Global prevalence of Staphylococcus aureus in food products and its relationship with the occurrence and development of diabetes mellitus
Tingting Liang, Zhuang Liang and Shi Wu contributed equally to this work.
Abstract
The worldwide distribution of Staphylococcus aureus across food types is an important food safety concern. This study aimed to estimate the prevalence of S. aureus in food products and its relationship with the occurrence and development of diabetes mellitus. A total of 55 articles were included. The pooled prevalence of S. aureus was 30.2%. The highest prevalence of S. aureus was observed in cereals, followed by meat and bean products, and the lowest in confectionery, egg products, and vegetables. The prevalence in dairy and seafood products was similar. Combinations of culture and molecular methods have been used for S. aureus detection. Furthermore, the prevalence of S. aureus in developed countries (Europe and North America) was higher than that in developing countries (Asia and Africa). In addition, the prevalence was higher in the provinces of Xinjiang and Shaanxi than that in Sichuan and Shandong in China, which may be due to the difference in climate and dietary habits. The results revealed that food type, bacterial detection methods, and location can influence the prevalence of S. aureus contamination. Resistance rates to preferred antibiotics against S. aureus were the highest for cephradine, polymyxin B, and penicillin at 82.9%, 82.0%, and 81.3%, respectively. In addition, 17 studies were system reviewed that the S. aureus infections are closely associated with the development of diabetes, and the treatment of probiotic, prebiotic, FMT, and bacteriophage can prevent and control S. aureus infections. This review emphasizes the high prevalence of S. aureus contamination in food, suggesting a potential diabetic infection risk and importance of observing principles of food safety and hygiene to reduce S. aureus.
1 INTRODUCTION
Foodborne pathogens and their toxins, which can cause disease and death, are of high concern for public health and food safety [1]. Among these, Staphylococcus aureus (S. aureus) is considered one of the most significant foodborne pathogens worldwide [2]. Although S. aureus is widespread in nature, food remains an important source of contamination. S. aureus causes foodborne illness in approximately 241,000 people in the United States every year [3]; moreover, S. aureus causes 20%–25% of the foodborne bacterial outbreaks in China [4]. S. aureus is a gram-positive, non-motile, facultative anaerobic pathogen. In general, S. aureus produces a wide range of enterotoxins, including staphylococcal enterotoxins, leukocidin, exfoliatin, and toxic shock syndrome toxin 1, which are mostly heat-resistant and can cause food poisoning, toxic shock syndrome, and dermatitis in humans [5]. S. aureus can survive in a low-temperature environment. Frozen food contaminated with these bacteria can cause food poisoning. Therefore, it is practically important to prevent S. aureus contamination in food because of the high levels of enterotoxins.
S. aureus is frequently reported to be isolated from various foods, such as egg products [6], vegetables [2], raw milk and dairy products [7, 8], cheese [9], meat and meat products [10], and seafood [11], thus constituting a risk to consumer's health. Previous studies have suggested the presence of S. aureus in ready-to-eat food in many countries, such as Korea, Brazil, and Vietnam [12]. Globally, S. aureus has caused several foodborne outbreaks from 2012 to 2022. According to a surveillance study in Japan, the poisoning incidents after consuming snow brand milk products, which infected tens of thousands of people due to the contamination of S. aureus, caused a huge burden on public health [13]. In the United States, one billion dollars every year is spent on medical costs due to food poisoning caused by S. aureus [14]; according to the centers for disease control and prevention, S. aureus was the second most common cause of outbreak from 1977 through 1981 [15]. In the European Union (e.g., France, Italy, and Hungary), 9.9% of pathogenic microorganism-induced outbreaks in 2015 were caused by staphylococcal toxins [14]. In China, microbial food poisoning accounted for 53.7% of food poisoning incidents; of these, 25% of outbreaks were reported to be caused by contamination with S. aureus [2]. Notably, the detection rate of S. aureus varied greatly among different regions, which may be related to the number of samples studied, local climate, dietary habits, food types, and detection methods. Hence, the prevalence of S. aureus in food must be monitored and controlled worldwide.
According to centers for disease control, two million people are infected by drug-resistant bacteria and 23,000 people die from these infections every year. Based on this current trend, it is estimated that 10 million people will die from food poisoning with drug-resistance organisms every year until 2050 with 47.3% in Asia. In general, the treatment of S. aureus infection involves the use of antibiotics. However, S. aureus is generally resistant to antibiotics and can produce exopolysaccharides to form a barrier to prevent antimicrobial agents. It can also make other foodborne bacteria resistant by carrying transferable resistance genes on plasmids or transposons. Therefore, monitoring the antibiotic resistance of S. aureus from different types of foods is of great importance to deal with this challenge.
Symptoms of S. aureus food poisoning have a rapid onset and may include vomiting, stomach pain, and diarrhea [16]. A previous study demonstrated that the incubation period and severity of symptoms depend on the type and amount of staphylococcal enterotoxins [17]. The initial nausea symptoms occur more rapidly than the incoercible characteristic vomiting (in spurts) syndrome, namely within 30 min to 8 h after consuming the contaminated food, and other symptoms, such as abdominal pain, diarrhea, dizziness, shivering, and general weakness syndrome, can also occur. It has been reported that most patients can recover with no specific therapy although diarrhea and general weakness can last 24 h or longer [18]. In general, S. aureus infections do not lead to death; however, they may occur in the most susceptible individuals, especially infants and the elderly [19]. Meanwhile, if the amount of S. aureus is greater than 104 colony-forming units per gram (cfu/g) of food, it is considered potentially hazardous for human [20].
Furthermore, it was reported that S. aureus infections caused the most deaths in 2019, which colonization occurs more frequently in patients with obesity and diabetes. S. aureus and its superantigens are causes of type 2 diabetes (T2D) [21], which can secrete a high-affinity insulin-binding protein that mediates insulin resistance in T2D [22]. Meanwhile, it was reported that S. aureus superantigens bind to the gp130 receptor stimulate human adipocytes to produce proinflammatory cytokines and affect normal insulin signaling and adipocyte functions, which is strongly associated with insulin resistance. Therefore, taking measures to prevent and control S. aureus infections is critical to the development and progression of T2D.
To date, multiple meta-analyses have been performed in the field of food safety to summarize study findings [23]. However, to the best of our knowledge, no meta-analysis has pooled the prevalence and antibiotic resistance of S. aureus isolates of food origin worldwide. Accordingly, this systematic review and meta-analysis aimed to evaluate the overall prevalence and antibiotic resistance of S. aureus worldwide and to investigate the effect of geographical distribution, types of foods, and bacterial detection methods on the prevalence of this bacterium. Meanwhile, the relationship of S. aureus infection and the development of T2D were system reviewed, and the prevention and control of S. aureus based on microecological therapy were also summarized. The results can provide epidemiological analysis and contribute to the reduction of risk factors for S. aureus, thereby improving public health.
2 MATERIALS AND METHODS
2.1 Search strategy
A systematic literature search was performed using three databases, PubMed, Scopus, and Science Direct, and accessed for studies between 2012 and 2022 with no language restrictions. The following search strategy was used: prevalence OR antibiotic-resistant OR epidemiology OR poultry OR beef OR pork OR eggs OR chicken OR turkey OR pig OR duck OR cow OR sow OR broiler OR poultry OR swine OR rabbit OR sheep OR equine OR water OR geese OR fish OR hens OR shrimp OR seafood OR goat OR cheese OR milk OR quail OR ostrich OR geese OR mussel OR mutton OR vegetables OR lettuce OR celery OR parsley OR artichokes OR oysters OR greenshell mussels OR abalone OR fruit OR salads OR meat OR dairy OR grains OR cereal OR bread OR rice OR pasta OR noodles OR Bean OR sandwich OR ice cream OR sausage OR Butter OR carcass OR yogurt AND Staphylococcus aureus AND S. aureus.
2.2 Study selection
After reading the title, abstract, and full text, 9002 studies were included, which were uploaded to Endnote X9.2, and duplicates were removed. Two reviewers (TTL and ZL) independently conducted title/abstract and full-text screening. Studies in foreign languages, review articles, clinical trials, and other unrelated studies were excluded. Moreover, studies not related to the prevalence of S. aureus and the original text referring to infected samples not related to food were excluded.
2.3 Data extraction
After study selection, data extraction was performed using a pre-specified Excel form, and the following data were extracted: the first author, publication year, number of samples, number of positive S. aureus samples, prevalence of S. aureus, type of food, location, and bacterial detection methods. Subgroup analysis was performed using the following potential moderator variables: food type, bacterial detection methods, country, and continent.
2.4 Statistical analysis
Meta-analysis of the prevalence of S. aureus was performed using the STATA software package, version 15.1 (StataCorp). A random-effects meta-analysis was used to generate forest plots, and the 95% confidence interval (CI) of S. aureus prevalence was obtained. Statistical heterogeneity was evaluated using the I2 statistic, and I2 categorized 0%–25%, 26%–75%, and 76%–100% were considered low, moderate, and high degrees of heterogeneity, respectively. Subgroup analysis was performed based on the type of food, continents, provinces, and bacterial detection methods. Funnel plots and Egger's tests were used to estimate the publication bias in this meta-analysis. Finally, a sensitivity analysis was performed by eliminating each study individually to evaluate the stability of the results.
3 RESULT AND DISCUSSION
To investigate the prevalence of S. aureus in food, as presented in Figure 1, we searched three databases from PubMed, Scopus, and Web of Science, which yielded 9002 studies, and one study was obtained by manual retrieval; among these, 3487 duplicate articles were identified and excluded. A total of 2733 studies were excluded after screening titles, including reviews, clinical trials, foreign language; 2728 studies were removed by full-text article screening; and 55 studies were considered eligible for full-text analysis and further qualitative and quantitative analyses. Ultimately, 55 studies were included in the meta-analysis. The prevalence of S. aureus was categorized according to food type, source region, and detection method.
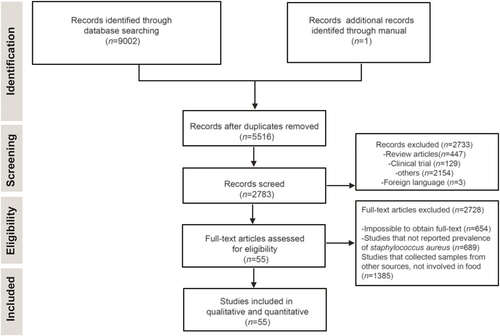
Flow diagram of database search, study selection, and articles included in the meta-analysis.
A detailed overview of the included studies is presented in Table 1, including the first author, publication year, sample size, prevalence of S. aureus, detection methods (culture, biochemical, and molecular), countries, pooled prevalence of S. aureus from every kind of food (with 95% CI), and weight of each study. Briefly, the sample size of these food types ranged from 7 to 3520, and the prevalence of S. aureus ranged from 1.7% to 75%. The most frequently studied food types included egg products, vegetables, dairy, meat products, seafood, confectionary, cereals, and bean products. Additionally, we found that most of the studies used culture alone or combined with molecular methods for detection; only one study used culture combined with biochemistry. Furthermore, it was observed that these research reports originated from 25 countries in five different continents, among which China and Turkey had the highest number of studies, followed by Ethiopia and India; countries such as Brazil, Bulgaria, Indonesia, Italy, Morocco, Romania, Serbia, and South Africa only had one study each.
| Study | n a | p (%)b | Effect (95% CIc) | Sample | Method | Country | % Weight | Resistance/enterotoxin gene |
|---|---|---|---|---|---|---|---|---|
| Amrita Pondit et al. 2018 | 220 | 10.45 | 0.105 (0.064, 0.145) | Chicken eggs | CMe | Mymensingh | 1.04 | mecA |
| 80 | 5 | 0.050 (0.002, 0.098) | Quail eggs | CM | Mymensingh | 1.03 | ||
| Wu et al. 2018 | 110 | 6.36 | 0.064 (0.018, 0.109) | Tomatoes | CBMf | China | 1.03 | mecA, sem, sec, sep, seg, sel, seh, seq, sej, seb, sen, ser |
| 128 | 1.56 | 0.016 (−0.006, 0.037) | Cucumbers | CBM | China | 1.06 | ||
| 84 | 15.48 | 0.155 (0.077, 0.232) | Lettuces | CBM | China | 0.97 | ||
| 87 | 2.30 | 0.023 (−0.009, 0.054) | Caraway | CBM | China | 1.05 | ||
| Qian et al. 2019 | 289 | 23.53 | 0.235 (0.186, 0.284) | Raw goat milk | CM | China | 1.03 | seb, tsst, sea |
| Mphahlele et al. 2020 | 2862 | 1.7 | 0.017 (0.012, 0.022) | Cow milk | CM | South Africa | 1.06 | – |
| Abdeen et al. 2020 | 90 | 44 | 0.444 (0.342, 0.547) | Raw milk | CM | Egypt | 0.91 | sea, seb, sed |
| 60 | 20 | 0.200 (0.099, 0.301) | Ice cream | CM | Egypt | 0.92 | ||
| Abdeen et al. 2020 | 50 | 46 | 0.460 (0.322, 0.598) | Minced meat | CM | Egyptian | 0.82 | – |
| 50 | 44 | 0.440 (0.302, 0.578) | Beef luncheon | CM | Egyptian | 0.82 | ||
| 50 | 44 | 0.440 (0.302, 0.578) | Karish cheese | CM | Egyptian | 0.82 | ||
| Stratev et al. 2020 | 33 | 6 | 0.061 (−0.021, 0.142) | Frozen sea fish | CM | Bulgaria | 0.96 | – |
| Rortana et al. 2020 | 204 | 31.2 | 0.314 (0.250, 0.377) | Pork | Cd | Cambodian | 1.00 | – |
| 408 | 41.9 | 0.419 (0.371, 0.467) | Chicken meat | C | Cambodian | 1.03 | ||
| Kanungpean et al. 2021 | 110 | 28.18 | 0.282 (0.198, 0.366) | Pork | C | Thailand | 0.096 | – |
| Bastam et al. 2021 | 35 | 37.1 | 0.371 (0.211, 0.532) | Raw milk | CM | Iran | 0.76 | – |
| 35 | 74.2 | 0.743 (0.598, 0.888) | Pasteurized milk | CM | Iran | 0.80 | ||
| 30 | 70 | 0.700 (0.536, 0.864) | Cheese | CM | Iran | 0.75 | ||
| Abimael et al. 2021 | 510 | 22.6 | 0.225 (0.189, 0.262) | Raw goat milk | CB | Brazil | 1.04 | – |
| Kou et al. 2021 | 144 | 43.1 | 0.431 (0.350, 0.511) | Retail raw milk | CM | China | 0.97 | see/sea/sec |
| Sivaraman et al. 2022 | 108 | 18.52 | 0.185 (0.112, 0.258) | Fresh fish | CM | Indian | 0.98 | – |
| 79 | 17.72 | 0.177 (0.093, 0.261) | Chilled fish | CM | Indian | 0.96 | ||
| 64 | 9.38 | 0.094 (0.022, 0.165) | Frozen fish | CM | Indian | 0.99 | ||
| 124 | 17.74 | 0.177 (0.110, 0.245) | Processed fish | CM | Indian | 0.99 | ||
| 76 | 5.6 | 0.053 (0.002, 0.103) | Water fish | CM | Indian | 1.02 | ||
| 47 | 4.26 | 0.043 (−0.015, 0.100) | Ice fish | CM | Indian | 1.01 | ||
| Sadat et al. 2022 | 700 | 41.1 | 0.411 (0.375, 0.448) | Raw cow milk | CM | Egypt | 1.04 | sea, seb, sec, hla, tst, blaZ, mecA |
| Lin et al. 2019 | 1192 | 2.6 | 0.026 (0.017, 0.035) | Meat products | C | China | 1.06 | – |
| 200 | 3.0 | 0.030 (0.006, 0.054) | Dairy | C | China | 1.05 | ||
| 409 | 2.2 | 0.022 (0.008, 0.036) | Fruit and vegetables | C | China | 1.06 | ||
| 348 | 2.3 | 0.023 (0.007, 0.039) | Desserts | C | China | 1.06 | ||
| Lemma et al. 2021 | 175 | 24.6 | 0.246 (0.182, 0.309) | Milk | CM | Ethiopia | 1.00 | mecA |
| 40 | 17.5 | 0.175 (0.057, 0.293) | Yogurt | CM | Ethiopia | 0.88 | ||
| 40 | 5 | 0.050 (−0.018, 0.118) | Cottage cheese | CM | Ethiopia | 0.99 | ||
| Akbar et al. 2013 | 209 | 18.18 | 0.182 (0.130, 0.234) | Poultry meat | CM | Thailand | 1.02 | – |
| Gücükoğlu et al. 2013 | 56 | 32.1 | 0.321 (0.199, 0.444) | Fruity ice cream | CM | Turkey | 0.86 | sea, seb, sed |
| 32 | 12.5 | 0.125 (0.010, 0.240) | Vanilla ice cream | CM | Turkey | 0.99 | ||
| 12 | 8.3 | 0.083 (−0.073, 0.240) | Chocolate ice cream | CM | Turkey | 1.02 | ||
| Saadat et al. 2013 | 100 | 9 | 0.090 (0.034, 0.146) | Milk samples | CBM | Iran | 0.86 | sea |
| 100 | 45 | 0.450 (0.352, 0.548) | Cheese samples | CBM | Iran | 0.88 | ||
| Shi et al. 2021 | 750 | 36.8 | 0.368 (0.333, 0.403) | Raw milk | CM | China | 0.77 | blaZ, dfrG, tetM |
| Siiriken et al. 2016 | 110 | 40 | 0.400 (0.308, 0.492) | Beef | CM | Turkey | 1.01 | mecA |
| 56 | 16.07 | 0.161 (0.065, 0.257) | Raw milk | CM | Turkey | 0.93 | ||
| 9 | 100 | 0.091 (0.063, 0.120) | Fish | CM | Turkey | 1.04 | ||
| Riva et al. 2015 | 383 | 9.14 | 0.750 (0.640, 0.860) | Raw milk | CM | Italy | 0.94 | sea, seb, sed, see |
| Gücükoğlu et al. 2012 | 60 | 75 | 0.375 (0.207, 0.543) | Raw milk | CM | Turkey | 0.93 | sea, seb, sec, sed |
| 32 | 37.5 | 0.300 (0.016, 0.584) | White cheese | CM | Turkey | 1.05 | ||
| 10 | 30 | 0.300 (0.016, 0.584) | Kashar cheese | CM | Turkey | 0.90 | ||
| 10 | 30 | 0.100 (−0.086, 0.286) | Butter | CM | Turkey | 0.74 | ||
| 10 | 10 | 0.108 (0.066, 0.151) | Ice cream | CM | Turkey | 0.47 | ||
| Amenu et al. 2019 | 203 | 10.8 | 0.278 (0.263, 0.293) | Raw milk and milk product | C | Ethiopia | 0.47 | – |
| Thapaliya et al. 2017 | 3290 | 27.8 | 0.320 (0.191, 0.449) | Raw meat | CM | USA | 0.69 | mecA |
| Liu et al. 2022 | 50 | 32 | 0.360 (0.172, 0.548) | Raw goat milk | CM | China | 1.03 | mecA, ant(6)-Ia, fexA, sec |
| 25 | 36 | 0.240 (0.073, 0.407) | Raw buffalo milk | CM | China | 1.06 | ||
| 25 | 24 | 0.200 (0.043, 0.357) | Raw camel milk | CM | China | 0.84 | ||
| 25 | 20 | 0.404 (0.327, 0.481) | Raw yak milk | CM | China | 0.69 | ||
| Ed-Dra et al. 2018 | 156 | 40.38 | 0.611 (0.452, 0.770) | Sausages | C | Morocco. | 0.74 | – |
| Sankomkai et al. 2020 | 36 | 60 | 0.313 (0.152, 0.473) | Sausage | CM | Thai | 0.77 | sea, seb, sec, sed, see, tsst-1 |
| Xing et al. 2014 | 32 | 31.3 | 0.276 (0.197, 0.355) | Cooked meats | CM | China | 0.97 | sea, seb, sec, sed, see,sef, seh |
| 123 | 27.6 | 0.231 (0.069, 0.393) | Vegetable salads | CM | China | 0.76 | ||
| 26 | 23.1 | 0.183 (0.111, 0.256) | Boiled peanuts | CM | China | 0.76 | ||
| 109 | 18.3 | 0.327 (0.199, 0.454) | Cold noodles | CM | China | 0.97 | ||
| 52 | 32.7 | 0.218 (0.161, 0.276) | Dried tofu | CM | China | 0.76 | ||
| Koláčková et al. 2014 | 197 | 21.8 | 0.380 (0.245, 0.515) | Raw pork meat | CM | Czech | 0.98 | mecA |
| Shawish et al. 2016 | 50 | 38 | 0.220 (0.105, 0.335) | Minced meat | CM | Saudi Arabia, Egypt | 0.85 | sea, seb, sec, sed |
| 50 | 22 | 0.300 (0.173, 0.427) | Beef burger | CM | Saudi Arabia, Egypt | 1.01 | ||
| 50 | 30 | 0.320 (0.191, 0.449) | Beef sausage | CM | Saudi Arabia, Egypt | 0.83 | ||
| 50 | 32 | 0.120 (0.030, 0.210) | Beef kofta | CM | Saudi Arabia, Egypt | 0.88 | ||
| 50 | 12 | 0.375 (0.269, 0.481) | Beef luncheon | CM | Saudi Arabia, Egypt | 0.85 | ||
| Bayomi et al. 2016 | 80 | 37.5 | 0.692 (0.441, 0.943) | Retail chicken products | CM | Egypt | 0.84 | mecA |
| Hao et al. 2015 | 13 | 69.2 | 0.714 (0.478, 0.951) | Lamb quick-frozen dumplings | CM | China | 0.95 | mecA, sea, seb, sec, see, seg, sej |
| 14 | 71.4 | 0.500 (0.217, 0.783) | Vegetarian quick-frozen dumplings | CM | China | 0.91 | ||
| 12 | 50.0 | 0.580 (0.473, 0.688) | Seafood quick-frozen dumplings | CM | China | 0.54 | ||
| 81 | 58 | 0.034 (0.009, 0.059) | Pork quick-frozen dumplings | CM | China | 0.57 | ||
| Wang et al. 2021 | 205 | 3.4 | 0.583 (0.459, 0.708) | Pasteurized milk | CM | China | 0.47 | blaZ, mecA, ermB, ermC, pbp2b, tetM, sec |
| Wardhana et al. 2021 | 60 | 58.3 | 0.039 (0.015, 0.062) | Chicken | CBM | Surabaya, East Java, Indonesia | 0.90 | – |
| Dai et al. 2019 | 258 | 3.9 | 0.391 (0.300, 0.482) | Pasteurized milk | CM | China | 1.05 | – |
| Sudhanthiramani et al. 2019 | 110 | 39.09 | 0.372 (0.319, 0.425) | Milk | CM | India | 0.94 | – |
| Rong et al. 2017 | 320 | 37.2 | 0.545 (0.447, 0.642) | Aquatic food | CM | China | 1.02 | mecA |
| Tsehayneh et al. 2021 | 101 | 54.45 | 0.064 (0.041, 0.088) | Raw beef | C | Ethiopia | 0.93 | – |
| Madahi et al. 2014 | 420 | 6.42 | 0.024 (0.016, 0.032) | Chicken nugget | C | Iran | 1.05 | sea, sec, sed, seg, sej |
| Wu et al. 2022 | 1463 | 2.4 | 0.399 (0.353, 0.445) | Retail food | C | China | 1.06 | lsa(E) |
| Sukanya et al. 2019 | 436 | 40 | 0.667 (0.582, 0.751) | Raw bovine milk | CM | Hokkaido, Japan | 1.03 | – |
| Savariraj et al. 2021 | 120 | 66.67 | 0.290 (0.177, 0.403) | Chicken meat | CBM | India | 0.96 | seb, seg, sei, sec, sed, sej |
| Sri Prabakusuma et al. 2022 | 62 | 29.03 | 0.081 (0.013, 0.148) | Rubbing cheese | C | China | 0.89 | mepA, tet(K), arlR, arlS, norA, mgrA, tet(38), LmrS, bacA, mecA, blaZ, APH(3′)-IIIa, aad(6), ErmB, SAT-4, mecR1, GlpT, murA |
| 62 | 8.06 | 0.279 (0.264, 0.294) | Rushan cheese | C | China | 0.99 | ||
| Ge et al. 2017 | 3520 | 27.9 | 0.755 (0.671, 0.838) | Retail meats | CM | USA | 1.06 | – |
| Tang et al. 2017 | 102 | 75 | 0.600 (0.385, 0.815) | Chicken | CM | Denmark | 0.96 | – |
| 20 | 60 | 0.522 (0.318, 0.726) | Pork | CM | Denmark | 0.62 | ||
| 23 | 52 | 0.895 (0.797, 0.992) | Turkey | CM | Denmark | 0.65 | ||
| Lika et al. 2021 | 38 | 89.5 | 0.354 (0.238, 0.470) | Retail raw chicken meat | C | Serbia | 0.93 | – |
| Tegegne et al. 2021 | 65 | 35.4 | 0.375 (0.337, 0.414) | Raw meat samples | M | Czech | 0.88 | sea, seg, sei, sak |
| Korpysa-Dzirba et al. 2019 | 610 | 37.5 | 0.667 (0.359, 0.975) | Raw cow milk | M | Poland | 1.04 | sea, seb, sec, sed |
| Kukułowicz et al. 2021 | 9 | 66.7 | 0.667 (0.428, 0.905) | Cephalopods | C | Poland | 0.43 | – |
| 15 | 66.7 | 0.571 (0.205, 0.938) | Crustaceans | C | Poland | 0.57 | ||
| 7 | 57.1 | 0.462 (0.191, 0.733) | Mollusks | C | Poland | 0.34 | ||
| 13 | 46.2 | 0.700 (0.610, 0.790) | Fish | C | Poland | 0.50 | ||
| Alghizzi et al. 2021 | 100 | 70 | 0.382 (0.253, 0.510) | Raw milk | M | Saudi Arabia | 0.95 | mecA, SCCmec II, seh |
| Mekhloufi et al. 2021 | 55 | 38.2 | 0.222 (0.126, 0.318) | Meat/fish-based foods | CM | Algeria | 0.85 | Tst, mecA |
| 72 | 22.2 | 0.163 (0.052, 0.273) | Vegetable-based foods | CM | Algeria | 0.93 | ||
| 43 | 16.3 | 0.176 (−0.005, 0.358) | Pastries | CM | Algeria | 0.89 | ||
| 17 | 17.6 | 0.050 (−0.046, 0.146) | Cereals | CM | Algeria | 0.70 | ||
| 20 | 5.0 | 0.355 (0.275, 0.435) | Milk and egg-based foods | CM | Algeria | 0.93 | ||
| Morar et al. 2021 | 138 | 35.5 | 0.032 (−0.004, 0.068) | Cheeses | C | Romania | 0.97 | mecA |
| Osada et al. 2022 | 93 | 3.2 | 0.091 (−0.029, 0.211) | Chicken | CM | Japan | 1.04 | fosB |
| 22 | 9.1 | 0.161 (0.032, 0.291) | Pork | CM | Japan | 0.87 | ||
| 31 | 16.1 | 0.086 (0.058, 0.114) | Beef and pork | CM | Japan | 0.84 | ||
| Gebremedhin et al. 2022 | 383 | 8.64 | 0.241 (0.086, 0.397) | Raw milk | C | Ethiopia | 1.05 | – |
| 29 | 24.14 | 0.149 (0.047, 0.251) | Curd milk | C | Ethiopia | 0.77 | ||
| 47 | 14.73 | 0.143 (0.013, 0.272) | Bulk tank milk | C | Ethiopia | 0.92 | ||
| 28 | 14.29 | 0.275 (0.248, 0.301) | Cottage cheese | C | Ethiopia | 0.84 |
- a Sample size.
- b Prevalence of S. aureus.
- c 95% confidence interval.
- d C is including culture methods of S. aureus detection in food.
- e CM is including culture/molecular methods of S. aureus detection in food.
- f CBM is including culture/biochemical/molecular methods of S. aureus detection in food.
The overall pooled prevalence of S. aureus in food was investigated in 55 studies, and the forest plots with the estimated pooled prevalence rates were 30.2% with a CI (95%) (0.266–0.337, I2 = 99.1%, p = 0.000), across all types of food, detection methods, and source regions (Figure 2).
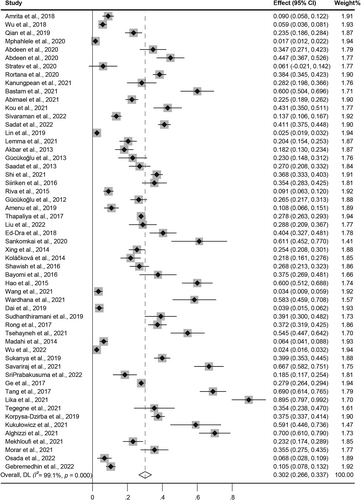
Forest plot of the meta-analysis of Staphylococcus aureus prevalence in all kinds of foods from included studies. Weights are from random-effects model.
To analyze the publication bias, a funnel plot (Figure S1) and Egger's test (Table S1) were performed. The funnel plot was not symmetrical, and Egger's regression test indicated the publication bias (p-value = 0.000). Finally, to explore the publication bias in the prevalence of S. aureus, the results of sensitivity analysis suggested that no single study likely affected the pooled results or total effect size, indicating robustness of the results (Figure S2).
Owing to the resistance to heat treatment, low pH, and proteolytic enzyme characteristics of S. aureus [24], it is abundant in meat, food, and the environment. It can lead to severe food poisoning, pneumonia, skin infections, enterotoxemia, and septicemia [25]. As shown in Table 1, we summarized various enterotoxin genes of S. aureus; different virulent genes were identified from S. aureus, including sea, seb, sec, sed, see, sef, seg, seh, sej, sel, sem, sen, sep, seq, ser, ser, and tsst. In addition, resistance genes, mecA, hla, tst, blaZ, dfrG, tetM, ant(6)-Ia, fexA, ermB, ermC, pbp2b, tetM, lsa(E), mepA, tet(K), arlR, arlS, norA, mgrA, tet(38), LmrS, bacA, APH(3′)-IIIa, aad(6), ErmB, SAT-4, mecR1, GlpT, murA, SCCmec II, and fosB, were identified from S. aureus in this review. As a result, S. aureus may cause periodic pollution in vegetable markets [26], slaughterhouses [27], dairy farms [28], the dairy industry [29], seafood markets [30], and other locations of interest. Therefore, S. aureus is ubiquitous in food production environments, such as those producing raw meat, cheese, infant foods, milk, vegetables, fruits, eggs, and cereals [31].
In this study, we classified these foods into nine categories according to their nature as shown in Table 2. The highest prevalence of S. aureus was found in cereals (46.3%), followed by meat and bean products (35.7% and 32.7%, respectively). The lowest prevalence of S. aureus was observed in confectionery (2.6%), followed by egg products (7.5%) and vegetables (10.0%). The prevalence of S. aureus in dairy and seafood products was similar (28.4% and 24.8%, respectively). The largest sample size was for meat products and dairy (10,989, and 9497), followed by vegetables and seafood (1039 and 959).
| G. food | Total sample size | Case number | Effect (95% CI) | I2 (%) | p for χ2 | % Weight |
|---|---|---|---|---|---|---|
| Egg products | ||||||
| Chicken eggs/quail eggs/milk- and egg-based foods | 320 | 28 | 0.075 (0.034, 0.116) | – | – | 3.00 |
| Vegetables | ||||||
| Tomatoes/cucumbers/lettuces/caraway/fruit and vegetables/vegetable salads/boiled peanuts/Vegetable-based foods | 1039 | 89 | 0.100 (0.056, 0.144) | 90.481 | 0.000 | 7.83 |
| Dairy | ||||||
| Goat milk/cow milk/pasteurized milk/yogurt/butter/milk buffalo/milk camel/raw milk yak/raw bovine milk/curd milk/bulk tank milk/cheese | 9497 | 1912 | 0.284 (0.232, 0.335) | 98.431 | 0.000 | 37.25 |
| Other foods | ||||||
| Ice cream/retail food/chocolate ice cream/vanilla ice cream | 1633 | 71 | 0.140 (0.034, 0.247) | 86.833 | 0.000 | 5.19 |
| Meat products | ||||||
| Minced meat/beef luncheon/pork/chicken meat/poultry meat/sausages/cooked meats/raw meat/turkey | 10,989 | 2919 | 0.357 (0.292, 0.422) | 98.732 | 0.000 | 29.13 |
| Seafood | ||||||
| Frozen sea fish/fresh fish/Chilled fish/Water fish/aquatic food/cephalopods/crustaceans/mollusks/fish-based foods | 959 | 245 | 0.248 (0.160, 0.335) | 92.168 | 0.000 | 10.63 |
| Confectionary | ||||||
| Desserts/Pastries | 391 | 15 | 0.026 (0.010, 0.041) | – | – | 1.95 |
| Cereals | ||||||
| Noodles/dumplings/cereals | 246 | 95 | 0.463 (0.248, 0.678) | 91.461 | 0.000 | 4.17 |
| Bean products | ||||||
| Dried tofu | 52 | 17 | 0.327 (0.203, 0.471) | – | – | 0.85 |
| Total | 25,126 | 5392 | 0.275 (0.248, 0.301) | 98.285 | 0.000 | 100.00 |
S. aureus is a common cereal contaminant [32], and several studies have reported its presence [33]. In this study, the overall prevalence of S. aureus in the cereals was 46.3%. Its high prevalence may be due to the presence of S. aureus in ready-to-eat cereals (e.g., lamb quick-frozen dumplings, vegetarian quick-frozen dumplings, seafood quick-frozen dumplings, pork quick-frozen dumplings, and cold noodles [34]) and the limited number of studies on cereals included in this study. Rice is one of the most widely consumed cereals worldwide [35], S. aureus is also present in rice cereals and is mostly consumed by children [32]. It is worth noting that S. aureus is the main cause of food poisoning associated with rice products [33]. In addition to the high prevalence of rice containing S. aureus, the fermented drink based on the cereal also showed high contamination with S. aureus.
Meat and meat products can be contaminated with S. aureus in various ways, including unsanitary slaughter [27] during meat processing and other faulty abattoir processes, which may increase the contamination of meat with livestock. This may partly explain the 35.7% prevalence of S. aureus in meat products in the present study. Nevertheless, it should be noted that meat contamination with S. aureus is also a serious issue in food production and processing environments. Several studies have shown a high prevalence of S. aureus in sausages (40.38%) in Morocco and (60%) in Thailand, depending on the season, sampling site, and origin of the raw material [36]. Therefore, the initial contamination of raw meat with S. aureus may be an important cause for contamination with S. aureus in meat products. To prevent the contamination of S. aureus in meat products, sanitary and hygiene practices in slaughterhouses or food production operations, including transportation, packaging, and storage, are required.
In this study, the prevalence of S. aureus in seafood was found to be 24.8%. Seafood is rich in proteins, which are broken down into low-molecular-weight peptides and amino acids and thus promote the growth of S. aureus [37]. However, in general, there is no S. aureus in marine water, and its high prevalence may be due to post-harvest contamination [38], including the infected person, improper hygiene practices, poor sanitary utensils, inadequate storage, as well as cross contamination [11]. A large proportion of contamination mainly occurred in the retail seafood and seafood-processing industries. Thus, the importance of enhanced monitoring of S. aureus and their enterotoxins in seafood should be emphasized to avoid risks to human health. Although seafood is usually consumed raw, adequate heat treatment is necessary to inactivate the S. aureus.
In this study, the prevalence of S. aureus in milk and dairy products was found to be 28.4%, including raw milk, pasteurized milk, yogurt, butter, and cheese. The bacterium can be introduced at almost every step of the production process in the dairy industry [39]. It should be noted that the udder (goat, cow, camel, and yak) and teat skin of dairy livestock, human handlers, and milking equipment can cause raw milk to be infected with S. aureus [40]. Thus, to prevent contamination of raw milk with S. aureus from cow udders before milking cows, appropriate methods are required to disinfect tanks, transport tubes, and milk-processing environments. Nevertheless, it was reported that although S. aureus can be inactivated in pasteurized milk, their enterotoxins retain their biological activity even after pasteurization [41], contributing to food poisoning and S. aureus diseases [42]. Moreover, it is worth noting that the largest consumers of milk and milk products are infants [43]. Therefore, more attention should be paid to S. aureus and its enterotoxins detection in milk and dairy products.
As S. aureus is extremely resistant to heat treatment, low pH, proteolytic enzymes, changes in temperature, and moisture extremes [44], it is challenging to prevent food contamination with S. aureus. A previous study has suggested that the toxins are secreted in food by enterotoxigenic S. aureus strains, are heat-stable, and do not degrade even when cooked, which can cause food poisoning [45]. In this review, in addition, S. aureus is a problematic issue in quick-frozen foods because it can survive for more than 700 days in the frozen state and even up to 7 years [34]. Another study confirmed that S. aureus can survive in frozen fish stored at −20°C [46]. Therefore, it is worth noting that concerns about foods with an extended shelf life in refrigerators should be raised.
In our study, we also compared the differences in the pooled prevalence of S. aureus among the bacterial detection methods (Table 3), which included culture, culture/biochemical, culture/molecular, and culture/molecular/biochemical. All four methods were similar, and the prevalence of S. aureus was 20%–30%. Nonetheless, the prevalence of S. aureus in each group detected using the culture/molecular method presented the highest incidence (0.292; 95% CI: 0.251–0.332), followed by culture/biochemical/molecular methods (0.248; 95% CI: 0.126–0.369). The lowest prevalence was reported for the culture/biochemical method with 22.5% (0.225; 95% CI: 0.190–0.264), followed by the culture method with 23.2% (0.232; 95% CI: 0.191–0.272). In terms of sample size, the highest numbers were detected using culture/molecular methods, followed by culture, and the lowest number was detected using culture/biochemical methods. It was found that these studies used culture combined with molecular methods more; conversely, the results may be due to the many advantages of culture methods, including specificity, reliability, low cost, and simplicity [47]. Nevertheless, it should be noted that molecular methods have the advantages of high sensitivity, strong specificity, simplicity, and speed and can be used for qualitative and quantitative detection at very low number of cells or nonculturable cells in the samples [48]. Therefore, considering the advantages of culture and molecular methods for the isolation, detection, and identification of S. aureus, culture combined with molecular methods is the preferred detection method for foodborne pathogen infections. However, the values of I2 for the four methods of bacterial detection were higher than 90%, demonstrating high heterogeneity among the results.
| Method | Total sample size | Case number | Effect (95% CI) | I2 (%) | p for χ2 | % Weight |
|---|---|---|---|---|---|---|
| Culture | 6045 | 705 | 0.232 (0.191, 0.272) | 97.789 | 0.000 | 21.48 |
| Culture/biochemical | 510 | 115 | 0.225 (0.190, 0.264) | – | – | 1.04 |
| Culture/molecular | 13,998 | 4115 | 0.292 (0.251, 0.332) | 98.357 | 0.000 | 69.61 |
| Culture/biochemical/molecular | 380 | 169 | 0.248 (0.126, 0.369) | 98.022 | 0.000 | 7.87 |
| Total | 25,126 | 5392 | 0.275 (0.248, 0.301) | 98.285 | 0.000 | 100 |
The prevalence of S. aureus based on the sample sources of the continents is shown in Table 4. We compared the differences in the pooled prevalence of S. aureus in different continents, including the five inhabited continents. The highest prevalence of S. aureus was observed in Europe with 46.5% (0.465; 95% CI: 0.331–0.598), followed by North America with 27.9% (0.279; 95% CI: 0.268–0.289). However, we observed that the prevalence of S. aureus in the remaining three continents was similar with a prevalence between 20% and 30%. The lowest reported prevalence rates were in South America with 22.5% (0.225; 95% CI: 0.190–0.264). We found that the prevalence of S. aureus was with 25.1% (0.251; 95% CI: 0.220–0.282) and 24.2% (0.242; 95% CI: 0.168–0.315) in Asia and Africa, respectively. In terms of sample size, the largest sample size was for Asia, including 10,552 samples from 10 countries, and the smallest samples were from South America with only 510 samples from Brazil.
| Continents | Total sample size | Case number | Effect (95% CI) | I2 (%) | p for χ2 | % Weight |
|---|---|---|---|---|---|---|
| Asia | ||||||
| Bangladesh/China/Cambodian/Thailand/Iran/Indian/Turkey/Indonesia/Japan/Saudi Arabia | 10,552 | 1996 | 0.251 (0.220, 0.282) | 97.525 | 0.000 | 60.56 |
| Africa | ||||||
| South Africa/Egypt/Ethiopia/Morocco/Algeria | 5351 | 776 | 0.242 (0.168, 0.315) | 97.671 | 0.000 | 20.08 |
| Europe | ||||||
| Bulgaria/Italy/Czech/Denmark/Serbia/Poland/Romania | 1653 | 541 | 0.465 (0.331, 0.598) | 97.520 | 0.000 | 11.83 |
| South America | ||||||
| Brazil | 510 | 115 | 0.225 (0.190, 0.264) | – | – | 1.04 |
| North America | ||||||
| The United States of America | 6810 | 1897 | 0.279 (0.268, 0.289) | – | – | 2.12 |
| Africa, Asia | ||||||
| Saudi Arabia and Egypt | 250 | 67 | 0.261 (0.165, 0.357) | 70.198 | 0.009 | 4.35 |
| Total | 25,126 | 5392 | 0.275 (0.248, 0.301) | 98.285 | 0.000 | 100.00 |
In this study, nine studies were included from Europe with food types including meat, seafood, and dairy. In contrast to previous studies, the results of this study [49] suggest a lower prevalence of food contamination by pathogenic bacteria in developed countries compared with those in developing countries. It is well known that high protein consumption is typical in Europe, which may be one of the reasons for the high prevalence of S. aureus compared to other continents. In the United States, S. aureus is considered one of the top five pathogens causing domestically acquired foodborne diseases and is responsible for estimated 241,000 illnesses per year [50]. We also found a very high prevalence of S. aureus in North America despite including developed countries, which is consistent with a previous study [1]. Recently, S. aureus, which infects food and causes poisoning, has become a growing concern in developed countries.
The pooled prevalence of S. aureus in different provinces of China, including six provinces, is summarized in Table S2. The highest prevalence of S. aureus was observed in Xinjiang with 43.1% (0.431; 95% CI: 0.348–0.516), followed by Shaanxi with 35.7% (0.357; 95% CI: 0.201–0.514). However, the lowest reported prevalence was in Sichuan and Shandong with 2.7% (0.027; 95% CI: 0.018–0.036) and 3.4% (0.034; 95% CI: 0.014–0.069), respectively. The major reason for the different prevalence of S. aureus in the six provinces is probably related to geographical conditions (climatic conditions) and dietary habits. In general, it is important to explore methods for monitoring and controlling S. aureus contamination in foods.
In addition, the antimicrobial resistance of S. aureus showed a statistically significant difference among the 77 types of antibiotics (Table 5). Based on the pooled rates of antimicrobial resistance, the highest rate of antibiotic resistance was observed for cephradine with 82.9% (0.829; 95% CI: 0.740–0.917). Cephradine is a first-generation antibiotic used more frequently than second- and third-generation cephalosporins. A previous study reported that cephradine can protect against S. aureus by its bactericidal activity. Next, we found that the highest antibiotic resistance rates were polymyxin B and penicillin with 82.0% (0.820; 95% CI: 0.714–0.926) and 81.3% (0.813; 95% CI: 0.711–0.915), respectively, which were assessed in one and nine studies, respectively. In S. aureus treatment, penicillin was the most preferred antibiotic. Wu et al. [2] reported that the resistance rate of penicillin for S. aureus isolated from food was 93.3%. Notably, polymyxin B is also used against S. aureus; thus, its resistance rate cannot be ignored. Meanwhile, we observed that S. aureus was not resistant to some antibiotics, such as rifampicin, cloxacillin, cephazolin, minocycline, ampicillin sulbactam, cefalotin, cefquinome, marbofloxacin, florfenicol, and tigecycline; nevertheless, it would not be realistic to discuss it because there was only one study for every antibiotic. Considering that these antibiotics have been increasingly used in animal breeding or human treatment, measures are needed to control the use of antimicrobials.
| Antibiotic | Number of total isolates | Number of positive isolates | Effect (95% CI) | I2 (%) | p for χ2 | % Weight |
|---|---|---|---|---|---|---|
| Amoxicillin | 436 | 270 | 0.664 (0.305, 1.024) | 99.4 | 0.000 | 2.80 |
| Oxacillin | 610 | 167 | 0.347 (0.163, 0.531) | 97.1 | 0.000 | 3.53 |
| Penicillin | 563 | 441 | 0.813 (0.711, 0.915) | 93.2 | 0.000 | 3.59 |
| Ciprofloxacin | 951 | 157 | 0.221 (0.123, 0.318) | 96.9 | 0.000 | 5.47 |
| Nalidixic acid | 67 | 37 | 0.595 (0.164, 1.026) | 94.1 | 0.000 | 0.75 |
| Erythromycin | 974 | 436 | 0.423 (0.276, 0.570) | 96.6 | 0.000 | 5.41 |
| Gentamycin | 220 | 33 | 0.147 (0.078, 0.216) | 51.2 | 0.000 | 1.57 |
| Tetracycline | 1051 | 421 | 0.412 (0.307, 0.517) | 93.4 | 0.000 | 6.53 |
| Vancomycin | 834 | 63 | 0.083 (0.036, 0.129) | 45.0 | 0.000 | 2.01 |
| Ampicillin | 777 | 507 | 0.627 (0.424, 0.829) | 98.7 | 0.000 | 5.52 |
| Cefepime | 278 | 18 | 0.062 (0.030, 0.094) | 12.2 | 0.000 | 1.22 |
| Cefoxitin | 746 | 170 | 0.318 (0.128, 0.508) | 98.3 | 0.000 | 3.59 |
| Ceftazidime | 314 | 27 | 0.090 (0.056, 0.123) | 0.00 | 0.000 | 1.58 |
| Amikacin | 417 | 16 | 0.164 (0.067, 0.260) | 96.3 | 0.000 | 1.95 |
| Gentamicin | 973 | 153 | 0.216 (0.138, 0.294) | 95.3 | 0.000 | 5.08 |
| Kanamycin | 406 | 98 | 0.252 (0.153, 0.351) | 82.4 | 0.000 | 2.64 |
| Streptomycin | 341 | 84 | 0.271 (−0.008, 0.550) | 98.4 | 0.000 | 1.97 |
| Chloramphenicol | 709 | 120 | 0.204 (0.071, 0.338) | 97.0 | 0.000 | 4.37 |
| Clindamycin | 682 | 170 | 0.257 (0.161, 0.354) | 90.3 | 0.000 | 3.87 |
| Erythromycin | 30 | 12 | 0.400 (0.225, 0.575) | 0.00 | 0.000 | 0.36 |
| Telithromycin | 72 | 22 | 0.230 (0.104, 0.356) | 0.00 | 0.000 | 0.69 |
| Norfloxacin | 388 | 44 | 0.114 (0.060, 0.169) | 63.7 | 0.000 | 2.33 |
| Linezolid | 210 | 14 | 0.099 (−0.002, 0.201) | 72.6 | 0.000 | 1.18 |
| Rifampicin | 199 | 20 | 0.189 (−0.062, 0.439) | 91.1 | 0.000 | 1.13 |
| Quinupristin dalfopristin | 259 | 14 | 0.046 (0.011, 0.081) | 33.9 | 0.000 | 1.61 |
| Teicoplanin | 62 | 5 | 0.250 (0.060, 0.440) | 0.00 | 0.000 | 0.36 |
| Nitrofurantoin | 315 | 4 | 0.011 (−0.002, 0.023) | 0.00 | 0.000 | 1.24 |
| Fusidic acid | 262 | 15 | 0.047 (0.009, 0.085) | 46.4 | 0.000 | 1.96 |
| Trimethoprim sulfamethoxazole | 800 | 132 | 0.191 (0.110, 0.273) | 92.8 | 0.000 | 4.38 |
| Linezolid | 249 | 26 | 0.197 (0.089, 0.305) | 60.0 | 0.000 | 0.80 |
| Cefazolin | 185 | 14 | 0.094 (−0.085, 0.272) | 93.0 | 0.000 | 0.82 |
| Piperacillin | 68 | 28 | 0.412 (0.295, 0.529) | 0.00 | 0.000 | 0.39 |
| Polymyxin B | 50 | 41 | 0.820 (0.714, 0.926) | 0.00 | 0.000 | 0.40 |
| Rifampicin | 50 | 0 | – | – | 0.000 | – |
| Cephradine | 70 | 58 | 0.829 (0.740, 0.917) | 0.00 | 0.000 | 0.40 |
| Cloxacillin | 52 | 0 | – | – | 0.000 | – |
| Cephazolin | 378 | 127 | – | – | 0.000 | – |
| Ceftriaxone | 186 | 53 | 0.477 (−0.320, 1.274) | 99.6 | 0.000 | 0.81 |
| Sulbactam | 98 | 11 | 0.112 (0.050, 0.175) | 0.00 | 0.000 | 0.41 |
| Levofloxacin | 313 | 19 | 0.034 (−0.008, 0.076) | 62.5 | 0.000 | 0.83 |
| Moxifloxacin | 118 | 4 | 0.041 (0.002, 0.080) | 0.00 | 0.000 | 0.41 |
| Daptomycin | 98 | 16 | 0.163 (0.090, 0.236) | 0.00 | 0.000 | 0.41 |
| Rifampin | 258 | 33 | 0.106 (0.058, 0.154) | 0.00 | 0.000 | 0.81 |
| Ceftaroline | 62 | 5 | 0.081 (0.013, 0.148) | 0.00 | 0.000 | 0.41 |
| Cephalothin | 274 | 51 | 0.296 (−0.105, 0.698) | 98.7 | 0.000 | 1.20 |
| Cefoperazone | 245 | 2 | 0.016 (−0.006, 0.037) | 0.00 | 0.000 | 0.42 |
| Co-trimoxazole | 43 | 2 | 0.047 (−0.016, 0.109) | 0.00 | 0.000 | 0.41 |
| Methicillin | 43 | 6 | 0.140 (0.036, 0.243) | 0.00 | 0.000 | 0.40 |
| Penicillin G | 359 | 202 | 0.693 (0.483, 0.902) | 94.2 | 0.000 | 1.91 |
| Enrofloxacin | 135 | 37 | 0.355 (−0.059, 0.770) | 98.3 | 0.000 | 1.20 |
| Cefotaxime | 157 | 33 | 0.402 (−0.374, 1.177) | 99.3 | 0.000 | 0.80 |
| Cefuroxime | 117 | 7 | 0.060 (0.017, 0.103) | 0.00 | 0.000 | 0.41 |
| Spectinomycin | 117 | 7 | 0.060 (0.017, 0.103) | 0.00 | 0.000 | 0.41 |
| Tobramycin | 117 | 10 | 0.085 (0.035, 0.136) | 0.00 | 0.000 | 0.41 |
| Midecamycin | 117 | 54 | 0.462 (0.371, 0.552) | 0.00 | 0.000 | 040 |
| Clarithromycin | 117 | 63 | 0.538 (0.448, 0.629) | 0.00 | 0.000 | 0.40 |
| Minocycline | 117 | 0 | – | – | 0.000 | – |
| Chloromycetin | 117 | 3 | 0.026 (−0.003, 0.054) | 0.00 | 0.000 | 0.42 |
| Telithromycin | 119 | 10 | 0.084 (0.034, 0.134) | 0.00 | 0.000 | 0.41 |
| Fosfomycin | 1077 | 156 | 0.010 (−0.007, 0.026) | 0.00 | 0.000 | 0.82 |
| Ofloxacin | 63 | 9 | 0.143 (0.056, 0.229) | 0.00 | 0.000 | 0.40 |
| Doxycycline | 63 | 26 | 0.413 (0.291, 0.534) | 0.00 | 0.000 | 0.39 |
| Amoxycillin clavulanic acid | 174 | 39 | 0.083 (0.005, 0.162) | 0.00 | 0.000 | 0.77 |
| Benzylpenicillin | 49 | 26 | 0.531 (0.391, 0.670) | 0.00 | 0.000 | 0.38 |
| Imipenem | 38 | 4 | 0.105 (0.008, 0.203) | 0.00 | 0.000 | 0.40 |
| Ampicillin sulbactam | 18 | 0 | – | – | 0.000 | – |
| Cefalotin | 11 | 0 | – | – | 0.000 | – |
| Ceftiofur | 11 | 8 | 0.727 (0.464, 0.990) | 0.00 | 0.000 | 0.31 |
| Cefquinome | 11 | 0 | – | – | 0.000 | – |
| Neomycin | 11 | 7 | 0.636 (0.352, 0.921) | 0.00 | 0.000 | 0.30 |
| Marbofloxacin | 18 | 0 | – | – | 0.000 | – |
| Tilmicosin | 11 | 4 | 0.364 (0.079, 0.648) | 0.00 | 0.000 | 0.30 |
| Tylosin | 11 | 2 | 0.182 (−0.046, 0.410) | 0.00 | 0.000 | 0.33 |
| Mupirocin | 18 | 1 | 0.056 (−0.050, 0.161) | 0.00 | 0.000 | 0.40 |
| Florfenicol | 11 | 0 | – | – | 0.000 | – |
| Tigecycline | 20 | 0 | – | – | 0.000 | – |
| Azithromycin | 40 | 2 | 0.050 (−0.018, 0.118) | 0.00 | 0.000 | 0.41 |
| Total | 20,030 | 4876 | 0.308 (0.279, 0.338) | 98.5 | 0.000 | 100 |
- Abbreviations: 95% CI, 95% confidence interval; I2, inverse variance index; Q, Q statistics.
In recent years, the concept of One Heath is increasingly gaining attention [51], one such pathogen is the S. aureus transmitted directly from contaminated food products to humans [52]. According to a report, S. aureus infections caused the most deaths in 2019, more than a million cases [53]. It is reported that S. aureus colonization occurs more frequently in some subgroups of obese individuals and patients with diabetes than in healthy individuals [54]. In this review, 17 papers published from the global diabetic patients with complications caused by S. aureus infections were summarized, including obese diabetes [55], prepregnancy diabetes [56], diabetic foot ulcer [57], diabetic urinary tract infection [58], diabetic pulmonary infection [59], and others [60] (Table 6). The results suggest as follows: (1) Obesity is a possible determinant for S. aureus nasal colonization independent of diabetes mellitus; (2) Prepregnancy diabetes, but not gestational diabetes, appears to be a risk factor for invasive S. aureus infection in the early postpartum period; (3) S. aureus was the common microorganisms in patients with diabetic foot infection, particularly the S. aureus infection, and it was found that there were frequently resistant to the usually prescribed antibiotics. Meanwhile, we also found that insulin-requiring diabetics carry S. aureus more frequently than non-diabetics, and diabetes is controlled by hypoglycemic agents can recover the normal S. aureus carriage rate. However, the precise mechanisms linking S. aureus infection and diabetes have not been identified.
| Authors | Country | n | Complications | Carrier location | Groups | Carrier rate (%) | Bacteria isolated | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Tuazon et al. (1975) | US | 35/36 | – | Skin, nose, throat | Injections of insulin/oral hypoglycémie agents | 34/11 | 15/4 | Daily parenteral self-medication is a principal factor that somehow predisposes to the increased carrier rate of coagulase-positive S. aureus |
| Smith et al. (1996) | Australia | 144/180 | – | Nare | Injections of insulin/oral hypoglycémie agents | 53.4/35 | 77/63 | Insulin-requiring diabetics carry S. aureus in the anterior nares more frequently than nondiabetics. Patients with diabetes mellitus that is controlled by hypoglycemic agents, however, have a normal S. aureus nasal carriage rate |
| Olsen et al. (2013) | Norway | 2169/1709 | – | Nare | Obese/lean (women/men) | – | – | Obesity is a possible determinant for S. aureus nasal colonization independent of DM, in particular for premenopausal women |
| Andrea et al. (2013) | US | 28,949/2376/185,514 | Prepregnancy diabetes/prepregnancy diabetes | Skin/urinary tract/genitourinary/wound infections/septicemia | Prepregnancy diabetes/diabetic complications/prepregnancy diabetes | 30.9/6.4/5.2/3.0/2.0 | – | Prepregnancy diabetes, but not gestational diabetes, appears to be a risk factor for invasive MRSA infection in the early postpartum period |
| Sowmya et al. (2013) | Karnataka | 570 | Urinary tract infections | Urethra | Different age ranges | 4.56 | 69 | The urinary tract infection was found to be highly significant in females with type 2 diabetes compared to men. The incidence of urinary tract infection was found to be increasingly high of about 82% among the patients with diabetes in Mysore |
| Estrella et al. (2015) | Mexico | 100 | Diabetic foot ulcer | Foot | – | 42 | 42 | The patients diagnosed with DM2 and with infected foot ulcers show a prevalence of S. aureus (42%), followed by Escherichia coli (36%) and, MRSA was predominant (34%) |
| Julie et al. (2015) | Australia | 258 | – | Nare/axilla | – | 39.1 (MRSA3.1%) | – | Persistent colonization may underlie the increased risk of hospitalization with S. aureus and MRSA |
| Jesper et al. (2016) | Denmark | 713 | – | – | – | 72 | – | Diabetes is associated with a substantially increased risk of S. aureus, particularly among patients with diabetes of long duration, poor glycemic control, and diabetes complications |
| Akshay et al. (2017) | India | 41 | – | Skin | – | 45 | 31 | The existence of cutaneous microbiome dysbiosis among patients with T2DM, which stem from the same activated innate immune response that is thought to be central to the development of T2DM, it can increase the risk of developing skin infections |
| Fraence et al. (2017) | Germany | 9401 | – | – | – | 1.08 | – | S. aureus is associated with a substantial healthcare burden and high mortality. Effective infection control measures should be considered to reduce post-surgical S. aureus infection risk in patients with T2DM |
| Muhammad et al. (2018) | Pakistan | 214 | Diabetic foot ulcer | Foot | – | 20.96 | 65 | This study may help to reduce the risk of complications in patients with infected diabetic foot ulcers and aid in the appropriate choice of antibiotics for maximum efficacy in their treatment and management |
| Mohammad et al. (2019) | India | 192 | Diabetic foot ulcer | Foot | MDR-DM/NMDR-DM (multidrug resistance) | – | 60 |
|
| Maram et al. (2020) | Sudan | 216 | Diabetic foot ulcer | Foot | – | – | 61 (28) | S. aureus was the common microorganisms in patients with diabetic foot infection, different isolated microorganisms showed to have different degrees of resistance and sensitivity to various antibacterial drugs |
| Ana et al. (2020) | Spain | 216,735 | Urinary tract infections | Urethra | UTIs was higher in T2DM patients/without this disease | – | – | The incidence of UTIs was over four times higher in T2DM than patients with nondiabetes, higher mortality rates in patients with T2DM were associated with the diagnosis of S. aureus isolation |
| Li et al. (2022) | China | 125 | Pulmonary infection | Sputum | Simple T2DM/infection group | – | 12 | Gram-negative bacteria are the main pathogens of T2DM complicated with pulmonary infection. Drug sensitivity test should be combined to understand the drug resistance of pathogenic bacteria and use drugs reasonably for patients |
| Mesmin et al. (2022) | Cameroon | 101 | Diabetic foot ulcer | Foot | – | – | 21 | Gram-negative bacteria were more frequently associated with diabetic foot infections and were frequently resistant to the usually prescribed antibiotics, but remain sensitive to imipenem and amikacin |
| Maria et al. (2022) | Poland | 863 | Diabetic foot ulcer | Foot | – | – | 201 (31MRSA) | Osteitis incidence is related to MRSA infection in patients with diabetic foot ulcers; thus, patients infected by S. aureus should be closely monitored in the course of using antibiotics and treated with narrow-spectrum antibiotics |
- Abbreviations: MDR-DM, multidrug resistance- diabetes mellitus; MRSA, methicillin-resistant S. aureus; N, Number of patients infected S. aureus; T2DM, type 2 diabetes mellitus; UTIs, urinary tract infections.
Currently, the main mechanisms linking S. aureus infection and T2D are described in Table 7. S. aureus produces a repertoire of Staphylococcal enterotoxins abbreviated SE-A, -B, -C, etc., which can cause food poisoning and toxic shock and are associated with several acute and chronic inflammatory diseases [61]. In S. aureus, the accessory gene regulator (Agr), consists of four genes (AgrA, AgrB, AgrC, and AgrD), an autoinducing peptide (AIP), is the primary quorum sensing system responsible for inducing the transcription of several virulence factors [62]. Meanwhile, it was reported that S. aureus superantigens bind to the gp130 receptor stimulate human adipocytes to produce proinflammatory cytokines and affect normal insulin signaling and adipocyte functions, which is strongly associated with insulin resistance [21, 63]. Besides, patients with T2D had a humoral immune deficit, possibly due to an immunoglobulin class switch defect (decreased S. aureus-specific IgG and increased IgM) during exacerbated S. aureus infection [64]. Moreover, we found that lack of nutritional immunity promotes S. aureus virulence in diabetic skin infections [65]. It is also noted that the S. aureus secreted protein, such as extracellular domain of LtaS (eLtaS), clumping factor A (clfA) that is associated with the human insulin, affects the glucose transporter GLUT4, and impairs glucose uptake in T2D [66]. Therefore, it can take measures to intervene the relevant target of S. aureus to achieve the goal of alleviating diabetes.
| Author | The precise mechanisms linking S. aureus infection and type 2 diabetes |
|---|---|
| Shu et al. (2008) | In S. aureus, the accessory gene regulator (Agr), consists of four genes (AgrA, AgrB, AgrC, AgrD), one of an autoinducing peptide (AIP), is the primary quorum sensing system responsible for inducing the transcription of several virulence factors |
| Banke et al. (2014) | S. aureus enterotoxin A binds to the gp130 receptor in adipocytes and affect normal insulin signaling and adipocyte functions, making them lower insulin sensitive |
| Schlievert et al. (2015) | S. aureus superantigens stimulate human adipocytes to produce proinflammatory cytokines. TSST-1 reduces levels of adipocyte anti-inflammatory genes for adiponectin and PPARγ, in which inactivation is strongly associated with insulin-resistance |
| Vu et al. (2015) | Chronically exposed to S. aureus superantigen TSST-1 experiences impaired glucose tolerance, systemic inflammation, and elevated endotoxin levels in the bloodstream, which facilitates the development of T2D |
| Christopher et al. (2015, 2018) | T2D had an impaired humoral immune response with reduced total IgG, decreased S. aureus-specific IgG, and increased IgM, and a humoral immune deficit, possibly due to an immunoglobulin class switch defect, in obesity and T2D during exacerbated S. aureus infection |
| Christopher et al. (2017) | An adaptation by S. aureus to obesity/T2D with an increased expression of mutation in clumping factor A (clfA) that is associated with the hypercoagulable state of the host and increased virulence of S. aureus |
| Liu et al. (2018) | The extracellular domain of S. aureus LtaS prevents insulin binding to the IR and abrogates signal transduction, which inhibits downstream IR signaling events involved in the promotion of glucose conversion, reduces recruitment of the glucose transporter GLUT4 to the cell membrane, and impairs glucose uptake |
| Lance et al. (2020) |
|
- Abbreviations: PPARγ, peroxisome proliferators-activated receptors γ; T2D, type 2 diabetes; TSST-1, toxic shock syndrome toxin-1.
With increasing antibiotic resistance and limited discoveries of novel antibiotics, it is imperative that need to explore other avenues for therapeutics [67]. Han et al. reported that regulating the relationship between phages and bacteria can maintain the health of the body and even reverse diseases [68]. The main papers reporting the effects of these supplements on the health of S. aureus infections are described in Table 8. The results reported that eight studies related to the effect of probiotic supplement on the S. aureus infections [69], and the probiotic administration might produce some metabolites, such as fengycin, lactic acid, and bacteriocin, and inhibit Agr quorum sensing, which can completely eliminate the intestinal colonization of S. aureus. Meanwhile, in our previous study, a strain of Lactiplantibacillus plantarum Lp84-3 with significant hypoglycemic and lipid-lowering was obtained through in vitro and in vivo models. By transcriptomic sequencing, we speculated that Lp84-3 might be induced by activating the expression of Akt and AKT2 genes in the insulin resistance signaling pathway, and GLUT4 expression is directly or indirectly regulated to inhibit the colonization of S. aureus, thus improving T2D. In addition, there were also two studies documenting that the prebiotic treatment also can decrease S. aureus colonization and lower proinflammatory signaling postinfection in obese/T2D mice [70]. Recently, fecal microbiota transplants (FMT) in the treatment of diseases related to intestinal and extra-intestinal flora disorders exhibited a promising prospect, which can also result in intestinal decolonization of extended S. aureus by producing some metabolities, such as short-chain fatty acids [71, 72]. Furthermore, with the increasingly serious problem of bacterial drug resistance, bacteriophage has come back to people's view [73]. It was reported that S. aureus's toxic shock toxin 1 and other superantigens are inserted into specific chromosomal sites, where they are excised and replicated by temperate phages. Collectively, it is very important to inhibit the colonization of S. aureus in intestinal tract and other tissues based on microecological therapy, However, the interaction and crosstalk relationships and its bacteriostatic mechanism need to be further clarified by using the multi-omics combined deep learning method.
| Authors | Country | Article type | Supplement | Outcomes of interest |
|---|---|---|---|---|
| David et al. (2018) | Singapore | Letter | Reprogramming probiotic (Lactobacillus reuteri) | Reprogramming probiotic L. reuteri as a biosensor for S. aureus-derived AIP-I detection |
| Pipat et al. (2018) | USA | Original | Probiotic (Bacillus) | The fengycin produced by Bacillus has a similar structure to AIP, a key factor in the Agr quorum sensing of S. aureus, and can interfere with Agr signal transduction, thus inhibiting Agr quorum sensing, which can completely eliminate the intestinal colonization of S. aureus |
| Li et al. (2018) | USA | Communication | Probiotic (Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2)+antibiotic | MRSA and Pseudomonas aeruginosa (PA) were eradicated using a combination of tobramycin and encapsulated probiotics |
| Liu (2020) | China | Original | Probiotic (Staphylococcus hominis S34-1) | In “probiotic” approaches, this strain led to reduced S. aureus infection and accelerated closure of S. aureus-infected wounds |
| Tan et al. (2020) | China | Original | Probiotic (Lactobacillus casei) | The inactivated L. casei biofilm shows excellent 99.98% antibacterial effectiveness against MRSA due to the production of lactic acid and bacteriocin |
| Wang et al. (2020) | China | Original | Probiotic (Bacillus subtilis) | With notable ability to survive and reside in the GI tract, coated Bacillus subtilis further shows a significantly enhanced decolonization effect in mice colonized with S. aureus |
| Wang et al. (2018) | China | Original | Probiotic (Lactobacillus rhamnosus)+brush sonication | Combination probiotic/brush sonication that can be developed to more effectively penetrate cracks and folds in the skin to remove S. aureus biofilms |
| Fang et al. (2019) | China | Original | Probiotic (Lactobacillus plantarum CCFM8610) | L. plantarum CCFM8610 treatment downregulated the functional genes of gut microbiota involving S. aureus infection |
| Hong et al. (2020) | China | Original | Prebiotic (galacto-oligosaccharides) | The wrinkle depth and S. aureus population decreased in the effects of a cosmetic serum containing galacto-oligosaccharides (GOS) |
| Tina et al. (2022) | USA | Original | Prebiotic (oligofructose) | Treatment with oligofructose significantly decreased S. aureus colonization and lowered proinflammatory signaling postinfection in obese/T2D mice |
| Amee et al. (2016) | Canada | Review | FMT | FMT resulted in intestinal decolonization of extended methicillin-resistant S. aureus |
| Hu et al. (2020) | China | Original | FMT | FMT reduces blood-milk barrier permeability by producing short-chain fatty acids, thus alleviating breast inflammation caused by S. aureus |
| Aleksandra et al. (2020) | Australia | Original | Bacteriophage | Three Myoviridae bacteriophages (AB-SA01) administered are safe in severe S. aureus infections, including infective endocarditis and septic shock |
| Andre et al. (2021) | USA | Original | Bacteriophage | Three Myoviridae bacteriophages (AB-SA01) administration significantly reduced S. aureus in axillary skin samples |
| Albac et al. (2020) | France | Original | Bacteriophage | Injection of bacteriophages showed significant antibacterial efficacy in two mouse models (nondiabetic and diabetic) of S. aureus foot infection |
- Abbreviations: AIP, autoinducing peptide; FMT, fecal microbiota transplants; MRSA, Methicillin-resistant S. aureus; T2D, type 2 diabetes; USA, United States of America.
There are several important limitations of our meta-analysis. First, our meta-analysis was not registered, for example, in a prospective register PROSPERO. Second, the sample sizes included were small. Third, different regimens, detection methods, and sampling sites were found in included studies. Thus, large, high-quality studies are required to validate our meta-analysis results.
4 CONCLUSIONS
This meta-analysis identified the pooled prevalence of S. aureus in various food types worldwide for the first time. The estimated S. aureus prevalence varied across the five continents and food types. In general, the prevalence of contamination with S. aureus indicates a higher prevalence of the bacterium in developed countries (e.g., Europe and North America) than that in Asia and Africa. Furthermore, the prevalence of S. aureus in cereals, meat products, and bean products was higher than that in dairy and seafood products; confectionery, egg products, and vegetables had the lowest prevalence of S. aureus. Contamination of these foods suggests that there is a potential risk for human health. Finally, it was observed that the culture method combined with molecular methods used to detect S. aureus increased the accuracy of the results. In addition, this study showed that the high antibiotic resistance rates of S. aureus isolates, from food, revealed that the rational use of antibiotics is of great importance. Meanwhile, it was suggested that S. aureus infection was closely associated with the development of T2D, and the treatment of probiotic, prebiotic, FMT, and bacteriophage can prevent and control S. aureus infections. It is believed that the results compiled herein will contribute to the epidemiological surveillance of the presence of S. aureus in foods worldwide. However, the mechanism linking S. aureus infection and T2D needs to be further clarified by using the multi-omics combined deep learning method.
Abbreviations
-
- AIP
-
- autoinducing peptide
-
- cfu
-
- colony-forming units
-
- CI
-
- confidence interval
-
- clfA
-
- clumping factor A
-
- eLtaS
-
- extracellular domain of LtaS
-
- FMT
-
- fecal microbiota transplants
-
- GLUT
-
- glucose transporter
-
- S. aureus
-
- Staphylococcus aureus
-
- SE
-
- staphylococcal enterotoxins
-
- T2D
-
- type 2 diabetes
AUTHOR CONTRIBUTIONS
Tingting Liang, Zhuang Liang, and Shi Wu analyzed the data and prepared the first draft of the manuscript. Tingting Liang, Zhuang Liang, and Shi Wu participated in the conception and design of the study, Yu Ding, Qingping Wu, and Bing Gu constructively revised the manuscript; Tingting Liang, Zhuang Liang, and Shi Wu participated in data collection and organization; Yu Ding, Qingping Wu, and Bing Gu participated in and supervised the study throughout, and they share the corresponding authorship. All authors commented on previous versions of the manuscript and approved the final version.
ACKNOWLEDGMENTS
We would like to thank Liang Wang and Ying Feng for the comments on the content of the article and also thank all participants for their time and involvement in this study. This work was supported by the Guangdong Province Academy of Sciences Special Project for Capacity Building of Innovation Driven Development (2020GDASYL-20200301002), National Natural Science Foundation of China (82072380), and Research foundation for advanced talents of Guangdong Provincial People's Hospital (KJ012021097).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
There is no data for this review.




