Recent Progress in Gene Delivery Systems Based on Gemini-Surfactant
Qianyu Zhang and Huali Chen contributed equally to this study.
ABSTRACT
Gemini surfactants (GSs) are two single-chain surfactant molecules covalently linked to their hydrophilic head groups via a spacer, resulting in a distinct structure with two hydrophilic heads and two hydrophobic tails. The GSs with cationic head groups have the potential for gene delivery by forming aggregates with negatively charged nucleic acids under the action of positive charge and self-assembly ability. Therefore, they have attracted increasing attention in the field of gene delivery. However, there remains a lack of systematic reviews summarizing various optimization strategies for GSs as gene delivery vectors in recent years. To address this gap, this review summarizes strategies for enhancing the transfection efficiency and biocompatibility of Gemini surfactant vectors, explores the relationship between their molecular structure and gene delivery performance, along with their delivery mechanism, highlights their applications in various gene delivery contexts, and discusses future development strategies and key challenges. This review provides a foundation for the further development of superior GSs, offering additional viable approaches for effective gene delivery and gene therapy of diseases.
1 Introduction
Gene therapy is a treatment strategy based on genetic medicines. It involves delivering nucleic acids, such as plasmid DNA (pDNA), messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA), or antisense oligonucleotides (ASOs), into target cells to regulate gene expression, modulate protein levels, or edit dysfunctional genes for disease-specific therapy [1-5]. In recent years, gene therapy received increasing attention in the treatment of cancers, infectious diseases, genetic disorders, and rare diseases [6], primarily because it offered a potential solution for diseases that could be treated but not completely cured using conventional approaches [7]. For example, gene-editing technologies such as CRISPR-Cas9 could precisely repair pathogenic gene mutations and achieve functional cures, such as in transfusion-dependent β-thalassemia [8], whereas conventional therapies such as blood transfusion and iron chelation therapy only temporarily relieved symptoms and maintained patient survival [9]. Furthermore, gene therapy could achieve long-term disease control through a single intervention. For example, adeno-associated virus (AAV) vector-mediated gene replacement therapy, represented by Zolgensma, could restore SMN1 gene expression through a single intravenous injection to functionally cure spinal muscular atrophy (SMA), and its therapeutic effects could last for years—achieving “one-time treatment, lifelong benefit” [10]. In contrast, conventional therapies often require frequent dosing. For instance, SMA patients need intrathecal injections of nusinersen every 4 months [11], and long-term treatment significantly reduces patient compliance. Therefore, gene therapy is a highly promising field in biomedicine, with broad application prospects. The efficient delivery of genes to target tissues or cells is essential to exert therapeutic effects [12]. However, such efficient gene delivery must overcome multiple critical extracellular and intracellular barriers [13]. In the extracellular space, negatively charged DNA and RNA faced electrostatic repulsion from cell membranes, were quickly hydrolyzed by nucleases, rapidly cleared by macrophages and the reticuloendothelial system, and exhibited short half-lives in vivo [14-17]. Additionally, in the intracellular space, these biomolecules encounter inefficient endosomal escape and limited nuclear entry [14-17]. Therefore, the key to the success of gene therapy is to use delivery vectors to help avoiding these intracellular and extracellular barriers and achieve effective delivery of target cells.
Gene delivery vectors have been classified into two primary categories: viral and nonviral vectors. Viral vectors have demonstrated high transfection efficiency and broad host cell compatibility. However, they also face challenges, including immunogenicity, random genomic integration, size constraints, production complexity, transportation instability, and high costs [18]. Compared with the viral vectors, nonviral vectors have attracted extensive research interest due to their favorable safety profile and low immunogenicity [19]. Currently, major types of nonviral vectors include lipid nanoparticles (LNPs), polymeric nanoparticles, natural polysaccharide polymers, inorganic nanoparticles, and Gemini surfactants (GSs).
Among these, LNP vectors, characterized by minimal toxicity, high safety, and efficient delivery, have demonstrated successful clinical translation, particularly as exemplified by the COVID-19 mRNA vaccines [20-22]. However, despite their advantages, LNPs still face several challenges, including poor colloidal stability, storage instability [23, 24], hepatotropism [25], and high costs. Polymer nanoparticles, on the other hand, exhibit strong gene binding ability [26, 27] and possess a “proton sponge effect” that could enhance endosomal escape [28]. They also offer flexible properties, such as surface functionalization with targeting ligands [29], and the ability to be engineered as responsive carriers, including pH-, enzyme-, or redox-sensitive systems for targeted drug release [30]. Nevertheless, their cytotoxicity at high concentrations limits their application [31]. Natural polysaccharide polymers are highly biocompatible, protect gene carriers, and dissociate rapidly after internalization, leading to reduced cytotoxicity [32]. However, their major drawback is the limited payload capacity [33]. Inorganic nanoparticles, known for their low cytotoxicity, low immunogenicity, good stability, and reduced susceptibility to biological reactions, offer considerable advantages. Nevertheless, their main challenge is the difficulty in ensuring batch-to-batch reproducibility [34, 35].
Gemini surfactant was first reported by Menger and Littau [36]. Due to its unique double-head and double-tail structure and the positive charge characteristics of the cationic head group, it exhibited a relatively low critical micelle concentration (CMC) and efficiently coagulated negatively charged nucleic acids through hydrophobic interactions and electrostatic binding [37-40], thereby inducing the formation of nanoscale nucleic acid complexes. Meanwhile, its structural flexibility allowed for diverse aggregation morphologies and conferred lysosomal escape ability [41, 42]. In comparison to LNPs, GSs do not require particularly complex components for gene delivery due to their dual cationic head groups and lower CMC, enabling more effective compression and neutralization of nucleic acids. Additionally, GSs have been shown to be capable of delivering a greater variety of nucleic acids, including pDNA, siRNA, and mRNA [43]. Although LNPs offered clear advantages in standardized production (microfluidics) [44] and clinical applications (mRNA vaccines) [45], researchers primarily focused on mRNA and siRNA delivery of LNPs, with limited studies on pDNA delivery [46, 47]. Compared with cationic polymers in research purposes and clinical trials, such as PEI [48, 49], GSs possess more independent modification sites, including head groups, spacer groups, and hydrophobic tail chains, whereas the modification sites of PEI are relatively limited [50]. In addition, although inorganic nanoparticles exhibited greater stability in terms of physical and chemical properties, they often relied on surface modification (e.g., PEI) to bind nucleic acids [51]. In contrast, GSs could bind without surface modification, which avoided additional toxicity and increased costs. Additionally, the structure of GSs was well-defined, and their quality control was relatively straightforward, whereas inorganic nanoparticles exhibited significant batch-to-batch variation, leading to unstable production [34, 35].
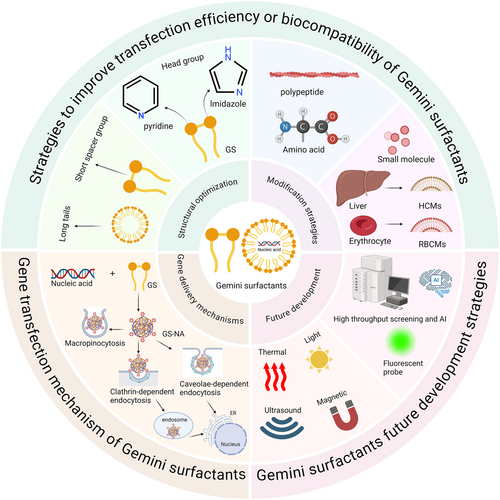
Despite these advantages, GSs have not yet been used in clinical applications in gene therapy. Moreover, despite the growing attention and increasing research on GSs, there has been no comprehensive review on GSs in the field of gene delivery in recent years. Therefore, the timely summarization of modification strategies and the in vivo fate of GSs holds significant guiding value for the subsequent development of GSs. In this review, as illustrated in Figure 1, we emphasized multiple strategic approaches to address the dual challenges of transfection efficiency and biocompatibility. Through a systematic summary of structural optimization, functional modification, and emerging biomimetic methods, combined with an analysis of delivery mechanisms, we provided a comprehensive reference and design strategy for Gemini surfactant vectors. In addition, we reviewed their applications in various gene delivery contexts and emphasized their potential for practical use in gene therapy. We also discussed future development strategies and key challenges and provided important insights for the clinical application of GSs. This review offers novel insights into overcoming the limitations of GSs in gene delivery. These insights contribute to advancing gene therapy for diverse diseases, proposing new directions for bridging the translational gap between vector design and clinical application, thereby accelerating the clinical translation of delivery systems, and establishing a theoretical foundation for future clinical endeavors.
2 Overview of Gemini Surfactant
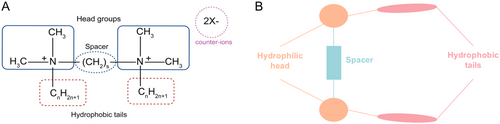
GSs represent a novel class of surfactants [36]. Their unique structure consists of two single-chain surfactant moieties linked by a spacer group at the hydrophilic head groups or between the head groups and hydrophobic tails [40, 52, 53]. As shown in Figure 2, the general formula for GSs is CnH2n+1(CH3)2N+(CH2)sN+(CH3)2CnH2n+1-2X−, abbreviated as n-s-n. Here, n and s denote the number of carbon atoms in the alkyl tail and polymethylene linker, respectively, and X− represents an anion (Figure 2) [54]. Most GSs are symmetric, consisting of two identical single-chain moieties, although asymmetric structures have also been reported. The unique molecular structure of GSs enables them to reach CMC more easily, form micelles efficiently, reduce interfacial tension, and exhibit excellent wettability [55]. Like conventional surfactants, GSs could incorporate nonionic, cationic, anionic, or amphoteric hydrophilic groups, which allow for tunable structural properties. According to current studies, GSs showed excellent corrosion resistance [56-59], broad-spectrum antibacterial activity [57, 58, 60, 61] and catalytic activity modulation ability [60-63]. In the field of medicine, cationic GSs, due to their unique positive charge characteristics, could bind to negatively charged nucleic acids via electrostatic interaction to form nanoscale complexes and make them suitable for gene delivery. In spite of these benefits, cationic GSs exhibited significant limitations, particularly poor biocompatibility and high cytotoxicity (even at 0.1 mM), which hindered their application in gene delivery [64]. Therefore, improving their biocompatibility and transfection efficiency is both critical for their further development. Current strategies to improve biocompatibility and transfection efficiency of GSs include the following: modifying the length and saturation of alkyl tails, adjusting spacer group length, altering hydrophilic head group types, incorporating biocompatible materials (e.g., peptides, amino acids, small molecules, or biomimetic materials as illustrated in Figure 3) and optimizing physicochemical properties like particle size, zeta potential, and N/P ratio [65].
3 The Influence of the Structure of Gemini Surfactants on Transfection Efficiency
The primary strategy for enhancing transfection efficiency of GSs is structural optimization, which could be achieved by adjusting the lengths of hydrophobic tail chains or spacer group, or by altering the head group (Table 1).
| Strategies | Specific structures | Improved results | Mechanisms | References |
|---|---|---|---|---|
| Altering spacer group lengths | 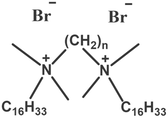 |
Shorter spacer groups (e.g., n = 2, 3) improve transfection efficiency | Shorter spacer groups improve transfection efficiency by forming more abundant multiphasic mixed structures and higher charge density | [66] |
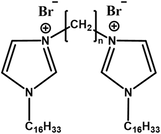 |
Shorter spacer groups (e.g., n = 2, 3,5) improve transfection efficiency | Shorter spacer groups improve transfection efficiency by forming more abundant multiphasic mixed structures and efficient DNA binding | [67] | |
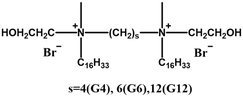 |
The longer spacer-group G12 enhances transfection efficiency | The long and flexible spacer region of G12 exhibits high hydrophobicity and curling ability, facilitating effective interaction with the cell membrane and enabling complementary binding to DNA | [68] | |
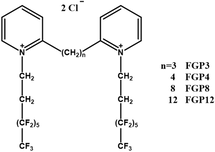 |
A spacer base length of n = 8 results in improved transfection efficiency | The fluorinated Gemini surfactant with an 8-carbon spacer group a “molecular clamp” conformation, efficiently binding with DNA. Meanwhile, the synergistic effect of DOPE facilitates membrane fusion, collectively enhancing transfection efficiency | [69] | |
| Altering hydrophobic tail lengths | 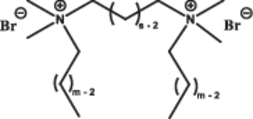 |
Long tails improve transfection efficiency | Longer hydrophobic tails improve transfection by forming non-lamellar structures and enabling efficient DNA binding | [70] |
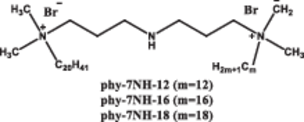 |
Long tails improve transfection efficiency | Longer hydrophobic tails improve transfection by forming multiphasic structures with DOPE and enabling efficient DNA binding | [71] | |
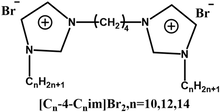 |
Long tail improves transfection efficiency | Longer hydrophobic tails enhance transfection by forming compact complexes with DNA via hydrophobic interactions and triggering DNA release under acidic conditions through π–π interactions | [72] | |
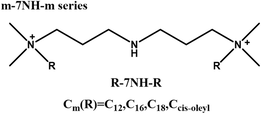 |
Long tails improve transfection efficiency | Longer hydrophobic tails improve transfection through both stable inverted hexagonal or cubic phase formation with DOPE and efficient DNA binding | [73] | |
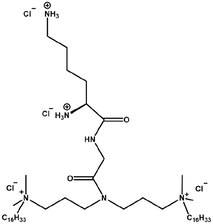 |
Longer carbon-16 tails improve transfection efficiency and reduce cytotoxicity | Gemini surfactants with C16 hydrophobic tails improve transfection via stable inverted hexagonal phase formation with DOPE and efficient DNA binding, while lowering cytotoxicity through reduced surfactant dosage (due to low CMC) and moderate hydrophobic interactions with the cell membrane | [74] | |
| Altering head group types | 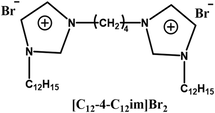 |
Improve transfection efficiency and reduce cytotoxicity | Imidazole headgroups reduce cytotoxicity by redistributing surface charge, stabilize DNA complexes via π–π interactions, and enhance transfection efficiency through proton sponge-mediated endosomal escape | [72] |
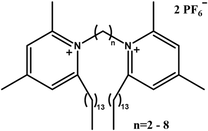 |
Improve transfection efficiency and reduce cytotoxicity | Pyridine headgroups reduce cytotoxicity by redistributing surface charge, stabilize DNA complexes via π–π interactions, and enhance transfection efficiency through proton sponge-mediated endosomal escape | [75] |
3.1 Spacer Group
In the structural optimization of GSs, the length of the spacer group influenced transfection efficiency. Some studies indicated that shorter spacer groups (n ≤ 5) generally resulted in higher transfection efficiency [66, 67]. This phenomenon was possibly attributed to the formation of abundant multiphasic mixed structures by shorter spacer groups in combination with the helper lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), which was believed to synergistically promote endosomal escape. In addition, the shorter spacer groups might provide higher charge density between the two cationic head groups, thereby enhancing their interactions with negatively charged DNA and facilitating the formation of more stable and uniform nanostructures. However, although the aforementioned studies supported the transfection advantages of shorter spacer groups, other research suggested that under certain conditions, longer (C12) [68] or intermediate (C8) [69] spacer groups might exhibit better transfection efficiency as well. Among these findings, the contradiction between the optimal performance of long and short spacer groups was possibly attributed to differences in carrier composition, delivery mechanisms, and cell types. In studies favoring shorter spacers, GSs with shorter spacer groups (n = 2/3) exhibited the highest charge densities, which facilitated effective DNA neutralization and condensation [66]. Furthermore, the incorporation of DOPE promoted the formation of multiphasic coexisting structures, enhancing endosomal escape and thereby improving transfection efficiency. This endocytosis–escape-based delivery mechanism was demonstrated to possess superior transfection outcomes across various cell types, including HEK293T, HeLa, and CHO cells. Conversely, hydroxyl modifications on Gemini surfactant might reduce cytotoxicity while weakening electrostatic interactions with DNA, thereby necessitating longer spacer groups (G12) to enhance hydrophobic interactions for efficient DNA binding and complex formation [68]. This result confirmed that DNA was tightly condensed by the hydrophobic domains of the longer spacers, forming densely packed nanoparticles. In addition, the hydrophobicity and curling ability of the longer spacer groups could enhance membrane fusion, which facilitated intracellular delivery and improved transfection efficiency. Such a hydrophobically driven membrane fusion mechanism might have been more effective in cell lines with high transfection susceptibility, such as 293T cells.
In another study, medium-length spacer groups exhibited the highest transfection efficiency compared to longer and shorter spacer groups, which was possibly attributed to the use of fluorinated tails instead of hydrocarbon tails in the Gemini surfactant [69]. The rigidity and steric hindrance of the fluorinated chains likely required longer spacers to enable intramolecular folding to form effective conformations, thereby promoting efficient DNA condensation. Shorter spacer lengths resulted in larger particle sizes and incomplete DNA condensation, which reduced transfection efficiency. At the same time, overly long spacer lengths led to looser structures and partial DNA condensation, which hindered the formation of effective transfection nanoparticles. Based on the contradictory results described above, it can be concluded that the spacer length was not the sole factor affecting the transfection efficiency, as other structural modifications also significantly affected transfection efficiency.
In addition to transfection efficiency, the spacer group also affected the biocompatibility and cytotoxicity of GS-based delivery systems. The research showed that longer spacer groups (s = 5 or 10) induced higher cytotoxicity, with these surfactants forming highly toxic complexes even at the lowest charge ratio (GS:pDNA = 2:1) [70]. Furthermore, adding helper lipids such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cholesterol (Chol) to reduce the zeta potential did not significantly alleviate this issue. This was possibly attributed to the disruption of the lipid components of the cell membrane by the long spacer groups, resulting in leakage of intracellular contents.
In summary, in the design of GSs for gene delivery, the selection of spacer group length should be considered in conjunction with molecular structural characteristics, target cell types, and the effects of co-components (such as DOPE). An appropriate spacer length would help regulate charge density, conformational flexibility, and interactions with DNA, thereby enhancing transfection efficiency and biocompatibility.
3.2 Hydrophobic Tail Chain
The length and saturation of the hydrophobic tail chains of GSs both played a critical role in their transfection efficiency. Several studies demonstrated that GSs with longer hydrophobic tails generally exhibited higher transfection efficiency [70-73]. This result was possibly attributed to the enhanced hydrophobicity and reduced CMC of long-tail GSs, which promoted stronger interactions with DNA and more efficient condensation, thereby protecting DNA from enzymatic degradation. Meanwhile, the non-lamellar structures formed with DOPE (e.g., inverted hexagonal phase) facilitated DNA release and endosomal escape through membrane fusion or lipid rearrangement. However, not all GSs with longer hydrophobic tails exhibited higher transfection efficiencies, as the saturation level of the hydrophobic tails still exerted a notable influence. One of the studies demonstrated that GSs with C18 hydrophobic tails exhibited lower transfection efficiency than those with C12 or C16 tails. This difference was possibly due to the saturation level of the hydrophobic tails [74]. Specifically, GSs containing unsaturated tails showed reduced tail flexibility, which led to looser packing during complex formation with helper lipids, decreased complex stability, and consequently, diminished transfection efficiency. Therefore, based on the contradictory results described above, the hydrophobic tail length was not the sole factor influencing the transfection efficiency, as other structural characteristics such as saturation level also exerted significant effects. In terms of cytotoxicity, some studies reported that longer hydrophobic tails exhibited reduced cytotoxicity. This was possibly attributed to the lower CMC of GSs with longer hydrophobic tails, which could have reduced the concentration of free surfactant molecules and thereby alleviated cytotoxicity [71, 74]. However, this relationship was not linear, as excessively long hydrophobic tails in certain cases caused severe leakage of intracellular contents and induced significant cytotoxicity.
Massa et al. demonstrated that a Gemini surfactant containing C16 hydrophobic tails, GP16_6, exhibited significant cytotoxicity at a concentration of 40 μM in both RD4 (rhabdomyosarcoma) and A549 (human lung adenocarcinoma) cell lines, and the incorporation of DOPE failed to mitigate this cytotoxicity. This was primarily attributed to the excessive interaction between the long C16 hydrophobic tails and the cell membrane, which disrupted the lipid bilayer structure and caused substantial leakage of intracellular contents, and ultimately resulted in marked cytotoxicity [76]. Notably, in a similar study, the 16-7N(GK)-16 (N = tertiary amine, G = glycine, K = lysine) Gemini surfactant exhibited minimal cytotoxicity, significantly lower than that of GP16_6, which contains C16 hydrophobic tails as well [74].
This discrepancy was possibly due to the incorporation of a dipeptide moiety in the spacer group of 16-7N(GK)-16, which might enhance the hydrophilicity of the Gemini surfactant, thereby improving biocompatibility and reducing cytotoxicity. Therefore, GSs with longer saturated hydrophobic tails (m ≥ 16) generally conferred higher transfection efficiency. However, their cytotoxicity needed to be considered as well. This trade-off between efficiency and toxicity could be mitigated through the incorporation of helper lipids or structural modifications such as amino acid conjugation.
3.3 The Alteration of the Head Group
The type of head group structure in GSs played a pivotal role in modulating both transfection efficiency and cytotoxicity. Several studies demonstrated that substituting the traditional quaternary ammonium head group with aromatic heterocyclic structures, such as pyridine [75] or imidazole [72], could significantly improve transfection efficiency while reducing cytotoxicity simultaneously. For example, Sharma et al. synthesized GSs featuring pyridine head groups [75]. The aromatic ring in pyridine enhanced complex stability through π–π stacking interactions with the bases in DNA, while its positively charged nitrogen facilitated electrostatic binding to the phosphate groups in DNA, leading to an efficient DNA condensation and nanoparticle formation. Furthermore, under acidic lysosomal conditions, the weakly basic pyridine ring became protonated, leading to osmotic pressure imbalance and promotion of lysosomal escape. Consequently, the π–π interactions between the head group and DNA, along with the protonation of the head group in the endosomal environment, collectively contributed to enhanced transfection efficiency. Notably, the delocalization of the positive charge over the aromatic ring weakened strong electrostatic adsorption to the cell membrane, thereby effectively reducing cytotoxicity. Therefore, based on these findings, the dual objectives of high transfection efficiency and low cytotoxicity could be achieved in the design of Gemini surfactant-based gene delivery systems by replacing the conventional quaternary ammonium head group with an aromatic heterocyclic head group.
3.4 Incorporation of Helper Lipids
In general, the incorporation of helper lipids, such as DOPE and cholesterol, into Gemini surfactant-based complexes has been proven to enhance transfection efficiency [70]. This enhancement was likely attributable to the interaction between GSs and the DOPE, which promoted the formation of non-bilayer structures. Additionally, cholesterol contributed to the stabilization of a fluid yet ordered lamellar phase. These structural transitions conferred both stability and biodegradability to the complexes, and facilitated efficient DNA uptake and release via endosomal escape, leading to an improved transfection efficiency.
However, not all Gemini surfactant-based complexes exhibited improved transfection efficiency upon the addition of helper lipids [77]. The discrepancy was likely due to differences in the composition and proportion of helper lipids, as well as the specific cell type involved. In the research conducted by Cardoso et al. [70], the helper lipid components included DOPE and cholesterol at a fixed molar ratio of 2:1, and the studies were conducted using the relatively easy-to-transfect HeLa cells. In contrast, Narsineni et al. [77] used only DOPE, without cholesterol, at a mass ratio of 1:0.5 with the Gemini surfactant. The results showed that varying amounts of DOPE might reduce the charge density by diluting the positive surface charge, leading to a reduced transfection efficiency. Moreover, the studies employed the relatively difficult-to-transfect A7 cells, as indicated by a low transfection efficiency of Lipofectamine 3000 (13.22 ± 0.30%). It could be concluded that slight variations in the complex composition could lead to decreased transfection efficiency in A7 cells. Therefore, the composition of each specific Gemini surfactant nanoparticle system needed to be optimized according to the particular experimental conditions to achieve optimal transfection efficiency and biocompatibility.
In a related study, researchers combined theoretical modeling with experimental data to demonstrate that DOPE concentration and charge ratio significantly influenced transfection efficiency. The results showed that, when Gemini surfactant cationic charge ratio was at 2.7 and DOPE-to-Gemini surfactant molar ratio was in the range of 2–3:1, they formed stable lamellar to hexagonal (HII) phase nanocomplexes [78]. These complexes readily underwent phase transitions in the acidic lysosomal environment (pH 5), which played a key role in endosomal escape and ultimately resulted in higher transfection efficiency. A further study involving another helper lipid monoolein (MO) demonstrated that MO-incorporation effectively enhanced transfection by inducing mixed vesicle formation, reducing particle size to promote endocytosis, enhancing membrane fusion, and facilitating siRNA escape from lysosomes [79].
In summary, the incorporation of helper lipids yielded different outcomes under varying conditions. Therefore, it was essential to comprehensively consider factors such as the type of helper lipid and its incorporation ratio to maximize transfection efficiency and biocompatibility.
4 Strategies to Improve Transfection Efficiency and Biocompatibility of Gemini Surfactants by Modification
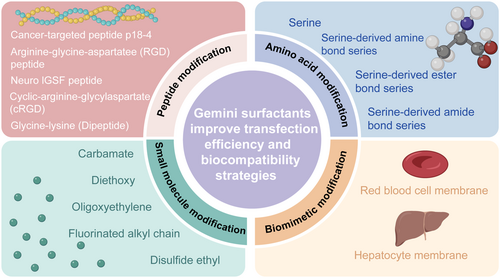
Functional ligands facilitated gene therapy by enhancing the binding of vectors carrying therapeutic genes to the target cells or tissues via specific recognition. This process might promote the distribution of therapeutic genes in target cells or the release of genes from vectors, leading to enhanced gene expression. Ligands such as antibodies, receptor-specific peptide ligands, nucleic acid aptamers, and affibodies have been incorporated into synthetic nonviral gene delivery vectors to enhance targeting, improve gene delivery efficiency, and reduce cytotoxicity [80]. Table 2 lists the strategies for Gemini surfactants modified with peptides, amino acids, small molecules, and biomimetic modifications.
| Strategies | Modification Methods | Improved results | Mechanisms | References |
|---|---|---|---|---|
| Peptides | Peptide types | |||
| Cancer-targeting peptide p18-4 | Improve transfection efficiency and biocompatibility | p18-4 cancer-targeting peptide binds overexpressed keratin in melanoma, enhancing transfection efficiency and reducing cytotoxicity by lowering zeta potential and nonspecific interactions. | [82] | |
| Glycine and lysine dipeptide | Improve transfection efficiency and maintain biocompatibility | Glycine-lysine dipeptides exhibit stronger buffering capacity and pH-dependent size increase, leading to enhanced protonation, promoting endosomal escape, and improving transfection efficiency. | [83] | |
| Cyclic-arginine-glycylaspartate (cRGD) | Improve transfection efficiency and maintain biocompatibility | cRGD peptide specifically targets overexpressed integrin α3/β1 receptors in melanoma cells, enhancing transfection efficiency. | [84] | |
| Integrin-bound RGD short peptides or neuro-IGSF-peptide | Improve transfection efficiency and maintain biocompatibility | neuro-IGSF-peptide (p3- FASNKL) may specifically bind to the immunoglobulin superfamily cell adhesion molecules (IgSF CAMs) on retinal cells, such as neuropilin-1 or signaling protein 3A | [85] | |
| Amino acids | Amino acid types | |||
| (12Ser)2N12 serine-derived amine bond series | Improve transfection efficiency and maintain biocompatibility | MO addition promotes endocytosis by reducing particle size, enhances membrane fusion, facilitates mixed vesicle formation, aids siRNA lysosomal escape, and ultimately improves silencing efficiency. | [79] | |
| serine-derived amine bond series | Improve biocompatibility | Serine-derived amine bond series exhibit high structural stability, are resistant to enzymatic degradation, and reduce cytotoxicity caused by concentration doubling after spacer group degradation. | [86] | |
| (12Ser)2COO5- serine-derived ester bond series | Improve transfection efficiency and biocompatibility | Introducing enzyme-degradable chemical bonds (e.g., ester) into Gemini surfactants facilitates gene release and expression. Meanwhile, it reduces the intracellular accumulation of Gemini surfactants, thereby lowering cytotoxicity. | [88] | |
| (14Ser)2N5- serine-derived amine bond series | Improve transfection efficiency and biocompatibility | Amino acid incorporation, such as serine, improves biocompatibility and maintains near-neutral surface charge, reducing serum binding. Helper lipids promote endosomal escape and enhance silencing efficiency. | [89] | |
| Add small molecules | Small molecule types | |||
| Oligo-oxyethylene | Improve transfection efficiency and biocompatibility | Two imidazolium cationic head groups combined with a short-to-moderate oligo-oxyethylene spacer optimize interactions with pDNA, enabling more efficient condensation, protection, and promoting its intracellular transport and release. | [90] | |
| Carbamate | Improve transfection efficiency | Carbamate linkers remain stable at neutral pH in plasma but degrade in acidic environments such as endosomes or lysosomes, releasing DNA and enhancing transfection efficiency. | [91] | |
| Fluoride | Improve transfection efficiency | Fluorinated compounds like FGP6 and FGP8 efficiently condense DNA into uniform nanoparticles and, with DOPE, promote membrane fusion to enhance transfection efficiency. | [92] | |
| Disulfide ethyl | Improve transfection efficiency | The protein corona masks extracellular EPT to prevent SS14 complex dissociation, while intracellular glutathione cleaves disulfide bonds inside the cell to release DNA, together leading to enhanced transfection efficiency. | [97] | |
| Biomimetic modification | Modification types | |||
| Red blood cell membrane/DOPE-PEG2000 | Improve transfection efficiency and biocompatibility | Biomimetic GS-based nanocomplexes efficiently deliver genes with excellent transfection efficiency and biocompatibility, both in vitro and in vivo, ensuring safety. | [94] | |
| Red blood cell membrane/hepatocyte membrane | Improve transfection efficiency and biocompatibility | Cell membrane biomimetic nanoparticles promote cellular uptake via Caveolae protein, enhance transfection efficiency, and exhibit favorable biocompatibility. | [95] |
4.1 Peptide Modified Gemini Surfactants
Peptide-modified gene vectors offered unique properties and the potential for enhanced efficiency, precise gene delivery to specific cells or tissues, thereby improving transfection efficiency and enhancing gene targeting and biological responsiveness [81]. This modification proved especially beneficial for achieving more efficient and precise gene delivery when targeting specific cells or tissues. Several studies [82-85] demonstrated that the incorporation of specific peptides into GSs could effectively improve delivery targeting, transfection efficiency, and biocompatibility. Mohammed Said et al. synthesized a novel gene delivery system by combining the cancer-targeted peptide p18-4 with the Gemini surfactant 12-7N(GK)-12 through either chemical coupling or physical mixing [82]. The results demonstrated that, compared with the Gemini surfactant complex without peptide modification, physical mixing of p18-4 with the Gemini surfactant led to a more than sixfold increase in gene expression in the human malignant melanoma (A375) cell line. Moreover, cell viability was also significantly improved in p18-4-incorporated conditions. In comparison, chemical coupling of p18-4 to the Gemini surfactant complex resulted in a threefold increase in gene expression, and also significantly improved cell viability. These findings suggested that, for this delivery system, both physical mixing and chemical coupling could enhance transfection efficiency and cytocompatibility. This might be due to the fact that the targeted peptide (p18-4) lipid complex specifically bound to the overexpressed keratin in melanoma, enhancing cellular uptake and transfection efficiency. Meanwhile, the peptide-modified lipid complex also had a lower zeta-potential, which might reduce the nonspecific interactions and thereby reduce cytotoxicity. Similarly, Narsineni et al. prepared integrin-bound RGD (arginine-glycine-aspartic acid) short peptides and neuro-IGSF-peptides such as the FASNKL peptide (p3), modified GSs (18-7N(pn)-18) [85]. In in vitro investigation, A7 astrocytes and 3D retinal spheres (MiEye8) were used to assess the specificity and efficiency of GFP reporter gene delivery mediated by peptide-modified GSs. In the in vivo investigation, therapeutic genes (BDNF-encoding plasmids) were injected into the eyes of CD1 mice to assess the gene transfection efficiency of the peptide-modified GSs-based gene delivery system in the retina. As shown in Figure 4, compared with the unmodified surfactant and Lipofectamine 3000 (Thermo Fisher Scientific, USA), the Gemini surfactant modified by p3 (18-7N(p3)-18) exhibited the highest transfection efficiency. The reason for the high transfection efficiency in vitro and in vivo might be that the p3 peptide specifically bound to the immunoglobulin superfamily cell adhesion molecules (IgSF CAMs) on retinal cells, such as neuropilin-1 or signaling protein 3A. This enhanced the interaction between the complex and retinal cells, and then promoted their specific uptake, leading to improved transfection efficiency. In addition, the modification of p3 peptide had no significant effect on the cytotoxicity of the complex. These results consistently indicated that peptide modifications not only enhanced gene delivery efficiency but also maintained excellent biocompatibility.
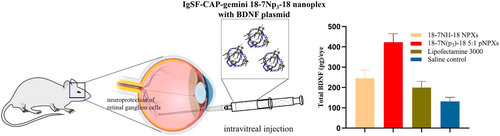
However, some studies also showed that under certain conditions, peptide-modified surfactants could not significantly improve the transfection efficiency. For instance, Waleed Mohammed-Saeid et al. prepared Gemini surfactant lipid complexes modified with cyclic-arginine-glycylaspartate (cRGD) through either chemical coupling or physical mixing, and evaluated the transfection efficiency and cytotoxicity using the A375 cell line [84]. The results indicated that the cRGD-modified Gemini surfactant in the chemical coupling manner(12-7N(RGD)-12) exhibited the highest transfection efficiency. The gene expression level reached more than twice that of unmodified Gemini surfactant (12-7N(GK)-12). However, the cRGD modified by physical mixing did not show a significant increase in transfection efficiency compared with the unmodified Gemini surfactant. Compared to physical mixing, chemical coupling might present the targeting ligands in a more stable and spatially favorable manner, which could promote receptor-mediated cellular uptake and improve transfection efficiency. This might indicate that the improved transfection efficiency was not due to the ineffectiveness of peptide modification itself, but rather the variations in the peptide modification methods.
Overall, peptide-modified GSs demonstrated substantial advantages in gene delivery systems, such as facilitating precise gene delivery to target sites, improving transfection efficiency, and reducing cytotoxicity. However, their transfection efficiency was affected by multiple factors, including the peptide type, modification method, and specific receptor expression on the target cells. Therefore, when designing peptide-modified GSs, it was crucial to choose an appropriate modification strategy based on the therapeutic objectives and the cell type. Additionally, it should be noted that the studies mentioned above only used the strategy of targeting peptide-modified GSs. However, effective strategies for peptidemodification for improving escape and nuclearlocalization in vivo were lacking. This might become a new strategy for GS modification in the future.
4.2 Amino Acid Modified Gemini Surfactants
Amino acids, the basic units of proteins, are well recognized by the human body, and commonly exhibit superior biocompatibility and degradability. In gene delivery, amino acids or their derivatives have been employed as carrier modification ligands to reduce cellular toxicity and enhance the biocompatibility of gene delivery. Currently, amino acids such as serine, lysine, arginine, glycine, and proline have been extensively utilized to enhance the biocompatibility and biodegradability of the carrier [54]. Table 3 summarizes the transfection efficiency, cell viability, and application fields of GSs modified with peptides and amino acids.
| Modification type | Specific strategy | Transfection efficiency | Cell viability | Application field | References |
|---|---|---|---|---|---|
| Peptide modification | Cancer-targeted peptide p18-4 | IFN-γ expression increased sixfold with physical mixing and threefold with chemical coupling. | Increased vitality | Human melanoma cells | [82] |
| Glycine-lysine modification | Significant increase | More than 90% | Epithelial cells | [83] | |
| Cycloarginine-glycine-aspartic acid (cRGD) | IFN-γ expression increased by more than twofold with chemical coupling. | More than 80% | Human melanoma cells | [84] | |
| neuro-IGSF-p3peptide (FASNKL) | In vitro GFP expression increased by 1.26-fold. In vivo expression of the BDNF plasmid increased by 3.4-fold. | 90%–95% | Retinal cells | [85] | |
| Amino acid modification | Serine | The lipid complex with added MO exhibited the highest silencing efficiency, ranging from 70% to 80%. | More than 70% | siRNA delivery | [79] |
| Serine | Unstudied | Serine-derived amine-bond spacer increased cell viability by 1078-fold. | HeLa cells | [86] | |
| Serine | The serine-derived ester-bond spacer (12Ser)2COO5 achieved a maximum transfection efficiency of 50%. | More than 80% | HeLa cells | [88] | |
| Serine | The serine-derived amine (14Ser)2N5 achieved a silencing efficiency of 50%. | More than 80% | Glioblastoma cells | [89] |
Amino acid-modified GSs demonstrated substantial advantages in gene delivery systems as well, especially with respect to their biocompatibility. Silva et al. [86] synthesized various serine derivatives-modified GSs, including amide bonds, ester bonds and amine groups. Their cytotoxicity was evaluated in HeLa cells. The results showed that, compared with conventional Gemini and single-chain surfactants, serine-derived GSs had superior biocompatibility. Among them, the serine-derived amine bond series exhibited the highest biocompatibility, with the median toxicity concentration (TC50) approximately 1078-fold higher than that of the unmodified GSs. The reason for lower cytotoxicity might be that surfactants with amides, ester bonds and amine groups were more susceptible to chemical or enzymatic degradation in the intracellular environment. Meanwhile, serine was well-known for its biocompatibility, so it was not surprising that its modified nanocarriers had lower toxicity [87]. In addition, the amino acid-modified Gemini surfactant-based gene delivery systems possessed high transfection efficiency compared with unmodified ones. There might be several reasons for this phenomenon. First, in amino acid-modified GSs, the incorporation of the helper lipids such as DOPE and cholesterol could promote membrane fusion and endosomal escape, which could enhance transfection efficiency [88].
Second, the ester bonds between amino acid and Gemini surfactant were easily chemically or enzymatically degraded, which could promote the release of DNA from the cells. Similarly, the combined action of amino acid-modified GSs and additives could also improve gene silencing efficiency. Gemini surfactant complexes containing MO achieved the highest silencing efficiencies compared with GSs alone [79]. This improvement was primarily attributed to the multifunctional role of MO: it reduced particle size to facilitate endocytosis, enhanced membrane fusion, and promoted the formation of mixed vesicles that aided in the lysosomal escape of siRNA. In summary, GSs modified with amino acids exhibited significant improvements in biocompatibility. However, their effect on enhancing transfection efficiency was somewhat limited. To achieve higher transfection efficiency, additional helper lipids were required [89]. Currently, amino acids commonly used in the modification of GSs were limited to serine, glycine, and lysine. Therefore, future studies could explore the use of other amino acid species to modify GSs to provide more effective carriers for gene therapy.
4.3 Small Molecule Ligands Modified Gemini Surfactants
Small-molecule modifications represented an effective strategy to markedly improve the transfection efficiency of GSs. The introduction of small-molecule modifications—such as oligo-oxyethylene spacers [90], carbamate [91], fluorinated groups [92], disulfide ethyl [93] and diethoxy [96]—into GSs has been shown to effectively enhance transfection efficiency. For example, Kumar et al. prepared a series of GSs containing imidazole-based (Im) head groups and different lengths of oligo-oxyethylene spacer groups [(C16Im)2(C2O)n] [90]. They validated the in vitro transfection efficiency and cytotoxicity of these surfactants by delivering GFP reporter genes to HEK293T, HeLa, CaCo-2 (Human colon adenocarcinoma), and A549 cells. It was found that, under both serum-free and serum-available conditions, the transfection efficiencies of each formulation in different cell lines were comparable to or better than those of Lipofectamine 2000 (Thermo Fisher Scientific, USA). The [(C16Im)2(C2O)3]/DOPE-pDNA lipid complex, with a molar fraction (α) of 0.2 and an effective charge ratio (ρeff) of 2.0, showed the highest transfection capacity. Moreover, cells treated with this optimally formulated lipid complex had better cell viability. This phenomenon was attributed to the two imidazole-cationic headgroups and short-to-moderately-long oligo-oxyethylene spacers, which optimized the interaction with pDNA. Compared to other cationic lipid structures, the [(C16Im)2(C2O)3]/DOPE-pDNA lipid complex could compress and protect pDNA more effectively, which facilitated the intracellular transport and release. Regarding cytotoxicity, the appropriate molar ratio and effective charge ratio helped to maintain the stability and biocompatibility of the lipid complexes, leading to reduced adverse effects on cells and ensuring their normal physiological functions while achieving highly efficient gene transfection. In addition, specific modifications, such as carbamate and diethoxy spacer groups, have also demonstrated significantly enhanced transfection efficiency. For example, Zhao et al. synthesized a novel class of Gemini surfactant with carbamate groups (G series) and single-headed cationic lipids (M series), and evaluated the transfection of pGFP-N2 plasmid in Hep-2 cells and silencing efficiencies of siRNA in firefly luciferase-expressing A549 cells [91]. The results indicated that the transfection efficiency of the G series GSs was higher than that of the single-headed surfactants, among which the G14 group (tail-chain length = 14) was the most effective. The G14 group achieved the highest transfection efficiency at an N/P ratio of 3:1, which was comparable to Lipofectamine 2000 (Thermo Fisher Scientific, USA). Furthermore, it silenced nearly 80% of luciferase expression in A549 cells, matching Lipofectamine 2000 (Thermo Fisher Scientific, USA) and outperforming other GSs and (1,2-dioleoyl-3-trimethylammonium-propane) (DOTAP). This enhanced efficacy was attributed to its suitable carbon chain length, which maintained liposome stability and improved cellular uptake and endosomal escape, thereby ensuring high transfection and silencing efficiencies. However, some special degradable small molecules were also reported. For example, Candiani et al. synthesized a triazine-based cationic Gemini surfactant with a bioreducible disulfide ethyl chain [93]. In this system, the protein corona masks exofacial protein sulfhydryls on the cell surface, protecting the Gemini surfactant complex from premature dissociation [97]. Subsequently, the disulfide ethyl chain was cleaved in the intracellular reductive environment (e.g., glutathione), releasing DNA. Together, these mechanisms synergistically enhanced transfection efficiency. Therefore, designing responsive degradation is an important strategy to increase the transfection efficiency of GSs.
In summary, small molecule modifications, such as fluorination, oligo-oxyethylene spacers, carbamate, disulfide ethyl, and diethoxy, significantly enhanced transfection efficiency. Further research was recommended to focus on the effects of spacer length and chemical properties on nucleic acid release and interactions with cells. Additionally, the development of responsive spacers (e.g., pH-sensitive or enzyme-responsive) was considered as a promising approach to enable precise control of nucleic acid release and intracellular behavior.
4.4 Biomimetic Modified Gemini Surfactants
Biomimetic modification refers to the use of biological membranes or biomolecules to modify gene delivery vectors, enabling them to mimic the structure and function of living organisms. Biomimetic modification showed significant advantages in Gemini surfactant-based carriers, as it not only enhanced the biocompatibility and targeting ability of the vectors, but also significantly improved gene delivery efficiency. Existing studies have shown that Gemini surfactant-based nanocomplexes modified with biomimetic coatings such as red blood cell membranes (RBCMs) or hepatocyte membranes (HCMs) could effectively enhance transfection efficiency, while exhibiting good biocompatibility in both in vitro and in vivo. For example, Lu et al. developed a biomimetic gene delivery complex based on Gemini surfactant, incorporating either red blood cell membrane (RBCM) or RBCM co-modified with DOPE-PEG2000 (DP) lipid material [94]. After a series of study in vitro and in vivo, as summarized in Figure 5A, it was found that the biomimetically modified GS nanocomplexes exhibited better transfection efficiency and biocompatibility than that of the unmodified GS complexes. Specifically, the transfection efficiency of GS-pDNA-DP-RBCM in Alpha Mouse Liver 12 (AML-12) cells and L-O2 cells was several times greater than that of Lipofectamine 2000 (Thermo Fisher Scientific, USA) and unmodified GS-pDNA. Besides, the GS-pDNA-DP-RBCM exhibited the highest fluorescence intensity and the longest duration of gene expression in the liver in vivo. Additionally, the incorporation of RBCM significantly improved cell viability. The reason for this improvement could be the co-incorporation of RBCM and DP, which enhanced vector stability. RBCM also facilitated cellular recognition and uptake of the complexes, which could promote efficient gene delivery and improve transfection efficiency. The RBCM modification facilitated the circulation of the complexes in vivo and reduced immune recognition and clearance. Meanwhile, the PEG modification of DP prevented aggregation and resisted protein adsorption, collectively reducing cytotoxicity. Building on these findings, in a similar study, as presented in Figure 5B, Wu et al. developed nanoparticles based on Gemini surfactant, modified for CRISPR/Cas9 delivery using either RBCM or HCM [95]. The physical and chemical properties, transfection efficacy, and safety profile of GS-based biomimetic nanoparticles were evaluated in vitro and in vivo. The results demonstrated that the luciferase (Luc) expression of GS-pGL3Luc-HCM was significantly higher than that of Lipofectamine 2000 (Thermo Fisher Scientific, USA) and unmodified GS-pGL3Luc in AML-12 and LX-2 cells. In in vivo study, GS-pDNA-HCM was more effective in targeting the liver compared to unmodified GS-pDNA. In conclusion, biomimetic modification of surfactants enhanced both biocompatibility and transfection efficiency, making them promising candidates for gene delivery applications. However, the biomimetic modification of cell membranes might cause some problems regarding sources, large-scale production, long-term storage and effects [98]. For instance, issues related to the donor source of cell membranes needed to be carefully considered. If blood cell membranes were used to wrap and form nanoparticles, we suggested that autologous sources should be used to manufacture biomimetic nanoparticles to avoid adverse immune responses in the human body to exogenous cell membranes. Furthermore, the large-scale production and extraction of blood cell membranes might be impractical. Meanwhile, other types of cell membranes (bacterial membranes) can be obtained sufficient cell membranes through large-scale culture methods, but obtaining high-purity cell membranes simultaneously required complex extraction procedures and also face significant challenges [99]. In terms of long-term storage, nanoparticles prepared through cell membrane modification might simultaneously have varying degrees of instability, which seriously hinder long-distance transportation and long-term storage [100]. At present, the comparison of long-term safety characteristics between biomimetic and traditional GSs had not been deeply studied. The long-term effects of these modified cell membranes on the human body, including potential immune responses and interference with normal physiological processes, remained largely unknown and required extensive research. The above aspects posed significant obstacles to the transformation of this biomimetic strategy into clinical applications.
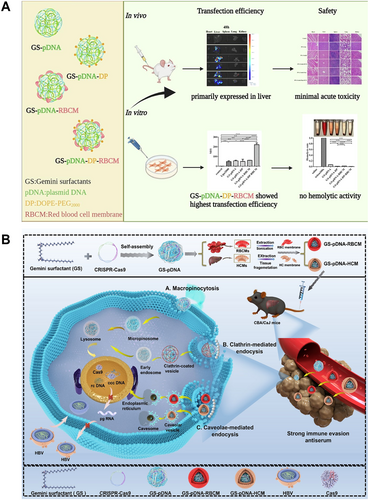
5 Gene Delivery Mechanisms of Gemini Surfactants
5.1 Cell Uptake
The phospholipid bilayer of the cell membrane constitutes a critical barrier that impedes the entry of exogenous substances into cells and represents a key obstacle that nonviral vectors must overcome to achieve efficient gene delivery. Nonviral nanocarriers typically range from 50 to 250 nm in size and are internalized primarily via membrane fusion or endocytosis. Among these, endocytosis is the predominant uptake pathway, encompassing clathrin-mediated endocytosis (CME), caveolin-mediated endocytosis (CvME), and macropinocytosis [101-104]. Figure 6 illustrates the various cellular uptake pathways of GSs.
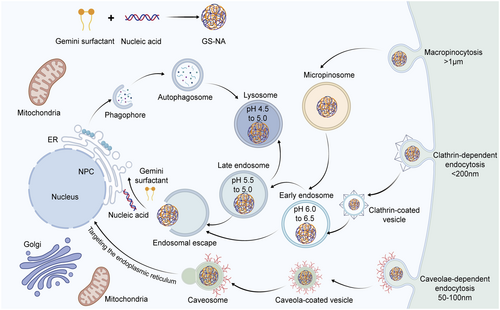
Distinct endocytic pathways possess specific molecular mechanisms and subcellular trafficking routes, which critically influence the intracellular fate of gene carriers and their transfection efficiency. CME, a classical transmembrane transport mechanism, is broadly involved in the uptake of nutrients and signaling molecules. Several studies had identified over 30 accessory proteins regulating CME, among which the adaptor protein complex AP-2 and clathrin coat proteins were essential for the formation of clathrin-coated vesicles (CCVs) [105-107]. In contrast, CvME depended on flask-shaped invaginations of 50–100 nm in diameter and the signature protein caveolin. Following internalization, caveolar vesicles bypassed lysosomal degradation by trafficking directly to the endoplasmic reticulum (ER) via the microtubule transport system, leading to enhanced delivery efficiency [108].
Recent investigations focused on the cellular uptake mechanisms of Gemini surfactant structure and surface modifications. Wu et al. demonstrated that GS-pDNA-HCM nanoparticles modified with hepatocyte cell membranes (HCMs), which carried a negative charge, primarily entered the ER through CvME, effectively avoided lysosomal degradation and markedly improved transfection efficiency [95]. In contrast, unmodified positively charged GS-pDNA mainly utilized CME or macrophage-mediated pathwaysand was ultimately localized within lysosomes, resulting in lower transfection efficiency. Similar findings were reported by Xing et al. [109] who noted that unmodified GS-pDNA predominantly relied on CME and CvME for cellular entry, with CME playing the dominant role, whereas TAT (cell-penetrating peptide) and PCM (cardiomyocyte-specific targeted peptide) dual-peptide-modification combining with RBCM-modification (GS-pDNA-TP-RBCM) favored CvME-mediated uptake. Additionally, folate-modified cationic Gemini surfactant FA-16-2-16 entered cells via lipid raft/CvME pathways, effectively circumvented lysosomal degradation and enhanced transfection [110]. Other studies showed that dipeptide-substituted GSs (e.g., 12-7NGK-12) were internalized through both CME and CvME routes and exhibited robust lysosomal escape capabilities [42].
Notably, there were studies suggesting that nanoparticle surface charge significantly influenced uptake pathways: negatively charged polyplexes and lipoplexes tended to enter cells via CvME [111], whereas positively charged particles, such as CeO₂ nanoparticles, preferentially utilized the CME pathway [112]. However, results pertaining to GSs did not fully conform to this pattern, suggesting that their uptake mechanisms were regulated by a combination of factors, including structural modifications, particle size, surface ligands, and membrane affinity, rather than surface charge alone. This complex interplay warranted further systematic investigation to elucidate the underlying principles.
5.2 Endosomal Escape
Most nonviral gene vectors, after entering the cell via endocytosis, follow the intracellular transport pathway from early endosomes to late endosomes and lysosomes. The low pH environment in the lysosome causes the degradation of exogenous genes, so nonviral vector design must consider whether the vector could escape from the lysosome, bypass the lysosomal pathway, or disrupt the lysosome directly. In 1997, Behr proposed the “proton sponge effect” [113]. This suggested that certain permanent polycations with strong buffering capacity at physiological pH (e.g., lipid polyamines, polyethyleneimine) enabled effective gene delivery in vivo and in vitro without lysosomotropic bases or membrane-disrupting agents. This was because polycations induced endosomal swelling and rupture, facilitating the escape of polycation–gene complexes.
Besides the proton sponge effect for lysosomal escape, an alternative intracellular pathway bypassing the lysosome can prevent the acidic degradation of exogenous genes by the lysosome. One such alternative pathway has been explored by Pollock et al. [114], who prepared an ER-targeted liposome. The intracellular transport of the ER-targeted liposome relied on caveolae proteins and microtubules. After 22–25 min of uptake, the liposome successfully reached the ER in an intact form. Subsequently, it fused with the ER membrane, thereby effectively avoiding lysosomal degradation.
Regarding GSs, after cellular uptake via CCVs, the complexes are typically transported to lysosomes. However, their lysosomal escape capabilities varied depending on molecular modifications. Donkuru et al. reported enhanced transfection efficiency through the design of a pH-sensitive Gemini surfactant-based carrier (m-7NH-m) [73]. Upon exposure to the acidic lysosomal milieu, this carrier absorbed protons, triggered an influx of aqueous ions, which led to endosomal swelling, membrane rupture, and release of the carrier into the cytoplasm, thus promoting lysosomal escape. Similarly, another study demonstrated that modification of GSs with short peptides (glycine–lysine) further enhanced lysosomal escape [42]. The peptide modification increased the acidic pH buffering capacity and particle size, allowing greater proton absorption and consequent chloride ion influx. This ion exchange induced lysosomal membrane destabilization and nanoparticle release into the cytoplasm. Furthermore, the increase in particle size might reflect nanoparticle relaxation, facilitating DNA release from the complex post-escape. Collectively, these findings underscored that enhancing the buffering capacity of GSs and strengthening their lysosomal escape abilities were critical strategies for improving gene delivery efficiency.
5.3 Nuclear Translocation
Once the Gemini surfactant complex is released into the cytoplasm, it needs to dissociate from the nucleic acid cargo, which must reach its site of action. For DNA, the entry into the nucleus is a prerequisite for initiating transcription. DNA may enter the nucleus through two distinct pathways: by passing through the nuclear pore complex (NPC) in non-mitotic cells or through nuclear envelope breakdown during mitosis [115]. In non-dividing cells, gene vectors typically rely on active transport via NPCs. In contrast, during mitosis, gene vectors may bind to chromatin-associated nuclear proteins and remain within the reassembled nuclear envelope [116].
Some researchers investigated the cellular uptake and subcellular distribution of three types of GSs: unmodified 16-3-16; 16-7NGK-16, modified with a glycine–lysine dipeptide in the spacer region; and 16(Py)-S-2-S-16(Py), modified with a pyridine headgroup and two sulfhydryl groups [117]. The results showed that 16(Py)-S-2-S-16(Py) exhibited the highest nuclear accumulation but also the highest cytotoxicity. In contrast, 16-7NGK-16 showed a more uniform distribution across cellular compartments, including the nucleus, and achieved the highest cellular uptake and transfection efficiency. These findings indicated that introducing polypeptides into the spacer region could help balance transfection efficiency and biocompatibility. Conversely, excessive nuclear accumulation of GS might be associated with increased cytotoxicity and reduced transfection efficiency. However, in most cases, it remained unclear at which stage DNA dissociated from the Gemini surfactant and whether nuclear entry occurred via the GS/DNA complex or as free DNA.
6 Therapeutic Applications of Gemini Surfactants-Based Gene Delivery Systems
6.1 Tumor Therapy
GSs were incorporated with tumor-targeting peptides to enhance gene delivery efficiency. For melanoma treatment [82], GS modified with the cancer-targeting peptide p18-4 via physical blending showed the highest transfection efficiency in human malignant melanoma (A375) cells. Similarly, GS chemically coupled with RGD peptide also showed optimal transfection performance in melanoma models [84]. These findings supported the potential application of GS-based vectors in melanoma gene therapy. Additionally, a serine-derived Gemini surfactant (14Ser) 2N5 was developed to deliver survivin siRNA, effectively silencing the survivin gene and enhancing glioblastoma (GBM) cell sensitivity to chemotherapy [89]. The combined “gene silencing plus chemotherapy” approach enhanced GBM cell chemosensitivity and showed synergistic antitumor effects in vitro, highlighting its potential for improving treatment outcomes.
6.2 Liver Diseases Therapy
A biomimetic Gemini surfactant (GS) coated with HCMs and RBCMs was developed as an efficient CRISPR/Cas9 delivery vector for hepatitis B virus (HBV) therapy [95]. In vitro evaluation, GS-pDNA@Cas9-HCM nanoparticles significantly inhibited HBV covalently closed circular DNA (cccDNA) in HepG2.2.15 cells. In vivo evaluation, intravenous administration of these nanoparticles markedly reduced hepatic cccDNA levels and other HBV-related markers in an HBV cccDNA mouse model. These results demonstrated the promising potential of GS-based systems for targeted gene therapy in liver diseases.
6.3 Heart Diseases Therapy
GS-based nanocarriers showed promising therapeutic potential for the treatment of heart diseases [109]. Researchers treated myocardial hypertrophy by adding TAT and PCM to biomimetic modified (erythrocyte membrane) GSs and synergized them with the heart-specific promoter cardiac troponin T promoter (cTnT). The results demonstrated that GS nanoparticles achieved high transfection efficiency in cardiomyocytes in vitro and effectively reversed isoproterenol (ISO)-induced cardiac hypertrophy. Furthermore, intravenous administration of the nanoparticles ameliorated histopathological alterations associated with myocardial hypertrophy and significantly improved cardiac function in hypertrophic hearts.
6.4 Glaucoma Diseases Therapy
Researchers developed GSs (18-7N(pn)-18) modified with integrin-targeting RGD peptides or neuro-IGSF peptides for glaucoma treatment [85]. In vitro studies demonstrated that the p3 peptide-modified Gemini surfactant (18-7N(p3)-18) exhibited the highest transfection efficiency in A7 retinal ganglion cells. Intravitreal injection of 18-7N(p3)-18 carrying the BDNF plasmid into CD1 mice resulted in the highest BDNF expression levels in vivo. These results indicated that the IGSF peptide (p3)-modified Gemini surfactant system effectively delivered the BDNF gene to retinal ganglion cells, enhanced neurotrophic factor expression and represented a promising gene therapy strategy for glaucoma.
6.5 Mucosal Diseases Therapy
In the field of mucosal diseases, researchers developed GSs modified with glycine and lysine dipeptides, combined with Poloxamer 407 and the penetration enhancer Diethylene Glycol Monoethyl Ether (DEGEE), for gene delivery targeting mucosal epithelial cells [83]. The results demonstrated that nanoparticles carrying the tdTomato plasmid significantly increased red fluorescent protein expression in rabbit epithelial (Sf1Ep) cells. These findings provided strong evidence that the Gemini surfactant-based system is a promising noninvasive gene delivery platform for mucosal epithelial cells.
6.6 Enamel Defect Disease
Researchers utilized a Gemini surfactant modified with glycine and lysine (16-7N(GK)-16) to deliver the T-box 1 (Tbx1) gene for the treatment of enamel defects [118]. The results demonstrated efficient delivery of Tbx1 into the dental epithelial stem cell line HAT-7, successfully inducing differentiation of odontogenic epithelial stem cells into ameloblasts, which promoted enamel matrix secretion and mineralization. These findings highlighted the potential of this Gemini surfactant-based platform as a novel strategy for regenerative therapy of enamel defects.
7 Future Development and Chanlleges for Gemini Surfactants-Based Gene Delivery
7.1 Artificial Intelligence (AI)-Assisted Structural Optimization
The high-throughput evaluation and screening methods offer a systematic and unified framework for data integration and analysis. By rapidly processing large volumes of data, these approaches enable the identification of potential trends and patterns. By combining AI-driven design strategies with leveraging extensive structure–activity relationship (SAR) data for GSs, the integrated approach aims at more accurate prediction of GSs properties, as well as efficient and targeted optimization of high-performance candidates. For example, some studies constructed computational libraries comprising hundreds of distinct GSs structures and parameterized their molecular features [119]. A membrane model of Escherichia coli was subsequently established, and molecular dynamics simulations were employed to quantify the interactions between different GSs structures and the bacterial membrane. The results indicated that spacer groups containing ether bonds and GSs incorporating pyridine rings exhibited strong membrane-disruptive capabilities. Moreover, spacer groups with polarity (e.g., amino, ether) and flexibility (e.g., extended methylene chains) enhanced both electrostatic interactions and membrane penetration. Additionally, longer hydrophobic tail chains contributed to a balance between membrane penetration and structural stability. These findings highlighted the deep integration of computational simulations with mechanistic insights into membrane disruption, provided actionable molecular design guidelines and significantly improved the efficiency of experimental validation and GSs development. Researchers implemented a systematic strategy that integrated high-throughput screening with machine learning to design formulations of LNPs with enhanced transfection efficiency [120]. By applying similar high-throughput screening and machine learning approaches to GSs, it was possible to construct large-scale GSs libraries, identify key structural parameters, and systematically optimize vector performance, which would provide a theoretical and technical foundation for the rational design of next-generation GSs with enhanced gene delivery capabilities. Although AI techniques show remarkable success in the rational design of LNPs, their application in the development of GSs for gene delivery faces significant challenges. One major limitation is that GS-related research is not as extensive or systematic as that of LNPs, particularly in the field of gene delivery. Currently, there is still a lack of comprehensive, high-quality data sets that link GSs structural features to key performance metrics such as transfection efficiency and biocompatibility. The scarcity of standardized and quantitative data hinders the effective training and generalization of AI models and limits their predictive power and practical applicability in GSs optimization. To overcome these challenges, future efforts can focus on generating high-quality, standardized GSs data sets through systematic structural modification, unifying experimental procedures, and standardizing evaluation criteria.
Besides, to date, the immunogenicity of GSs is largely overlooked, with limited studies addressing their potential immune responses in vivo. Given the crucial role immunogenicity plays in the safety and efficacy of gene delivery vectors, future research needs to focus on systematically investigating the immunological effects of GSs. A thorough understanding of their immunogenic profile will be vital for optimizing their design and advancing their clinical translation.
7.2 The Metabolic Process and the Fate In Vivo Need Further Investigate
Currently, studies of the metabolic fate and internalization pathways of GSs remain limited, with most research focused on preliminary explorations of their metabolic pathways. These studies were primarily conducted in vitro conditions, which were typically short-term and did not fully capture the complexity of in vivo metabolic processes and biodistribution. For example, Jin et al. investigated the metabolism of three binary surfactants with different structural models using high-resolution quadrupole orbital trap mass spectrometry (Q-Exactive) [121]. Their findings revealed that the unsubstituted GS (16-3-16) did not undergo metabolism in cells, while GSs containing a pyridine head group (16(Py)-S-2-S16(Py)) were mainly metabolized through phase I reactions, such as oxidation and dealkylation, and produced metabolites potentially associated with its high toxicity. In contrast, GSs modified with glycine-lysine dipeptides (16-7N(GK)-16) were primarily metabolized through phase II reactions, including methylation, acetylation, glucose conjugation, and palmitoylation/stearoylation. These results suggest that the metabolic pathways of GSs are closely related to their structural features, and the metabolites produced may contribute to varying degrees of toxicity, warranting further attention to their metabolic toxicity.
In addition, a key challenge in developing Gemini surfactant nanocarriers lies in the limited understanding of their degradation, metabolic pathways, and biodistribution in vivo. Although some studies revealed in vitro metabolic pathways of GSs, these studies were generally restricted to short-term observations under laboratory conditions, leaving the complexities of in vivo metabolism and individual differences insufficiently addressed. Therefore, further research into the cellular internalization pathways and cellular fate of Gemini surfactant nanocarriers will be crucial for optimizing their performance in vivo.
Recent advances in eco-responsive bioimaging techniques provided new perspectives for monitoring the in vivo fate of nanocarriers. These techniques can respond to changes in the physiological environment, enabling real-time tracking of nanocarrier dynamics in vivo. Well-established bioimaging methods, including aggregation-induced emission (AIE), fluorescence resonance energy transfer (FRET), and aggregation-induced quenching (ACQ) probes, have been proven effective in this context [122, 123]. When encapsulated within a nanocarrier, these probes will emit strong fluorescence, which can be rapidly suppressed upon carrier leakage as they aggregate. Researchers modified tetraphenylethylene (TPE) into the spacer groups of GSs and utilized the AIE properties of TPE to track the fate of GS/DNA complexes within cells in real-time [124]. The results revealed that processes such as endocytosis, nuclear localization, and DNA release could be observed through variations in fluorescence intensity and color. These techniques enable real-time monitoring of Gemini surfactant nanocarriers' behavior in vivo, providing valuable insights for optimizing their structure and improving transfection efficiency.
Although research on the metabolic toxicity and in vivo fate of GSs faces several challenges, ongoing advancements in metabolic pathway analysis and bioimaging technologies hold promise for a more comprehensive understanding of the metabolic processes and biodistribution of these nanocarriers. Such insights will provide deeper theoretical support for the design and clinical application of future nanomedicines.
7.3 GS Delivery System Combining Physical Assistive Methods
Current developments of Gemini surfactant vectors remain focused on traditional modification strategies. Developing novel bio-responsive and photo-, thermal-, ultrasound-, and magnetic-responsive delivery vectors for controlled gene release will enable on-demand gene delivery and precise disease treatment. For example, under an external magnetic field, magnetic-responsive surfactant carriers induce directional migration and aggregation of DNA, resulting in more compact DNA structures even at low concentrations [125, 126]. On the other hand, under an external magnetic field, the carrier can be accurately transported to the target site to achieve targeted therapy [127]. Due to the synergistic effect of the double hydrophobic chain and the bipolar head group, GSs may theoretically have stronger DNA condensation ability, better magnetic response targeting and surface activity after introducing magnetic groups. Therefore, magnetic GSs may have great application value and research prospects in the direction of gene delivery.
8 Conclusion
Gene delivery vectors play a pivotal role in the realization of gene therapy. In recent years, GSs have emerged as promising nonviral carriers due to their excellent DNA condensation ability, efficient DNA compaction, ease of synthesis, high stability, and multifunctionality. This review systematically summarized recent advances in the structural optimization, functional modification, and emerging biomimetic strategies of GSs, highlighted their applications in various gene delivery contexts, and discussed their intracellular trafficking mechanisms. Current findings suggested that transfection efficiency could be significantly improved by extending hydrophobic tail length, shortening spacer groups, or introducing aromatic/heterocyclic headgroups. Moreover, functional modifications using peptides, amino acids, small molecules, and biomimetic moieties were demonstrated to be potential methods to enhance both transfection performance and biocompatibility. In addition, we provided insights into future development strategies and the key challenges associated with clinical translation. Despite the breadth of this review, there are certain limitations. Due to the structural diversity of GSs and variations in experimental conditions, direct comparison of transfection efficiency across different studies is still challenging. Furthermore, some emerging functionalization strategies and their impact on immunogenicity cannot be fully discussed due to limited available data. In spite of this, this review offered new perspectives on overcoming the limitations of GSs in gene delivery, provided a theoretical framework to bridge the gap between vector design and clinical translation, and contributed to advancing gene therapy for disease treatment. Ultimately, it will lay a solid foundation for accelerating the development of gene delivery systems toward future clinical applications.
Author Contributions
Peng Qian: data curation (equal), formal analysis (equal), investigation (equal), visualization (equal), writing – original draft (equal). Yuxin Chen: data curation (equal), investigation (equal), visualization (equal). Yangchen Xing: data curation (equal), investigation (equal). Kexin Wu: data curation (equal), investigation (equal). Qianyu Zhang: conceptualization (equal), funding acquisition (equal), project administration (equal), supervision (equal), writing – review and editing (equal). Huali Chen: conceptualization (equal), funding acquisition (equal), project administration (equal), supervision (equal), writing – review and editing (equal). All authors have read and approved the final manuscript.
Acknowledgments
All original figures in this article were created through www.biorender.com, for which we would like to express our gratitude. We are grateful for the financial support received from various sources, including the Natural Science Foundation of Chongqing Municipal Science and Technology Commission (Grant nos. CSTB2024NSCQ-MSX0621 and CSTB2023NSCQ-MSX0086), Science and Technology Research Program of Chongqing Municipal Education Commission (Grant nos. KJQN202200451 and KJQN202500406), and Chongqing College Student Innovation and Entrepreneurship Project (Grant no. S202410631050).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.