Long COVID across SARS-CoV-2 variants: Clinical features, pathogenesis, and future directions
Laurence Si Chong Lok and Shuvam Sarkar contributed equally to this study.
Abstract
Long coronavirus disease (COVID) is characterized by persistent symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and has emerged as a significant health concern. As SARS-CoV-2 evolved from the wild-type strain to the Alpha, Beta, Delta, and Omicron variants, there may be a variant-specific influence on long COVID akin to the acute disease. This review aims to summarize our current knowledge of variant-specific influences in long COVID incidence, symptom profile as well as mechanisms of pathogenesis. We highlight that long COVID incidence may be lower with the Omicron variants. The symptom profile of long COVID may also show some dependence on the different variants, with a reduction in cardiopulmonary symptoms with more recent SARS-CoV-2 variants. This heterogeneity of long COVID may also be related to the variant-specific differences in affecting the immune system, viral persistence, and autoimmunity. However, emerging data also suggest that vaccinations may play a big role in shaping the presentation of long COVID. We also highlight ongoing work on long COVID incidence and symptom profiles in populations infected only by the Omicron variants. This will be beneficial toward more useful disease definitions and the development of effective diagnostic and therapeutic strategies.
1 INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1-3 In December 2019, the initial instances of this novel coronavirus were identified, and its transmission swiftly extended to various nations worldwide leading to the declaration of a Public Health Emergency of International Concern, and subsequently a pandemic. The COVID-19 pandemic has affected hundreds of millions of people worldwide since its emergence, with more than 775 million confirmed cases globally as of May 2024.4 Most acute COVID-19 infections cause a mild flu-like illness that subsides within 1–2 weeks. However, early in the pandemic, multiple reports emerged of COVID-19 patients who have recovered from the acute infection but continue to suffer from the consequences of COVID-19.5 These long-term effects of COVID-19 are heterogeneous and multisystemic. They are collectively referred to as postacute sequelae of COVID-19 (PASC) or “long COVID.”6
The wide-ranging set of symptoms associated with long COVID has resulted in a lack of unifying disease definition.7 The current clinical definitions of long COVID by the World Health Organization, the Center for Disease Control and prevention, the National Institute for Health and Care Excellence, and the National Academies of Sciences, Engineering, and Medicine8 are summarized in Table 1. Overall, the definition of long COVID is the development or persistence of symptoms following suspected (or confirmed) COVID-19 infection. The nature of such symptoms can be continuous or in a relapsing or remitting pattern. The symptoms span multiple organ systems. The difficulty in establishing a unified definition for long COVID is also extended to the diagnosis of long COVID, and it is very likely that the disease is underdiagnosed. Nevertheless, it was estimated that by the end of 2023, long COVID has a cumulative incidence of over 400 million.9 The time course with which long COVID develops means many risk factors can contribute to the disease course,10 such as age, gender, vaccination status, underlying disease processes, as well as multiple SARS-CoV-2 infections with different variants (Figure 1).
| CDC | WHO | NICE | NASEM | |
|---|---|---|---|---|
| Duration of symptoms | At least 3 months | At least 2 months | At least 3 months | At least 3 months |
| Nature of symptoms | Continuous, relapsing, and remitting or progressive disease state | Continuation or development of new symptoms | Signs and symptoms that develop during or after COVID-19 infection, not explained by an alternative diagnosis. Symptoms can fluctuate over time | Continuous, relapsing, and remitting or progressive disease state |
| Confirmation of COVID-19 infection | No | No | No | No |
| Stated symptoms | Bloating/constipation/diarrhea, difficulty concentrating, tachycardia, lightheadedness, memory change, persistent fatigue, postexertional malaise, smell/taste problems, headaches, shortness of breath, sleep disturbances | Fatigue, shortness of breath, cognitive dysfunction, and over 200 other symptoms | Shortness of breath, persistent cough, pain in breathing, palpitations, variations in heart rate, chest pain | Shortness of breath, cough, persistent fatigue, postexertional malaise, difficulty concentrating, memory changes, recurring headache, lightheadedness, fast heart rate, sleep disturbance, problems with taste or smell, bloating, constipation, and diarrhea |
| Interference with daily life | Yes | Yes | Yes | Yes |
- Abbreviations: CDC, Center for Disease Control and prevention; COVID-19, coronavirus disease 2019; NASEM, National Academies of Sciences, Engineering, and Medicine; NICE, National Institute for Health and Care Excellence; WHO, World Health Organization.
- Source: CDC, https://www.cdc.gov/covid/hcp/clinical-overview/index.html; WHO, https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-COVID-19-condition; NICE, https://www.nice.org.uk/guidance/ng188; NASEM, Reference.8
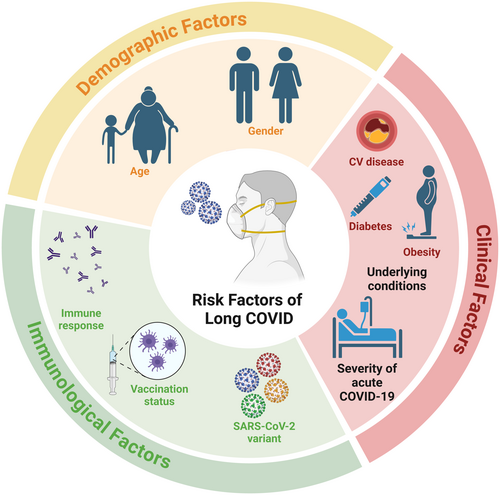
The COVID-19 pandemic was driven by the emergence of different SARS-CoV-2 variants of concern (VOCs), which allowed the virus to continually escape host- and vaccine-mediated immune responses (reviewed in Ao et al.11). At the time of writing this review, the persistence of COVID-19 in the general population is mainly driven by the Omicron JN.1 variant of interest and several JN.1 descendent variants under monitoring.12 While the influence of different SARS-CoV-2 variants on the disease process of acute COVID-19 is well documented, summaries of their potential influence on long COVID are lacking. In this review, we will summarize the current knowledge of the epidemiology, clinical features, risk factors, and mechanisms of long COVID in the context of different SARS-CoV-2 variants. We will also discuss the influence of re-infections and vaccinations on long COVID and how this creates difficulties in defining long COVID as well as the difficulties in conducting long COVID research.
2 COVID-19 AND SARS-COV-2 VARIANTS
SARS-CoV-2 is an enveloped single-stranded positive RNA virus.13 The genome is composed of multiple open reading frames (ORFs), encoding proteins involved in the disruption of host cell processes and viral replication.14 Some ORFs contain genes for structural proteins, such as the nucleocapsid, membrane, envelope, and spike glycoproteins.15 The spike protein mediates receptor binding and fusion of the viral and host membranes.16 It is composed of two subunits (S1 and S2), with the S1 subunit containing the receptor-binding domain (RBD) that binds directly to the human angiotensin-converting enzyme 2 (ACE2) receptor.17 The S2 subunit enables the fusion of the viral and host cell membranes, allowing the viral genome to enter the host cell.18
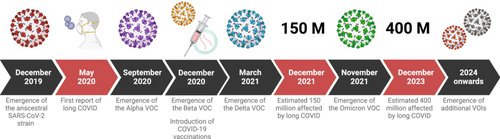
Mutations occur relatively frequently in RNA viruses, due to an error-prone RNA-dependent RNA polymerase. As a result, SARS-CoV-2 has undergone many mutations since its emergence, with one estimate suggesting that circulating lineages acquire up to two nucleotide mutations per month.19 Although most mutations have a minimal impact on viral fitness, some can provide a selective advantage enabling a strain to become more prevalent. This results in the emergence of VOCs. Most mutations found in SARS-CoV-2 VOCs are concentrated in the RBD of the spike protein, which is not surprising since this is the region responsible for the virus's ability to spread and evade neutralizing antibodies. These mutations therefore drove sequential waves of infections during the COVID-19 pandemic.20 In addition, the COVID-19 pandemic was also shaped by the introduction of multiple mass vaccination programs (Figure 2). Indeed, the real-world pathogenicity and transmissibility of each VOC were determined not only by the phenotype of the accumulated mutations but also by the population's past infection and vaccination history.20 This creates difficulties in evaluating the clinicopathological impact of each SARS-CoV-2 variant, which can deviate from data obtained in animal models and controlled laboratory experiments.21-23
The major SARS-CoV-2 VOCs can be categorized into Alpha, Beta, Delta, and Omicron.24 The Alpha variant was first detected in September 2020 and has been associated with significant increases in COVID-19 cases and hospitalizations, particularly among younger individuals.25, 26 The Alpha variant carries a higher risk of hospitalization, severe COVID-19, and death compared to the ancestral strain.27, 28 This variant contains N501Y mutation in the RBD which affects the pathogenicity.29 Two other mutations, D614G and P681H, are located near the cleavage site between the S1 and S2 domains, enhancing the cleavage efficiency and potentially increasing transmissibility.30, 31 Other changes in the Alpha variant include the deletion of amino acids 69–70 in the N-terminal domain of the spike protein which contributes to immune evasion.32
The Beta variant had a higher rate of transmission than the Alpha variant,33, 34 caused by a number of spike protein mutations (e.g., E484K, K417N, and A701V) which increase ACE2 binding affinity and immune evasion.35-37 In particular, the E484K/N501Y double mutation confers a selective advantage due to increased transmissibility and the ability to evade immune responses.38, 39
The Delta variant was characterized by a high viral load and a prolonged duration of infection, as a result of mutations that increase replication rates (e.g., P681R).40 Other mutations in the RBD (e.g., T19R, G142D, R158G, L452R, and T478K) contribute to higher ACE2 affinity and therefore transmissibility.41-44 The overall manifestation of these mutations was an increased risk of disease severity, hospitalization, ICU admission, and death.45 Importantly, the protection offered by a single dose of the mRNA vaccine was notably lower in people infected with the Delta variant.46 While the efficacy of two doses of vaccination conferred comparable protection when compared with the Alpha variant, this represented the beginning where vaccination efficacy was impaired.
The Omicron variant was unusual because of the substantial number of unique mutations.47 It demonstrates a higher rate of transmission and reinfections,48 though it was generally less severe in terms of hospitalization and mortality compared to the Delta variant.49 Shortly after Omicron was first detected, it evolved into three sublineages, BA.1, BA.2, and BA.3. BA.1 initially dominated but was soon overtaken by BA.2, while BA.3 remained rare.50 These three sublineages have 21 shared spike protein mutations, with some unique mutations present only in BA.1 and BA.2.51 Overall, these mutations enhance binding to ACE2, fitness, transmission, and immune evasion.52 Furthermore, serum from people infected with earlier variants or vaccinated with mRNA or protein-based vaccines targeting the wild-type strain showed reduced neutralization effectiveness against these new variants.53, 54
BA.2 further evolved into other sublineages (e.g., BA.2.12.1, BA.4, and BA.5), with BA.4 and BA.5 triggering another wave of global infections.55 They differ from BA.2 by a 69–70 deletion, two mutations (L452R and F486V), and an R493Q reversion. These mutations allowed these new sublineages to evade nearly all antibody classes targeting the RBD.56 The mutations also confer enhanced immune evasion, resulting in frequent breakthrough infections.57 As the BA.4 and BA.5 wave peaked, a new sublineage, BA.2.75, emerged in early June 2022,58 with additional mutations (e.g., G446N and N460K) which enhanced immune evasion.59 Other sublineages emerged from BA.5. BQ.1 and BQ1.1 had two additional spike mutations (K444T and N460K), while BQ.1.1 has three (K444T, N460K, and R346T).58 XBB and XBB.1, which were recombinants of BA.2.10.1 and BA.2.75 carried additional spike protein mutations beyond those seen in BA.2.60 These sublineages exhibited strong immune evasion, with sera from BA.5 bivalent vaccination showing limited neutralization against them.61 The later dominant strain (JN.1) was derived from the BA.2 (via an intermediate BA.2.86) and contains over 30 additional spike protein mutations compared to the parent strain,62 including the spike protein L455S substitution.63 This substitution alone conferred additional immune evasion capabilities.64 The current circulating variant (at the time of writing this review) is the KP.2 which descended from JN.1 with additional spike protein mutations (R346T and F456L), enhancing viral fitness.65
3 LONG COVID INCIDENCE ACROSS SARS-COV-2 VARIANTS
The acute COVID-19 disease shows marked differences between SARS-CoV-2 variants, with a tendency for enhanced transmission as the virus evolves, continued immune evasion, and an overall reduction in disease severity. The evolving acute disease may also impact the prevalence of long COVID. Indeed, two large systematic reviews found differences in the prevalence of long COVID in patients infected with the ancestral, Alpha, Delta, and Omicron variants.66, 67 Pooled calculations found that the prevalence of long COVID was highest following infection with the ancestral and Alpha variants, with a gradual reduction to the Delta and Omicron variants.67 However, there is substantial heterogeneity in how long COVID was reported, making it difficult to evaluate true prevalence.66 A number of studies have attempted to specifically compare the incidence of long COVID in patients infected with different SARS-CoV-2 variants. The aggregate findings from these studies also indicate a trend of decreasing severity and prevalence of long COVID, particularly with the Omicron variant.68-71 One large study found that the cumulative incidence (per 100 unvaccinated persons) of long COVID decreased from 10.4 in the pre-Delta period to 9.5 in the Delta period, to 7.8 in the Omicron period.72 Importantly, the study found that the cumulative incidence of long COVID substantially decreased with vaccinations across all the variant periods. Studies in the United Kingdom, Netherlands, Spain, and Poland have also shown reduced long COVID incidence following infection with Omicron when specifically compared to Delta 69, 73 or Alpha 68 variants. Similar results were obtained when comparing long COVID incidence between Omicron and “pre-Omicron” variants.74 One longitudinal study in Belgium showed that the odds of experiencing long COVID were significantly higher during the periods dominated by the Alpha and Delta variants compared to the Omicron period.75 Another study on hematological patients found a reduction in the prevalence of long COVID in successive waves of infection.76 Indeed, while the majority of ancestral or Alpha infections were associated with long COVID,70, 71 the data were much more variable for Omicron with estimates ranging from 4.5% 69 to 48%.74 Similar results were noted in pediatric populations, finding that children infected with the Omicron variant had a significantly lower risk of developing long COVID compared to those infected with earlier variants, suggesting a broader, age-spanning trend of long COVID risk.77, 78
There are a number of suggested reasons for the link between the SARS-CoV-2 variant and the incidence of long COVID. One study highlighted the effectiveness of vaccination across different variants in reducing the severity of long COVID symptoms, particularly noting a marked improvement with Omicron.79, 80 An enhanced immune response against SARS-CoV-2 through multiple past infections may also contribute to a reduction in prevalence.81 Other suggestions include a viral evolution toward less pathogenic forms 82 and the nature of the immune response, including antibody production following infection, which varies between variants and influences the prevalence and severity of long COVID symptoms.83 Nevertheless, there is a lot of difficulty in the evaluation of long COVID. As discussed above, there is no standardized definition of long COVID, and self-reported data contain inherent bias. Variation in the evaluation of long COVID caused by different variants may also arise from difficulties in the determination of the identity of the SARS-CoV-2 variant, with some studies distinguishing on the basis of sequencing,68, 70 while others were based on the most dominant circulating variant at the time of recruitment.69, 71, 73, 74 These methodological differences, together with the potential of multiple infections and vaccination, may account for some of the variation in the reported prevalence of long COVID and provide significant challenges to conducting systematic reviews and meta-analyses.
4 LONG COVID SYMPTOMS DIFFERENCES BY THE SARS-COV-2 VARIANT
In general, long COVID symptoms are vague and nonspecific, and multisystem involvement is seen (Figure 3A). In an early systematic review, constitutional symptoms such as fatigue, weakness, and general malaise were the most common,84 occurring in approximately 25%–40% of people with long COVID.85 This is likely to be even higher in people with existing comorbidities.86 Another systematic review also found fatigue was the most common symptom reported by long COVID patients.66 This is consistent with individual early case reports of long COVID highlighting fatigue as a predominant symptom.87 Cardiopulmonary symptoms such as breathlessness and exertional dyspnea were also seen. A more recent large systematic review and meta-analysis (conducted in 2023), which included 194 studies and over 700,000 participants, provided a more comprehensive evaluation of the prevalence of different symptoms of long COVID.88 Consistent with earlier systematic reviews, fatigue, and weakness were the most prevalent, occurring in 25% of long COVID cases. The same meta-analysis also revealed respiratory system dysfunction and dyspnea were also common in long COVID sufferers (18% of long COVID cases). Interestingly, it was reported in a study of patients with post-COVID dysfunctional breathing that their pulmonary function tests including gas transfer capacity were within normal limits and that their exercise capacity during cardiopulmonary exercise testing was normal.89 This raises the possibility that dyspnea in long COVID may result from dysfunctional breathing patterns, such as hyperventilation, rather than parenchymal lung damage.
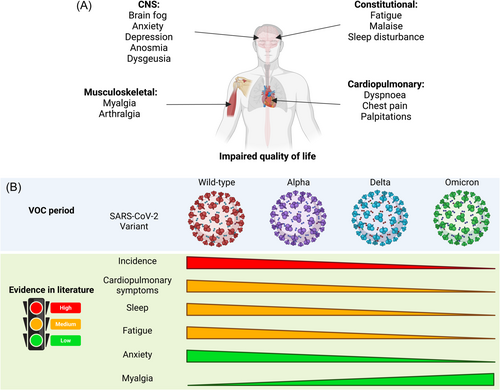
In many long COVID cases, impaired daily or usual activity was reported. This is usually characterized by impairments in movement (e.g., muscle or joint pain) and cognitive impairment. Indeed, “brain fog” is one of the most debilitating symptoms of long COVID, occurring in up to 20% of sufferers.88, 90 In addition, long COVID is also associated with other neurological and neuropsychiatric symptoms such as anosmia, ageusia, depression, and anxiety.91 The impact of long COVID on quality of life may be a marker of the symptomatic burden placed on patients' physical and mental health. It has been estimated that over 50,000 disability-adjusted life years (DALY) were lost over a 4-month period during the Omicron wave in Australia and up to 10% of this was attributed to long COVID.92 In a large cohort of COVID-19 survivors, there was a loss of 10 DALY per 1000 persons 3 years after COVID-19 infection in nonhospitalized survivors. This increased to 90 lost DALY for those who were hospitalized. Indeed, the risk of long COVID as well as death at 3 years persisted among those who were hospitalized.93
The long COVID symptoms and prevalence described above span multiple waves of COVID-19 infections, driven by different SARS-CoV-2 variants (Figure 3B). A number of studies have attempted to delineate the symptom profiles of infection waves attributed to the different dominant circulating strains of SARS-CoV-2. Comparing the symptomatology against the pre-Omicron period, there were large reductions in the incidences of cardiopulmonary, mental health, neurologic, and fatigue symptoms, but increases in gastrointestinal, metabolic, and musculoskeletal disorders in the Omicron-dominant period.72 Many variant-specific differences were negated when vaccination was taken into account, but the reductions in cardiopulmonary, mental health, and neurologic symptoms in the Omicron period were still seen when vaccinations were taken into account, providing crucial support that variant-specific effects do play a part in shaping long COVID symptomatology.
SARS-CoV-2-specific changes in long COVID symptomatology, particularly the reductions in cardiopulmonary symptoms, are supported by other studies. One prospective longitudinal study on UK adults evaluated self-reported symptoms of long COVID between March 2020 and December 2021, during which COVID-19 infections were driven sequentially by the ancestral, Alpha, and Delta variants.94 Long COVID during the ancestral strain period was characterized predominantly by cardiopulmonary symptoms. Another retrospective study also showed that cardiopulmonary symptoms characterized the long COVID profile caused by earlier variants.68 A number of other studies found variant-attributable changes in central nervous system symptoms in long COVID, but there was substantial heterogeneity in the findings. Central nervous system manifestations of long COVID were mainly caused by fatigue, sleep disturbances, pain, and cognitive impairment. In terms of fatigue, one study found the Alpha variant was also associated with a higher prevalence when compared with the Delta or Omicron variants.68 Similarly, in another prospective cohort study, fatigue, and anxiety were also more associated with earlier strains.79 A meta-analysis showed that the frequency of sleep difficulty was highest with the wild-type strain, fatigue was highest with the Alpha variant, and myalgia was highest with the Omicron variant.67 Another observational study in Italy found pain was more strongly associated with the ancestral variant.95 However, a number of studies also found central nervous system symptoms increased in the Alpha and Delta variant predominant periods.71, 75, 96, 97
A number of studies, however, found no association between long COVID symptomatology and the SARS-CoV-2 variant. One systematic review of fatigue in long COVID found that while poor sleep was noted in approximately 60% of patients, there were no variant-attributable differences.98 Another study in the United Kingdom found no differences in the long COVID symptom profile associated with the ancestral or Alpha variants.70 Another study found that the symptomatology profile was consistent across all variants.81 Some studies even suggested a tendency for later variants to cause more severe long COVID. One study found increased severity of long COVID in patients infected with Delta or Omicron variants, versus ancestral or Alpha variants.99 Another study reported quality of life and mental health measures were lower in patients with long COVID after Omicron infection.100 The reported symptomatology profile is similarly heterogeneous in the pediatric and adolescent population with long COVID. The most reported symptoms in children with long COVID were fatigue, difficulty concentrating, and dyspnea.83 Additionally, tiredness, low mood, poor sleep, dyspnea, and anxiety were also noted to be persistent sequelae of COVID-19 infection.101, 102 However, evidence on the prevalence of fatigue in children is conflicting. One meta-analysis suggests that the risk for developing long COVID was higher in children aged 10 and over.103 This could be attributed to variable clinical presentations across different age groups. Children under the age of 3 years presented with upper respiratory tract symptoms while adolescents were more likely to display systemic symptoms such as fatigue and exertional dyspnea.78 Symptoms may also differ between variants as evidence shows children affected by the Delta variant were more likely to develop fatigue, weakness, anxiety, and gastrointestinal upset compared to Omicron.104 One prospective cohort study conducted in under 18-year-olds in Russia found that the wild-type strain was associated with long COVID characterized by fatigue and dermatological, gastrointestinal, sensory, and sleep manifestations when compared with the Omicron variant.82, 104
5 MECHANISM OF LONG COVID
The wide-ranging symptomatology of long COVID points to a number of possible mechanisms of pathogenesis (Figure 4), some of which may be dependent on the SARS-CoV-2 variant. Detailed summaries of the pathogenesis of long COVID either generally,6, 105 or related to specific body systems such as the nervous system106, or the vasculature,107 are available. We aim to provide a more general overview of long COVID pathogenesis in the context of different variants in this section.
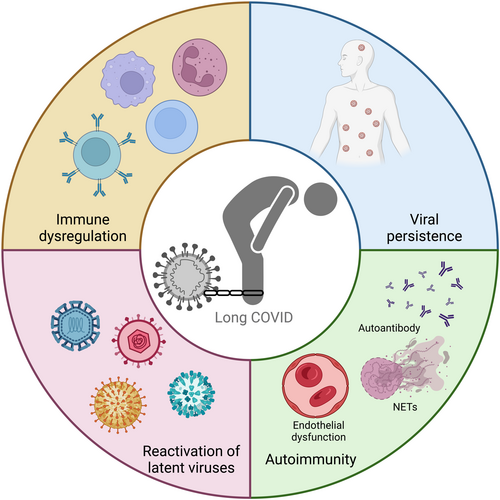
5.1 Immune dysregulation
Immune dysregulation is observed following other acute viral infections such as parvovirus B19 and influenza H7N9.108, 109 In people with long COVID, alterations in T cell function, such as exhausted T cells and reduced effector memory cell numbers, are seen110, 111 for as long as 1 year after infection.112 There is also a reduction in naïve B cell numbers. Alterations in the gut microbiota may be involved in some of these long-term changes in immune cell function.113, 114 In addition to the immunomodulatory effects, alterations in the gut microbiota may also impact the production of gut-derived neurotransmitters (e.g., serotonin), which is implicated in some of the neurological sequelae of long COVID.115, 116 In terms of cytokines, there is an increase in interferons from convalescent samples taken from individuals with long COVID-19 versus healthy controls.117 This may not be surprising, given dysfunctional interferon responses have been shown to characterize the development of severe COVID-19.118, 119 Other cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma-induced protein 10, may also be elevated in long COVID.120 The production of interferons appears to be SARS-CoV-2 variant-specific, with the Omicron variant stimulating a slower time course of interferon,121 which could contribute to a milder long COVID phenotype. Indeed, one study found that patients with earlier variants had elevated proinflammatory cytokines like IL-1β and TNF-α, which were less prominent in those infected with Omicron variants.122 The Omicron and indeed the Alpha variants have also evolved mechanisms to reduce their sensitivity to the antiviral effects of interferon.123, 124 Nevertheless, variant differences in modulating T cell function may be small and related to a wide-ranging number of epitopes targeted by the T cell response.125
Most individuals mount an antibody response following infection with SARS-CoV-2,126 which generally correlates with disease severity.127 However, protection against reinfection or severe disease from similar variants can occur in those with a mild initial illness.128 It is rather the extent and breadth of the quantitative neutralizing antibody response that correlates with protection.129, 130 Interestingly, an insufficient immune response during the acute COVID-19 infection is associated with the development of long COVID 131, 132 Indeed, it was observed that individuals with long COVID generally exhibit lower neutralizing antibody titers compared to recovered individuals.133 Such observations are interesting because those with breakthrough infections are also at a higher risk of long COVID.134
5.2 Viral persistence
Viral persistence in tissues is present following infection with several RNA viruses, including Polio, Chikungunya, Ross River, and Measles viruses.135 The relapsing and remitting symptom pattern seen in long COVID may also be due to viral persistence.136 One potential source of viral persistence is the gut, where SARS-CoV-2 nucleocapsid protein was detected both in intestinal biopsies,137 and in the feces of patients post-SARS-CoV-2 infection, even with negative nasopharyngeal PCR.138 However, SARS-CoV-2 spike protein has also been detected in blood,139 brain,140 circulating monocytes,141 heart tissue,142 lung tissue,143 gut and liver tissue,144 eye145, and urine146 weeks to months after initial SARS-CoV-2 infection. One systematic review found evidence for viral shedding in bodily fluids associated with sexual activity (saliva, semen, vaginal secretions, and feces) for up to 210 days.147 In a population following the Omicron wave of infections, viral RNA was detected in a wide range of tissues up to 4 months after the initial infection.148 The presence and the copy number of viral RNA found in recovered patients were also significantly correlated with the development of long COVID symptoms. Viral persistence may provide a reservoir of SARS-CoV-2 to drive long-term sequelae, causing sustained innate immune activation and/or leading to the development of autoimmunity.149 Indeed, one study on breakthrough Omicron BA.2 infections found evidence of ongoing immune activation 6 months postacute infection.150
A number of factors may influence the persistence of SARS-CoV-2 following recovery. The severity of the acute disease as well as the presence of co-morbidities may prolong the duration of viral persistence.151, 152 The duration of viral shedding also differs depending on the SARS-CoV-2 variant. Analysis of Ct values from respiratory specimens suggests that the Delta variant showed longer persistence of viral RNA than the ancestral strain,153 while there was no difference in the presence or absence of SARS-CoV-2 RNA between Delta and Omicron BA.1 infections.154 A change in viral tropism may also influence persistence. There is a shift in SARS-CoV-2 tropism from the ancestral strain through to Omicron.155 The ancestral strain and Delta variants target the olfactory epithelium, while the Omicron variant shows a reduced infection rate in the olfactory epithelium and increased infection in the respiratory epithelium. Consistent with this, it was observed in mouse models that Omicron variants show less spread to CNS tissues.156 It is possible that such differences may account for some of the differences in symptom profile, as well as the effectiveness of viral clearance. However, the true influence of viral persistence caused by different variants may be complicated by the prolonged pandemic and the associated factors, such as re-infections, pre-existing immunity, and vaccinations.157
5.3 Reactivation of latent viruses
Reactivation of latent viruses, such as Epstein–Barr virus (EBV), cytomegalovirus, herpes simplex virus, varicella zoster virus, and human herpes virus 6, have been implicated in long COVID.110, 158-162 It is possible that the immune response against these reactivated viruses plays a role in long COVID. Indeed, higher levels of anti-EBV antibodies have been found in those with long COVID compared to those without.110, 163 This chronic dysregulation of the immune system due to viral persistence and reactivation may result in low-grade inflammation and multiple organ dysfunction.164 Other mechanisms proposed for latent virus reactivation and long COVID include host/viral miRNAs interactions, histone modifications, oxidative and cellular stress responses, and cytokine-mediated reactivation.165-167
Reactivated viruses have also been observed in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).168 There is a remarkable similarity between ME/CFS and long COVID. ME/CFS also tends to follow an infectious illness. Many of the immune markers found to characterize long COVID patients are also found in people with ME/CFS. Indeed, one meta-analysis of 21 studies integrated symptoms reported by individuals with both ME/CFS and long COVID.169 Fatigue characterizes both syndromes, in addition to sleep disturbances, brain fog, and respiratory problems. Decreased taste and smell are found more often in long COVID patients, while pain is often a feature of ME/CFS. Given the similarities, it is possible that the two also share similar mechanisms and pathogenesis, such as mitochondrial fragmentation and energy metabolism disturbances.170 Elucidation of the mechanisms in either of these syndromes will undoubtedly shed light on the other.
5.4 Autoimmunity
During the acute phase of SARS-CoV-2 infection, autoantibodies against a range of targets have been described, including antinuclear antibodies, antibodies associated with myositis, systemic sclerosis, connective tissue diseases, and other immunomodulatory proteins.171, 172 Multiple studies have identified elevated levels of autoantibodies in long COVID patients,159 including autoantibodies to ACE2, β2-adrenoceptor, muscarinic M2 receptor, angiotensin II AT1 receptor, and the angiotensin 1–7 MAS receptor.173-175 However, associations between specific autoantibodies and symptoms in long COVID have not been clearly demonstrated.110, 156, 169, 174, 175
Innate immune cells have potential pathogenic roles in long COVID. A small study of primary care COVID patients showed blood neutrophil and eosinophil activation 3–6 months following infection.176 A study in Italy, in a period covering wild-type, Alpha, Delta, and Omicron waves, of unvaccinated patients showed symptoms such as fatigue, insomnia, memory impairment, concentration impairment, and anxiety/depression present in 30%–40% of patients 6 months postinfection, and long COVID was associated with increased serum markers of neutrophil degranulation (matrix metalloproteinase-8 and myeloperoxidase) and endothelial dysfunction (P-selectin and l-selectin).177 Another study with similar prevalence of long COVID symptoms also demonstrated upregulation of multiple genes, including those involved in neutrophil activation, platelet activation, and thrombosis.178
A study of COVID patients with persistent interstitial lung changes following severe infection (with a study period spanning wild-type, Alpha, and Delta waves) showed increased blood neutrophils, myeloperoxidase, and neutrophil extracellular traps (NETs).179 NETs are potentially antimicrobial structures released by activated neutrophils, consisting of DNA, antimicrobial proteins such as myeloperoxidase, and structural proteins such as histones. NETs may be involved in the pathogenesis of autoimmune diseases such as vasculitis, for example, via transfer of pathogenic myeloperoxidase.180 Similarly, it has been suggested that components of NETs could act as neoantigens and contribute to the generation of autoantibodies, driving chronic inflammation, thrombosis, endothelial dysfunction, and fibrosis, leading to cardiovascular, pulmonary, and neurological complications seen in long COVID.181
6 DIAGNOSTIC TOOLS, TREATMENT, AND PREVENTION OF LONG COVID
6.1 Diagnostic tools
There is a lack of objective diagnostic criteria for long COVID. Therefore, current diagnostic approaches are vague and based on the exclusion of other underlying pathologies that could explain the symptoms. The lack of routine biomarkers for long COVID adds an additional level of difficulty.182 Serum levels of cortisol seem to be predictive of long COVID,110 but the circadian fluctuations in endogenous cortisol level complicates interpretation. Lymphocyte markers may have some utility but the changes in long COVID are often subtle.183 Imaging techniques to detect coagulopathy,184 and magnetic resonance imaging to detect pulmonary gas exchange abnormalities may show some promise.185 Biomarkers for ME/CFS may hold some promise, given the similarity in the two syndromes. Developing and validating biomarkers for long COVID is crucial for diagnosis, but those which require specialist instruments may not be widely adopted. The heterogeneity in presentation and diverse changes in physiology seen in long COVID patients may implicate artificial intelligence (AI) and machine learning approaches in its diagnosis. Noninvasive approaches, such as using fundus, tongue, and facial imaging, to predict type 2 diabetes and chronic kidney diseases have shown promise.186 Differences in chronological age and AI-predicted “bioage” may also be useful as markers of long COVID,187 alongside other diagnostic criteria. Similar approaches can be conceivably used to develop machine learning algorithms to detect patients with long COVID. The challenge with the development of AI long COVID diagnostics is to find suitable populations as training cohorts. Populations that have been largely shielded from infections with early variants may be useful for training AI to detect long COVID caused by Omicron variants only.
6.2 Current and potential treatments
Currently, there are no broadly effective treatments for long COVID. Antivirals against SARS-CoV-2, such as nirmatrelvir-ritonavir have shown no effect in preventing long COVID in a randomized controlled trial,188 or in a retrospective cohort study.189 However, in another retrospective cohort study conducted in Hong Kong, patients who were prescribed nirmatrelvir-ritonavir during the acute phase of COVID-19 had a significantly lower hazard of developing cardiovascular problems, acute respiratory distress, end-stage renal disease, or death in long-term follow-up.190
Nevertheless, a number of clinical trials found in ClinicalTrials.gov are currently exploring potential treatments for long COVID. We categorized these trials and summarized them in Table 2. These trials encompass a wide range of treatments from pharmacological, herbal, nutritional, and behavioral approaches. In addition, symptom-specific or ME/CFS-specific treatments may have efficacy in subsets of patients. These include pacing,191 β-blockers for postural-orthostatic hypotension,192 antihistamines,193 low-dose naltrexone for neuroinflammation, metformin,194 and intravenous immunoglobulin for immune dysfunction.195 Treatments that target the autoimmune and coagulopathy aspects of long COVID have also shown some promise.196 Convalescent plasma and early antibody treatment may also be helpful for patients with long COVID.197 There are also nonpharmacological options, such as exercise, which have benefited some patients.192 An observational study of pulmonary rehabilitation for long COVID in Germany showed that the majority of patients, whether having had mild or severe COVID, achieved benefits such as reducing breathlessness and improving performance and health status following an inpatient pulmonary rehabilitation program.198 Nevertheless, given the similarity between ME/CFS and long COVID, it is important to prioritize research into ME/CFS treatment since it may also benefit long COVID patients.
| Treatment | Ongoing clinical trials | Summary of treatment |
|---|---|---|
| CBD treatment | NCT04997395 | Cannabidiol for managing long COVID symptoms |
| Clinical protocols/medication | NCT05595369 | Application of standardized protocols for long COVID symptoms |
| Herbal/traditional medicine | NCT05668104 NCT05684952 |
Herbal and/or traditional Chinese medicines for long COVID symptoms |
| Psychological/behavioral therapy | NCT05705154 NCT05815693 |
Psychological and behavioral interventions for fatigue and smell loss |
| Nutrition and diet | NCT05630339 | Nutritional supplementation (e.g., vitamin D, Zinc) and dietary interventions for long COVID symptoms |
| Drug repurposing | NCT05874037 NCT05764538 NCT06316843 NCT05576662 NCT04904536 |
Fluvoxamine, ivermectin, and other drugs for long COVID symptoms |
| Physical therapy | NCT05012826 NCT06142240 |
Physical therapy such as exercises and rehabilitation programs for fatigue, muscle pain, and mobility issues |
| Alternative therapies | NCT05848401 NCT05926505 NCT05677932 |
Acupuncture and biosound therapy for pain, fatigue, and psychological distress |
- Abbreviations: CBD, cannabidiol; COVID, coronavirus disease.
6.3 Vaccination
Antibody responses to coronaviruses (SARS-CoV and middle eastern respiratory syndrome [MERS]-CoV) have been studied in detail. Protection against reinfection and/or severe disease correlates with the neutralizing antibody response.128 However, the waning of the antibody responses over time, as well as the emergence of new variants, leads to increased reinfections, but the development or recurrence of severe disease remains rare. In the context of long COVID symptomatology and severity, our review has highlighted the immense heterogeneity within the literature. We argue that the reason for such heterogeneity lies in the infection history of the population, as well as vaccination.79 For example, a prospective cohort study comparing symptoms 3 months following acute COVID-19 infections found differences in the proportion of people with myalgia, brain fog, and fatigue during pre-Delta, Delta, and Omicron periods of the pandemic.199 Importantly, these differences were no longer statistically significant when the data were adjusted for vaccinations. In another large study of over 400,000 health records, vaccinations accounted for a large proportion of the reduction in long COVID incidence and risk from the pre-Delta to Omicron predominant periods of the pandemic.72 The same study also identified that vaccination was responsible for a reduction in disease severity across multiple categories. The effects of vaccinations on long COVID are also supported by other large meta-analyses that show vaccinations against COVID-19 consistently lower the risk of developing long COVID.200, 201 The findings suggest that vaccination contributes more significantly to the observed reduction in long COVID risk compared to other era-related changes, such as changes in the characteristics of the virus or improved medical care over time.
Interestingly, there is also some evidence that vaccination in the convalescent setting may ameliorate symptoms, although reports are conflicting.202 It is unknown why vaccination may prevent or improve long COVID symptoms. One hypothesis is that vaccination attenuates acute disease severity, and thus subsequent immune derangements and organ damage. A limitation to this hypothesis is that long COVID occurs frequently even in those with a mild initial illness.203 A second theory is that long COVID is due to a dysfunctional antibody response, which either fails to clear viral remnants or leads to the induction of self-reactive antibodies or proinflammatory antibodies. Vaccination before natural infection may prime the immune system and avoid this dysfunctional response. The presence of distinct immunoglobulin signatures during the acute infection which predicts long COVID suggests that approaches that modify the immune system, such as vaccination, can affect long COVID.204
7 CONCLUSIONS AND PERSPECTIVES
The emergence of long COVID as a significant public health concern highlights the complexities and long-term implications of SARS-CoV-2 infections. This condition, characterized by a wide range of symptoms affecting multiple organ systems, underscores the need for continued research and targeted healthcare strategies. Long COVID symptoms can persist for months, significantly impacting the quality of life and daily functioning of affected individuals. Variability in symptom prevalence across different SARS-CoV-2 variants, such as Alpha, Delta, and Omicron, suggests that viral evolution plays a crucial role in the manifestation and severity of long-term sequelae. Vaccination remains a critical tool in reducing the incidence and severity of COVID-19 and its long-term effects. Continued efforts to promote vaccination and booster campaigns are vital, particularly in light of emerging variants.
Moving forward, it is imperative to focus on several key areas to mitigate the impact of long COVID. Establishing clear, standardized diagnostic criteria is essential for accurately identifying and categorizing the condition across diverse populations and healthcare settings. This includes the development of reliable biomarkers and diagnostic tools that can provide a consistent and objective basis for diagnosis. Furthermore, further research into the variant-specific characteristics of long COVID is needed to understand how different variants influence the severity and type of long-term symptoms. One of the primary difficulties lies in the fact that many populations have experienced repeated infections with different SARS-CoV-2 variants over the course of the pandemic. These repeated exposures complicate the ability to pinpoint the specific impacts of any single variant on the development of long COVID. As the virus has evolved from its original strain through Alpha, Delta, and various Omicron subvariants, the symptoms and severity of long COVID have also appeared to shift, making it difficult to establish clear, consistent patterns in the condition's manifestation. This variability poses a major obstacle in defining long COVID, identifying the precise mechanisms, and developing targeted treatment strategies.
In contrast to populations with mixed-variant exposure, the Macau Special Administrative Region (SAR) population offers a unique opportunity to study long COVID in a more controlled context. Due to strict lockdown restrictions and a stringent zero-COVID policy, Macau SAR remained largely naive to COVID-19 until December 2022. During this time, the population had minimal exposure to earlier variants of the virus, such as Alpha and Delta, which heavily impacted other regions. It was only after these restrictions were lifted that the Omicron BA.4 and BA.5 variants began to circulate widely in Macau. The LOng COvid in MACau (LOCOMAC) project leverages this unique epidemiological situation to focus exclusively on individuals who have been infected with the Omicron BA.4 and BA.5 variants, without prior infections from earlier variants. This isolated exposure scenario provides a rare and valuable opportunity to examine the symptom profile and biochemical characteristics of long COVID associated specifically with these Omicron subvariants. The LOCOMAC project is poised to offer critical insights into the evolutionary clinical picture of long COVID, free from the confounding effects of repeated infections with different variants. In this context, the LOCOMAC project provides a unique and invaluable opportunity to deepen our understanding of long COVID.
AUTHOR CONTRIBUTIONS
Daniel Tomas Baptista-Hon and Olivia Monteiro conceived the study. Laurence Si Chong Lok, Shuvam Sarkar, Calista Chi In Lam, Chak Fun Law, Sin Teng Chau, Chun Yip Thomas Leung, Wai Hin Cheang, and Ting Li drafted the manuscript. Laurence Si Chong Lok, Shuvam Sarkar, Daniel Tomas Baptista-Hon, and Olivia Monteiro produced the final version of the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank the Fundo para o Desenvolvimento das Ciências e da Tecnologia (FDCT 0109/2020/A3, FDCT 0106/2021/A, and FDCT 0055/2022/A1) and the Macau University of Science and Technology Faculty Research Grants (FRG-22-023-FMD and FRG-21-037-FMD) for funding.
CONFLICT OF INTEREST STATEMENT
Daniel T. Baptista-Hon is an editorial staff of MedComm-Future Medicine. He was not involved in the journal's review of, or decisions related to, this manuscript. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




