BMPR1B gene in brachydactyly type 2–A family with de novo R486W mutation and a disease phenotype
Abstract
Background
Brachydactylies are a group of inherited conditions, characterized mainly by the presence of shortened fingers and toes. Based on the patients’ phenotypes, brachydactylies have been subdivided into 10 subtypes. In this study, we have identified a family with two members affected by brachydactyly type A2 (BDA2). BDA2 is caused by mutations in three genes: BMPR1B, BMP2 or GDF5. So far only two studies have reported the BDA2 cases caused by mutations in the BMPR1B gene.
Methods
We employed next-generation sequencing to identify mutations in culpable genes.
Results and Conclusion
In this paper, we report a case of BDA2 resulting from the presence of a heterozygous c.1456C>T, p.Arg486Trp variant in BMPR1B, which was previously associated with BDA2. The next generation sequencing analysis of the patients’ family revealed that the mutation occurred de novo in the proband and was transmitted to his 26-month-old son. Although the same variant was confirmed in both patients, their phenotypes were different with more severe manifestation of the disease in the adult.
1 INTRODUCTION
Brachydactylies belong to a large group of genetically determined dysostoses (Hall, 2002), inherited in an autosomal dominant pattern. The disease symptoms encompass variable shortening of bones within the hands or feet and can exist as isolated or syndromic disorders. Isolated brachydactyly, which was subdivided into 10 distinct subtypes (listed in the OMIM database), is a rare disease, except for the relatively prevalent types A3 and D (David et al., 2015). Brachydactyly type A2 (BDA2, OMIM #112600) is characterized by hypoplasia/aplasia of the second middle phalanx of the index finger, sometimes little finger and anomalies of the second toe. Affected individuals have a triangular-shaped middle phalanx in the index fingers and second toes. The end of the index finger usually is curved. Deformity of the second toes are more consistent finding than deformity of the index finger. The big toes show malformation of the proximal phalanx resulting in fibular deviation of the distal phalanx (Su et al., 2011; Temtamy & Aglan, 2008), without other accompanying abnormalities. BDA2 is a very rare syndrome, with only few cases reported in the literature, all of which have been summarized in OMIM. Based on the Orphanet, the prevalence of the disease is less than 1 in 1,000,000. The disease is caused by pathogenic variants in one of three genes: members of the bone morphogenetic protein signaling group, BMPR1B (OMIM #603248) and BMP2 (OMIM #112261) or their corresponding ligand, GDF5 (OMIM #601146) (Dawson et al., 2006; Kjaer et al., 2006; Ploger et al., 2008; Seemann et al., 2005).
Bone morphogenetic proteins (BMPs) belong to the TNF-β family and play important roles in in morphogenesis. BMPs are active during early development and their function is important in the neurological, cardiovascular, gastrointestinal, urinary, adipose, and musculoskeletal systems; thus these proteins were proposed to be called body morphogenic proteins (Gomez-Puerto et al., 2018; Wagner et al., 2010). BMPs signal might be transduced either through the canonical or non-canonical pathways. In the canonical pathway, BMPs bind to the serine/threonine kinase type receptors on the cell surface, what leads to the formation of active complexes and phosphorylation of the downstream proteins (Horbelt et al., 2012). The BMP receptors (BMPRs) have been classified in two groups: type I, containing the activin receptor-like kinases, and type II, containing three distinct receptors: BMPR-II, ActR-II, and ACTR-IIB. The type I receptors are subdivided into three sub-groups: the BMPR1 and ALK1 groups, activating Smad1/5/8 proteins and the TβR-I group, which interacts with SMAD2/3 (Miyazono et al., 2010). The BMPR1 group comprises the BMPR-IA and BMPR-IB receptors; though other receptors are expressed in various cell types, the expression of BMPR-IB, encoded by the BMPR1B gene, is significant in the adrenal, brain, endometrial, ovarian and prostate tissue (NCBI Gene database (O'Leary et al., 2016). Aberrances in the BMPR1B gene have been associated with brachydactyly type A, as well as with pulmonary arterial hypertension and autosomal recessive acromesomelic dysplasia. Current research shows that BMPR1B is also indispensable for genetic control of the reproductive performance (Zhang et al., 2017). Due to rarity of the disease, the association of BMPR1B with brachydactyly type A2 has been shown in only two studies (Lehmann et al., 2003, 2006) and no broader epidemiologic data are available.
In this study, we describe a family with the c.1456C>T, R486W variant in BMPR1B, presenting with the brachydactyly type A2 phenotype and lacking other systemic abnormalities that could potentially result from aberrant BMPR1B signaling in other tissues and organs. The disease symptoms differ from the previously described, indicating variable spectrum of the disease and its different expression depending on the developmental status of the patient.
2 MATERIALS AND METHODS
2.1 Ethical compliance
All the participants or their parents signed an informed consent form before entering the study.
2.2 Patient samples
The proband and other family members were examined at the Department of Surgery, Orthopedics, Rescue Medicine and Polytrauma at the University Hospital in Cracow and at the Department of Medical Genetics, Jagiellonian University Medical College.
2.3 Genetic testing
Total genomic DNA was isolated from the peripheral blood leukocytes according to Miller et al (Miller et al., 1988). The complete coding sequence of the genes associated with brachydactyly and syndactyly (BMPR1B; ESCO2; GDF5; GNAS; HOXA13; HOXD13; IHH; NOG; RECQL4; ROR2; SOX9; TP63) was analyzed in next-generation sequencing (NGS) on the NextSeq500 instrument (Illumina), according to the standard protocol. The mean depth coverage was 95,4x and the quality threshold 100%. The identified genetic variants were classified following the guidelines on NGS variant interpretation (Richards et al., 2015).
3 RESULTS AND DISCUSSION
The proband, a 31-year-old male, was admitted to the Department of Surgery, Orthopedics, Rescue Medicine and Polytrauma of the University Hospital in Cracow due to a post-traumatic fracture of the left tibia. The physical examination revealed the presence of shortened middle phalanx of index fingers in both hands and abnormal position of the thumbs, with a slight cutaneous syndactyly. In the feet, physical examination revealed bilateral shortening and dorso-lateral deviation of the first toe and elongated second toe. All toes and fingernails were present, but the nail plates of the thumbs showed abnormal shape. Subsequent hands radiographs showed bilateral underdevelopment of the distal part of the metacarpal bone in the thumb and bilateral absence of the middle phalanx in the index fingers (Figure 1). Additionaly, the image showed symphalangism between the proximal and middle phalanx in the right hand. The bones of first, fourth, and fifth fingers, metacarpals and carpals appeared to be normal. Overall, the hand phenotype resembled BDC (Brachydactyly type C, OMIM #113100) with additional features of SYM1A (Symphalangism, proximal, OMIM #185800). In the feet, the radiograph showed bilateral shortening of the distal part of proximal phalanx in the first toe, with the lack of inter-phalangeal joint and a resulting dorso-lateral deviation (Figure 1). It also revealed the presence of a bilateral elongation of the proximal phalanx with an absence of the middle phalanx. The body proportions, height, weight as well as head and thorax circumferences were normal.
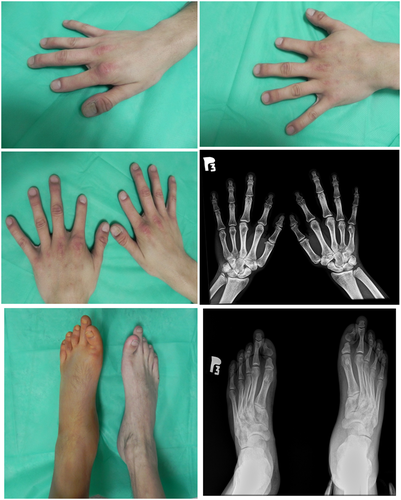
The observed phenotype had no negative effect on the patient's every day functioning. Due to the suspicion of brachydactyly, the patient was referred for genetic testing. Next-generation sequencing analysis of the BMPR1B; ESCO2; GDF5; GNAS; HOXA13; HOXD13; IHH; NOG; RECQL4; ROR2; SOX9; TP63 genes; revealed a heterozygous, pathogenic, variant in the BMPR1B gene (NM_001203.3: c.1456C>T, p.Arg486Trp, rs121434418). The variant is very rare, with a MAF <0.00001 in the PAGE and ALFA Project studies.
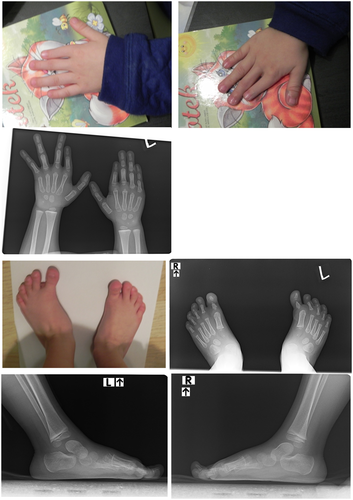
Further interview revealed that the patient's 26-month-old son (patient 2) presented with similar symptoms. The child's examination and radiological imaging revealed presence of a similar phenotype to observed in his father (Figure 2). The hand radiographs confirmed malformations of the index fingers, including absence of the middle phalanx in the left hand and a very small middle phalanx in the right hand together with bilateral deformation of the distal phalanx in the thumb. Compared to the father, the child presented with additional bilateral ulnar subluxation of index fingers, which may, however, result from developmental stage of the skeletal system. A summary of phenotypic changes in both patients is presented in Table 1.
| Observation | Proband (age at diagnosis 31 years) | Patient 2 (age at diagnosis 26 months) |
|---|---|---|
| Hands | ||
| Morphological malformation | Thumb:
Index fingers:
|
Thumb:
Index fingers:
|
| Radiological malformation | Thumb:
Index finger:
|
Thumb:
Index finger:
|
| Feet | ||
| Morphological malformation | Toe I:
2nd toe
|
Toe I:
2nd toe:underdevelopment of MTP, setting in the medial position, more expressed on the left side 3rd toe:
|
| Radiological malformation | Toe I:
2nd toe:
|
Toe I:
|
| Body proportion | ||
| Body proportion | Normal | Normal |
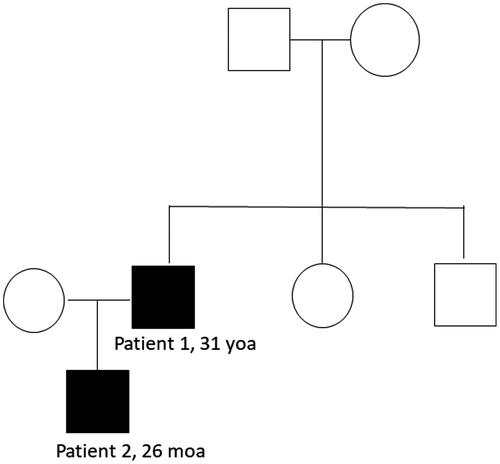
Further genetic diagnostics performed in the patient's family members confirmed that the mutation occurred de novo in the proband and was further transmitted to his son (Figure 3). Neither of the probands’ parents or siblings carried the mutation nor presented with the disease phenotype.
In this study, we identified the p.Arg486Trp, c.1456C>T variant in the BMPR1B gene as a de novo mutation, which occurred in a patient presenting with brachydactyly type A2 symptoms. The mutation was previously described in patients with a suspected brachydactyly and was shown to inhibit chondrogenesis (Lehmann et al., 2003). The identified variant is located in a highly conserved sequence of BMPR1B gene, termed the nonactivating-non-downregulating box. This region is important for the endocytosis of the receptor and inactivation by transphosphorylation. Its high conservativeness among various species confirms the importance for the proper functioning of the protein. Aberrances in that region lead to the loss of biological function of the protein due to inhibition of the intracellular signaling pathways (Garamszegi et al., 2001). The variant is also predicted as deleterious by in silico algorithms (Choi et al., 2012). Presence of the variant within the receptor inhibits chondrogenesis (Lehmann et al., 2003), and this mechanism is responsible for most of the observed phenotypic aberrances. Interestingly, other aberrances in the BMPR1B have been associated with brachydactyly type A (Lys325Asn) (Racacho et al., 2015), as well as with childhood pulmonary arterial hypertension (Chida et al., 2012), suggesting its important role in development and pathogenesis of human diseases.
Other studies revealed a family carrying a germline mutation at the same amino acid. The c.1456C>T missense mutation leads to an arginine to glutamine substitution (R486Q), resulting in additional features of SYM1 (Lehmann et al., 2006), which are not present in the members of the family analyzed within this study. Interestingly, the BMP signaling is also involved in the heterotopic ossification (Ranganathan et al., 2015). However, the long-term follow-up of patient 1, performed 12 months, after the surgery showed normal bone fusion without ossification. The knee flexion was 90°, instead of 100–110°, the knee extension was in normal range. No instability of the knee was noticed. Comparison of the features of both patients revealed that malformations presented in hands and feet of father and son are similar.
In contrast to previous studies, the patients described in this study did not present with clinodactyly (Ploger et al., 2008), and syndactyly was only cutaneous. In contrast to previously described patients, no hypoplasia within fifth fingers was observed (Lehmann et al., 2003). The previously described patients revealed deviation of first and second toe, with a broadening of the first toe and medial deviation of second toe. In our study, patient 1 had bilateral underdevelopment of first toe and shortening of second, while patient 2 did not show any malformation in the length of the second toe. This information indicates that BMRP1B gene could have variable expression among family members. Moreover, the patients described within this study did not present with a triangular shaped middle phalanx of the second finger, as described in other BDA2 patients (Lehmann et al., 2006). Underdevelopment of metacarpal bones is also not typical feature for brachydactyly type A2 which indicate overlapping with brachydactyly type B. Interestingly, other authors indicated no family specific or sex correlated phenotypes in diagnosed patients. The exact mechanism leading to aberrances in chondrogenic differentiation, caused by this specific mutation remains unknown. Still, as indicated by the observed phenotypic differences, the disease outcome has to be influenced by other, yet unknown factors.
ACKNOWLEDGMENTS
This research was supported by Warsaw Genomics, Uniwersytet Jagielloński Collegium Medicum research grant no K/ZDS/006278. Members of Warsaw Genomics are supported by the POIR.04.04.00-00-41DB/17-00, TEAM TECH 4/2017 grant awarded by the Foundation for Polish Science.
CONFLICT OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
MB, MT, MK, BK-W, AD: collection and analysis of clinical data. MK, MK-Ł, AK-D, EU-G, JS-R, PG, IG, MŚ, KS, AK, KJ: sequencing and genetic analysis. AW: study design, analysis of genetic data, and preparation of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




