Clinical decision making: Evolving from the hypothetico-deductive model to knowledge-enhanced machine learning
Graphical Abstract
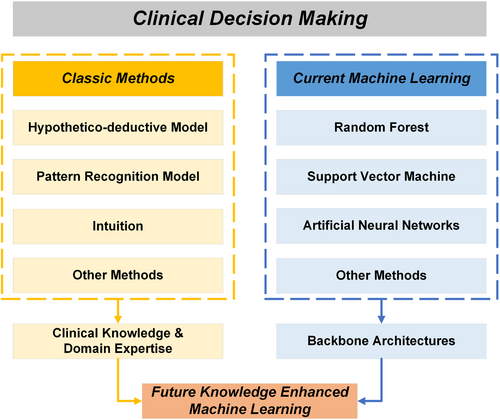
Knowledge-enhanced machine learning can be conceptualized as a fusion of clinical knowledge and domain expertise extracted from traditional clinical decision making methods alongside powerful machine learning architectures. Knowledge-enhanced machine learning significantly improves current machine learning methods in terms of interpretability, generalizability, accuracy, and equity.
Abbreviations
-
- CDM
-
- clinical decision making
-
- HDM
-
- hypothetico-deductive model
-
- KEML
-
- knowledge-enhanced machine learning
-
- ML
-
- machine learning
-
- PRM
-
- pattern recognition model
Clinical decision making (CDM) is a process that healthcare professionals undertake when making assessments about patients' conditions and decisions about the care to provide [1, 2]. Traditional CDM is founded on either unconscious intuition or conscious inference frameworks with well-defined logic [3]. Intuition, defined as understanding without a rationale, integrates tacit knowledge and pertinent experience developed over years of practice to automate cognitive processing devoid of formalized rules [4, 5]. However, its unconscious nature obscures the precise identification of initiating cues and inference logic, limiting its application in CDM [6]. Distinct from intuition, conscious inference frameworks possess well-defined logic and execution steps, predominantly encompassing the hypothetico-deductive model (HDM) [7] and pattern recognition model (PRM) [8].
1 CLASSIC CDM METHODS
The HDM involves four indispensable steps: cue acquisition, hypothesis generation, cue interpretation, and hypothesis evaluation [7]. Initially, cue acquisition systematically collects patient medical information per requirement by clinicians. Subsequently, multiple preliminary hypotheses are derived from the retrieved information, enabling clinicians to either use an established theory based on gathered information or propose hypotheses according to their clinical knowledge and domain expertise [9]. This is followed by cue interpretation, in which clinicians discern cues pertinent to their initial hypotheses and accordingly refine these hypotheses [10]. The HDM culminates in hypothesis evaluation, whereby the hypotheses are either corroborated or refuted based on the amassed evidence. Should all hypotheses be rejected, another round of the HDM will commence. Ng et al. present an application of the HDM in a real-world CDM task [11]. When diagnosing patients with acute chest pain, clinicians gather cues related to cardiovascular risk factors, smoking history, recent viral infections, and other relevant information. Based on this information, they generate diagnostic hypotheses, such as acute coronary syndrome, myocarditis, pericarditis, or pneumonia. They interpret these cues and evaluate the hypotheses, refining and ruling in or out possibilities through further investigations, such as electrocardiograms, complete blood counts, and chest radiographs. In contrast to the analytical HDM, PRM employs nonanalytical matching of new clinical cases with similar patterns stored in memory, specifically for patients encountered previously or documented within established guidelines [8, 10]. In routine clinical encounters, PRM outpaces CDM at an exceptional rate. Notably, in instances of ambiguity, the analytical HDM approach retains its superiority as a more effective solution [8, 10].
2 CONTEMPORARY CDM METHODS
Although these traditional methods are widely implemented in real-world practice and some of them, including the HDM, have been recognized as gold standards [12], machine learning (ML), as demonstrated in Figure 1, is increasingly adopted to handle the unprecedented volume of information generated by advanced medical instruments and electronically recorded by medical systems [13]. This abundance of data poses challenges for traditional CDM methods relying on manual effort, but ML techniques hold promise because they enable computers to automatically learn projection functions between raw data and targets of interest without explicit instructions from human experts [14]. For example, support vector machine, random forest, and k-nearest neighbor have been used to diagnose Alzheimer's disease [15], breast cancer [16], and Parkinson's disease [17], respectively. In addition to these conventional techniques, deep learning, a specialized ML subset focusing on design and training strategies of artificial neural networks, has emerged as the state-of-the-art approach for various CDM tasks owing to its extensive parameterization and intricate pattern recognition capability [13].
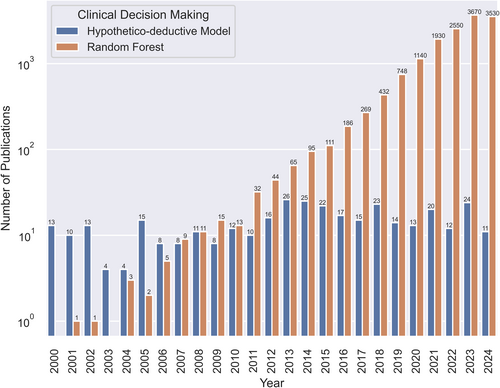
Comparison of a traditional method (hypothetico-deductive model) versus a contemporary machine learning approach (random forest) in scholarly publications over the last 25 years. Specific numbers were retrieved through a systematic inquiry on Google Scholar employing the search terms “clinical decision making” in conjunction with either “hypothetico-deductive model” or “random forest” on August 21, 2024.
3 FUTURE CDM METHODS
Though purely data-driven ML has demonstrated superior accuracy over traditional CDM methods [18], ML exhibits drawbacks in interpretability because of its complex architectures for information processing [19]. Interpretability stands as a pivotal characteristic within CDM to rectify potential erroneous decisions that endanger patient lives. To address this challenge and further augment other capabilities of ML, including its exceptional accuracy, researchers collaborate with clinicians to incorporate clinical knowledge and domain expertise into ML methodologies [20]. As depicted in Figure 2, this integration, termed knowledge-enhanced ML (KEML) [21], can be conceptualized as a fusion of clinical knowledge and domain expertise extracted from traditional CDM alongside powerful ML architectures, encompassing both conventional models and deep learning approaches [22].
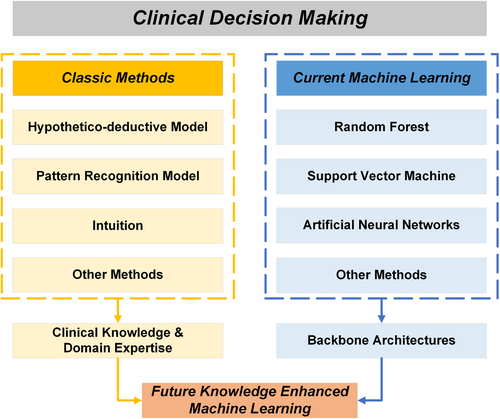
Schematic plot depicting the fusion of classic and machine learning methods leading toward future knowledge-enhanced machine learning for clinical decision making.
The foremost advantage of KEML over contemporary ML is its ability to significantly improve interpretability [23]. Although various explainable artificial intelligence techniques have been proposed to supplement ML with interpretability, these data-driven explanations frequently suffer from logical inconsistencies stemming from noise within datasets or limited applicability to particular cohorts [24]. For instance, a previous study showed that data-driven ML classifiers for pneumothorax often rely on irrelevant regions beyond the lesion area for diagnosis, resulting in overfitting to specific data sources [23]. Integrating clinical knowledge of disease occurrence can enhance both the generalization and interpretability of these classifiers. Another illustrative example is the knowledge-guided interpretable disease prediction method, which showcases the application of medical knowledge graphs to modeling personalized patient data and improving CDM interpretability by extracting crucial graph paths as prompts for ChatGPT to generate clinician-comprehensible natural language explanations [24]. In addition, KEML leverages external clinical knowledge to improve CDM accuracy further. Dynamic gated recurrent neural network exemplifies the enrichment of the representation of a specific medical event with additional information about its adjacent events [25]. KEML also alleviates the biases of data-driven ML through the involvement of healthcare professionals and their medical expertise. Chen et al. systematically summarized the potential of KEML to mitigate disparity and inequity in each stage of the ML life cycle [26]. Hence, the integration of clinical knowledge into ML not only amplifies interpretability and accuracy but also mitigates biases, ultimately advancing the real-world deployment of ML-driven healthcare solutions [27].
In this commentary, we summarized the evolution of CDM from traditional to contemporary ML techniques and elucidated the underlying rationale driving this paradigm shift, emphasizing the imperative of adapting to the era of big data. While embracing ML represents a crucial advancement in modeling clinical information, knowledge and expertise embodied in traditional methods should not be disregarded. Therefore, we advocate for KEML, a novel paradigm capitalizing on the strengths of both methodologies, to propel CDM to an exceptional level of interpretability, accuracy, and fairness [28].
AUTHOR CONTRIBUTIONS
Han Yuan: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing—original draft; writing—review & editing.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data was generated or analyzed during the current study.