New observation of severe tooth malformation in a female patient with ectodermal dysplasia due to the EDA splice acceptor variant c.742-2A>G
Abstract
Background
Ectodermal dysplasias are inherited disorders, which are characterized by congenital defects in two or more ectodermal structures such as skin, sweat glands, hair, nails, teeth, and mucous membranes.
Method
Here, we describe a new observation of significant oligodontia in a female patient with the EDA gene variant c.742-2A>G.
Results
The results strongly suggest that the EDA gene variant c.742-2A>G is pathogenic. The oligodontia in the proband was exceptionally severe.
Conclusion
We demonstrate that the very rare splice acceptor variant EDA c.742-2A>G is associated with severe oligodontia even in females. Our study points that this variant is pathogenic. An early identification of this variant is crucial for planning adequate treatment and follow-up in time by a multidisciplinary team.
1 INTRODUCTION
Ectodermal dysplasia (ED) is a hereditary disease comprising of more than 150 phenotypes derived from ectodermal tissues (Chee et al., 2014; Itin & Fistarol, 2004; Mues et al., 2009; Srivastava, 2011). EDs include symptoms in hair, nail, teeth, and sweat glands (Desmuch & Prashanth, 2012). In addition, congenital abnormalities may be detected in the external ear, apocrine or exocrine glands, melanocytes, mammary glands, the CNS, sebaceous glands, cornea, conjunctiva of eye, the lacrimal apparatus, and in the nasal, oral, and pharyngeal mucosa (Itin & Fistarol, 2004; Kargül et al., 2001; Montonen et al., 1998). Furthermore, dysphonia has been reported in ED patients (Daniel et al., 2002).
EDs are classified based on their symptoms into subgroups. Each subgroup includes a different set of primary ED symptoms. The primary ED symptoms are: (1) Trichodysplasia (hair dysplasia), (2) Dental dysplasia, (3) Onychodysplasia (nail dysplasia), (4) Dyshidrosis (sweat gland dysplasia), (5) other ectodermal defects (Itin & Fistarol, 2004). Hypohidrotic ectodermal dysplasia (HED) is the most common group of EDs (Gopinath et al., 1999). Moreover, X-linked HED (XLHED) is the most common HED and is associated with symptoms such as hair dysplasia, dental dysplasia, and dyshidrosis, and nails may also be affected. In XLHED, hypodontia (up to five missing teeth), oligodontia (missing more than five teeth), and anodontia (a total loss of all teeth) may occur (Clauss et al., 2008). Due to the X-linked recessive trait, severe dental malformations are more often described in male patients than in female patients (Crawford et al., 1991; Nguyen-Nielsen et al., 2013; Sholapurkar et al., 2011).
Diverse groups of ED are caused by different types of pathogenic variants (PVs) located in various genes. HED has been associated with pathogenic variants in the EDA, EDAR, WNT10A, and EDARADD genes. Approximately, 60% of HEDs are attributed to PV in EDA. EDA is the only gene known to be associated with XLHED (OMIM 305100). In addition, few EDA PVs are associates with non-syndromic oligodontia (Lee et al., 2014; Song et al., 2009).
Ectodysplasin A (EDA) is a type II transmembrane protein of the TNF family encoded by the EDA gene located on Xq13.1 and it plays a decisive role in the development of ectodermal tissues (Kere et al., 1996). Proteolytic release of EDA is required to regulate the differentiation and morphogenesis of ectodermal appendages.
Here, we report an exceptionally severe tooth malformation in ectodermal dysplasia patient with the variant c.742-2A>G in EDA gene. This variant has not been reported at ClinVar database. The patient shows severe dental symptoms in addition to manifestations in the skin, hair, and sweat glands. The findings of our study strongly suggest that the variant is pathogenic.
2 METHODS
In 2018, the 5-year-old girl, visited the Department of Clinical Genetics, Turku University Hospital, with her father after referral from a pediatrician at a local health care center. The referral was due to abnormal symptoms including very thin skin, atopic eczema, hair loss, dental manifestations, and oligodontia. Based on a clinical examination by a clinical geneticist, the suspicion of XLHED confirmed. Thus, the ectodermal dysplasia gene panel was ordered to analyze known genes associated with ED from the patient's DNA sample. The gene panel was ordered from Blueprint Genetics and included the following genes: ABCC9, BCS1L, DSP, EDA, EDAR, ERCC2, EVC, EVC2, GJB2, GJB6, IFT122, IKBKG, JUP, PORCN, RMRP, SHOC2, TP63, WDR35, and WNT10A.
Due to skin manifestations, she was also referred to the Dermatology Outpatient Clinic at the Turku University Hospital. The patient presented with smooth, soft, dry, and thin skin in the face and neck region, hyperkeratosis of the palms and soles of the feet. In axillar region, an itch-marked region was found. Skin ulcer was detected behind the ears.
Furthermore, the patient was referred to the Ophthalmology Outpatient Clinic at the Turku University Hospital due to eye manifestations. Keratoconjunctivitis, keratitis and an erosion in the right eye and a blur of the cornea was detected. The eyelid's conjunctivas were composed bloodshed.
The patient is in regular follow-up at the Department of Oral and Maxillofacial Diseases, Turku University Hospital, where the dental examination was done.
3 RESULTS
3.1 Result of dental examination
At the age of 7 years, a thorough oral examination was performed at the Department of Oral and Maxillofacial Diseases. Significant oligodontia was detected by panoramic imaging (Figure 1). The pantomogram showed that four permanent first molars, D16, D26, D36, D46 were erupted, while permanent incisors D11, D21, in the maxilla and the permanent incisor D32 and the permanent molar D37 in mandible were still developing. The developmental stage D for D32 corresponds to the age of 5 years in females. Moreover, the stage E for DD11, 21 corresponds to the age of 5 years in females, and stage G for DD16, 26, 36, 46 corresponds to the age of 7 years in females (Demirjian, 1986). The patient's teeth in anterior region of the maxilla and mandible were conical shaped (Wright et al., 2022). The primary molars d55, d65, d75, primary canines, d53, d63, d73, d83 and primary incisive d51 were observed. On the left side, there was an AII bite defect, which might lead to mandibular retrognathia in future. The mucous membranes and gingiva of the mouth were in good condition.
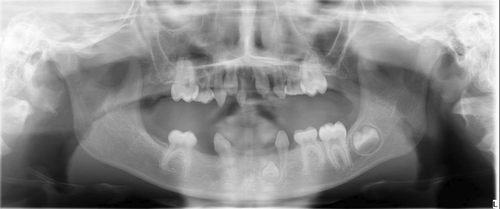
3.2 Result of clinical examination
At the age of 5 years, the patient displayed the following clinical features (Figure 2) at a visit in the Department of Clinical Genetics: a protruding forehead, fine linear wrinkles around the eyes and increased periorbital hyperpigmentation, a depressed nasal bridge, reduced lower facial height and midface hypoplasia caused by lacking development of maxillary and mandibular alveolar bone heights, sparseness of scalp hair, and both fine eyebrows and eyelashes. The patient showed a wide midline diastema and conical anterior teeth. In addition to this sparseness of body hair, the patient presented with sweating deficiency and severe atopic eczema.
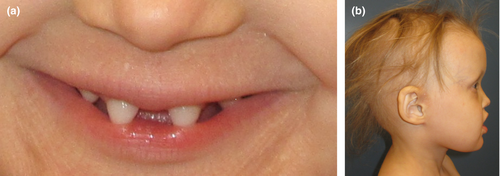
3.3 Result of genetic studies
The ectodermal dysplasia gene panel analyzed from the patient's DNA sample showed a heterozygous splice acceptor variant c.742-2A>G in the EDA (Ectodysplasin A) gene (GenBank reference sequence NM_001399.4). The variant was confirmed by bidirectional Sanger sequencing. This variant has not been observed in the large reference population cohort of the Genome Aggregation Database (gnomAD) nor in Finland, as the variant has not been observed in SISu (The Sequencing Initiative Suomi) database. Moreover, this variant is not reported at the ClinVar database. The in silico analysis using the MutationTaster predicted that this splice acceptor variant is disease causing. Additionally, SpliceSiteFinder-like (SSF) tool predicted a shift of four bases towards exon-to-exon boundary causing abnormal protein production.
An association is established between the EDA gene and the patient's phenotype. The identified EDA variant explains the features seen in Figure 2 as well as atopic eczema, hypohidrosis, hypotrichosis, and the oral manifestation of severe oligodontia and conical teeth. The same variant has been identified in the mother with a mild oral malformation. The patient's mother presented with a milder phenotype including hypodontia and/or conical teeth and additionally spottily reduced sweating (Pääkkönen et al., 2001).
The described patient's EDA variant was classified as likely pathogenic by the analyzing laboratory due to the patient's phenotype, the rarity of this variant in control populations, the type of splice acceptor variant, and because the variant has been previously found in an individual with a HED phenotype. The clinical geneticist discovered that the individual in whom the variant has been previously found was the patient's mother (Pääkkönen et al., 2001).
3.4 Family
Prior to the visit at the Department of Clinical Genetics, the patient's parents had been asked for background information on the family and possible hereditary diseases. At that time, the patient's mother was not aware of the previously identified EDA variant during her childhood. The grandparents had both been asymptomatic. No DNA sample had been obtained from the grandparents. The family pedigree is presented in Figure 3. The mother with same variant in the EDA gene showed only focal sweating deficiency and minor dental manifestations, for which she had received orthodontic treatment. The mother's pantomogram at the age of 25 years also revealed hypodontia, since the DD 18, 17, 15, 14, 12, 44, 45, 48 teeth were missing and only dental roots of DD 21, 28, 37 were left. The primary lower molar, d85 persisted (Figure 4). The hypodontia of the mother was congenital, since there was no record of tooth extractions. Additionally, the patient's mother presented with xerostomia.
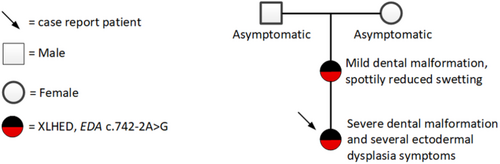
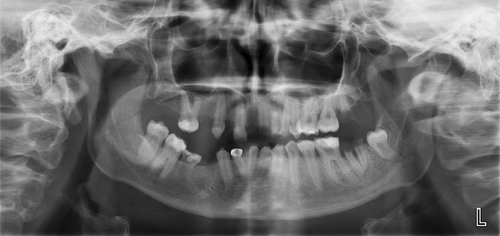
4 DISCUSSION
In the present study, we describe a very rare splice acceptor variant EDA c.742-2A>G, which is associated with a manifestations in the skin, hair, eyes, sweat glands, and teeth, and may also lead to severe oligodontia even in females. The results of our study point that this variant is pathogenic, since this variant segregates in the patient and her mother. Additionally, they both show suitable HED phenotypes, the variant is exceptionally rare in control populations (not found), both the in silico MutationTaster analysis and SpliceSiteFinder-like (SSF) predicted that the variant is disease causing, and the variant is a conserved splice site acceptor variant. In EDA, altogether 325 likely pathogenic and pathogenic variants have been reported by ClinVar, of which 17 are splice site mutations (ClinVar, Nov 2022). The splice site variant discovered in our study is not reported by ClinVar.
After further investigation, it was discovered that the case published by Pääkkönen et al. (2001), which was referred to by the laboratory, was the patient's mother. According to the mother, her symptoms had been perceived as mild during her childhood. Therefore, she has not received regular follow-up. The patient's symptoms are severe with many ectodermal symptoms that strongly correspond to the XLHED phenotype. Interestingly, the dental malformations detected in our female patient resembled a severe phenotype typically seen in male patients. Our study showed that the variant c.742-2A>G in the EDA gene can cause exceptionally severe teeth malformations with severe oligodontia, conical teeth, and abnormal timing of dental maturation. The same EDA variant has been found in the mother with mild oral malformations and she exhibit minimal expression in the form of hypodontia (Figure 4). The patient had also several ectodermal dysplasia symptoms in contrast to her mother who had only focally reduced sweating. Dysmorphic features previously observed in XLHED were seen in our patient such as periorbital fine linear wrinkles and hyperpigmentation, midface hypoplasia, a depressed nasal bridge and a protruding forehead, and hypotrichosis (Figure 2). Skewed X-chromosomal inactivation often explains exceptionally severe symptoms in some females in X-linked hereditary diseases. However, in the study of Körber et al. (2021), no evidence for preferential X-chromosome inactivation in female XLHED patients was found and it was proposed that other genetic or epigenetic factors explain the phenotype variability in EDA families (Körber et al., 2021). Further investigations are warranted to discover other causes of variable expressivity than skewed X-chromosomal inactivation in XLHED patients.
The pantomogram of the mother indicates that the mother's teeth are poorly maintained. Hypodontia was detected, as DD 18, 17, 15, 14, 12, 44, 45, 48 teeth were missing. Also, only roots of DD 21, 28, 37 were seen. The primary lower molar, d85 is persistent. (Figure 4). The teeth were poorly maintained, to which XLHED may contribute (Kieri et al., 2014). For example, XLHED causes xerostomia, which increases risk for caries (Quilici & Zech, 2019). As enamel is derived from the ectoderm, enamel disorders might be common in ED (Kurisu & Tabata, 1997). Enamel can be thinner and softer than average predisposing teeth for caries, decaying, and abnormal wearing (Kurisu & Tabata, 1997). In the present case, however, the pantomogram showed a marked contrast between the dentin and enamel supporting the normal maturation of the enamel. Furthermore, no dilation of pulpal area (taurodontia) was present.
The pediatricians treating the patient had no information that she had a 50% risk of inheriting the EDA splice site acceptor variant. Knowledge of family hereditary risk makes it possible to examine at an early stage if offspring have the family mutation and plan appropriate follow-up (Wright et al., 2022). Many patients with ectodermal dysplasia have problems with psychological and physiologic development, which result from unacceptable esthetics and abnormal functions of craniofacial structures. Therefore, early intervention and treatment is recommended (Clauss et al., 2008; Wright et al., 2022). Optimal treatment is provided by a multidisciplinary collaborative team of specialists in pediatrics, psychology, otorhinolaryngology, and speech therapist. Patients require symptom-related instructions and the possibility to consult a doctor when needed.
Dentists have the responsibility to rehabilitate these patients to improve appearance, mastication, and speech and also prophylactically treat teeth to prevent dental decay (Finn, 1967; Lexner et al., 2008; Oliver et al., 1975; Suprabha, 2002). Furthermore, dentists can play a decisive role in the early diagnosis of ED, when referring children to further specialists after the detection of significant dental abnormalities as described here. Based on the degree of oligodontia, a treatment plan is formed. In this case, oligodontia was identified at the age of 7 years. A thorough treatment plan including reconstruction due to the patient's bite defect and oligodontia was made. Oral treatment will be performed by a close collaboration of various clinical specialties (specialists in orthodontics, prosthetics, surgeries, specialists in dentistry including orthodontia, prosthetic dentistry and pediatric dentistry, and oral surgery).
Hair manifestation also present an esthetics problem, and can be partly corrected by hairpiece or hair transplantation. Severe atopic eczema in the whole-body area found in our patient has been partly treated by skin emulsion (Paschos et al., 2002). To treat severe atopic eczema, topical pimecrolimus or tacrolimus and ultraviolet-A therapy can be considered (Chee et al., 2014; Srivastava, 2011). Eye symptoms need regular medical contact and control.
Currently, several EDA1 replacement therapies with recombinant protein (Fc-EDA) administered prenatally are in trial phase I-II. Results have been mixed with some treatments providing good outcome alleviating EDA symptoms (Körber et al., 2020).
To the best of our knowledge, this is the first report of severe dental malformation associated with the EDA c.742-2A>G variant and we propose a pathogenic classification of this variant. Furthermore, this is the first study to describe the ectodermal symptoms in teeth, skin, hair, eyes, and sweat glands associated with this EDA slice acceptor variant.
AUTHOR CONTRIBUTIONS
Vivian Reinhold: Contributed to conception, design, data acquisition and interpretation, drafted and critically revised the manuscript. Stina Syrjänen: Contributed to conception, design, data acquisition and interpretation, performed all statistical analyses, drafted and critically revised the manuscript. Minna Kankuri-Tammilehto: Contributed to conception, design, and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
Special thanks to Andreina Kero, MD, PhD for proofreading the manuscript and to Minna Toivonen, M.Sc., PhD, for assistance with SpliceSiteFinder-like.
FUNDING INFORMATION
No funding was received.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
ETHICAL COMPLIANCE
The study complies with the Declaration of Helsinki regarding the use of human samples and identifiable information. This study is a case report and informed consent was obtained from the patients regarding the use of information obtained during clinical treatment. In the study, analyzed data were from patients who had been treated at the hospital. As no new samples were required, a separated ethics board permit was not required.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




