Congenital central hypoventilation syndrome in Chinese population: Analysis of three new cases and review of the literature
Abstract
Background
Congenital central hypoventilation syndrome (CCHS) is a rare autosomal dominant disease that is mainly caused by PHOX2B mutations. The purpose of this study is to analyze and summarize the clinical and genetic characteristics of CCHS patients in the Chinese population from our study and previous literature.
Methods
The potential pathogenic gene mutations of CCHS were identified and verified by next generation sequencing combined with Sanger sequencing, fluorescent probe PCR and capillary electrophoresis. The clinical characteristics and gene mutations of CCHS cases in Chinese population were summarized from our study and previous literature to explore the genotype–phenotype correlations.
Results
We identified 48 CCHS cases including three new cases from our report in China. Overall, 77.1% of the patients had PHOX2B polyalanine repeat expansion mutations (PARMs), and the remaining 22.9% had 10 distinct PHOX2B non-polyalanine repeat expansion mutations (NPARMs). Compared to those with PARMs, patients with NPARMs were more likely to have premature birth (54.5% vs. 2.8%, p < 0.001) and lower birth weight (33.3% vs. 3.2%, p = 0.030), with statistical significance. The patients with PARMs were more likely to have cardiovascular defects (64.9% vs. 27.3%, p = 0.063), cerebral hemorrhage (29.7% vs. 9.1%, p = 0.322) and seizures (37.8% vs. 9.1%, p = 0.151) than those with NPARMs, with no statistical significance.
Conclusions
CCHS patients with PHOX2B NPARMs were more likely to have premature birth and low birth weight, while PHOX2B PARMs tended to be positively associated with the risk of cardiovascular defects, cerebral hemorrhage and seizures in Chinese population.
1 INTRODUCTION
Congenital central hypoventilation syndrome (CCHS, OMIM 209880) is a rare autosomal dominant genetic disease that affects the autonomic nervous system, featuring the deficient autonomic control of breathing. It is estimated that the incidence rate is 1/148000–1/200000 live births (Shimokaze et al., 2015). Mutations in the paired-like homeobox 2B (PHOX2B, OMIM 603851) are responsible for CCHS (Miura et al., 2019). In most CCHS patients, the syndromes start in the neonatal period, while they may start in infancy, childhood or even adulthood some patients (Trang et al., 2020). Due to the primary defect of chemoreceptors in the respiratory center, patients have reduced sensitivity to carbon dioxide (CO2) and hypoxemia. As a widespread autonomic nervous system disorder, CCHS can be accompanied by some nervous system disorders, including dysphagia, Hirschsprung's disease (HSCR), neural crest tumors (NCT) and so on. Arrhythmia, eye regulation disorders and thermoregulation disorders are common manifestations of CCHS (Laifman et al., 2020; Saiyed et al., 2016; Vanderlaan et al., 2004; Weese-Mayer et al., 2010), and some CCHS individuals also have unique facial features (Todd et al., 2006).
Located on human chromosome 4p13, the PHOX2B (OMIM 603851) encodes a transcription factor crucial to the development of autonomic nervous system (ANS), which consists of 314 amino acids (Bachetti & Ceccherini, 2020). Compared to 20 alanine repeats (genotype 20/20) in the PHOX2B allele of non-CCHS people, most (about 90%) CCHS patients have abnormal expansions of the alanine repeat tracts (genotype 20/24–20/33), which are known as the polyalanine repeat expansion mutations (PARMs). The remaining 10% of patients have heterozygous non-PARMs (NPARMs) in the polyalanine repeat region or elsewhere in the PHOX2B coding sequence, including frameshift, nonsense and missense mutations, while less than 1% of patients have exon or whole gene deletion of PHOX2B. A PHOX2B mutation would be always required for a diagnosis of CCHS, however, in individuals with CCHS or phenotypes similar to CCHS and negative for PHOX2B mutations, homozygous mutations of two new genes, MYO1H (OMIM 614636) and LBX1 (OMIM 604255) have recently been found (Hernandez-Miranda et al., 2018; Spielmann et al., 2017). In particular, LBX1 cooperates with PHOX2B in the development of the retrotrapezoid nucleus (RTN), and its frameshift mutation interferes with this cooperativity, probably by blocking the recruitment of co-activator and/or a possible interaction with PHOX2B, thus changing the way their target genes are regulated. According to previous studies, the majority of NPARMs have been reported to cause severe phenotypes with the need for continuous auxiliary ventilation, and increase the risks of HSCR and NCT. However, a few patients with NPARMs have relatively mild phenotypes (Byers et al., 2018; Kasi et al., 2017; Unger et al., 2017). Some patients with NPARMs may be asymptomatic and are only identified by genetic studies due to an affected family member (Janssen et al., 2018).
Since PHOX2B gene was identified as the pathogenic gene of CCHS in 2003, approximately 3000 cases of PHOX2B mutation-confirmed CCHS have been reported worldwide (Weese-Mayer et al., 1993). The clinical diagnosis of CCHS in the neonatal period is sometimes challenging because the symptoms and signs of CCHS often overlap with other diseases, such as neuromuscular diseases. At present, there are only sporadic reports on individual cases in China, and the comprehensive analyses of the clinical characteristics and mutations of CCHS patients in China are scarce. In addition, although some reported Chinese patients showed symptoms of CCHS, including persistent hypoventilation and hypercapnia during sleep after excluding possible pulmonary or neuromuscular and cardiac pathogens within 1 year after birth, no PHOX2B gene detection was performed. These patients were not included in the study. In this study, we analyzed three new CCHS cases in China and searched the literature to summarize the previously reported CCHS cases in China confirmed by genetic testing. The purposes of this study are to summarize the clinical and genetic characteristics of CCHS cases in Chinese population, analyze the correlations between genotypes and phenotypes, and broaden our understanding of clinical characteristics of CCHS so as to promote the accurate diagnosis and timely treatment of patients with this disease.
2 METHODS
2.1 Ethical compliance
This study was approved by the Ethics Committee of Qingdao Municipal Hospital, the Affiliated Hospital of Qingdao University and Qingdao Women and Children's Hospital.
2.2 Patients
Three female neonates with genetic confirmed CCHS evaluated at Qingdao Women and Children's Hospital from January 2018 to December 2021 were included in this study. Inclusion criteria: (I) patients with typical or suggestive clinical findings of CCHS in line with the Statement of American Thoracic Society; (II) patients with PHOX2B mutations identified by genetic testing. Exclusion criteria: subjects had symptoms of CCHS without PHOX2B mutations or not had genetic testing. After obtaining the informed consent from patients' guardians, the blood samples of patients were collected for genetic testing.
2.3 Molecular analysis
2.3.1 Fluorescent probe PCR and capillary electrophoresis for dynamic mutations detection
Genomic DNA extraction kit was used to extract genomic DNA from the peripheral blood of patient1(P1) and patient3(P3) in strict accordance with the manufacturer's process. Primer premier 5 was used to design upstream and downstream primers, and the forward primers were labeled with FAM fluorescence. Then, the product was obtained by polymerase chain reaction(PCR) (reaction system consisting of: (I) Gold start Best Mix 2X 12.5 μL. (II) Forward Primer (10 uM) 1.0 μL. (III) Reverse Primer (10 μ M) 1.0 μL. (IV) DMSO 1.2 μL, gDNA 100 ng. (V) adding ddH2O to 25 μL. PCR reaction procedure consisted of: 95°C, 10 min; 94°C, 50 s, 70°C, 50 s, 72°C, 90 s, 3 cycles; 94°C, 50 s, 67°C, 50 s, 72°C, 90 s, 3 cycles; 94°C, 50 s, 64°C, 50 s, 72°C, 90 s, 3 cycles; 94°C, 50 s, 61°C, 50 s, 72°C, 90 s, 28 cycles; 72°C, 5 min 4°C, Hold. The PCR products were detected by capillary electrophoresis and the number of trinucleotide repeats was obtained according to the preset parameters.
2.3.2 NGS for mutations detection
The qualified genomic DNA samples of patient 2 (P2) were divided into 180-250 bp. After the end modification of DNA fragments, the DNA library and library preparation kit were prepared. Then, the whole exon region was effectively enriched by liquid chip capture system. The exon library was enriched by PCR, and then the library quality was evaluated. Finally, the high-throughput sequencing of the qualified enriched libraries was performed on an Illumina NextSeq 500 sequencer for paired-end reads of 150 bp. The coverage of the target regions was 99.9%, and average sequencing depth was 254×.
2.3.3 Sanger sequencing validation
The mutations of PHOX2B(NM_003924.4) detected by NGS were confirmed by Sanger sequencing. After extracting the genomic DNA of P2, the upstream and downstream sequences involving mutation sites were amplified by PCR. Primers were designed by Primer Premier5 software (primer sequences: Forward −5′ - AGAACGGCTCCTCGGGCAAAA-3′ and Reverse −5′- ACTGCTCTTCACTAAGGCGGC-3′). The PCR-amplified products were analyzed by 1% agarose gel electrophoresis followed by purification of the products and mutational analyses on an ABI 3730 analyzer (Applied Biosystems).The variants were identified by comparison with the reference sequence on the National Center Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/).
2.4 Literature review
We reviewed articles of Chinese CCHS cases with genetic information published in PubMed, CNKI and Wan Fang before April 2022. We recorded the following data: gender, gestational age, birth weight, perinatal history, family history, age of onset, the modes of PHOX2B mutation, genotypes, clinical manifestations, ventilation modes, treatment results and other relevant characteristics. Cases with unknown genetic information, negative PHOX2B sequence or insufficient description of clinical data were excluded.
2.5 Statistics analysis
The statistical analysis was performed with SPSS software, version 26. Descriptive statistics were used to analyze demographic variables. Categorical variables were presented as frequencies and percentages. The Chi-squared test or Fisher's test was used to compare the categorical variables when appropriate. A p-value of <0.05 was considered to be statistically significant.
3 RESULTS
3.1 Analysis of clinical data from the three new cases of CCHS
All the three new cases of CCHS were female neonates. The average birth weight was 3046.7 ± 98.7 g and the average gestational age was 38.7 ± 1.5 weeks. P1 was a premature infant. Although no facial abnormalities were observed, P1 had single palmar crease. P1 and P2 had abnormal perinatal history, including premature rupture of membranes and meconium-stained amniotic fluid. All the patients developed clinical signs of CCHS in the neonatal period. They required repeated endotracheal intubation for invasive mechanical ventilation due to their clinical manifestation of respiratory depression. All patients showed pneumonia and bronchopneumonia on chest CT examinations. The abdominal plain film of P3 showed an inflated and dilated intestinal tube, as well as thickened intestinal space. In addition, lower gastrointestinal radiography indicated possible Hirschsprung's disease (HSCR) (long segment type). P1 and P3 had seizures, intracerebral hemorrhage, anemia and cardiovascular defects. Cardiovascular defects included atrial septal defect (ASD), patent ductus arteriosus (PDA), pulmonary hypertension (PH), mitral regurgitation (MR) and patent foramen ovale (PFO). P1 and P2 had nervous system abnormalities such as hypotonia. In addition, P2 had temporary hypoglycemia and hyperglycemia. However, she had no neural crest tumor or other autonomic nerve dysfunction symptoms, such as abnormal heart rate variability or thermoregulation. No patient underwent polysomnography since their condition was not stable enough to undergo such study, but continuous electrocardiogram monitoring and timely blood gas analysis were conducted to monitor the patients' condition. Guardians of P2 and P3 gave up treatment and leave the hospital automatically with nasal continuous positive airway pressure ventilation and invasive assisted ventilation through tracheal intubation, respectively, the reason for their decision is that they do not want their children to live in pain, while guardians of P1 chose to transfer to another hospital with bi-level positive airway pressure ventilation. The detailed clinical feature and laboratory examination results of three patients are shown in Table 1.
| Patients | Sex | Birth weight (g) | Gestation (weeks) | Perinatal history | Family history | Age of onset | Mutation type | Genotype | Respiratory manifestation | Digestive system manifestation | Neurological complication | Other clinical manifestations | HSCR | Chest CT | Echo | Brain imaging | EEG | Ventilation type | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 2980 | 37 + 3 | Premature rupture of membranes | NA | 30 min | PARM | 20/24 |
Shallow and slow breathing Apnea Inflammation of tracheobronchial intima |
Cholestasis | Low muscle tone |
Anemia Low citrulline level Right hand through the palm |
N |
Pneumonia Pleural effusion |
ASD PDA PH MR PFO |
Subarachnoid hemorrhage | Abnormal | Endotracheal intubation | Transfer to another hospital |
| P2 | F | 3160 | 40 + 2 | Amniotic fluid pollution | NA | 30 min | NPARM | c.741-758dup | Shallow slow breathing apnea | (−) |
Low muscle tone |
Hyperglycemia Low blood sugar |
N |
Bronchopneumonia Decreased lung permeability |
(−) | Decreased white matter density | NP | Endotracheal intubation | Give up treatment, Automatic discharge |
| P3 | F | 3000 | 39 + 2 | (−) | NA | 2d | PARM | 18/27 | Shallow slow breathing apnea |
Abdominal distension Peritoneal effusion Inflatable dilatation of intestine |
(−) |
Slightly higher proportion of benzene and cheese Anemia |
Y |
Pneumonia |
PH PFO PDA |
Lateral intraventricular hemorrhage Subarachnoid hemorrhage |
(−) | Endotracheal intubation | Give up treatment, Automatic discharge |
- Abbreviations: (−), normal; ASD, atrial septal defect; Echo, Echocardiogram; F, female; GDM, gestational diabetes mellitus; HSCR, Hirschsprung's disease; M, male; N, no; NA, not available; NP, not performed; MR, Mitral regurgitation; PDA, patent ductus arteriosus; PFO, patent foramen oval; PH, Pulmonary hypertension; Y, yes.
3.2 Analysis of PHOX2B gene mutations in the three new cases of CCHS
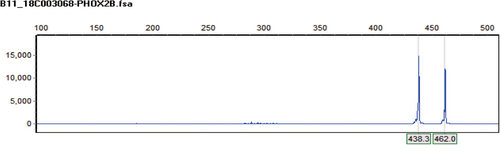
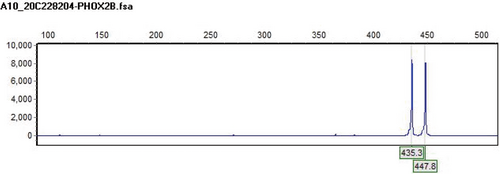
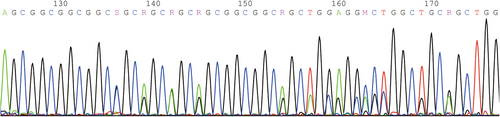
Among the three new cases, P1 and P3 had repeat mutations of PHOX2B detected by fluorescence probe PCR and capillary electrophoresis, with genotypes of 20/24 and 18/27 respectively; P2 had c.741_758dup heterozygous mutation (NM_003924.3) on exon 3 of PHOX2B. 18/27 and c.741_ 758dup were new genotypes caused by PHOX2B mutations in China. The DNA fragments analysis diagram and test results of P1 and P3 are shown in Figures 1 and 2 respectively. The genetic testing results of P2 are shown in Table 2 and the sequence diagram is shown in Figure 3.
| Full name | Family relationship | Gene | Exon/ intron | Chromosome position | Transcript number |
|---|---|---|---|---|---|
| Patient 2 | / | PHOX2B | Exon3 | Chr4:41748010–41748011 | NM_003924.3 |
| Nucleotide changes | Amino acid change | Heterozygous/Homozygous | Variation type | Source of variation | Frequency in population |
| c.741_758dup | p.255_A260dup | Heterozygous Mutation | Pathogenic Mutation | / | / |
3.3 Literature review of CCHS cases in China
Our initial search identified 76 cases of CCHS in China from 34 reports. After reviewing for inclusion criteria and data quality, 31 cases were excluded from our analysis due to lack of genetic or other important information. Finally, we further analyzed the remaining 45 Chinese CCHS cases (Hong et al., 2013) including our three new cases. Full phenotypic and genotypic details of the 45 cases can be found in Table 3.
| Author | Year | Sex | Birth weight (g) | Gestation (weeks) | Mutation type | Genotype | Perinatal history | Family history | Age of onset | Respiratory manifestation | Digestive system manifestation | HSCR | Seizure | Neurological complication | Other clinical manifestations | Intracranial hemorrhage | CHD | Arrhythmia | Ventilation type | Withdrawn of treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ren et al. (2013) | 2013 | M | 4000 | Full term | PARM | 20/25 | (−) | (−) | 23 days | Apnea; Pleural effusion; Pneumonia; Atelectasis | Fatty liver like changes; Hepatomegaly | N | N |
Ventricular enlargement; Sulcus deepening; Subdural effusion;Abnormal EEG |
(−) | Y |
PH; Pericardial effusion |
Sinus bradycardia; Incomplete right bundle branch block |
Endotracheal intubation; BiPAP; CPAP |
N |
Breathing and circulation are stable; Backward growth and development |
| Li et al. (2013a, 2013b) | 2013 | F | 3600 | 39 + 2 | NPARM | c.758-759Ins18 | Vaginitis during pregnancy | (−) | 1 day |
Apnea; Shallow and slow breathing; Respiratory failure; Pneumonia |
(−) | N | Y | (−) | (−) | N | (−) | (−) |
Endotracheal intubation |
N |
Noninvasive ventilation during sleep; Normal growth and development |
| Wang et al. (2013) | 2013 | F | 2600–3700 | 39 | PARM | 20/26 | (−) | NA | 2 h | Dyspnea; Slow and irregular breathing; Pneumonia; Laryngeal edema | (−) | N | Y | Decreased muscle tone; Widened lateral ventricle; Widened subarachnoid space | Anemia | N | PH | (−) |
Endotracheal intubation; CPAP |
Y | Death |
| Wang et al. (2013) | 2013 | M | 2600–3700 | 38 | PARM | 20/25 | (−) | NA | 28 days | Dyspnea; Slow and irregular breathing; Pneumonia | Low sucking force; Choking milk | N | Y | Decreased muscle tone | (−) | N | PDA | (−) |
CPAP |
N |
Noninvasive ventilation during sleep; Generally in good condition |
| Wang et al. (2013) | 2013 | M | 2600–3700 | 40 | PARM | 20/25 | (−) | NA | 30 days | Dyspnea; Slow and irregular breathing; Pneumonia; Airway inflammation | Low sucking force; Choking milk | N | Y | Decreased muscle tone; Bilateral lacunae in basal ganglia | (−) | N | ASD;PH | (−) | CPAP | N | Noninvasive ventilation during sleep; Generally in good condition |
| Han et al. (2013) | 2013 | M | 3600 | 37 | PARM | 20/26 | (−) | (−) | 1 day | Respiratory depression; Pneumonia | Low sucking force; Delayed meconium excretion | N | N | Decreased muscle tone; Decreased white matter density | (−) | N | PDA; PFO | (−) | BiPAP | N | Normal growth and development |
| Han et al. (2013) | 2013 | M | 3170 | 39 + 4 | PARM | 20/27 | Polyhydramnios | (−) | 5 h | Respiratory depression; Pneumonia | Low sucking force; Delayed meconium excretion | N | N | Decreased muscle tone | (−) | N | PFO | (−) | Endotracheal intubation; DUOPAP | Y | Death |
| Han et al. (2013) | 2013 | M | 3640 | 38 | PARM | 20/25 | (−) | (−) | 1 day | Respiratory depression; Pneumonia | Low sucking force; Delayed meconium excretion | N | N | Widened extracerebral space | (−) | N | PDA; PFO | (−) | Ventilator ventilation | Y | Death |
| Han et al. (2013) | 2013 | F | 2900 | 39 | PARM | 20/25 | (−) | (+) | 1 day | Respiratory depression; Pneumonia | Low sucking force; Delayed meconium excretion | N | N | (−) | (−) | N | PFO | (−) | DUOPAP; Ventilator ventilation | N | Generally in good condition |
| Mei et al. (2014) | 2014 | M | 2500 | 35 + 6 | NPARM | c.722-759 het-del | Polyhydramnios | (+) | 6 days | Respiratory depression |
Intestinal obstruction Abdominal distention |
Y | N | Decreased brain white matter density | (−) | N | PFO | (−) | NA | Y | Death |
| Mei et al. (2014) | 2014 | M | 3500 | 39 | PARM | 20/27 | Polyhydramnios | (+) | 1 day | Respiratory depression; Pneumonia | (−) | N | N | Decreased brain parenchymal density | Reduced free carnitine | N | PFO | (−) | NA | Y | Death |
| Yan et al. (2015) | 2015 | F | 3060 | 39 | PARM | 20/29 | Premature rupture of membranes | (−) | 1 day | Laryngeal softening | Low intestinal motility; Abdominal distension | N | Y | (−) | (−) | N | (−) | (−) | CPAP; Nasal catheter oxygenation | Y | Death |
| Yin et al. (2021) | 2021 | M | 3300 | 38 | PARM | 20/25 | (−) | NA | 24 days | Shallow and weak breathing | (−) | N | N | Frontal lobe abnormal signal shadow | (−) | N | PFO | (−) | Endotracheal intubation;BiPAP | N | Normal growth and development |
| Yin et al. (2021) | 2021 | M | 3360 | 38 + 4 | PARM | 20/27 | (−) | NA | 36 h | Shallow and weak breathing | Abdominal distension | N | N | (−) | (−) | N | PFO | (−) | Endotracheal intubation | Y | Death |
| Yin et al. (2021) | 2021 | F | 2400 | 35 + 4 | PARM | 20/27 | (−) | NA | 2 h | Apnea | Abdominal distension; Delayed meconium excretion | N | N | (−) | (−) | N | PFO | (−) | Endotracheal intubation | Y | Death |
| Yin et al. (2021) | 2021 | F | 3030 | 41 + 2 | PARM | 20/27 | (−) | NA | 30 min | Apnea | Delayed meconium excretion | N | N | Decreased muscle tone | (−) | Y | ASD | (−) | Endotracheal intubation | Y | Death |
| Yin et al. (2021) | 2021 | M | 3150 | 39 + 4 | PARM | 20/26 | (−) | (+) | 23 days | Apnea | Abdominal distension | Y | N | (−) | (−) | N | (−) | (−) | Endotracheal intubation | Y | Death |
| Yu and Cui (2021) | 2021 | M | 3410 | Full term | PARM | 20/27 | Polyhydramnios | (−) | 14 days | Respiratory depression; Pneumonia | (−) | N | Y | Brain edema | (−) | Y | PFO;PDA | (−) | Endotracheal intubation; NCPAP | Y | Death |
| Lee et al. (2009) | 2009 | M | NA | NA | PARM | 20/25 | (−) | (+) | 30 year | Pneumonia | (−) | N | N | N | Secondary polycythemia; Pestle finger | N | TR;PH | Ventricular ectopic beat | ;BiPAP | N | Generally in good condition |
| Mei et al. (2021) | 2021 | M | 2500 | 35 + 6 | NPARM | c.722_759del38 (p.A241Gfs*106) | Polyhydramnios | (+) | 0–7 days | Localized patchy clouding opacity | Multiple dilated loops of bowel | Y | N | (−) | (−) | N | (−) | (−) | Invasive | N | Death |
| Mei et al. (2021) | 2021 | M | 3500 | 39 | PARM | 20/27 | Polyhydramnios | (+) | 0–7 days | (−) | (−) | N | Y | EEG Abnormal | (−) | N | ASD | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | M | 2160 | 35 + 6 | NPARM | c.684dup (p.P229Afs*131) | Decreased fetal movements | (−) | 0–7 days | (−) | Unremarkable | N | N | (−) | (−) | N | (−) | (−) | Invasive | Y | NA |
| Mei † et al. (2021) | 2021 | F | 3650 | 39 + 2 | PARM | 20/26 | Polyhydramnios | (+) | 0–7 days | Left pulmonary whiteout, right pulmonary consolidation | Unremarkable | N | Y | (−) | (−) | N | (−) | (−) | Invasive | Y | Death |
| Mei et al. (2021) | 2021 | M | 2440 | 34 | NPARM |
c.202G > T (p.G68C) |
(−) | (−) | 0–7 days | Reduced lungs lucent;Diffuse reticular granule pattern | Multiple dilated loops of bowel | Y | N | (−) | (−) | N | (−) | (−) | Invasive | Y | Death |
| Mei et al. (2021) | 2021 | M | 3250 | 41 + 2 | PARM | 20/27 | (−) | (−) | 0–7 days | (−) | (−) | N | N | EEG Abnormal | (−) | Y | PDA | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | F | 3150 | 41 + 6 | PARM | 20/26 | (−) | (−) | 0–7 days | (−) | (−) | N | N | (−) | (−) | N | (−) | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | M | 3200 | 39 + 2 | PARM | 20/26 | (−) | (−) | 0–7 days | Localized lobar infiltrates | (−) | N | N | EEG Abnormal; Periventricular encephalomalacia | (−) | N | (−) | (−) | Invasive | Y | Death |
| Mei et al. (2021) | 2021 | M | 3240 | 41 | NPARM | c.422G > A (p.R141Q) | (−) | (−) | 0–7 days | (−) | (−) | N | N | (−) | (−) | N | (−) | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | F | 3840 | 38 + 3 | PARM | 20/26 | Polyhydramnios; GDM | (+) | 0–7 days | Localized lobar infiltrates | (−) | N | N | White matter lesions | (−) | N | (−) | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | F | 2575 | 39 | PARM | 20/27 | Polyhydramnios | (−) | 0–7 days | Lobar infiltrates with superimposed air bronchograms | Small bowel distension | N | N | EEG Abnormal | (−) | N | ASD;PDA; PLSVC | (−) | Invasive | Y | Death |
| Mei † et al. (2021) | 2021 | M | 3240 | 38 + 5 | PARM | 20/26 | (−) | (−) | 0–7 days | (−) | Unremarkable | N | Y | (−) | (−) | Y | PDA | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | F | 3500 | 40 | PARM | 20/26 | (−) | (−) | 0–7 days | Lobar infiltrates | (−) | N | N | (−) | (−) | N | (−) | (−) | Invasive | Y | NA |
| Mei et al. (2021) | 2021 | F | 3400 | 39 + 1 | PARM | 20/27 | (−) | (−) | 0–7 days | (−) | (−) | N | N | Hypotonia | (−) | N | (−) | (−) | Invasive | Y | Death |
| Li et al. (2018) | 2018 | M | NA | 39 + 2 | NPARM | e.756-776del21bp | (−) | (−) | 18 days | Shallow and slow breathing; Apnea; Pneumonia; Endobronchial inflammation | Difficult defecation | N | N | Widened extracerebral space; EEG Abnormal | (−) | N | PFO | (−) | Endotracheal intubation; CPAP | Y | Death |
| Li et al. (2018) | 2018 | F | 2600 | 40 + 6 | PARM | 20/27 |
Fetal distress; Amniotic fluid pollution; Placental calcification |
NA | 9 h |
Shallow and slow breathing; Apnea Pneumonia; Endobronchial inflammation |
(−) | N | N | Brain MR abnormalities | (−) | N |
PDA;PFO; PH;TR |
Atrial premature contraction; Supraventricular tachycardia |
Endotracheal intubation; CPAP | Y | Death |
| Li et al. (2018) | 2018 | F | NA | Full term | PARM | 20/26 | (−) | (−) | 2 days | Shallow and slow breathing; Pneumonia; Consolidation of lung; Softening laryngeal cartilage | (−) | N | N | (−) | (−) | Y | PFO | (−) | Endotracheal intubation | Y | Death |
| Li et al. (2018) | 2018 | F | 3200 | 37 + 2 | PARM | 20/26 | (−) | NA | 10 days |
Shallow and slow breathing; Pneumonia; Consolidation of lung |
(−) | N | Y | Widened extracerebral space; HIE | (−) | N | (−) | (−) | Ventilator ventilation | Y | Death |
| Zhang et al. (2020) | 2020 | M | NA | Full term | PARM | 20/27 | Polyhydramnios | (−) | 10 h |
Shallow and slow breathing Apnea |
Vomit; Abdominal distention; Less defecation |
N | Y | Low muscle tone | (−) | N | (−) | (−) | Invasive | Y | Death |
| Zhang et al. (2020) | 2020 | M | NA | Full term | PARM | 20/26 | (−) | (−) | 11 h |
Shallow and slow breathing Apnea |
Poor gastric emptying; Difficulty in adding milk |
N | Y | Low muscle tone | (−) | Y | (−) | (−) | Invasive | Y | Death |
| Zhang et al. (2020) | 2020 | F | NA | Full term | PARM | 20/26 | Polyhydramnios | (−) | Immediately after birth |
Shallow and slow breathing Apnea |
(−) | N | N | Low muscle tone | (−) | N | (−) | (−) | Invasive | Y | Death |
| Zhang et al. (2020) | 2020 | F | NA | Full term | NPARM | c.836-843del | (−) | (−) | Immediately after birth |
Shallow and slow breathing Apnea |
Poor gastric emptying; Difficulty in adding milk |
N | N | Low muscle tone | (−) | N | (−) | (−) | Invasive | Y | Death |
| Zhang et al. (2020) | 2020 | F | NA | Full term | PARM | 20/30 | Polyhydramnios | (−) | Immediately after birth |
Shallow and slow breathing Apnea |
(−) | N | N | Low muscle tone | (−) | Y | (−) | (−) | Invasive | Y | Death |
| Xu et al. (2018) | 2018 | M | 3600 | 34 | NPARM | c.202G>T (p.G68C) | Fetal distress | NA | 3 days | Apnea; Pneumonia |
Abdominal distention; Intestinal dilatation |
N | N | (−) | (−) | N | (−) | (−) |
Endotracheal intubation; NCPAP |
Y | Death |
| Hu et al. (2021) | 2021 | F | 3400 | Full term | PARM | 20/25 | (−) | (−) | 9 months | Shallow and weak breathing; Pneumonia |
Hepatomegaly Peritoneal effusion |
N | Y | (−) | Eyelid and facial edema | Y |
PH;ASD; PFO;TR |
(−) |
Endotracheal intubation; BiPAP |
N |
Nocturnal noninvasive BiPAP Good growth and development |
| Ren et al. (2022) | 2022 | F | 1090 | 33 + 1 | NPARM | c.440 A > G | Polyhydramnios; Subclinical hypothyroidism of mother | (−) | Immediately after birth |
Dyspnea Apnea |
Abdominal distention; Gastrointestinal bleeding; Intestinal obstruction |
N | N | Widened lateral ventricle | (−“) | Y | AFO | NA |
Endotracheal intubation; BiPAP |
Y | Death |
- Abbreviations: (−), normal; †, sibling; AFO, patent foramen oval; ASD, atrial septal defect; BiPAP, bilevel positive airway pressure; CHD, congenital heart disease; CPAP, continuous positive airway pressure; F, female; GDM, gestational diabetes mellitus; HIE, hypoxic ischemic encephalopathy; HSCR, Hirschsprung's disease; N, no; NA, not available; NCPAP, nasal continuous positive airway pressure; NPARM, non-polyalanine repeat expansion mutation; M, male; PARM, polyalanine repeat expansion mutation; PDA, patent ductus arteriosus; PH, Pulmonary hypertension; PLSVC, persistent left superior vena cava; TR, Tricuspid regurgitation; Y, yes.
Among the 45 cases, there were 25 males and 23 females (sex ratio: 1.1). About 14.9% of the patients were born prematurely with the youngest gestational age of 33 + 1 weeks, the median gestational age was 39.1 ± 3.3 weeks. Nearly half (44.7%) of the patients had abnormal perinatal history, among whom 13 cases had fetal polyhydramnios, 28.6% of the patients had abnormal family history, 93.8% of the patients developed clinical signs of CCHS in the neonatal period, and the birth weight of 90.0% patients was within the normal range. In terms of phenotypes, 28 patients had respiratory abnormalities, such as pneumonia, bronchitis and laryngomalacia; 26 patients had digestive system abnormalities, including dysphagia, abdominal distension, intestinal obstruction, hepatomegaly, peritoneal effusion, cholestasis and delayed meconium excretion; five patients were diagnosed with HSCR; 15 patients had seizures; 12 patients had intracerebral hemorrhage; and 29 patients also had nervous system abnormalities such as hypotonia and abnormal EEG; and in terms of the cardiovascular system diseases, 27 patients showed atrial septal defect (ASD), patent ductus arteriosus (PDA), patent foramen ovale (PFO), pulmonary hypertension (PH) and tricuspid regurgitation (TR), while only 6.4% of the patients had arrhythmia. In addition to the above common systemic manifestations, we also found some abnormal manifestations in a few individuals, such as anemia, temporary hypoglycemia, hyperglycemia and single palmar crease.
3.4 Analysis of overall characteristics of 48 patients with CCHS in China
In this study, 37 patients were detected with PHOX2B PARMs, of which the ratio of male and female was about 1.0 (18/19). There were about 38 patients who needed endotracheal intubation for ventilation, other patients only needed supplemental oxygen for treatment. About 37 patients' guardians gave up treatment, among them, 27 patients were confirmed to have died in the end, and 10 patients had unknown outcomes. In this study, only nine patients were ultimately confirmed to have survived under assisted ventilation.
Our analysis of PHOX2B mutations revealed that the small alanine expansions (+ 5 to +7 alanines) were the most frequent mutations identified in this series (Figure 4), which is consistent with the previous studies reporting that 20/25, 20/26 and 20/27 genotypes were the most common mutation types of PHOX2B (Weese-Mayer et al., 2010; Zhou et al., 2021). More than half (37/48) of patients had PARMs in PHOX2B (one with 24 PARM, seven with 25 PARM, 14 with 26 PARM, 12 with 27 PARM, one with 29 PARM, one with 30 PARM and one with a novel genotype 18/27), among whom 28 patients had larger PARMs (alanine expanded from +6 to +13) and the remaining nine patients had smaller PARMs. The remaining 11 patients had 10 different NPARMs.
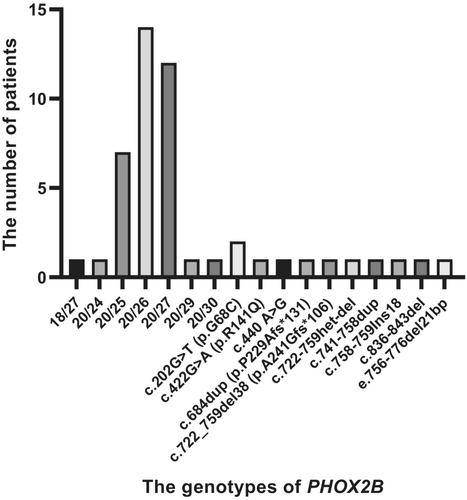
Genotype–phenotype correlation analysis was performed on these 48 CCHS cases in China (Table 4). Patients with NPARMs had preterm births (54.5% vs. 2.8%, p < 0.001) and low birth weight (33.3% vs. 3.2%, p = 0.030) than those with PARMs. Patients with NPARMs were more likely to have HSCR (27.3% vs. 5.4%, p = 0.128), and less likely to have cardiovascular defects (27.3% vs. 64.9%, p = 0.063), cerebral hemorrhage (9.1% vs. 29.7%, p = 0.322) and seizures (9.1% vs. 37.8%, p = 0.151) than those with PARMs, with no statistically significant differences. Among the PARM group, no occurrence of HSCR has been reported in the cases with the 20/25 genotype, and the occurrence of HSCR has been reported in a few cases (5.4%) with the 20/26 and 18/27 genotypes. In addition, we found that patients with lower birth weight had a higher incidence of HSCR than patients with normal weight (42.9% vs. 5.0%, p = 0.019).
| Variable | PARMs (n = 37) | NPARMs (n = 11) | p value |
|---|---|---|---|
| Sex (male) | 18 (48.6%) | 7 (63.6%) | 0.382 |
| Preterm | 1 (2.8%) | 6 (54.5%) | <0.001 |
| Birth weight (g) | 1 (3.2%) | 3 (33.3%) | 0.043 |
| Perinatal history | 14 (38.9%) | 7 (63.6%) | 0.272 |
| Hirschsprung disease | 2 (5.4%) | 3 (27.3%) | 0.128 |
| Intracranial hemorrhage | 11 (29.7%) | 1 (9.1%) | 0.322 |
| Neurological complication | 24 (64.9%) | 5 (45.5%) | 0.421 |
| Seizure | 14 (37.8%) | 1 (9.1%) | 0.151 |
| CHD | 24 (64.9%) | 3 (27.3%) | 0.063 |
| Endotracheal intubation | 28 (77.8%) | 10 (100.0%) | 0.179 |
| Bad outcome | 21 (72.4%) | 7 (82.5%) | 0.649 |
4 DISCUSSION
CCHS is a rare disorder of autonomic nervous system, mostly present in the neonatal period. At present, more than 3000 cases have been reported abroad (Weese-Mayer et al., 1993) (Amiel et al., 2003), and 76 sporadic cases have been described in China. However, most of them had a poor prognosis. CCHS patients exhibit respiratory dysregulation, which is most severe during sleep, and do not have normal physiologic responses to hypercapnia or hypoxia (Zaidi et al., 2018). The severity of symptoms in CCHS varies from person to person. Patients with moderate control of breathing deficits have normal ventilation when awake and mild hypoventilation during sleep. However, Patients with severe control of breathing deficits have severe hypoventilation in both sleep and awake states. CCHS patients also have some secondary manifestations resulting from hypercapnia and hypoxemia, such as headache in the morning, fatigue in the daytime, drowsiness, trance, mental impairment, pulmonary hypertension and right ventricular dysfunction. Therefore, early diagnosis, timely treatment and effective management of CCHS patients can facilitate the prevention of the above adverse events.
Since CCHS has an extremely low incidence rate and atypical clinical manifestations, genetic testing plays a vital role in the diagnosis of this disease, which was first used by the study of Weese Mayer team in 2003 showing that the PHOX2B mutations were the main cause of CCHS (Weese-Mayer et al., 2003). Currently no multigene panel exists for the diagnosis of CCHS. Most multigene panels are NGS-based assays that are unable to detect PHOX2B PARMs (Slattery et al., 2022). The recommended stepwise testing with molecular methodologies to detect PHOX2B PARMs include Step 1 fragment analysis, and PHOX2B fragment analysis uses electrophoresis to detect all PARMs, NPARMs with nucleotide deletions and duplications that change the length of the coding sequence, and mosaicism (Jennings et al., 2012). If Step 1 is negative, then Step 2 Sanger sequencing can detect all PARMs and all NPARMs, but no low-level mosaicism (Weese-Mayer et al., 1993). If Steps 1 and 2 are negative but clinical suspicion of CCHS remains high, the Step 3 multiplex-ligation dependent probe amplification testing is implemented to identify patients missing the whole PHOX2B gene with or without neighboring genes (Slattery et al., 2022). In this study, we used NGS combined with Sanger sequencing to detect the PHOX2B gene. However, we do not recommend the routine use of NGS for PHOX2B gene mutation detection, current NGS-based technologies do not always detect PHOX2B PARMs and can miss pathovariants in PHOX2B,so Sanger sequencing is recommended if fragment analysis is not used as a first step in the testing process.
In this study, we retrospectively reviewed our three new CCHS cases and 45 previously reported Chinese CCHS cases diagnosed by genetic testing. This study has included the largest number of Chinese CCHS cases with PHOX2B mutations so far. Using QF-PCR and capillary electrophoresis, our study showed that P1 and P3 had the genotypes of 20/24 and 18/27, respectively. Our study is the first to report the 18/27 genotype in China. Using NGS and Sanger sequencing, c.741_758dup heterozygous mutation on exon 3 of PHOX2B was identified in P2. c.741_ 758dup heterozygous mutation is a novel pathogenic mutation of CCHS. The novelty of the two genotypes suggests that there may be more PHOX2B genotypes that have not been found before.
Among the currently reported cases of CCHS, most are caused by new heterozygous variation; a few may inherit the disease from their own symptomatic parents; and about 25% of the cases inherit it from their asymptomatic parents (Rand et al., 2012). Mosaicism for the PARMs has been documented as a source of phenotypic variability in 5%–25% of parents who may harbor PHOX2B PARMs, with up to 50% risk of passing the PHOX2B PARM to their offspring (Bachetti et al., 2014). Therefore, accurate molecular identification of PHOX2B PARMs in CCHS patients is crucial for informing patient management, and in their parents to determine reproductive risk.
It is noteworthy that in this Chinese cohort, 28.6% of patients were reported to have abnormal family history. In addition, perinatal history plays an important role in the diagnosis of CCHS. Nearly half (44.7%) of the patients had abnormal perinatal history, among whom 61.9% had fetal polyhydramnios (Shimokaze et al., 2015). In addition, many patients had delayed meconium excretion, which might be related to excessive amniotic fluid.
Previous studies have described the relationships between CCHS phenotypes and PHOX2B genotypes. Patients with more PARMs or NPARMs appear to have more severe phenotypes with continuous ventilator support, as well as a higher risk of HSCR (13% - 20%) and neural crest tumors (Weese-Mayer et al., 2017). Our results showed that there was no significant associations between genotypes and HSCR, which might be confounded by our small sample size, but HSCR was indeed more common in NPARMs subjects than in PARMs subjects in China. We also found that subjects with PHOX2B NPARMs were more likely to have premature births and lower birth weight compared with PARMs subjects, we think the reason for this phenomenon is that the patients with PHOX2B NPARMs are likely to have intestinal obstruction due to HSCR, resulting in excessive amniotic fluid and growth restriction. Besides, HSCR was more likely to occur in patients with low birth weight than those with normal weight. These findings may explain why patients with PHOX2B NPARMs are more likely to have severe phenotypes and HSCR, because children with premature and low birth weight are more likely to have adverse events and dysplasia including immature respiratory center, bronchopulmonary dysplasia and so on. Therefore, we strongly recommend early genetic testing for premature and low birth weight children clinically suspected of CCHS. A previous study by Lombardo et al. on the association between cardiovascular defects and PHOX2B mutations showed that 30% of CCHS patients had congenital heart disease (CHD), which is much higher than that of the general population (0.8%) (Lombardo et al., 2018), and most CCHS patients had abnormalities involving the proximal aortic arch and/or the proximal coronary artery. In our cohort, we found that more than half of Chinese CCHS cases showed cardiovascular defects such as ASD, PDA, PFO, PH and TR. We also found that about 75% of patients showed abnormalities of nervous system, including hypotonia, seizures, intracerebral hemorrhage, EEG abnormalities and so on. Among them, seizures could result from a primary neurological problem, asystole-induced hypoxemia, and/or hypoglycemia, they are more often due to complications in management of hypoventilation (Weese-Mayer et al., 1993). They are a sign of hypoxia or anoxic brain injury or chronic overventilation most commonly in children with CCHS. Participants in PARM group were more likely to have cardiovascular defects (64.9% vs. 27.3%, p = 0.063), intracerebral hemorrhage (29.7% vs. 9.1%, p = 0.322) and seizures (37.8% vs. 9.1%, p = 0.151) than those in NPARM group, with no statistical significance. Considering the small sample size of our study, future large-scale and multi-center studies are warranted.
CCHS is a lifelong disease. There is no effective drug treatment at present and the main treatment method is respiratory support, including positive pressure ventilation via tracheostomy (PPV-T), noninvasive positive pressure ventilation (NPPV) and/or diaphragm pacing. There are few reports on the treatment of negative pressure ventilation and diaphragm pacing. The American Thoracic Society suggests that children with CCHS should first choose tracheotomy positive pressure ventilation to ensure effective ventilation and optimal oxygenation, and improve the prognosis of the nervous system. Noninvasive assisted ventilation can be considered when they are 6 ~ 8 years old (Weese-Mayer et al., 2010). Lifelong assisted ventilation and multidisciplinary management are still the main treatment methods for CCHS. In our Chinese cohort, the existing data showed that about 80% of patients needed endotracheal intubation for auxiliary ventilation after having the symptoms of hypopnea. In the face of CCHS, about 37 patients' guardians gave up treatment, among them, 27 patients were confirmed to have died in the end, and 10 patients had unknown outcomes. In this study, only nine patients were ultimately confirmed to have survived under assisted ventilation. According to Mei's previous report, the possibility of deciding to give up treatment and patients' mortality showed significant associations with the severity of this disease (Mei et al., 2021). In China, by asking the thoughts of the guardians who have abandoned treatment for their CCHS children, we summarized the reasons why family members give up treatment as follows: (1) economic burden (2) a pessimistic attitude towards the treatment outcome (poor prognosis or bad quality of life).
Due to our literature review, methodological problems such as selection bias may limit our conclusions. First of all, this study is only for those Chinese CCHS cases with genetic diagnosis. We may have omitted the relevant information of CCHS cases without genetic data. Secondly, although these cases have genetic data, most of them are sporadic cases. We are unable to obtain relevant clinical information which was not reported. Thirdly, since CCHS has the possibility of mosaicism, it is very necessary to detect the mosaicism of parents for patients with abnormal family history. However, we did not detect the genes of the parents for three local new cases. Considering these bias factors, these results should be verified in the future large-scale studies with long follow-up time in China.
In conclusion, we analyzed 48 CCHS cases in China, including three new CCHS cases in this study. Our findings not only help to expand the mutation spectrum of PHOX2B in China but also improve our understanding of CCHS, facilitating accurate diagnosis and timely treatment for CCHS patients. In addition, this study is also conducive to the development of genetic counseling for CCHS patients.
AUTHOR CONTRIBUTIONS
Xiuxiang Liu and Xiaoying Chen contributed to providing and sorting out cases. Lina Wang and XinjuanYu contributed to literature search and collation. YaoyaoWang and Jiahui Wang contributed to writing the manuscript. Wei Han, Xipeng Cao and ShiguoLiu revised the manuscript.
ACKNOWLEDGMENTS
The authors thank the patients and contributors for their participation.
FUNDING INFORMATION
No funding was received for this research.
CONFLICT OF INTEREST STATEMENT
All authors certify that they have no affiliations.
ETHICS STATEMENT
The study was approved by the Ethics Committees of Qingdao Municipal Hospital, the Affiliated Hospital of Qingdao University and Qingdao Women and Children's Hospital and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained for this study from all the patients prior to their inclusion in the study.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors




