no apparent association between the number of affected brain regions and the age of onset or diagnosis of patients with MYORG-PFBC. In reported cases of MYORG-PFBC, both biallelic and some monoallelic carriers exhibit calcifications, with biallelic carriers experiencing more severe calcification (Taglia et al., 2019). Among monoallelic carriers, we found that 10 mutation sites, including c.103A>G, c.191G>A, c.348_349insCTGGCCTTCCGC, c.679C>G, c.782_783 GC>TT, c.893G>C, c.1333C>T, c.1511G>C, c.1530delG, and c.1967T>C, are associated with significant brain calcifications (Chen, Cen, et al., 2020; Ramos et al., 2019). Interestingly, most of the monoallelic carriers with mutations in the above mutation sites have calcifications in the brain stem, except for those with c.103A>G and c.679C>G mutations. Additionally, three out of five monoallelic carriers with 1060_1062del GAC mutation revealed punctuated calcification limited to the basal ganglia, and their family members with biallelic MYORG mutations also had calcification in the brain stems (Arkadir et al., 2019). Therefore, we speculated that the presence of calcification among monoallelic MYORG carriers is not only related to a dose-effect (Chen, Cen, et al., 2020) but also to the mutation site. Pontine calcifications are highly specific to MYORG-PFBC and are relatively rare in patients with AD-PFBC. Among the reported cases of PFBC with biallelic MYORG mutations, 45 cases showed brainstem calcification (Arkadir et al., 2019; Chelban et al., 2020; Chen, Cen, et al., 2020; Chen et al., 2019; Ferreira & de Oliveira., 2019; Forouhideh et al., 2019; Gao et al., 2022; Grangeon et al., 2019; Kume et al., 2020; Liu et al., 2021; Ramos et al., 2019; Saranza et al., 2020; Taglia et al., 2019;Tsai et al., 2022; Zeng et al., 2021) and 24 cases showed pontine calcification (Arkadir et al., 2019; Chelban et al., 2020; Ferreira & de Oliveira, 2019; Gao et al., 2022; Grangeon et al., 2019; Kume et al., 2020; Ramos et al., 2019; Taglia et al., 2019; Tsai et al., 2022; Zeng et al., 2021). However, the brainstem tropism of lesions associated with MYORG mutations remains unknown.
Along with genetic heterogeneity, PFBC can present with a wide range of neurological and psychiatric symptoms, which can vary in severity and age of onset. Common manifestations include dysarthria, movement disorders (such as tremors, rigidity, dystonia, and ataxia), cognitive impairment (such as memory loss, confusion, and dementia), and mood disturbances (such as depression, anxiety, and psychosis). Other possible symptoms include headaches and seizures. Compared with AD-PFBC, patients with MYORG-PFBC have approximately 100% clinical penetrance (Chen, Cen, et al., 2020). Similar to previous studies (Grangeon et al., 2019; Taglia et al., 2019; Zeng et al., 2021), we observed that dysarthria is a prominent feature and the most common manifestation in patients with MYORG-PFBC, as well as other motor signs, including ataxia, dystonia, dyskinesia, Parkinsonism, and akinetic hypertonic syndrome. Ischemic stroke was also reported in five cases, and none of them had risk factors for stroke (Gao et al., 2022; Grangeon et al., 2019; Li et al., 2022; Yang et al., 2022). Central neuropathic pain was also observed in a 43-year-old Chinese woman (Peng et al., 2018). Regarding nonmotor signs, cognitive deficits were the most common manifestation, followed by depression, headache, and psychosis. In patients with AD-PFBC, Parkinsonism, bradykinesia, cognitive deficits, and headache are the most common symptoms, while dysarthria is uncommon (Batla et al., 2017). In this study, the patient also had paroxysmal dysarthria as one of the chief complaints, similar to the results of the above studies. To date, 57% of all reported MYORG-PFBC cases are homozygous, and 43% are compound heterozygous. The clinical symptoms of most MYORG biallelic carriers vary, whereas most monoallelic carriers are clinically asymptomatic. In addition, we found that children and young adults always have mild symptoms, and the severity of these symptoms may increase with age.
The mechanisms by which MYORG mutations cause brain calcification are not well understood. Histopathological examinations have demonstrated that brain calcification is mainly associated with small blood vessels, glial cells, and neurons (Xu et al., 2023). MYORG is specifically expressed in astrocytes, mainly located in the endoplasmic reticulum and nuclear envelope (Meek et al., 2022; Yao et al., 2018; Zarb et al., 2019). Astrocytes are functionally associated with endothelial cells and pericytes of the blood–brain barrier, neurons, cardiomyocytes, and the extracellular matrix. They participate in the common formation of the neurovascular unit (NVU), which is related to four autosomal dominant genes and is involved in the pathogenesis of PFBC (Betsholtz & Keller, 2014; Xu et al., 2023). Dysfunction of the NVU impairs the normal substance exchange between the blood and cerebral cortex, leading to a high amount of blood Pi leaking into the brain, which in turn causes calcification (Xu et al., 2023). Although recent studies have demonstrated the functions of MYORG in animals, its pathophysiology in the human brain remains unclear. Meek et al. (Meek et al., 2022) used X-ray crystallography to obtain the structure of MYORG and suggested that these mutations may cause MYORG dysfunction, resulting in the disruption of the folding or maturation of one or more protein products of genes linked to PFBC, including SLC20A2, PDGFB, PDGFRB, and XPR1. The novel frameshift mutation identified in our patient was located in the unknown function domain. To date, 24 mutations in this domain have been reported (Chelban et al., 2020; Chen, Lin, et al., 2020; Chen, Cen, et al., 2020; Chen et al., 2019; Ferreira & de Oliveira, 2019; Gao et al., 2022; Grangeon et al., 2019; Kume et al., 2020; Li et al., 2022; Malaquias et al., 2020; Sadok et al., 2023; Taglia et al., 2019; Tekin et al., 2021; Yang et al., 2022; Yao et al., 2018; Zeng et al., 2021), suggesting that this region plays an important role in MYORG, leading to PFBC. Therefore, more studies are recommended to investigate the correlations between mutation sites, pathogenicity-implicated imaging findings, and clinical phenotypes. Notably, induced pluripotent stem cell lines have been generated from patients with PBFC (Yada et al., 2021) and may be a powerful tool to reveal the pathogenesis of PBFC and explore potential therapeutic candidates.
Currently, no specific prevention or treatment for PFBC exists, and existing treatment regimens are primarily used to control the disease symptoms. These may include medications to manage seizures, movement disorders, and cognitive impairment, as well as therapies to support and improve the quality of life. However, no effective therapy has been developed to control the progression of brain calcification. Therefore, ongoing research is focused on developing new therapies that target the underlying mechanisms of the disease to control the progression of brain calcification. For example, recent studies have explored the potential use of agents to inhibit nonsense mutation (Peters et al., 2020) or stabilize mutant proteins and facilitate their proper folding (Meek et al., 2022). Other promising research avenues include reducing Pi intake, restoring normal Pi transport in the brain, and inhibiting cerebral cell ossification.
5 CONCLUSION
We report a novel pathogenic homozygous mutation (c.743delG: p.G248Afs*32) of the MYORG gene. We highlighted that screening for MYORG mutations in patients with primary brain calcification is important, especially younger patients without a family history. Moreover, MYORG monoallelic carriers may have brain calcifications, which may affect their health. Therefore, long-term follow-up and genetic counseling are recommended for these individuals.
AUTHOR CONTRIBUTIONS
Siyue Liu and Juan Xu performed the data collection and collation. Tianxue Zhao and Shaokun Xu collected the patient's information, analyzed and interpreted the data, and drafted the manuscript. Yuhong Zhan and Xianfeng Zhang designed the study and reviewed the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank the patient and her family members for participating in this study. In addition, we would like to thank Editage (www.editage.cn) for English language editing.
FUNDING INFORMATION
This work was financially supported by grants from the Medical Science and Technology Project of Zhejiang Province (2021KY879), Hangzhou, China and the Medical and Health Technology Project of Hangzhou (A20230111 and A20200753), Hangzhou, China.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
The protocol was followed in accordance with Declaration of Helsinki and approved by the local ethical committee of Hangzhou First People's Hospital. Written informed consent was obtained from the patient and her family.
Open Research
DATA AVAILABILITY STATEMENT
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.




