Stem Cell-Derived Exosomes as Nanotherapeutics for Inflammatory Diseases
Xinyu Wei, Qingyi Wang, and Wen Wen contributed equally to this study.
ABSTRACT
Inflammation, as a complex biological response, can lead to tissue damage and pathological physiological changes, forming the basis for many chronic diseases. Stem cell-derived exosomes (SC-Exos), a type of nanoscale extracellular vesicle, possess advantages such as small volume, low immunogenicity, and drug-carrying capacity, demonstrating immense potential in the field of disease diagnostics and therapeutics. Current studies indicate that SC-Exos can not only alleviate inflammatory diseases by suppressing inflammatory cytokines and modulating the activation of macrophages through their immunomodulatory and regenerative properties but also show significant potential as carriers for anti-inflammatory drugs, presenting a promising therapeutic approach for inflammatory conditions. However, the current lack of systematic summaries of SC-Exos in the treatment of inflammatory diseases has impeded the development of standardized therapies and clinical applications. This review elucidates the methods of SC-Exo sourcing, isolation, characterization, and engineering, as well as their application, mechanisms of action, and efficacy in the treatment of inflammatory diseases such as periodontitis, osteoarthritis (OA), and inflammatory bowel disease. Integrating these findings, this review highlights that SC-Exos can attenuate a variety of inflammatory diseases by transporting a diverse range of molecules to modulate immune responses, thereby providing foundations for subsequent standardization of production and clinical trials.
1 Introduction
Inflammation is a natural response of the organism to injury, infection, or irritation, involving the regulation and interaction of various cell types, signal transduction pathways, and inflammatory mediators. In the context of chronic diseases, inflammation may lead to tissue damage and pathological physiological alterations. Prolonged inflammatory responses result in tissue destruction and impairment of organ function, and can also provide a conducive environment for the development of tumors [1, 2]. Therefore, the study of inflammation and its therapeutic strategies is crucial for a profound understanding of disease pathogenesis, progression, and treatment. Current management and treatment of chronic inflammatory diseases include drug delivery systems, combination therapies, and integrated tissue-targeting and pathway-selective strategies, among others. Among these, exosome-mediated drug delivery has gained significant attention due to its inherent advantages, such as targeted delivery and low immunogenicity.
Stem cells (SCs) have emerged as a focal point in biomedical research in recent years, demonstrating significant potential in the treatment of human diseases, antiaging interventions, and tissue regeneration. Generally speaking, SCs are a group of adult SCs that can be isolated from a variety of organs, including the brain, liver, lung, and pancreas, as well as from tooth pulp, adipose tissue, bone marrow, cord blood, and umbilical cord [3]. Studies have indicated that SCs exert their therapeutic effect not only by differentiating into desired cells but also through factors they secrete [4]. Additionally, they are beneficial in cell treatment for autoimmune and inflammatory illnesses, which significantly impact immune cells such as dendritic cells, T lymphocytes, and macrophages [5]. There is growing evidence that SCs can secrete extracellular vehicles (EVs) with lipid bilayer structures, including exosomes (Exos), microvesicles and apoptotic bodies. Exos are the most important component among EVs, which have a diameter of 30–150 nm and were first identified by their endocytic origin. They are essential for intercellular communication because they transport heavy loads of proteins, lipids, microRNAs, mRNAs, and medications [6]. The key to activating the body's signal transduction machinery is the lipid bilayer, which serves as the entry point for these bioactive molecules after being selectively sorted and packaged by parental cells. stem cell-derived exosomes (SC-Exos), as a pivotal medium for intercellular communication, possess distinct biological characteristics. Their bilayer lipid membrane structure endows them with remarkable stability under both physiological and pathological conditions and enables them to carry a variety of bioactive molecules, including proteins, lipids, and nucleic acids, thereby facilitating the transmission of complex signals between cells [6]. The biogenesis and secretion of SC-Exos are intricately regulated by multiple intracellular signaling pathways, which exhibit significant differences across diverse physiological and pathological states [3]. Moreover, the biological functions of SC-Exos are not only determined by the type of stem cells from which they originate but are also profoundly influenced by the microenvironment in which the cells reside [7]. Therefore, the therapeutic potential of SC-Exos in disease treatment is a result of the synergistic influence of their biological properties, origin, and microenvironment. Previous studies have indicated that SC-Exos show considerable promise in anti-inflammation and immunomodulatory effects with the advantages of relative small size, low degree of immune response, and penetrating the blood–brain barrier (BBB).
However, although the application of natural SC-Exos still has some shortcomings such as impurity, lack of targeting, and low drug delivery rate, it is still an important development direction. In particular, the emergence of novel technologies to modify or construct stem cell-derived engineered SC-Exos, such as physical technology, chemical technology and biotechnology, points out the way for its subsequent development. To create SC-Exos with advantageous characteristics, numerous modification techniques have been devised, including altering parent cells and processing SC-Exos directly. Co-incubation, genetic engineering, electroporation, ultrasound, and artificial manufacture of modified SC-Exos are some of these techniques that provide them with anti-inflammatory properties and allow for the targeted stimulation of effective soft tissue healing [7]. For example, SC-Exos carrying nucleic acids (miR-155-5p, miR-1246, miR-375, miR-21, miR-17-5p) and drugs such as melatonin and deferoxamine, can be derived by transfecting SC-Exos-secreting cells with recombinant viruses or plasmids to obtain SC-Exos carrying specific genes traditionally. Nucleic acid-carrying exos can also be produced by processing physical materials, such as by applying static magnetic fields and magnetic nanoparticles to the donor cells. The benefits of SC-Exos modification are greater than those of parent cell modification. Although the current research is in the initial stage and relevant reports are not rich, it is still an important branch of nanomedicine development.
In this review, as illustrated in Figure 1, we have reviewed the derivation, isolation, and engineering methods of SC-Exos, as well as their applications in the treatment of inflammatory diseases (Figure 1). SCs have attracted a lot of interest because of their potential for immune regulation and tissue regeneration. As a crucial secretory product of SCs, SC-Exos can exert anti-inflammatory effects by modulating immune responses and promoting tissue repair. As a key secretory product of SCs, SC-Exos exert anti-inflammatory effects by regulating macrophage polarization, suppressing pro-inflammatory cytokines, and promoting tissue repair. These properties make SC-Exos a promising therapeutic strategy for inflammatory diseases such as periodontitis, OA, and inflammatory bowel disease. Furthermore, advancements in the separation, characterization, and engineering technologies of SC-Exos have laid the foundation for their clinical application. Although research on SC-Exos is still in its infancy, they show great promise in the treatment of inflammatory diseases and are expected to become a novel therapeutic strategy in the future. This comprehensive review systematically encapsulates the state-of-the-art advancements of SC-Exos in the realm of inflammatory disease therapeutics and delves into the intricate potentialities and hurdles associated with their clinical translation. By meticulously elucidating the underlying mechanisms of action and advanced engineering strategies of SC-Exos, this manuscript not only furnishes novel insights and methodological paradigms for the domain of inflammation treatment but also provides crucial theoretical foundations and practical directives that are poised to catalyze the evolution of SC-Exos research and inform future clinical trials.
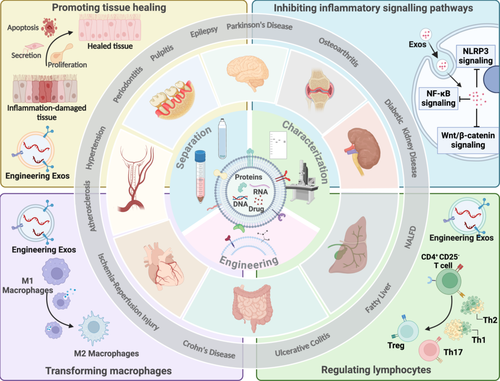
2 Anti-Inflammation Mechanisms of SC-Exos
Inflammation is the body's natural and crucial response to signals arising from invading pathogens and toxins [8]. However, if the inflammation response is overly responsive or uncontrolled, it can ultimately lead to the growth and progression of diverse inflammatory diseases, such as neuronal, periodontitis, cardiovascular, inflammatory bowel disease and autoimmune diseases [9].
Therefore, modulating inflammatory homeostasis is a critical therapeutic goal. Current anti-inflammatory treatments are primarily categorized into four major classes: dietary regulation, pharmacological anti-inflammation, surgical intervention, and lifestyle modification. Dietary modulation can attenuate inflammatory responses by optimizing nutritional intake and incorporating specific nutrients [10, 11]. Additionally, the maintenance of a healthy lifestyle is a straightforward yet efficacious approach that can augment immune function and metabolic efficiency, thereby promoting overall health [12, 13]. Surgical intervention is a key method for regulating inflammatory homeostasis. It can remove pathological tissues and reduce inflammatory responses. However, its therapeutic efficacy for inflammatory diseases is limited and may not achieve complete disease resolution. Pharmacological anti-inflammation is one of the mainstays of current inflammatory disease treatment. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are widely used to mitigate inflammatory responses [14, 15]. In addition, in recent years, some novel drugs, such as interleukin-1β (IL-1β) antagonists and TNF inhibitors, have also been employed in the treatment of inflammation-related diseases [16]. However, the efficacy of drug therapy is often limited by factors such as drug delivery efficiency, highlighting the need for more effective targeted delivery systems. SC-Exos have garnered significant attention from scientists due to their unique characteristics, and are considered a potential breakthrough in the research for efficient drug delivery.
The primary mechanisms of SC-Exos in the treatment of inflammatory diseases are summarized in Figure 2. SC-Exos, which are widely distributed in bodily fluids, can carry and release a variety of bioactive molecules, inhibiting the production of inflammatory mediators and thereby reducing inflammation. Additionally, SC-Exos enhance the phagocytic function of macrophages and other immune cells, accelerating the clearance of inflammatory mediators (Figure 2).
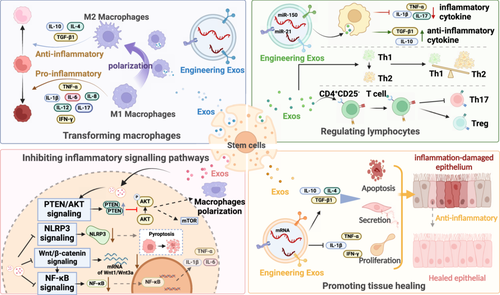
SC-Exos exert anti-inflammatory effects by modulating the activity of key inflammatory cells. Macrophages have a detrimental effect on the induction of inflammation. These macrophages increase the release of inflammatory cytokines and chemokines that draw pro-inflammatory M1 macrophages, including interferon-γ (IFN-γ), multiple interleukins, and tumor necrosis factor alpha (TNF-α). SC-Exos derived from SCs have been shown to promote the polarization of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages, thereby reducing inflammation [17]. SCs concurrently secrete immune-suppressive cytokines to promote the generation of regulatory T (Treg) cells, a process that is dependent on antigen-presenting cell (APC)-mediated T cell activation [18]. And it prevents T helper 1 (Th1) and T helper 2 (Th2) cells from differentiating, which helps to control the harmful inflammation.
More specifically, it can be done by affecting inflammatory signaling pathways. Several signaling pathways, including PTEN/AKT, NF-κB, Wnt/β-catenin, and pyrin domain-containing 3 (NLRP3) signaling pathways, are regulated in the anti-inflammatory process of SCs and their generated SC-Exos. For instance, SC-Exos can influence macrophage polarization via the PTEN/AKT signaling pathway [19, 20]; subsequently, they can modulate inflammation through the NF-κB and Wnt/β-catenin pathways [21]; furthermore, by affecting the NLRP3 inflammasome and thereby controlling the recruitment of pro-inflammatory cytokines, SC-Exos can exert anti-inflammatory effects [22, 23]. The current research findings on the mechanisms of SC-Exos in treating inflammatory diseases are primarily centered around these aforementioned pathways.
3 Separation, Characterization and Engineering of SC-Exos
SC-Exos play a critical role in intercellular communication and hold immense potential for disease diagnosis and treatment. The isolation, characterization and engineering transformation of SC-Exos are the frontiers of regenerative medicine and nanobiotechnology. However, the inherent heterogeneity of SC-Exos poses significant challenges in establishing standardized protocols for their preparation and characterization. To address this issue, researchers have designed and implemented several strategies aimed at optimizing the acquisition, characterization, and engineering of SC-Exos. Figure 3 illustrates the various acquisition, characterization, and engineering methods of SC-Exos that are currently well-established in research (Figure 3).

SCs can be isolated from numerous tissues, including bone marrow, adipose tissue, cord blood, and various adult tissues, each offering unique advantages for therapeutic applications. The exceptional ability of umbilical cord MSCs (UC-MSCs) to proliferate and their low immunogenicity have made them popular in clinical research [24]. With the advent of induced pluripotent stem cell (iPSCs) technology, the reprogramming of adult cells into SCs has gained increasing attention. These SCs have the ability of self-renewal and multidirectional differentiation, and are considered to be highly potential therapeutic cells in the field of regenerative medicine. In terms of SCs standardization, the International Society for Cell Therapy (ISCT) has developed a set of minimum standards for SCs that include requirements for the expression of cell surface markers, such as CD105, CD73, CD90, and the absence of specific markers, such as CD45, CD34, HLA-DR [25, 26]. However, the practical utility of these minimum standards in clinical applications may be limited, so more precise evaluation criteria need to be further developed according to the characteristics of different SCs types. In addition, the multidirectional differentiation potential of SCs must also be verified by in vitro experiments.
Currently, the engineering of SCs that has been comparatively well-studied primarily involves the modification of SCs through genetic or physical means to endow them with specific therapeutic functionalities. Common approaches include gene editing using the CRISPR/Cas9 system, and the use of synthetic biology tools to design specific genetic circuits that enable SCs to respond and perform therapeutic functions in specific microenvironments [27]. These tactics present fresh opportunities for the treatment of SCs and are anticipated to be significant in clinical practice in the future.
Exos are nanoscale vesicles secreted by cells. Their surfaces are rich in lipids, proteins and RNA, and have a bilayer lipid membrane structure, so they show a certain stability under pressure and temperature changes. Due to the unique properties of Exos, a variety of extraction methods have been developed. Taking MSC-Exos as an example, currently, they can be isolated from SC culture super nuances by ultrafast centrifugation, polymerization precipitation, density gradient centrifugation, column chromatography, and immune magnetic beads. The most commonly used methods at present are ultracentrifugation and density gradient centrifugation [28].
Ultracentrifugation, also known as differential centrifugation, is a standardized method for Exos separation. This method relies on controlling the centrifugal speed to exploit the size differences of Exos. Initially, cell debris, microvesicles, or apoptotic bodies are removed using an ultrafiltration filter or low-speed centrifugation. Subsequently, ultracentrifugation is performed to separate large vesicles and precipitate Exos. Finally, the supernatant is removed, and the Exos are resuspended in a smaller volume of buffer for further research [29]. This method is simple and cost-effective and is widely used. However, multiple high-speed centrifugations may cause Exos to rupture, thereby reducing the purity and stability of the final product.
Density gradient centrifugation is an optimization based on ultracentrifugation, which introduces centrifugal media (such as sucrose, iodixanol) to improve the purity of Exos. After centrifugation, Exos are transferred to a layer of liquid with a suitable density gradient to achieve purification. Although this method improves the purity of Exos, the operation process is more complex.
With the rapid development of biotechnology and the deepening of the understanding of Exos, new methods and technologies for Exos separation have been emerging, such as microfluidic chip technology [30]. These techniques are optimized on the basis of traditional methods to more quickly obtain Exos that are stable and suitable for sensitive downstream applications. It is expected that more efficient Exos separation methods will be discovered in the future, which will greatly promote the in-depth study and clinical application of Exos.
The characterization of Exos is specific and similar, and the effective identification of Exos after isolation is the premise and basis for subsequent research or clinical application, and it is also the difficulty of the current research on Exos. The characteristics of Exos were mainly studied from the aspects of size, morphology, protein concentration and purity [31]. The main identification methods include flow cytometry, transmission electron microscopy (TEM) imaging, biological immunization such as SDS-PAGE/Western blot (WB)/antibody array techniques, and dynamic light scattering techniques. In 2014, the International Extracellular Vesicle Society (ISEV) proposed that the isolated Exos need to be identified from three levels: (1) WB identification of Exos surface markers; (2) Exos morphology was identified by TEM; and (3) nanoparticle tracer analysis (NTA) was used to identify Exos size. According to the requirements of the Vesicle Society, the characterization of single vesicles requires at least two different methods to complement each other for identification [32].
Exosome engineering aims to enhance drug loading capacity and targeting specificity through modifications of their source cells or direct manipulation of exosome properties [33, 34]. For example, Exos can be engineered by cell engineering to induce SCs to secrete Exos containing specific therapeutic molecules, or by chemically modifying the Exos surface to target ligands. These methods primarily consist of loading therapeutic medicines into Exos, modifying the surface of Exos to improve targeting, and preconditioning parental cells to increase the intrinsic therapeutic impact [35]. Furthermore, the duration of Exos action can be extended by employing methods that protect Exos with carriers. For instance, using chitosan hydrogel (CS) to encapsulate Exos can reduce the clearance of exosomes by the immune system [36].
Parental cell preconditioning techniques involve the manipulation of parental cells under specific culture conditions, such as hypoxia, three-dimensional culture systems, and serum starvation [37-39]. Exo-based drug delivery technology is a crucial component of drug delivery systems, wherein Exos can influence recipient cells by releasing their naturally formed contents as well as therapeutic agents they carry. Drug loading strategies are primarily categorized into endogenous and exogenous approaches [40]. Endogenous methods involve the collection of drug-carrying Exos following the preconditioning of parental cells, which can be further divided into two strategies: coculturing drugs with parental cells and using chemical techniques, like liposomal transfection, to transfer medications into parental cells [41, 42]. Exogenous methods, on the other hand, involve loading exogenous drugs into isolated Exos and primarily include three techniques: coculturing drugs with extracted Exos, using physical methods (a common physical method is electroporation) to transfer drugs onto Exos, and directly importing drugs into Exos through chemical transfection techniques [43-45]. Furthermore, surface modifications of Exos confer unique functionalities, such as the introduction of targeting peptides or sites through genetic manipulation and chemical modification. Surface functionalization can be categorized as either endogenous or exogenous, just as drug loading. Endogenous approaches involve the genetic modification of cells through the transfection of vectors encoding the desired molecules, thereby engineering the exosomes to carry the specified cargo. Additionally, bioorthogonal chemistry can be employed for endogenous modification, allowing for the selective labeling or functionalization of biomolecules within the exosomes without interfering with native cellular processes [46]. Exogenous methods, on the other hand, directly modify the exosome membrane through physical or chemical means. These include sonication to disrupt the membrane structure, facilitating the incorporation of new molecules, and covalent attachment of lipid or protein constructs to the exosome surface [47, 48]. These techniques enable the introduction of targeting ligands, fluorescent tags, or other functional groups to enhance the therapeutic potential and tracking capabilities of exosomes.
As an important intercellular communication medium, Exos play a critical function in regulating physiological processes and disease diagnosis and treatment [49, 50]. With the deepening of Exos research, researchers are committed to developing standardized methods for Exos acquisition, identification and engineering to be widely used in clinical practice. Optimizing and improving existing Exos technologies, especially increasing the yield and purity of Exos, as well as achieving efficient production of engineered Exos, are key to achieving their clinical applications.
4 SC-Exos Treatment for Oral Inflammations
Oral inflammatory diseases, such as periodontitis, peri-implantitis, and dental caries, are infectious diseases that impact the oral cavity's soft and hard tissues. These conditions not only diminish the quality of life for affected individuals but can also lead to complications in the maxillofacial region and systemic health, including cardiovascular, gastrointestinal, diabetes, pulmonary, and neurological disorders [51]. Therefore, it is crucial to diagnose and treat oral inflammatory illnesses as soon as possible. Presently, a growing amount of research indicates that SC-Exos might be essential in the management of inflammatory oral disorders. SC-Exos are capable of modulating the homeostasis of the inflammatory microenvironment by regulating immune cell activity and signaling pathways. Additionally, they facilitate the repair of damaged tissues through their regenerative properties.
4.1 SC-Exos in Periodontitis
Periodontitis is a widespread chronic inflammatory disease that progressively destroys periodontal tissues. These tissues include cementum, the periodontal ligament (PDL), and alveolar bone [52]. Globally, severe periodontitis has affected more than 11% of world's population, which can eventually lead to tooth loss and seriously endanger quality of life [53]. The major induction factor in the pathological events is Lipopolysaccharides (LPS) released by bacteria such as Porphyromonas gingivalis (P. gingivalis), leading to disorder of immune-inflammatory responses and overexpressing of inflammatory factors [54]. Hence, it is significant to timely alleviate the inflammation and control periodontal damage caused by the infection. SC-Exos derived from GMSCs (gingival mesenchymal SCs), PDLSCs (periodontal ligament SCs) and DPSCs (dental pulp SCs) can be a prospective and effective approach to periodontitis treatment.
Previous studies have demonstrated that GMSC-Exos enhance the healing of periodontitis by modulating the nuclear-factor kappaB (NF-κB) and Wnt/β-catenin signaling pathways and downregulating the expression of inflammatory markers [21, 55]. PDLSCs play an essential role in repairing periodontal tissue damage caused by periodontitis because of their excellent osteogenic differentiation potential [56, 57]. But that capacity was dramatically attenuated in the inflammatory environment. The pro-inflammatory factors TNF-α and IL-1β were overexpressed in P.g-LPS-induced inflammatory conditions, but the expression of the anti-inflammatory factor IL-10 was inhibited. Researchers discovered that the GMSC-Exos treatment has reversed the change that downregulated TNF-α and IL-1β and upregulated IL-10 expression in LPS-induced PDLSCs through NF-κB and Wnt/β-catenin pathways. Co-treatment with GMSC-Exos alleviated the inhibition on β-catenin and dramatically decreased the expression of both NF-κB/p65 and IκBα. Following P.g-LPS induction, a quantity of NF-κB/p65 complex in the cytoplasm of untreated PDLSCs was translocated to the nucleus, as demonstrated by the nuclear and cytosolic protein fractions. Treatment with GMSC-Exos partially halted this progression. Taken together, the results suggested that GMSC-Exos treatment promoted the recovery of periodontitis by mediating NF-κB and Wnt/β-catenin pathways in LPS-induced PDLSCs. Furthermore, another research revealed that after GMSC-Exos were administered to macrophages, the level of the pro-inflammatory marker CD86, which was high expressed on pro-inflammatory macrophages, was significantly reduced (Figure 4A) [58]. The result suggested that GMSC-Exos could regulate the transition of macrophage phenotype from pro-inflammatory to anti-inflammatory phenotype.

In the realm of periodontitis therapy, a microencapsulation technique involving SCs has been identified to exhibit promising therapeutic efficacy. Zhao et al. recently created a SCs microsphere with exceptional biocompatibility and universal adhesion that is encased in an metal-phenolic network (MPN) capsule and serves as a delivery vehicle for PDLSCs [59, 60]. A new approach called cell encapsulation involves encasing living cells in a semipermeable membrane. In this work, spheroid@[FeIII-TA (tannic acid)] microcapsules were created by coating the spheroid of PDLSCs with a FeIII/TA coordination network. A naturally occurring antioxidant polyphenol, TA was the primary component of the FeIII-TA shell and has the ability to reduce the oxidative damage brought on by ROS. Additionally, the FeIII-TA encapsulation controlled the immunomodulatory activity of PDLSC spheroids, which markedly reduced the LPS-induced mRNA and protein production of IL-6 and IL-8. These indicate that spheroid@[FeIII-TA] microcapsules can be an excellent alternative in periodontitis therapy. This methodology encapsulates SCs within microcapsules, leveraging the protective barrier to mitigate and delay the deleterious effects on the SCs. It is worth mentioning that, although this study did not directly use SC-Exos, it provided a feasible approach to modify and protect cells and their biologically derived functional components through the use of artificially synthesized materials, thereby enhancing their performance.
Lei et al. found that h-PDLSCs, or healthy PDLSCs, also released a lot of SC-Exos, which may help PDLSCs recover from periodontitis and continue to differentiate osteogenically [61]. As mentioned above, Wnt signaling pathway was indispensable in inflammatory response. Previous study has revealed that inflammatory factors could over activate the Wnt/β-Catenin pathway, worsening the inflammatory PDLSCs' (i-PDLSCs) osteogenesis defect [62]. Studies revealed that i-PDLSCs had an over-activated Wnt signaling pathway in comparison to h-PDLSCs, and that h-PDLSCs-Exo therapy reduced the mRNA levels of canonical Wnt signals, such as Wnt1, Wnt3a, Wnt10a, and the downstream signal β-Catenin (Figure 4B) [61]. Therefore, by blocking the over-activated Wnt signaling, h-PDLSCs Exos assisted in lowering the inflammatory stimulation to i-PDLSCs and promoting osteogenic differentiation of i-PDLSCs. In another vitro study, PDLSC-Exos showed their capacity to control miR-155-5p in chronic periodontitis, hence influencing Th17/Treg balance [63]. According to the study, patients with chronic periodontitis had increased Th17 and decreased Treg, indicating an imbalance in the Th17/Treg ratio in vivo. However, when CD4+ T cells treated with SC-Exos from normal PDLSCs (N-EXO) and LPS-stimulated PDLSCs-Exos (L-EXO) were compared, Th17 expression was significantly lower in the L-EXO group compared to the N-EXO group, while Treg expression was higher. The overexpression of miR-155-5p in PDLSC-Exos coincided with this occurrence. All things considered, PDLSC-Exos with miR-155-5p can affect the inflammatory milieu by controlling Th17/Treg differentiation and balance.
Shen et al. also have confirmed that DPSC-Exo-incorporated chitosan hydrogel (DPSC-Exo/CS) has demonstrated efficacy in the treatment of periodontitis (Figure 4C) [36]. Because it shields SC-Exos from immune system clearance, CS is a perfect carrier for loading SC-Exos and can extend their residence period in wounded locations [64]. The levels of inflammatory markers, such as IL-23, IL-1α, TNF-α, IL-12, IL-1β, IL-27, and IL-17, were significantly lower in the periodontal tissues of mice with experimental periodontitis treated with DPSC-Exo/CS than in the control groups. This could be because DPSC-Exo/CS downregulates the NF-κB p65 and p38 MAPK signaling pathways [64, 65]. Additionally, the research showed that the most prevalent miRNA in DPSC-Exo, miR-1246 could induce polarization of M2 macrophages and regulate the release of inflammatory and pro-inflammatory factors. Therefore, by reducing the periodontal inflammatory response and enabling macrophages to change from a pro-inflammatory to an anti-inflammatory phenotype in the periodontium both in vitro and in vivo, the study showed that DPSC-Exo/CS can speed up periodontitis repair [36].
4.2 SC-Exos in Other Oral Inflammatory Diseases
Not only periodontitis, SC-Exos have shown great ability to alleviate inflammation in other oral inflammations, including periodontitis, pulpitis, gingival wound, and radiation/chemical-induced oral mucositis.
Another prevalent inflammatory oral condition is pulpitis. According to histopathologic results, necrotic tissue, a disrupted odontoblast layer, and inflammatory cell infiltration were seen beneath the wounded area. Yu et al. discovered that by encouraging the conversion of CD4+CD25− T cells to Tregs, SC-Exos from the apical papilla (SCAP-Exos) may successfully reduce the local inflammation in rats with experimentally produced pulpitis [66]. This alteration was specifically caused by SCAP-Exos upregulating Foxp3 expression in vitro. Vasodilation appeared to be less severe in the pulp's center when SCAP-Exos were injected into the pulp chamber, and the degree of inflammatory cell infiltration started lower below the damaged area. As a result, SCAP-Exos offer a potentially innovative treatment for early dental pulp inflammation in the clinic.
What's more, GMSCs-Exos can be applied therapeutically to enhance the healing of gingival wounds [67]. In comparison to skin MSCs-Exos, GMSCs-Exos were found to produce noticeably more interleukin-1 receptor antagonist (IL-1RA), an inhibitor of the pro-inflammatory cytokine IL-1. Meanwhile, both in vitro research and in vivo research revealed that TNF-α was able to activate IL-1RA containing GMSCs-Exos release via upregulating Fas/Fap-1 expression, which was also regulated by the NF-κB pathway. These findings showed that GMSCs-Exos with elevated IL-1RA might encourage gingival wound healing.
The radiation/chemical-induced oral mucositis is also a major concern in oral diseases. Researchers have discovered that in mice models of radiation- or chemical-induced oral mucositis, CXCR2-overexpressing MSCs (MSCsCXCR2) shown the promise of therapeutic effects. MSCs that overexpressed the chemokine receptor CXCR2 enhanced cell survival and enhanced migration to the inflammatory mucosa [68]. Additionally, by blocking the synthesis of pro-inflammatory chemokines and radiogenic reactive oxygen species (ROS), MSCCXCR2 transplantation accelerated the healing of ulcers. This provides new insights into cell-based therapy for oral mucositis brought on by chemicals or radiation.
5 SC-Exos Treatment for Osteoarthritis
Osteoarthritis (OA) characterized by cartilage damage, synovial inflammation and subchondral bone sclerosis, is the most common joint disease worldwide with musculoskeletal degeneration [69, 70]. This disease exerts a significant detrimental effect on the patients' life quality by suffering approximately 250 million people around the world from severe pain, stiffness and reduced mobility [71]. And the incidence of OA increase with age, the number of patient may reach 400 million by 2030 in an aging population [71-73]. Clinically, pharmaceutical agents such as NSAIDs and painkillers have been widely used, but these treatments can only alleviate symptoms without preventing the prothistdisgress of the disease, plus they also have many side effects [69, 73]. Therefore, the application of stem-cell therapy has become a focus in recent years.
MSC-Exos derived from various sources, including adipose-derived MSCs (ADMSCs), bone marrow-derived MSCs (BMMSCs), synovial-derived MSCs (SMSCs), and umbilical cord-derived MSCs (UMSCs), have demonstrated significant potential in the treatment of OA [74]. The following are MSCs' and MSC-Exos' primary repair processes in the management of OA: (1) the chondrogenic characteristics of MSCs which promote chondrogensis and reduce apoptosis of chondrocytes; (2) the anti-inflammatory and immunomodulatory effects such as decreasing the releasing of pro-inflammatory cytokines and regulating macrophage polarization; and (3) the delivering capacities of SC-Exos or extracellular vesicles [75]. Moreover, researchers have explored the drug delivery ability of SC-Exos in OA therapy, and they can be loaded with a variety of cargos, including medications, proteins, and nucleic acids, which increases their versatility and intensifies their effects [76].
ADMSCs have been broadly investigated for their advantages of abundant availability and isolation simplicity, and ADMSC-Exos show their abilities in cartilage repairing and inflammation inhibition. Guillén et al. found that Exos and microvesicles from ADMSCs regulate the production of oxidative stress in OA chondrocytes during inflammation [77]. Chang et al. also showed that hypoxia-ADSC (adipose-derived SCs)-Exos with strong miRNA enhance the function of normal human articular chondrocytes (HACs), reduce HAC inflammation, and slow the progression of OA by suppressing normal cartilage markers and increasing fibrous or degenerative cartilage markers, matrix degradation enzymes, and inflammatory cytokines like TNF-α and IL-6 [78]. Upon sequencing, the authors discovered that hypoxia-ADSC-Exos harbor seven unique miRNAs, which confer upon these exosomes an enhanced therapeutic efficacy in the context of inflammation. It has been proposed that BMMSCs contribute to the therapy of OA mostly through their Exos [74]. Vonk et al. investigated how BMMSC-Exos suppressed TNF-α-induced collagenase activity and reversed the TNF-α-mediated elevation of COX2 and pro-inflammatory interleukins. Furthermore, BMMSC-Exos increased the synthesis of type II collagen and proteoglycans in OA chondrocyte cells [79]. Likewise, Jin et al. demonstrated that BMMSC-Exos preserve the chondrocyte phenotype by promoting type II collagen synthesis and preventing apoptosis and senescence brought on by IL-1β [80]. Likewise, SMSCs represent an attractive source for Exos-based therapies in OA recently, but they have a stronger chondroprotective function compared with the two above [75]. Research showed that SMSC-Exos altered by various mi-RNAs improved chondrocyte migration and proliferation without negatively affecting ECM secretion [81, 82]. Besides, Xu et al. utilized SMSC-Exos to transport kartogenin to improve cartilage regeneration [83]. In addition, there are plenty of other MSCs applied to OA treatment. A study was carried out by Cao et al. that examined the restorative effects of (umbilical cord mesenchymal stem cell-Exos (UCMSC-EXOs) on chondrocytes in OA. The research indicated that the p53 signaling pathway within UCMSC-EXOs is a key element in the rejuvenation of aged chondrocytes. By reversing the senescent phenotype of OA chondrocytes, this mechanism presents a possible therapeutic strategy for OA treatment [84]. Zhang and colleagues found that Exos from human embryonic stem cell-derived MSCs (hESC-MSCs) reduce inflammatory cytokines and M1 macrophages while increasing M2 macrophage infiltration in the treatment of OA [85]. Further, they also found the positive effects of these Exos in the use of Temporomandibular joint OA, which enhance sulfated glycosaminoglycan (s-GAG) synthesis impeded by IL-1β, and suppressed IL-1β-induced nitric oxide (NO) and matrix metalloproteinase (MMP) 13 production [86].
Although extensive research has been conducted on the therapeutic effects of MSC-Exos in OA, the majority of studies are limited to animal models, with limited clinical evidence to support specific therapeutic recommendations [75]. Therefore, additional verification research is required to determine the best course of treatment for OA with MSC-Exos in the future.
6 SC-Exos Treatment for Inflammatory Bowel Disease
Ulcerative colitis (UC) and Crohn's disease (CD) are the two most prevalent subtypes of inflammatory bowel disease (IBD), a chronic, nonspecific, recurrent illness marked by idiopathic inflammation of the gastrointestinal tract [87]. The primary clinical manifestations of IBD include gastrointestinal symptoms such as hematochezia, diarrhea, and abdominal pain, which significantly impact patients' quality of life [88]. Although the precise pathophysiology is yet unknown, genetically predisposed hosts' improper immune responses to infections aid in its progression and further cause problems with the integrity of the intestinal barrier [89]. IBD has already become a worldwide disease with increasing incidence, but the current therapeutic approaches are very limited [90]. Thus, new safe and effective treatments is very urgent, which brings out the use of MSCs and their Exos for therapy.
One of the primary mechanisms by which MSC-Exos alleviate inflammation is through the modulation of the Th17/Treg balance. By promoting the differentiation of regulatory T cells (Tregs) and suppressing the activity of pro-inflammatory Th17 cells, MSC-Exos effectively reduce inflammatory responses in IBD. By regulating the ratio of Treg cells to Th17 cells, where Treg cells decrease the inflammatory response, Yan et al. found that hUCMSC-Exos and fetal placenta MSC produced Exos (hFP-Exos) can reduce inflammation [91]. Both Heidari et al. and Yang et al. found MSC-Exos increase Treg population and down-regulate the level of pro-inflammatory cytokins [92, 93]. Further, Zhang et al. explain the relationship of periodontitis and IBD, and they use 3D-cultured MSC-Exo enriched with miR-1246, which suppressing the expression of Nfat5, to restore the Th17 cell/Treg balance [94].
Recent research has highlighted the critical roles of inflammasome activation and macrophage activities in the pathogenesis of IBD, emphasizing their potential as therapeutic targets [95]. Wang et al.'s experiment illustrated the therapeutic role of BMMSCs-Exos in IBD, in which miR-539-5p contained in the Exos inhibits pyroptosis by directly targeting NLRP3 [96]. Another example carried out by Cai et al. indicated that hucMSC-Exos carrying miR-378a-5p suppress NLRP3 inflammasome and regulate macrophage pyroptosis on colitis repair [97]. According to Xu et al., hucMSC-Exo with miR-203a-3p.2 most likely reduces colitis by preventing caspase 11/4-induced macrophage pyroptosis [98]. Except for ameliorate macrophage pyroptosis, MSC-Exos could also polarize M2 macrophages and induce production of IL-10 from macrophages to downregulate inflammatory responses in colitis, according to Liu's research [99].
Kou et al. discovered that miR-23a carried by hAESCs-Exos (human amniotic epithelial stem cell-Exos) can reduce the expression of tumor necrosis factor receptor 1 (TNFR1), thereby preventing the NF-κB signaling pathway in colon epithelial cells. This technique eventually led to a decrease in inflammatory responses and helped experimental colitis patients heal colonically [100]. Li et al. found that Hy-Exos (hypoxia-preconditioned Hair follicle mesenchymal stem cell-Exos) can suppress oxidative stress responses and alleviate inflammation-associated damage in UC through the inhibition of the PI3K/AKT/mTOR signaling pathway mediated by miR-214-3p, maintaining mitochondrial dynamic stability, alleviating mitochondrial dysfunction, and enhancing mitophagy [101, 102]. In addition, According to a recent study, hucMSC-Exo can alleviate IBD by using miR-129-5p to target acyl-CoA synthetase long-chain family member 4 (ACSL4) and prevent ferroptosis and lipid peroxidation (LPO) [103].
7 SC-Exos Treatment for Neurological Disorders
Numerous indications suggest that neuroinflammation is becoming a key pathogenic mechanism in neurological conditions [104-106]. A long-standing issue is that the majority of powerful medications that could enhance treatment for a number of central nervous system (CNS) disorders are not available in clinics due to their large size, which prevents them from passing through the BBB [107]. SC-Exos hold great promise for treating neurological conditions such as epilepsy, Alzheimer's disease, stroke, and Parkinson's disease (PD). Their small size and ability to cross biological barriers like the BBB make them particularly effective [108]. However, research focused solely on SC-Exos is relatively scarce compared to other conditions, with the majority of studies concentrating on both SCs and their derived Exos. SCs and their derived exosomes, which deliver mRNA and miRNA, exhibit significant anti-inflammatory effects, making them promising new approaches for treating neurological disorders.
As MSCs are confirmed to have immunoregulatory effects, amount of investigations have revealed the therapeutic effect of MSC-Exos on epilepsy and PD. BMMSCs and MSC derived from adipose tissue downregulated pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α in the brain and blood, while upregulating anti-inflammatory cytokines, such as transforming growth factor-beta1 (TGF-β1), prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), Indole amine 2,3 dioxygenase (IDO), NO, IL-4, and IL-10, in the animal models [106, 109-111]. These immunoregulatory effects may be mediated by the Nrf2–NF-κB signaling pathway, which plays a key role in modulating inflammation [106]. More importantly, Giunti et al. found that MSCs might switch microglia from a detrimental phenotype to a neuroprotective one via the release of fractalkine CX3CL1 [112]. The inhibition of microglial activation effectively protected dopaminergic neurons, which raised the possibility of the clinical application of MSCs as a method of treatment for PD. The study of the mechanisms of SCs in neuroinflammation has provided a foundation and thought process for subsequent research on the functional role of SCs-Exos in the treatment of CNS diseases.
Moreover, MSC-Exos also possesses the immunomodulatory properties. In the status epilepticus, the BMMSC-Exos also ease inflammation by reducing the activation of microglia in the hippocampus, consequently, aberrant neurogenesis and memory impairment during status epilepticus are prevented [113]. Furthermore, it was discovered that BMMSC-Exos can suppress T cell development into interleukin 17-producing effector T cells (Th17) and encourage the conversion of Th1 cells into Th2 cells in AD mice. Additionally, there was an increase in Treg and cytotoxic T lymphocyte-associated protein 4 [114]. Through the suppression of reactive astrocytes, activated microglia, and cytokine production, MSC-Exos also help to induce anti-inflammatory effects, which significantly improve learning and memory functions while lowering plaque formation and Aβ levels in AD. Through the regulation of enzyme activity, MSC-Exos can reduce the inflammatory response. Glial cells that have been stimulated by Aβ to produce NO synthase (NOS) release a lot of NO, which causes neurotoxicity. Neuronal cell death results from NO's inhibition of mitochondrial respiration [115]. In this regard, administration of MSC-derived EVs restored the impairment of CA1 synaptic transmission in APP/PS1 mice and reduced the production of Aβ-induced NOS mRNA and protein in vitro [116].
What's more, by significantly lowering the expression of glial fibrillary acidic protein (GFAP) and inhibiting activated microglia/macrophages, the mRNA and miRNA that the BMMSC-Exos supplied enhanced brain angiogenesis, neurogenesis, and neurite remodeling [117]. Furthermore, MSC-Exos expressing miR-21 have been shown to suppress STAT3 expression and NF-κB activation in APP/PS1 mice [118].
8 SC-Exos Treatment for Ischemia-Reperfusion Injury
Ischemia-reperfusion (I/R) injury is a biphasic phenomenon that leads to apparent functional and structural changes in multiple tissues and organs. During the ischemia phase, the limited blood flow supply results in hypoxia, leading to cellular damage and tissue dysfunction. The subsequent restoration of blood flow and reoxygenation exacerbates the injury by triggering oxidative stress and inflammatory responses [119]. Infarction, sepsis, and organ transplantation are the main causes of I/R-induced tissue damage [120]. which raises morbidity and mortality rates in a variety of conditions such myocardial infarction, ischemic stroke, acute kidney injury, liver injury, and trauma [121, 122]. The physiopathological mechanisms of I/R injury (IRI) are numerous and complex, among which inflammation plays a vital role. Inflammatory mechanisms involve the generation of ROS and its cascade response [123], the NLRP3 inflammasome pathway [124, 125], macrophage polarization [126] and different signaling that produce inflammatory cytokines [120]. To prevent ischemia-induced endothelial dysfunction and vascular damage, MSCs differentiate into functional endothelial cells (ECs) and release angiogenic factors, promoting tissue repair and vascular regeneration [127]. Through paracrine actions, MSCs have been shown to shield ECs from oxidative stress and apoptosis [128]. SC-Exos have been shown to play a significant role in the paracrine effects of MSCs [129]. and SC-Exos have been proposed as novel treatments for cardiovascular and cerebrovascular disorders due to their ease of passage through the tissue barrier, decreased risk of rejection response, and decreased oncogenesis [130, 131]. The therapeutic effects of SC-Exos in IRI are mainly exerted by inhibiting inflammatory responses at different targets.
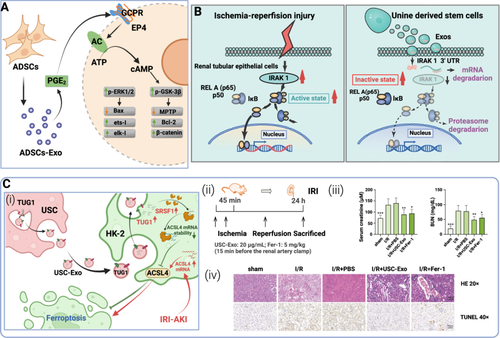
Several studies have demonstrated the significance of overexpression of ROS and induction of inflammation in IRI [121, 132, 133]. The generation of ROS is associated with the reperfusion of ischemia tissue, reoxygenation stimulates these toxic molecules, leading to cell dysfunction or death [121, 134]. MSC-Exos, a kind of MSC extracellular vesicles (MSC-EVs), were discovered to be enriched in a large number of antioxidant enzymes that controlled the redox status [135], suggesting that MSC-Exos may be employed to treat oxidant-related disorders. For example, both Zhang et al. and Piao et al. found in their therapeutic research on hepatic ischemia-reperfusion injury (IRI) animal models that ADSCs and ADSC-Exos can reduce inflammatory factors' expression and enhance the activity of antioxidant enzymes in tissues, thereby alleviating liver IRI (Figure 5A) [136, 138]. Additionally, MSC-Exos modified by microRNAs (miRs) are more successful in controlling the activities of target cells. Pan et al. discovered that miR-132-3p amplifies the beneficial effects of MSC-Exos on brain IRI by decreasing ROS production, apoptosis, and tight junction disruption in hypoxia/reoxygenation (H/R)-injured ECs [139]. When coculturing H/R murine renal tubular epithelial cells (RTECs) with MSCs, Yuan et al. also demonstrated that miR-223 expression in MSCs plays a favorable influence in RTEC viability and apoptosis rates [127]. Besides, ADSC-Exos could increase miR-221/222 expression to decrease I/R-induced cardiomyocyte apoptosis and hypertrophy by reducing H2O2 and its following products [140]. Moreover, hypoxic BMMSCs transfected with an adenovirus expressing miR-98-5p have been shown by Zhang et al. to protect against myocardial IRI by inhibiting myocardial enzyme levels, oxidative stress, inflammation response, and macrophage infiltration [141].
In addition to the overexpression of ROS, the reperfusion phase can also lead to inflammatory injury, in which NLRP3 inflammasome plays a vital role [142]. Several research show that the NLRP3 inflammasome is closely related with myocardial IRI [143-145]. Meng et al. [145] claim that NLRP3 KO decreased IRI-induced TNF-α, IL-1β, myocardial enzyme levels (LDH, AST, and CK), and apoptosis. After proving that the NLRP3 inflammasome was essential in triggering inflammation in the early stages of liver graft injury, Liu et al. hypothesized that the NLRP3 inflammasome is the cause of steatotic liver grafts' vulnerability to IRI [146]. By directly influencing the renal tubular epithelium, NLRP3 may possibly be a factor in renal IRI [147]. Thus, treatments against NLRP3 inflammasome could be efficient for IRI. Since miR-223 negatively regulates NLRP3, Yuan et al.'s study found that the miR-223/Notch signaling pathway was linked to MSCs’ therapeutic effect on I/R-induced kidney damage, as exosomal miR-223 produced by MSCs suppressed NLRP3 expression [127]. Another study by Liu et al. showed that BMMSC-Exos might reduce NLRP3 inflammasome-mediated inflammation and pyroptosis by altering microglial polarization, hence improving cerebral IRI [148]. There are also many other ways to treat I/R by reducing inflammation. As mentioned above, macrophage polarization plays a vital role in inflammation regulation. Following the reperfusion phage, there is a rise in M1 macrophage infiltration to eliminate cell debris and promote inflammation. By releasing a combination of growth factors and anti-inflammatory cytokines, M2 macrophages gradually proliferate to repair wounds and create scars [149]. Hence, a suitable balance between M1 and M2 macrophages that promotes earlier and greater M2 macrophage infiltration may be a treatment approach for IRI [150]. Zhao et al. found that MSC-Exos attenuate cerebral ischemia by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization [151, 152]. Several other studies have explored the positive role of different micro-RNAs in MSC-Exos in the treatment of I/R [149, 150, 153].
Inflammation is also triggered by certain signaling pathways by producing inflammatory cytokines. Therefore, SC-Exos therapeutic approaches targeting either signal pathway or inflammatory cytokines have been elaborated by many researchers. For example, Human urine-derived SCs (USCs) have been shown by Li et al. to provide protection against renal IRI through exosomal miR-146a-5p, which may target IRAK1 and hence prevent NF-κB signaling activation and inflammatory cell infiltration (Figure 5B) [120]. Additionally, it has been discovered that USC-Exos with lncRNA TUG1 (Taurine Upregulated Gene 1) protects IRI-induced AKI via regulating ASCL4-mediated ferroptosis through its interaction with SRSF1 (Figure 5C) [137]. Pu et al. explained the indirect role of IL-6 secreted by ADSC in protecting skin flaps during IRI [154]. Likewise, Zhang et al. ADSCs-Exos treatment in hepatic IRI significantly downregulated TNF-α, IL-1β, and IL-6 levels while enhancing IL-10 levels [155]. Furthermore, Lim et al. discovered that Exos derived from induced pluripotent SC (iExo) exert powerful therapeutic effects in renal IRI by attenuating tubular necrosis, apoptosis, oxidative stress and inflammatory cytokine production [156].
9 SC-Exos Treatment for Metabolic Disease
SC-Exos are proved to work well in metabolic diseases that associated with inflammation, including diabetes [157], atherosclerosis [158], fatty liver, and hypertension.
The autoimmune inflammatory destruction of pancreatic beta cells leads to type 1 diabetes, which accounts for 5%–10% of all diabetes cases. This condition results in complete insulin insufficiency [159]. Islet transplantation may be used to treat this metabolic disorder. Immune rejection and inadequate islet function are two obstacles to islet transplantation success that can be overcome by the hBMMSC and its Exos-based therapy. Wen et al. discovered that hBMMSC-Exos transfected with plasmids encoding shFas and anti-miR-375 overexpressed siFas and anti-miR-375. These molecules inhibited Fas and miR-375 in human islets, enhancing their survival and function against inflammatory cytokines [160]. After inhibiting Fas and miR-375 at the same time, inflammatory cytokine therapy dramatically reduced islet apoptosis and increased insulin release. However, intravenous injection of hBMMSC and peripheral blood mononuclear cell (PBMC) cocultured Exos could inhibit further immunological activation. Therefore, hBMMSCs and their Exos might be a potent treatment method for Type 1 diabetes by simultaneously silencing Fas and miR-375 under inflammatory challenging of autoimmune inflammation [160]. With their remarkable capacity for repair and regeneration, human endometrial stem cells (hEnSCs) may also be a novel and alluring option in the field of regenerative medicine [161]. In a different study, scientists discovered that injecting hEnSCs-Exo into a diabetic rat model could decrease the release of NF-κB and IL-1β, two crucial inflammatory mediators, and increase the anti-inflammatory cytokine IL-10' expression. This would resolve the negative effects of inflammation, restore beta cells' ability to produce and secrete insulin, and improve diabetes [162].
The prevalence of type 2 diabetes and its plenty of complications continue to increase annually, seriously endangering quality of life and health. Diabetic wounds are the most common complications of type 2 diabetes. Liu et al. revealed that melatonin-stimulated MSC-Exos can inhibit inflammation in diabetic wounds by increasing the ratio of M2 polarization to M1 polarization, which was achieved by targeting the PTEN/AKT pathway. Additionally, the study demonstrated that melatonin-stimulated MSC-Exos promote PTEN expression while blocking AKT phosphorylation, which further promotes angiogenesis and collagen formation in vivo [20]. One of the most dangerous side effects of diabetes is diabetic nephropathy (DN), for which there are presently no viable medications. Notable results of treatment with human UC-MSCs included decreases in renal interstitial fibrosis, inflammatory cell infiltration, and renal vacuole degeneration. The kidney and blood of DN rats seemed to have lower levels of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and pro-fibrotic factor (TGF-β), and the number of UC-MSCs recruited to the injured kidneys was greater than in the controls. Large volumes of Exos released by UC-MSC reduced the cytokine production in renal tubular epithelial cells and renal glomerular endothelial cells damaged by high glucose. Therefore, UC-MSCs and their derivative Exos may be a viable DN therapy option [163].
The greatest cause of death globally, atherosclerosis is a chronic inflammatory blood vessel disease that can result in peripheral vascular disease, cerebral infarction, and coronary heart disease. MSC-Exos promoted M2 macrophage polarization in atherosclerotic plaques and inhibited atherosclerosis in ApoE−/− mice by blocking macrophage invasion [164]. Additionally, by specifically targeting junction adhesion molecule A (JAM-A), exosomal miRNA-145 produced from MSCs can prevent atherosclerosis [165].
A potentially fatal fibrotic condition is nonalcoholic fatty liver disease (NAFLD). When free fatty acid supplementation is administered, it can lead to cirrhosis, fibrosis, steatohepatitis with pro-inflammatory cytokines released, and irritated tissue. Researchers showed that in all experimental groups, UC-MSC-Exo and anti-miR17-5p reduced TGF-β1, interleukin-1β, and interleukin-6. LX2 activation was blocked after the TGF-β1 pathway was suppressed, and extracellular matrix proteins, such as COL I and α-SMA, were decreased. Additionally, fibrosis conditions increased the expression of miR-17-5p. Anti-miR17-5p and MSC-Exos may be viable therapeutic approaches for treating liver fibrosis, since the study also demonstrated that both MSC-Exos and MSC-Exos + anti-miR17-5p therapies could lower ROS production [166].
Hypertensive chronic kidney disease is a common complication of hypertension, which eventually leads to end-stage renal failure. BMMSC-Exos can exert cytoprotective and immunomodulatory effects in the disease context, modulating selective cellular pathways in recipient renal tubular cells to achieve renoprotection. Li et al. discovered that the anti-fibrotic medication serelaxin (RLX) can enhance the viability and therapeutic efficacy of BMMSC-Exos. While RLX and BMMSC-Exos treatment did not reduce renal tubular epithelial injury and renal fibrosis, the study demonstrated that this treatment can mitigate renal inflammation to a certain extent, reduced macrophage infiltration, and ameliorate some measures of kidney NLRP3 inflammasome activation [167].
10 Summary and Prospect
SC-Exos have been the focus of biomedical research in recent years, and have great potential in improving human diseases, delaying aging, tissue regeneration, including their role in the treatment of inflammatory diseases. This review delineates the recent research advancements and clinical applications of SC-Exos in a spectrum of inflammatory diseases, including periodontitis, OA, inflammatory bowel disease, neurological disorders, and ischemia-reperfusion injury. The discussion encompasses the types, isolation methods, loading strategies, mechanisms of action, and therapeutic efficacies of SC-Exos, thereby highlighting their roles and functions in various inflammatory conditions within the human body. SC-Exos are a promising therapeutic tool in nanomedicine because of their biocompatibility, ability to target specific cells, and ability to encapsulate therapeutic molecules.
SC-Exos play a crucial role in regulating inflammatory reactions and promoting tissue healing. Their anti-inflammatory mechanisms primarily involve the regulation of immune responses, promotion of tissue regeneration, and modulation of key inflammatory pathways. They exert their anti-inflammatory effects through multiple mechanisms, including the modulation of immune cell activity and signaling pathways, as well as the direct carriage and release of anti-inflammatory molecules. Exosomes derived from diverse sources and engineered via distinct methodologies exert dissimilar impacts on inflammatory diseases. Table 1 systematically encapsulates the mechanisms of action and outcomes of various exosome types in response to a spectrum of inflammatory conditions.
| Disease | SC-Exos | SC type | Treatment | Function | Reference |
|---|---|---|---|---|---|
| Periodontitis | GMSC-Exos | GMSC | Coculture with PDLSC | Inhibiting the expression of inflammatory markers, thereby improving the healing of periodontitis; regulating the transition of macrophages from a pro-inflammatory to an anti-inflammatory phenotype | [55, 58] |
| h-PDLSC-Exos | PDLSC | Reduce the inflammatory stimulation to i-PDLSCs and promote osteogenic differentiation of i-PDLSCs by inhibiting the over-activated Wnt signaling | [61] | ||
| PDLSC-Exos | PDLSC | Loading miR-155-5p | Control the differentiation and balance of Th17/Treg to influence the inflammatory environment | [63] | |
| DPSC-Exo | DPSC | CS; loading miR-1246 | Decrease inflammatory markers; miR-1246 induces M2 macrophage polarization, regulates the release of inflammatory and pro-inflammatory factors, and accelerates the healing of periodontitis | [36, 64, 65] | |
| Pulpitis | SCAP-Exos | SCAP | Promote the conversion of CD4+CD25− T cells to Tregs, reducing local inflammation | [66] | |
| GMSCs-Exos | GMSC | Produce IL-1RA, inhibit the pro-inflammatory cytokine IL-1, and facilitate the healing of gingival wounds | [67] | ||
| OA | MSC-Exos | MSC | Promote cartilage formation and reduce chondrocyte apoptosis; decrease the release of pro-inflammatory cytokines and regulate macrophage polarization | [74] | |
| ADMSC-Exos | ADMSC | Transfected with human Prdx6 siRNA | Reduce HAC inflammation, inhibit normal cartilage markers, increase fibrotic or degenerative cartilage markers, matrix-degrading enzymes, and pro-inflammatory cytokines, slowing the progression of OA | [77, 78] | |
| BMMSC-Exos | BMMSC | Hypoxia | Promote the synthesis of type II collagen, prevent chondrocyte apoptosis and senescence, and maintain phenotype | [79, 80] | |
| SMSC-Exos | SMSC | Modified by miRNAs | Improve the migration and proliferation of chondrocytes | [81, 82] | |
| SMSC-Exos | SMSC | Loading kartogenin | Enhance cartilage regeneration | [83] | |
| UCMSC-EXOs | UCMSC | Surface-designed Targeting peptides, encapsulated in a two-stage release system | Reverse the senescent phenotype of OA chondrocytes for the treatment of OA | [84] | |
| IBD | MSC-Exos | MSC | Regulate the Th17/Treg balance to reduce inflammatory responses; polarize M2 macrophages to decrease inflammatory reactions in colitis | [92, 93, 99] | |
| hUCMSC-Exos | UCMSC | Reduce inflammatory responses | [91] | ||
| hUCMSC-Exos | UCMSC | Loading miR-378a-5p | Inhibit the NLRP3 inflammasome, regulate macrophage pyroptosis, and repair colitis | [97] | |
| hUCMSC-Exos | UCMSC | Loading miR-203a-3p.2 | Suppress macrophage pyroptosis to alleviate colitis | [98] | |
| hUCMSC-Exos | UCMSC | Loading miR-129-5p | Target ACSL4 to alleviate IBD | [103] | |
| hFPMSC-Exos | FPMSC | Reduce inflammatory responses | [91] | ||
| BMMSC-Exos | BMMSC | Loading miR-539-5p | Target NLRP3 to inhibit pyroptosis | [96] | |
| hAESCs-Exos | AESC | Loading miR-23a | Reduce the expression of tumor necrosis factor receptors, inhibit the NF-κB signaling pathway, decrease inflammatory responses, and aid in the healing of the colon in experimental colitis patients | [100] | |
| Hy-Exos | HFSC | Hypoxia | Alleviate oxidative stress responses and inflammation-related damage in UC | [101, 102] | |
| Neurological disorders | MSC-Exos | MSC | Downregulated pro-inflammatory cytokines and upregulated anti-inflammatory factors for the treatment of epilepsy and PD | [115] | |
| MSC-Exos | MSC | Loading miR-21 | Inhibited the expression of STAT3 and the activation of NF-κB | [118] | |
| BMMSC-Exos | BMMSC | Alleviated inflammation by reducing the activation of microglia in the hippocampus; regulated T cell development and transformation; enhanced cerebral angiogenesis, neurogenesis, and neurite remodeling | [113, 114, 117] | ||
| IRI | MSC-Exos | MSC | Protect ECs from oxidative stress and apoptosis; Reverse microglial M1 polarization to alleviate cerebral ischemia | [128, 129, 151, 152] | |
| MSC-exos | MSC | Loading miR-132-3p | Decrease EC damage caused by ROS | [139] | |
| MSC-exos | MSC | Loading miR-223 | Be beneficial to the survival and apoptosis of RTECs; inhibit the expression of NLRP3, which is beneficial for IRI treatment | [127] | |
| ADSC-Exos | ADSC | Reduce the expression of inflammatory factors and enhance the activity of antioxidant enzymes to alleviate liver IRI | [136] | ||
| ADSC-Exo | ADSC | Loading miR-221/222 | Reduce I/R-induced cardiomyocyte apoptosis and hypertrophy | [140] | |
| BMMSC-Exos | BMMSC | Transfected miR-98-5P | Change microglial polarization to reduce inflammation and pyroptosis, improving cerebral IRI | [141, 148] | |
| USCs-Exos | USC | Loading miR-146a-5p | Prevent NF-κB signaling activation and inflammatory cell infiltration, protecting against kidney IRI | [120] | |
| USCs-Exos | USC | lncRNA TUG1 | Regulate ASCL4-mediated ferroptosis to protect against IRI-induced AKI | [137] | |
| iExo-Exos | iPSC | Alleviate tubular necrosis, apoptosis, and oxidative stress, downregulate inflammatory cytokines, and treat kidney IRI | [156] | ||
| Type 1 diabetes | hBMMSC-Exos | BMMSC | Silencing Fas and miR-375; coculture with PBMC | Enhance the survival and function against inflammatory cytokines | [160] |
| hEnSCs-Exo | EnSC | Decrease the release of inflammatory mediators, increase the expression of anti-inflammatory cytokine IL-10, restore the ability of β-cells to produce and secrete insulin, and improve diabetes | [162] | ||
| Type 2 diabetes | MSC-Exos | MSC | Melatonin stimulation | Regulate macrophage polarization to suppress inflammation in diabetic wounds | [20] |
| DN | UCMSC-Exos | UCMSC | Reduce renal tubular degeneration, inflammatory cell infiltration, and renal interstitial fibrosis, treating DN | [163] | |
| Atherosclerosis | MSC-Exos | MSC | Promote M2 macrophage polarization, block macrophage invasion, and inhibit atherosclerosis | [164] | |
| MSC-Exos | MSC | Target JAM-A; loading miRNA-145 | Prevent atherosclerosis | [165] | |
| NAFLD | UCMSC-Exos | UCMSC | Silencing miR17-5p | Decrease the release of pro-inflammatory cytokines, treating liver fibrosis | [166] |
| Hypertensive chronic kidney disease | BMMSC-Exos | BMMSC | Coculture with RLX | Alleviate renal inflammation, reduce macrophage infiltration, and improve NLRP3 inflammasome activation | [167] |
To reduce inflammation, SC-Exos can first change the polarization of macrophages, causing them to change from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype. To reduce inflammation through the NF-κB and Wnt/β-catenin signaling pathways, Exos generated from MSCs can, for example, increase the expression of the anti-inflammatory cytokine IL-10 while suppressing the expression of inflammatory factors like TNF-α and IL-1β. Second, by transporting particular microRNAs (miRNAs), including miR-155-5p, miR-1246, and miR-375, SC-Exos directly contribute to the control of inflammation. These miRNAs can affect how immune cells operate, which includes promoting the release of anti-inflammatory cytokines and inhibiting the synthesis of pro-inflammatory cytokines. Furthermore, SC-Exos can also suppress inflammatory responses by modulating the NLRP3 inflammasome, a multi-protein complex within cells, whose activation is associated with a variety of inflammatory diseases. MiR-223 within SC-Exos can directly target NLRP3, inhibiting its expression and consequently reducing the activation and release of the inflammatory factor IL-1β. These anti-inflammatory properties of SC-Exos offer novel therapeutic strategies for inflammatory diseases. They are not only capable of acting as drug delivery systems, transporting therapeutic molecules directly to sites of inflammation, but also have the potential to modulate the inflammatory microenvironment through their own bioactive molecules, demonstrating significant potential in nanomedicine.
In addition, the characterization and bioengineering of SC-Exos have also made remarkable progress. A variety of SC-Exos separation methods developed at present have realized the efficient separation of SC-Exos from different sources and different types, and together with the characterization and identification requirements of SC-Exos, have built the cornerstone of SC-Exos research and clinical application. At present, the research of engineering SC-Exos methods also has good results, and a variety of engineering methods have been developed to modify SC-Exoss to improve their drug loading capacity and targeting. However, the road from research to clinical practice is fraught with challenges. Standardizing methods for SC-Exos acquisition, identification, and engineering, ensuring batch-to-batch consistency, and scaling up clinical use are key obstacles to be ad(dressed. Follow-up studies must rigorously evaluate the safety and efficacy of engineered SC-Exos in humans through comprehensive clinical trials to ensure safe and effective clinical use.
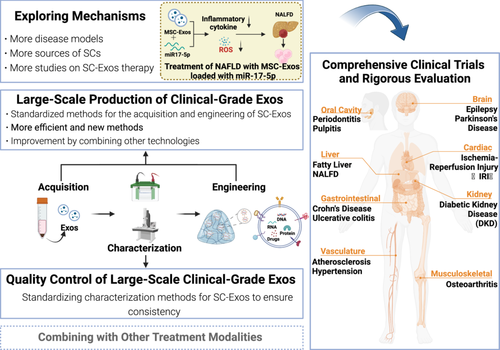
Figure 6 highlights the multiple aspects that future research on SC-Exos should focus on (Figure 6): First, explore the therapeutic mechanisms of Exos from various SC sources in different disease models, with the aim of better guiding clinical applications. Second, achieve large-scale production of clinical-grade Exos. There is an urgent need to standardize the acquisition and engineering methods of SC-Exos, and based on this, develop newer and more efficient methods or attempt to combine with other technologies to improve technical methods, enhancing and stabilizing their therapeutic effects. Third, standardize Exos characterization methods to achieve quality control of large-scale clinical-grade SC-Exos, ensuring batch-to-batch consistency. Only after achieving the above aspects can we more effectively conduct comprehensive clinical trials and rigorously evaluate the therapeutic effects and safety of SC-Exos, providing a scientific basis for clinical treatment. In addition, whether the combination of SC-Exos with other treatment modalities has a stronger therapeutic effect also needs to be studied. In the future, SC-Exos are expected to become a new strategy for the treatment of inflammatory diseases, bringing more effective and safer treatment options to patients.
Author Contributions
Xinyu Wei: conceptualization (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Qingyi Wang: writing – original draft (equal). Wen Wen: writing – original draft (equal). Lingxiao Yang: visualization (equal). Hao Chen: methodology (equal), writing – review and editing (equal). Gang Xu: conceptualization (equal), funding acquisition (equal), resources (equal), writing – review and editing (equal). Yongjie Zhou: conceptualization (equal), writing – review and editing (equal). Jiayin Yang: conceptualization (equal), funding acquisition (equal), resources (equal), supervision (equal), writing – review and editing (equal). Zhenyu Duan: conceptualization (equal), funding acquisition (equal), supervision (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). All authors read and approved the final version of the manuscript.
Acknowledgments
All original images in this article were created through www.biorender.com, for which we would like to express our gratitude. This work was supported by the National Natural Science Foundation of China (52203182, 82300663), 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (ZYGD24002), the Key R&D Projects of Sichuan Province (No. 23ZDYF2182), and Sichuan Science and Technology Program (2024NSFSC1022, 2023NSFSC1630).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




