The antisickling agent, 5-hydroxymethyl-2-furfural: Other potential pharmacological applications
Abstract
For the last two decades, the aromatic aldehyde 5-hydroxymethyl-furfural (5-HMF) has been the subject of several investigations for its pharmacologic potential. In 2004, the Safo group reported that 5-HMF has potent antisickling activity by targeting and ameliorating the primary pathophysiology of hypoxia-induced sickling of erythrocytes (red blood cells [RBC]). Following the encouraging outcome of the preclinical and phase I/II clinical studies of 5-HMF for the treatment of sickle cell disease (SCD), there have been multiple studies suggesting 5-HMF has several other biological or pharmacologic activities, including anti-allergic, antioxidant, anti-hypoxic, anti-ischemic, cognitive improvement, anti-tyrosinase, anti-proliferation, cytoprotective, and anti-inflammatory activities. The wide range of its effects makes 5-HMF a potential candidate for treating a variety of diseases including cognitive disorders, gout, allergic disorders, anemia, hypoxia, cancers, ischemia, hemorrhagic shock, liver fibrosis, and oxidative injury. Several of these therapeutic claims are currently under investigation and, while promising, vary in terms of the strength of their evidence. This review presents the research regarding the therapeutic potential of 5-HMF in addition to its sources, physicochemical properties, safety, absorption, distribution, metabolism, and excretion (ADME) profiles.
1 INTRODUCTION
1.1 Overview
5-Hydroxymethyl-furfural (5-HMF) (Figure 1) is a small aromatic aldehyde molecule with a molecular weight of 126.11 Da, log Po/w of 0.19, and possesses an absorption maximum at 283 nm. A by-product of sugar metabolism, it is found in sugar/carbohydrate-containing foods as a natural product. It has an odor of chamomile flowers.1 5-HMF has an acidic pKa of 12.8 and is neutral at the physiological pH range (SciFinder Scholar®). It is a polar molecule, highly soluble in water across the entire pH range (54 g/L), and is also soluble in methanol, ethanol, acetone, ethyl acetate, dimethyl formamide, ether, benzene, and chloroform but less soluble in carbon tetrachloride and sparingly soluble in petroleum ether.1 It is almost liquid at room temperature (MP: 31.5°C) and should be stored away from light, moisture, air, and heat. It has a boiling point of 110°C at 0.02 mmHg and 114–116°C at 1 mmHg atmospheric pressure.1 Chemically, it is quite stable under physiological conditions, at least 6 months at 21°C in 5 mM phosphate buffer at pH 7.4, but amenable to enzyme-mediated metabolic degradation, for example, by aldehyde dehydrogenase.2-5
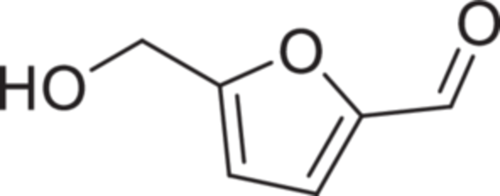
The existence of 5-HMF has been known for decades, where in industry it is used in the synthesis of dialdehydes, glycols, ethers, amino alcohols, acetals, and phenol/furfural novolak-type resins. However, it was not until the early 2000s that its therapeutic potential was discovered, first for sickle cell disease (SCD) and later for other diseases.6, 7 SCD is characterized by a primary pathophysiology of hypoxia-induced polymerization of human sickle hemoglobin (HbS) and subsequent sickling of erythrocytes or red blood cells (RBCs) that lead to several secondary adverse pathological effects.8-14 5-HMF was found to interact with hemoglobin (Hb) and increase the oxygen affinity of HbS, thereby reducing the polymerization and sickling.6, 7 Following the successful outcome of the preclinical and phase I/II clinical studies for SCD, 5-HMF became the subject of several other investigations for its potential pharmacologic activities for a variety of diseases or conditions (Table 1), which include cognitive disorders, gout, allergic disorders, hypoxia, cancers, anemia, hemorrhagic shock, liver fibrosis, oxidative injury, and so forth.15-29 Some of these conditions share a similar underlying cause of hypoxia as that of SCD.
| Disease | 5-HMF mode of action | References |
|---|---|---|
| Sickle cell disease | Increase Hb oxygen affinity to reduce hypoxia-induced polymerization of sickle Hb and the concomitant sickling of erythrocytes | [6, 7] |
| Hypoxia | Increase oxygen delivery to hypoxic tissues; activation of phosphorylation- extracellular signal-regulated kinase (p-ERK) | [15-18] |
| Anemia | Increase oxygen delivery to tissues with enhanced microvascular function during acute anemia | [19] |
| Oxidative stress | Protective role against oxidative stress by scavenging free radicals | [20] |
| Liver fibrosis | Suppress oxidative stress and scavenge free radicals and reactive oxygen species (ROS) to attenuate liver fibrosis | [21] |
| Alcohol-induced liver oxidative injury | Protective role against alcohol-induced liver oxidative injury due to antioxidant property | [21] |
| Ischemic brain damage | Antioxidative effect by increasing superoxide dismutase (SOD) content and decreasing free radical levels | [22] |
| Antiproliferative effect | Induce apoptosis of cancer cells and arrest G0/G1 cell cycle | [20, 23] |
| Hemorrhagic shock | Increase Hb oxygen affinity to enhance microvascular function | [24, 25] |
| Gout | Noncompetitive inhibition of xanthine oxidase to block its catalytic action in uric acid production | [26] |
| Allergic disorders | Interfere with antigen-antibody cross-linking and antibody-receptor binding | [27] |
| Cognitive disorders | Block scopolamine-induced learning deficits and improve cognitive function by activating N-methyl-D-aspartate (NMDA) receptor signaling | [28, 29] |
Although 5-HMF has been hypothesized to target several macromolecules that may be consistent with its proposed multiple pharmacologic actions, the most well-studied and validated target is Hb, where this compound forms a Schiff-base interaction with the αVal1 amine of the two α-subunits of Hb, which in addition to other noncovalent interactions, mostly via the 5-methylhydroxy substituent, leads to stabilization of R-state oxygenated Hb (KD of 0.37 mM).6, 7, 30 Formation of 5-HMF-calf thymus DNA complex in vitro has also been studied, where the binding constant of 103 L/mol suggests a weak affinity between 5-HMF and DNA.31 The binding of 5-HMF to DNA has been suggested to induce some damage to the DNA structure.31
This article aims to review studies that have reported potential applications of 5-HMF in treating various diseases or conditions, which vary in terms of their strength. We also provide a review of the source of 5-HMF, its physicochemical properties, and absorption, distribution, metabolism, and excretion (ADME) besides its safety profiles.
1.2 Sources of 5-HMF
5-HMF is a by-product of the Maillard reaction, which occurs during thermal treatment or thermal decomposition or dehydration of sugars and carbohydrates.18, 32-35 It is present naturally in many foods and drinks such as sweet potato, fruits, and fruit juices, honey, spirits, milk, beer, breakfast cereals, baked foods, pharmaceuticals, parenteral solutions, and cigarette smoke. Water extraction of artichoke and lemon peel at 180°C for 45 min has been reported to contain 58.83 and 231.21 mg/L of 5-HMF, respectively.36 Extraction of flaxseed meal at 200°C for only 15 min is known to produce 222.94 mg/L of 5-HMF.36 In addition to temperatures and time of extraction, other factors, such as cellulose and other plant contents, affect the amount of 5-HMF obtained from food.36 Water content and pH have also been shown to influence the amount of 5-HMF produced from foods.18, 34, 35 Commercially, 5-HMF is prepared from fructose with sulfuric acid as a catalyst.37
Humans are exposed to 5-HMF through the consumption of a number of commonly available beverages and foods, pharmaceutical preparations, cigarette smoke, and chewing tobacco.38-40 It is estimated that the daily consumption of 5-HMF ranges between 10 and 150 mg,41, 42 which is within the reported acceptable daily intake of 2.5 mg/kg.43 The International Federation of Fruit Juice Processors (IFFJP) recommends 5–10 and 25 mg/L 5-HMF concentrations in fruit juice and fruit concentrate, respectively.44, 45 Some diets, however, could result in as high as 1 g intake of 5-HMF.41 For example, a meal with instant coffee, a salad with balsamic vinaigrette dressing, toasted bread, wine, and dried pears with caramel for dessert has been reported to contain over 1 g of 5-HMF.40, 46-49 It is even reported that certain dried food products, such as coffee or caramel, may contain concentrations of 5-HMF that sometimes exceed 6 g/kg.47 Invertose- and glucose-containing parenteral solutions have been reported to have 5-HMF concentrations ranging from 3 to 56 mg/L and 1 to 4 mg/L, respectively.50 In a 2021 study on species-dependent 5-HMF formation in dried fruits, including apricot, gooseberry, cherry, cornel, blackcurrant, plum, and apple, it was found that the antioxidant activity for blackcurrant was highest, but lowest in apple, leading to the conclusion that higher antioxidant capacity is correlated with higher 5-HMF content.51
5-HMF is a nutraceutical in drinks.52 In Japan, 5-HMF is present in the common traditional barley tea drink.53 5-HMF is an active ingredient of a micronutrient supplement, Sanopal® (C.Y.L. Pharmazeutika GmbH) at a serving size of about 12 mg/kg.54 In addition, 5-HMF has been identified as a therapeutic moiety in traditional Chinese medicines.55-57
1.3 Safety profile of 5-HMF
Several studies have reported on the safety profile of 5-HMF, some of which are conflicting and unconfirmed. The EPA has determined the oral LD50 for 5-HMF for male and female rats of 2500 and 5000 mg/kg, respectively (US EPA, 1992; https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/hydroxymethyl_508.pdf), while other studies confirm an LD50 in mice of ~2000 mg/kg.50, 58 In comparison, the aromatic aldehyde vanillin, which has also been studied for its potential as an antisickling agent and considered nontoxic, has an acute oral LD50 of 1580 mg/kg in rats.59 The chronic oral dosing of 5-HMF at 375 mg/kg/day in rodents (rats and mice) over a 2-year period showed no serious toxicity (NTP, US Dept of Health and Human Services; https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr554.pdf?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=tr554). A study by Klimmek et al. with Beagle dogs who received an I.V. infusion of 397 mg/kg/h for 2 h reported an increase in heart rate, femoral blood flow, respiratory minute volume, and blood lactate, and a decrease in blood pCO2, pH, and bicarbonate.60 These results are expected for an overdose of 5-HMF, which would lead to an excessive increase in Hb affinity for oxygen and prevent O2 release, consequently leading to tissue hypoxia and anaerobic metabolism. The same study also reported no appreciable change in blood pressure, Hb, hematocrit, electrolytes, various-enzymes activities, urea nitrogen, creatinine, or total bilirubin. A study by Rasmussen et al. with rabbits that were given two daily subcutaneous injections of 400 mg 5-HMF for 6 days reported no significant effects on their mean body weight, Hb, leukocytes, platelets, serum-protein, serum-alanine-aminotransferase, or alkaline phosphatase.61 Histological examination of the liver also did not reveal any treatment-related effects.61 A 11-month study by Zaitsev et al. with rats (80 mg/kg/day oral dose) reported no observable change in protein and lipid metabolism, adrenal ascorbic acid content, hepatic succinate dehydrogenase activity, internal organ morphology, general physical condition, and body weight.62 However, by increasing the dose to 160 mg/kg, the investigators observed a temporary rise in the serum γ-globulin level, increase in spleen weight, and a tendency toward increased hepatic tributyrinase activity. Although elevated, these activities were reported to be within the tolerable range. Lang et al. also reported no toxic effects of 5-HMF when rats were given an oral dose of 250 mg/kg/day for 40 weeks as the animals showed normal weight gain, food consumption, and food-utilization efficiency, as well as normal histopathology of the liver, kidney, heart, spleen, and testis.63
In a study of 5-HMF by Abdulmalik et al. for its antisickling activity, incubation of sickled cells (SS cells) for 5 h with as high as 5 mmol/L of 5-HMF did not cause hemolysis of RBCs, nor promoted oxidation or denaturation of intracellular HbS.7 The nystatin loading test showed that 5-HMF had no notable effects on the Na–K pump, Na–H exchange, and Na–K–2Cl cotransport activities.7
Studies involving humans also found 5-HMF to be relatively safe. A 2007 study by Matzi et al. reported that 16 randomized patients who received the micronutrient supplement Sanopal® (720 mg/day 5-HMF) or placebo for 10 days before lung resection surgery showed no adverse effects, with a reported reduction in oxidative stress in the experimental group.54 In another study with Sanopal® by Matzi et al.,54 17 patients with metastatic thoracic cancer who received 5-HMF via I.V. for 26 days also did not report any adverse effects. Earlier, Herwig et al.64 had also reported that 7 patients with metastatic prostate cancer who received I.V. KARAL® solution (a combination of 5-HMF, α-ketoglutaric acid, N-acetyl-L-methionine and N-acetyl-L 5-selenomethionine) for 28 days did not have any adverse effect.64 A phase I/IIa clinical trial of 5-HMF with individuals with SCD, given an ascending dose up to 4 g, or placebo, reported no severe adverse events and only a few mild/moderate events, which include frequent vomiting by two patients.65 In a 2014 study of exercise performance and endurance in hypoxia, single doses of 5-HMF (1000 or 3000 mg) or placebo given to 11 patients per group were well tolerated by all patients, with only episode of nausea in one patient in 3000 mg 5-HMF group (NCT01871142).66
Despite the reports that suggest 5-HMF to be relatively nontoxic, several studies report contrary findings. In a recent 2020 study by Li et al., the authors reported that 5-HMF (7 and 35 mg/kg, I.V.) induces anaphylactoid reactions in vivo by elevating histamine and glutathione peroxidase (Gpx-1) levels in rats.67 The investigators also reported that 5-HMF resulted in the release of human β-hexosaminidase (β-Hex), and secretion of the inflammatory cytokines IL-4 and IL-6 from both RBL-2H3 and P815 cells, activating mast cell/basophil degranulation in the process. The authors concluded that 5-HMF represents a severe safety issue with more research required to control and monitor 5-HMF levels in food.67 Decades previous to these data, Simonian, et al. had reported that oral administration of 5-HMF at 310 mg/kg/day dose to rats for 2 months led to disruption of liver function, with elevation of hepatic tributyrinase, intestinal phosphomonoesterase 1 and enterokinase activities.58 A study where mice were treated with 250 mg/kg 5-HMF reported DNA damage, although it was noted that the toxicity may vary depending on the dose, animal species, and route of administration.38 The study also reported that overall protein, albumin, and globulin contents were decreased, which is thought to reflect the degenerative effect of 5-HMF on the liver.38 In addition, aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) released by damaged hepatocytes were found at elevated levels.38 The research speculated on the mechanism for the toxic effect: sulfonation of the allylic hydroxyl group on 5-HMF,38 which subsequently causes genome damage.38, 68, 69 Chi et al. suggested that 5-HMF is both cytotoxic and genotoxic, leading to the accumulation of toxins within humans.70 Additional studies have similarly suggested that 5-HMF irritates the upper respiratory tract, eyes, skin, and mucous membranes,71 while being potentially harmful to viscera and striated muscle, organs, and genetic material.71, 72 Of particular interest, a study by Delgado-Andrade et al. suggested that 5-HMF causes carcinogenesis in rats, and raised a concern regarding the ability of 5-HMF to diffuse passively through the monolayer of the cell membrane in Caco-2 cells.73
Other recent studies also describe the potential side effects of 5-HMF. One such study with C57BL/6 mice suggested that 5-HMF could trigger persistent and widespread inflammation, expediting the advancement of frailty in mice by promoting cell senescence and elevating the levels of inflammatory markers such as IL-6, TNF-α, and CRP.74 A study by Jiang et al. with zebrafish larvae suggested that high concentrations of 5-HMF caused an increase in reactive oxygen species, disruption of the Wnt signaling pathway, and subsequent cardiovascular abnormalities in zebrafish larvae.75 The same group reported studies in which high concentrations of 5-HMF led to developmental toxicity in zebrafish larvae by inhibiting cartilage development and reducing bone mineralization.76 Together, these data indicate that there may be toxic effects of 5-HMF, and any future clinical trials with this compound should be well-monitored for such adverse effects.
1.4 ADME properties of 5-HMF
Several studies have been conducted on the ADME of 5-HMF in animals and humans. In a study with rats and mice following oral administration of 5, 10, 100, or 500 mg/kg of 5-HMF, Godfrey et al. showed that 5-HMF was rapidly absorbed in the tissue of both animals.2 5-HMF was primarily excreted through urine, with 60% to 80% being excreted within 48 h. Three metabolites were identified (Figure 2), with 5-hydroxymethyl-2-furoic acid (HMFA) accounting for 80% of the metabolites. Furan-2,5-dicarboxylic acid (FDCA) and the glycine conjugate (N-5-hydroxymethyl-2-furoyly-glycine, HMFG) were reported as secondary metabolites, with the parent 5-HMF found in negligible quantity. It is not surprising that these three metabolites, which are more polar than the parent 5-HMF molecule, are excreted in the urine.2 The oxidation of 5-HMF has been suggested to be mediated by aldehyde dehydrogenase (ALDH), which is most prevalent in the liver.3 Germond et al. also reported that oral and intravenous administration of [14C]-5-HMF in rats resulted in rapid elimination of 5-HMF and its metabolites in urine, with 95% to 100% being recovered in 24 h.43 The investigators identified two metabolites—HMFA and HMFG—similar to the ones reported by Godfrey and colleagues. Higher 5-HMF doses led to similar rapid elimination with proportionally declining HMFG levels. Autoradiography confirmed presence of radioactive 5-HMF in the liver, with higher amounts found in the kidney and bladder.43 Oral and I.V. administration routes showed similar results, except for higher radioactive material in the brain tissue of injected rats.
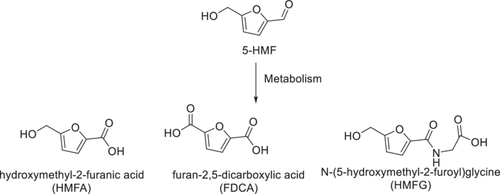
Mirochek and Rainey reported human ADME to be similar to the rodent data, with HMFA, FDCA, and HMFG present in all human urine samples that were analyzed using MS.77 A later study by Prior et al. (2006) used HPLC-MS/MS to identify 5-HMF metabolites in human subjects following consumption of dried plums or dried plum juice.78 This group identified four metabolites of 5-HMF instead of the previously reported three. Two of the metabolites, 5-carboxylic acid-2-furoyl) glycine (CFG); and 5-carboxylic acid-2-furoyl aminomethane (CFA), were not reported in the previous rodent studies. Lastly, a recent study also confirmed the formation of the three major metabolites of 5-HMF (HMFA, FDCA, and HMFG) in human urine after oral dosage.79
2 THERAPEUTIC POTENTIAL OF 5-HMF
This section reviews current literature on the therapeutic potential of 5-HMF, starting with its application in treating sickle cell disease. Other possible applications of 5-HMF (mostly in laboratory animals or cell lines) reviewed in this article include treatment of type I allergic diseases, Alzheimer's disease, gout, anemia, hypoxia, cancers, anemia, hemorrhagic shock, liver fibrosis, and oxidative injury.
2.1 Sickle cell disease
Sickle cell disease (SCD) is an inherited disorder that affects over 20 million people worldwide.80, 81 In SCD, the patient's red blood cells (RBC) have abnormal hemoglobin (Hb) due to a pathogenic mutation that replaces βGlu6 of the β-chains of normal Hb with βVal6, forming sickle hemoglobin (HbS). When deoxygenated HbS polymerizes and several downstream adverse effects are initiated, including but not limited to sickling of RBC, hemolysis of RBC, vaso-occlusion of the micro-vasculature, painful vaso-occlusive crises (VOC) crises, decreased vascular NO bioavailability, oxidative stress, inflammation, morbidity, organ damage, and early mortality.8-13 Two-thirds of individuals with SCD have the more severe, homozygous (HbSS) genotype, while one-third have the milder, heterogeneous genotype (HbSC).82 Potential curative therapies for SCD include bone marrow transplantation, stem cell transfusions, and gene replacement therapy. However, these are very costly and unaffordable to most patients, especially in the developing world. The FDA has approved four drugs, including hydroxyurea (Droxia and Siklos; manufactured by Bristol-Myers Squibb Company and Addmedica Laboratories, respectively),83 l-glutamine (Endari; Emmaus Life Sciences),84-86 crizanlizumab (Adakveo; Novartis),87 and Voxelotor® (Oxbryta; Global Blood Therapeutics/Pfizer)88-90 for the treatment of SCD. Voxelotor, like 5-HMF, is an aromatic aldehyde and is the first of this class of compounds to be approved for treating SCD. Aromatic aldehydes have been studied since the 1970s for their potential to treat SCD, though modern development of aromatic aldehydes for this disease started with the seminal work by collaborative groups from Virginia Commonwealth University (Safo and Abraham) and the Children's Hospital of Philadelphia (Asakura and Abdulmalik) with 5-HMF.6, 7 Other groups, including Global Therapeutics, who developed Voxelotor,88 Kato and Du groups who developed a state-of-the-art microfluidic system to study and quantify the antisickling effect of aromatic aldehydes, have also contributed immensely to the development of antisickling aromatic aldehydes.91, 92
The investigation of 5-HMF and several of its analogs, including 5-methyl-2-furfural (5-MF), 5-ethyl-2-furfural (5-EF), and furfural (FUF), for their antisickling properties began in the early 2000s.6 These compounds form Schiff base-adducts with Hb by interacting with the two α-chain αVal1 amines that lead to a substantial shift of the Hb allosteric equilibrium to the high-O2 affinity R-state, thus increasing the concentration of the non-polymer forming oxygenated HbS and preventing hypoxia-induced RBC sickling. The lower toxicity of 5-HMF, its greater partitioning into the RBC, and its more potent effect compared to its analogs and the previously studied aromatic aldehyde, vanillin, made it the most promising agent for treating SCD.6, 7 5-HMF also inhibited RBC hemolysis under sheer stress in vitro, while tissue and plasma proteins did not appear to have any inhibitory effect on the binding of 5-HMF to HbS.7 Further, using 5-HMF, the group demonstrated the molecular basis of aromatic aldehyde antisickling activities for the first time. Aromatic aldehydes preferentially bind to oxygenated Hb in the relaxed R2-state conformation, and not the previously proposed classical R-state conformation, to allosterically shift the equilibrium to the R-state Hb, increasing overall Hb affinity for oxygen.6 Additionally, 5-HMF binds to deoxygenated Hb and destabilizes the T-state, leading to an equilibrium shift to the R-state that increases its affinity for oxygen. In a study with transgenic (Tg) sickle mice under severe hypoxic conditions, the investigators showed that a single oral dose of 100 mg/kg 5-HMF was able to prolong the survival time of the mice.7 These results confirmed the success of the in vitro results, as 5-HMF administered to mice did not cause hemolysis, denaturation of HbS, nor formation of methemoglobin.7
In the phase I clinical study, a single oral dose of 5-HMF to healthy normal volunteers was absorbed rapidly, tolerated well, and absorbed preferentially into RBC relative to blood plasma.93 5-HMF was also well tolerated in SCD patients with promising improvements in reducing pain, decreased RBC hemolysis, improved diastolic blood pressure, and increased blood oxygen saturation (ClinicalTrials.gov identifier NCT01597401).66, 93-95 Nonetheless, the subsequent phase II study failed due in part to extensive oxidative metabolism of the 5-HMF, significantly shortening its pharmacologic effect via short half-life (~1 h) and low bioavailability.2, 4, 5, 93, 96
The outcomes of the preclinical and clinical studies for the treatment of SCD spurred further investigations into potential secondary antisickling mechanisms of 5-HMF beyond increasing Hb affinity for oxygen. One of the pathophysiological hallmarks of SCD is the dehydration of RBC which contributes to their rigidity and brittleness. The Gardos channel (Ca2+-activated K+ channel) has been shown to play an important role in this pathophysiology by contributing to potassium loss from sickling RBC and their subsequent dehydration.97 Hennemann and colleagues showed that 5-HMF was able to inhibit the Gardos channel and prevent RBC dehydration,98 supporting the hypothesis that 5-HMF could be developed to treat or prevent SCD through promoting RBC ion and water hemostasis.
Most individuals with sickle cell trait (heterozygous) do not usually experience any significant complications; however, blood flow velocity in these individuals decrease in regions of low oxygen tension due to higher blood viscosity as a result of the increased polymer formation that causes cell sickling.99 Hansen et al., using sickle trait blood, studied the ability of 5-HMF to restore such low-oxygen rheology in vitro.99 Blood obtained from six individuals with sickle cell trait was measured for blood flow velocity using a microfluidic device. 5-HMF treatment had a positive effect even at 0% oxygen tension, where at 1 mmol/L the drug significantly increased blood flow velocity by sixfold. The authors speculated that the effectiveness of 5-HMF at such low oxygen could be due to its direct influence on polymer stability via allosteric binding.99
2.2 Hypoxic injury
Hypoxia is known to play critical roles in the pathogenesis of several diseases, such as SCD, pulmonary hypertension, congestive heart failure, myocardial infarction, inflammatory diseases, chronic obstructive pulmonary disease, cerebrovascular diseases, and so forth.100 Like SCD, 5-HMF has also been studied by investigators for its pharmacologic activity against hypoxia to potentially treat some of these diseases. Yalcin and Cabrales conducted a study with hamsters to test whether increasing Hb oxygen affinity with 5-HMF would augment O2 transport during severe hypoxia (10% and 5% inspired O2) compared with normal Hb oxygen affinity.15 Male Golden Syrian Hamsters were infused with 20 mg/kg of 5-HMF or vehicle, and the effects of increasing Hb oxygen affinity were studied in the hamster window chamber model to monitor the systemic and microvascular hemodynamics and partial pressures of O2 (pO2). Pimonidazole binding to hypoxic areas of hamster heart and brain were also analyzed. The results showed that even though 5-HMF decreased the P50 [pO2 at which 50% of Hb is saturated with oxygen (sO2)] by 12.6 mmHg during 10% and 5% O2 hypoxia, arterial blood O2 saturation was increased by 35% and 48% compared to the vehicle group, respectively. The heart rate and blood pressure of the hamsters at 5% O2 hypoxia were 58% and 30% higher, respectively, for 5-HMF compared to the vehicle. 5-HMF preserved microvascular blood flow, whereas blood flow decreased to 40% of baseline in the vehicle group, resulting in perivascular pO2 that was three times higher in the 5-HMF group compared with the control group at 5% O2 hypoxia. 5-HMF-treated hamsters also showed reduced heart and brain hypoxic areas. The authors concluded that increased Hb oxygen affinity by 5-HMF resulted in hemodynamics and oxygenation benefits during severe hypoxia, which may have implications in treatment during severe environmental hypoxia when logistic constraints prevent chronic acclimatization.15
The protective effect of 5-HMF against hypoxia was also studied by Li et al. using ECV304 cells as an in vitro model. In the study, cells were pretreated with or without 5-HMF for 1 h, exposed to hypoxic condition (0.3% O2) for 24 h, and then investigated for cell apoptosis, necrosis, changes of mitochondrial membrane potential (MMP), and the expressions of phosphorylation-extracellular signal-regulated kinase (p-ERK).16 Cells treated with 5-HMF showed significant attenuation of hypoxia-induced necrosis and apoptosis at late stage, as well as the ability to rescue the decline of the MMP and an increase of p-ERK protein under hypoxia conditions.
In a follow-up study by the same group (Li et al.), they utilized Kunming mice in acute hypobaric hypoxia to verify the pharmacologic activity of 5HMF.16 The survival time and rate of mice were significantly increased when the animals were treated with 100 mg/kg (IP) 5-HMF for 1 h and exposed to acute hypobaric hypoxic condition for 6 h, when compared to untreated mice. 5-HMF also markedly attenuated acute hypobaric hypoxia-induced permeability of the blood brain barrier (BBB), attenuated cellular damage extent of the hippocampus and the cortex, especially in the hippocampus CA1 region. The authors concluded that 5-HMF enhanced the survival of mice through the decreased acute hypoxic damage to the brain, which may be linked to its effects on the BBB and p-ERK.16
5-HMF has been examined in several studies for its cardiovascular effects during hypoxia. In one such study, Lucas et al. explored the therapeutic potential of 5-HMF on left ventricular (LV) cardiac function (LVCF) during hypoxia. The investigators administered 5-HMF at 100 mg/kg by IV to Golden Syrian Hamsters at stepwise increased hypoxia (5%, 10%, and 15%) every 30 min. 5-HMF improved cardiac indices, including stroke volume (SV), cardiac output (CO), ejection fraction (EF), and stroke work (SW) compared to the vehicle group. At 5% O2, SV, CO, EF, and SW were increased by 53%, 42%, 33%, and 51%, respectively, with 5-HMF relative to vehicle. No significant change in heart chronotropic activity was observed, prompting the suggestion that differences in LV-CF during hypoxia by 5-HMF were driven by volume-dependent effects. Based on the fact that the coronary blood flow and cardiac muscle metabolism showed no direct pharmacological effects from 5-HMF, the authors attributed the results to 5-HMF-dependent increase in Hb-O2 affinity, and further concluded that high-O2 affinity compounds like 5-HMF have therapeutic potential by increasing O2 delivery during hypoxia.18
Mahon et al. studied the effect of 5-HMF on cardiovascular parameters during the exposure of a swine model to acute normobaric hypoxia (NH).17 The animals were randomly assigned to one of three treatment groups and received an equal volume of normal saline (VEH), 20 mg/kg 5-HMF, or 40 mg/kg 5-HMF, followed by the animals breathing 10% FiO2 (amount of oxygen inhaled) for 120 min. The 5-HMF-treated swine showed elevated oxygen affinity, oxygen saturation (sO2), and pulmonary artery pressure (PAP) during hypoxia. 5-HMF decreased the P50 (thereby increasing Hb affinity for oxygen), lowered heart rate (HR), and cardiac output (CO). 5-HMF at both concentrations also resulted in significantly smaller decrement in both arterial O2 saturations (sO2) and mixed venous oxygen saturation (sVO2), as well as smaller increases in mean pulmonary artery pressure (PAP), compared to the saline group. The authors concluded that 5-HMF could be used for acute NH conditions.17
Wolkart explored the potential cardioprotective effects of 5-HMF by subjecting isolated hearts to ischemia/reperfusion (I/R) injury in the presence and absence of the compound.101 The effects of 5-HMF on action potential and L-type Ca2+ current (ICa,L) were also studied by patch-clamping guinea pig isolated ventricular cardiomyocytes. The results showed that 5-HMF relaxed coronary arteries in a concentration-dependent manner and exerted negative inotropic, lusitropic, and chronotropic effects in the rat-isolated perfused hearts. On the other hand, 5-HMF improved recovery of inotropic and lusitropic parameters in the isolated hearts that were subjected to I/R. The patch clamp study also showed 5-HMF to be an inhibitor of L-type Ca2+ channels, prompting the suggestion that the cardioprotective effect of 5-HMF in I/R is mediated by its inhibition of L-type Ca2+ channels. The investigators concluded that 5-HMF could be a beneficial additive to cardioplegic solutions, though adverse effects and contraindications of Ca2+ channel blockers would need to be considered in therapeutic application of the drug.101
Ciarlone et al. conducted a study to test the hypothesis that 5-HMF would improve muscle performance in rats subjected to a simulated high altitude of ~5500 m.102 Fisher 344 rats were given either 40 mg/kg/day of 5-HMF or a placebo while being exposed to sea level or high-altitude conditions for 72 h. High altitude is known to reduce blood oxygen levels, elevates superoxide concentrations, and diminishes mitochondrial membrane potential. At sea level, 5-HMF was found to increase isometric force production and lower oxidant production. In rats that were exposed to high altitude, 5-HMF prevented a decrease in isometric force at some submaximal contractions by curbing superoxide levels. The investigators concluded that 5-HMF reduced hypoxic increase in skeletal muscle superoxide levels and prevented decreased plantar flexor isometric force production. Since continuous delivery of 5-HMF, did not improve arterial oxygen saturation (SaO2), the investigators suggested that 5-HMF was mainly acting as an antioxidant when continuously delivered.
The ability of 5-HMF to ameliorate hypoxic effects has also been tested in humans. Matzi et al. examined the potential of 5-HMF to reduce complications and shorten the recovery time of lung surgery patients.54 In an observer-blinded, prospective randomized clinical trial, 16 patients were given 7.2 mg of α-ketoglutaric acid (α-KG) and 720 mg of 5-HMF for 10 days, diluted in either orange juice or water. No side effects were observed. The investigators reported significant reduction in the hospitalization (9.9 ± 3.6 vs. 16.2 ± 5.5 days) and intensive care unit (ICU) (0.6 ± 0.5 days vs. 2.6 ± 2.0 days) of the study group compared to the control. They concluded that using a combination of α -KG and 5-HMF of preoperative micronutrition may be a step in a multimodality approach to improve recovery in fast-track lung surgery patients.
2.3 Microvasculature and tissue oxygenation during acute anemia
Though not as extensively as in hypoxia or SCD, studies have been conducted using 5-HMF to treat the symptoms of acute anemia. Cabrales et al. studied the effect of increasing Hb O2 affinity with 5-HMF on microvascular perfusion and tissue oxygenation during acute anemia in hamsters.19 The hamsters were infused by exchange transfusion of 35% of blood volume with 5-HMF-modified high-O2 affinity RBC with P50 lowered to 10 or 25 mmHg (normal P50 is 32 mmHg). Systemic parameters, microvascular perfusion, capillary perfusion (functional capillary density, FCD), and microvascular pO2 levels were measured. The RBCs modified by 5-HMF caused normal/optimal microvasculature and systemic conditions. A comparative experiment with the low-O2 affinity allosteric effector, inositol hexaphosphate (IHP), resulted in a P50 increase from 45 to 50 mmHg, which negatively impacted microvascular and systemic conditions by decreasing O2 delivery and extraction, reducing FCD, and/or lowering microvascular flow.19
2.4 Ischemic brain damage
Ischemia is associated with decreased blood flow, and it follows that 5-HMF might be effective at improving outcomes. Ya et al. studied the effect of 5-HMF on survival and oxidative stress in a mouse model of forebrain ischemia.22 The mice were intraperitoneally injected with 4 or 12 mg/kg 5-HMF (IP) for 30 min before ischemia or 5 min after ischemia. Low and high doses of 5-HMF prolonged the survival time by 29% and 33%, respectively, compared to untreated mice. The authors also reported increased superoxide dismutase (SOD) activity and reduced malondialdehyde content, similar to the effects of the positive control Edaravone, a hydroxyl radical scavenger used for the treatment of stroke. The investigators concluded that intraperitoneal injection of 5-HMF prolongs the survival of mice with permanent forebrain ischemia, due to its antioxidative effects through increasing SOD activity and decreasing free radical levels.22
2.5 Liver diseases
5-HMF has been studied by various groups for its protective effect against liver diseases or liver injury. Li et al. studied the effect of 5-HMF on acute alcohol-induced liver oxidative injury in mice.21 In this 2015 study, 5-HMF isolated from the fruits of Schisandra chinensis was used to dose (through gavage feeding) ICR mice at 7.5, 15, and 30 mg/kg for 7 days, followed by an examination of biochemical markers and enzymatic antioxidants from serum and liver tissues. Significant decrease in the activities of alanine aminotransferase (ALT) and aspartate transaminase (AST), as well as a decrease in the levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein (L-DLC), and malondialdehyde (MDA), were observed in the 5-HMF-treated group when compared with the group receiving alcohol. Additionally, the enzymatic antioxidants catalase (CAT), glutathione peroxidase (GSH-Px), and GSH and SOD were markedly elevated in 5-HMF-treated mice. The investigators also reported significant suppression of pro-inflammatory response marker tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Histopathological examination revealed that pretreatment with 5-HMF (30 mg/kg) notably prevented alcohol-induced hepatocyte apoptosis and fatty degeneration of the liver. The investigators suggested that the hepatic protective effects exhibited by 5-HMF on alcohol-induced liver oxidative injury may be due to its potent antioxidant properties.21
Testing 5-HMF with a known mouse model of liver fibrosis induced by carbon tetrachloride (CCl4) and alcohol showed beneficial treatment with this compound.103 Mice treated with the CCl4 and alcohol were gavaged with 5-HMF (7.5, 15, and 30 mg/kg B.W.) or a traditional Chinese medicine, Huganpian (350 mg/kg B.W.) daily, starting in the fourth week and lasting for 4 weeks. The positive control group (mice receiving only drinking water with or without alcohol) was similarly treated with 5-HMF or Huganpian. Results showed a significant reduction of hyaluronic acid (HA), laminin (LN), collagen type IV (CIV), and malondialdehyde (MDA), as well as a decrease in alanine transaminase (ALT) and aspartate transaminase (AST) enzymatic activities. High levels of ALT (an enzyme involved in cellular energy production) and AST (an enzyme involved in amino acid metabolism) are often indicative of liver damage. On the other hand, the enzymatic antioxidants SOD, CAT, and GSH-Px showed significant elevation. Additional histopathology analysis suggested that 5-HMF prevented hepatocyte apoptosis, fatty degeneration, and inflammatory cell infiltration on liver fibrosis. This led the authors to conclude that 5-HMF had a protective effect against liver injury and reduced liver fibrosis through suppression of oxidative stress.103
Ding et al. evaluated the protective effects of 5-HMF isolated from Fructus Corni on the human hepatocyte cell line, LO2, against cytotoxicity induced by hydrogen peroxide (H2O2) in vitro.104 5-HMF-treated LO2 cells (0.5 and 1 μg/mL) relieved cell damage induced by H2O2 in a dose-dependent manner. While H2O2 typically induces DNA damage and apoptosis, the 5-HMF treated cells (1 μg/mL 5-HMF for 24 h) showed a significant decrease in DNA degradation and apoptosis, thereby recovering the cell cycle by promoting the S phase into G2/M phase. Overall, 5-HMF caused a significant increase in the viability of LO2 cells, decrease of cell apoptosis, and recovery of the cell cycle in LO2 cells injured by H2O2, which was accompanied with a decrease in nitric oxide levels and caspase-3 activity. Confirming these results in a separate experiment, Jiang et al. investigated the protective effect of 5-HMF in human L02 hepatocytes injured by d-galactosamine (GalN) and tumor necrosis factor-α (TNF-α).105 These experiments show that 5-HMF caused significant increase in the viability of the injured L02 cells through a dose-dependent decrease in apoptotic cell death. Overall, these experiments suggest that 5-HMF protects LO2 cells by inhibiting apoptosis.
2.6 Wound healing
Kong et al. have investigated the ability of 5-HMF in wound healing.106 In this study, 5-HMF and silver nanoparticles (Ag-NPs) were incorporated in multifunctional poly(vinyl alcohol)/sodium alginate (PVA/SA) hydrogels. 5-HMF-embedded PVA/SA hybrid accelerated the wound healing process while pure PVA/SA hydrogels and Ag-NPs-embedded PVA/SA hydrogels wound healing effects were marginal. 5-HMF was found to stimulate fibroblast proliferation and migration as well as collagen synthesis in vitro, which is indicative of fast wound healing. 5-HMF also facilitated vascular endothelial growth factor (VEGF) and cluster of differentiation (CD31) expression for neovascularization, wound closure for reepithelization, and collagen deposition for wound remodeling to expedite the wound healing process in a large full-thickness wound excision rat model. The authors concluded that 5-HMF-embedded PVA/SA hydrogels could be an effective and affordable wound dressing candidate as an antioxidant/anti-inflammation-based tool for accelerating wound healing.106
2.7 Antiproliferative and anticancer
5-HMF has been identified by several groups as an antiproliferative agent. Some of the studies are reviewed here. Zhao et al. investigated the inhibitory effect of 5-HMF on human cancer cell proliferation by MTT assay, flow cytometric analysis, and the TUNEL and DAPI-containing assays.20 The results showed that 5-HMF displayed higher antiproliferative activity on human melanoma A375 cells than other cell lines. Mechanistically, 5-HMF appeared to induce A375 cell apoptosis and G0/G1 cell cycle arrest. The investigators suggested that 5-HMF has the potential to be developed as a novel natural antioxidant with applications in cancer chemoprevention.
The membrane channel, aquaporin-1 (AQP1), acts as dual water and ion channels and is also known to enhance migration and invasion in cancer cells.107 Chow et al. published a study that suggested 5-HMF was able to inhibit AQP1 ion channels. In the study, 5-HMF and several related analogs, 5-nitro-2-furoic acid (5NFA), 5-acetoxymethyl-2-furaldehyde (5AMF), and methyl-5-nitro-2-furoate (M5NF), were analyzed for their effects on AQP1 activity in Xenopus oocytes and cell migration, invasion, and cytoskeletal organization in a differentially expressing cancer cell lines (HT29—high-AQP1 colon cancer line, MDA—AQP1-expressing breast cancer line, and SW480—low-AQP1–expressing cancer line). The results indicated that three compounds 5-HMF (IC50 0.43 mM), 5NFA (IC50 1.2 mM), and 5AMF (IC50 ∼3 mM) showed dose-dependent blockage of ion conductance, but not water flux, through AQP1 channels. The investigators concluded that 5-HMF, along with furans, could be considered a new class of AQP1 ion channel inhibitors for the development of therapeutic agents for controlling cancer metastasis targeting aquaporin channel activity.107
Additional data suggest that 5-HMF inhibits interleukin-1β and tumor necrosis factor-α expression, which has led to a phase II clinical study of 5-HMF in combination with other substances as a cytostatic agent.108 This cytostatic effect of 5-HMF and its structural analogs was postulated to be related to the ability of the compound to selectively inhibit both terminal deoxynucleotidyltransferase (TdT) with an IC50 of 5.5 µM and DNA polymerase λ with an IC50 of 26.1 µM in a competitive manner.108
In another investigation, Greilberger and colleagues demonstrated that the introduction of a combined solution containing α-KG and 5-HMF to leukemic cells led to antitumoral, antiproliferative, and apoptotic outcomes.109 These effects appeared to stem from the antioxidative capabilities of these substances, which not only prevent oxidative changes in proteins but also counter protein nitration and oxidative DNA damage. This is closely associated with elevated nitrotyrosine levels, the release of cytochrome c, activation of caspase 3, and modification of the p53 gene. Importantly, these compounds did not exhibit any adverse impacts on human fibroblasts. In a subsequent investigation, the researchers found that among the two substances, 5-HMF demonstrated significant antioxidative potential in effectively neutralizing peroxynitrite and tyrosine residue nitration. As a result, it more robustly hindered cell growth and mitochondrial activity compared to α-KG.110
2.8 Anti-inflammatory
Several studies have investigated the anti-inflammatory properties of 5-HMF. Kong et al. used lipopolysaccharide (LPS)-stimulated RAW 264.7 cells to understand the mechanism of how 5-HMF acts as an anti-inflammatory compound.111 LPS-stimulated cells that were pretreated with 5-HMF (31.5–126.0 μg/mL) significantly reduced the production of nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) when compared with a control of only LPS-stimulated cells. Additionally, the pretreated cells significantly downregulated the mRNA expression of the major inflammatory mediators, nitric oxide synthase (NOS) and cyclooxygenase-2 (COX-2), and suppressed the production of pro-inflammatory cytokines when compared with the untreated cells. 5-HMF also suppressed the phosphorylation of extracellular regulated protein kinases (ERK1/2), c-Jun N-terminal kinase (JNK), IκBα, NF-κB p65, the mammalian target of rapamycin (mTOR), and protein kinase B (Akt). Finally, 5-HMF inhibited NF-κB p65 translocation into the nucleus to activate inflammatory gene transcription. Based on these results, the authors concluded that 5-HMF could mitigate the inflammatory response in LPS-stimulated RAW 264.7 macrophages by suppressing the protein phosphorylation of MAPK, NF-κB, and Akt/mTOR signal pathways, and has the potential to be a therapeutic component in food products.111
Gorczynski, et al. also investigated the protective effect of 5-HMF on a mice model of severe colitis with enhanced colonic expression of inflammatory cytokines.112 5-HMF (100 mg/kg/day, orally) was able to attenuate the disease in the mice, prompting the suggestion that 5-HMF may be of clinical value as an adjunctive for the treatment of colitis and attenuating inflammatory cytokine production.
In a study aimed to evaluate the impact of 5-HMF on multiple sclerosis (MS), it was shown that 5-HMF promoted IFN-γ-stimulated microglial M2 polarization, reducing inflammation in a IFN-γ-stimulated murine microglia (BV2 cells) as a model for MS.113 Network pharmacology and molecular docking results suggested a 5-HMF had a binding site for migratory inhibition factor (MIF). By binding to MIF and disrupting the MIF-CD74 interaction, 5-HMF impedes microglial M1 polarization and enhances the anti-inflammatory response. Following this, an experimental autoimmune encephalomyelitis (EAE) mouse model was established, followed by injecting 5-HMF. In vivo, 5-HMF improves EAE, inflammation, and demyelination. This study underscores 5-HMF's role in advancing microglial M2 polarization through the inhibition of MIF-CD74 interaction, consequently alleviating inflammation and demyelination in EAE mice.
2.9 Hemorrhagic shock
The potential application of 5-HMF in protecting against hemorrhagic shock has been demonstrated by Villela et al.24 The investigators introduced the high-O2-affinity 5-HMF (low P50) and the low-O2-affinity Hb allosteric effector IHP (high P50) into RBCs through electroporation to increase and decrease the oxygen affinity of hemoglobin, respectively, resulting in the modification of P50 to between 10 and 50 mmHg (the normal P50 was 32 mmHg). Hamsters were hemorrhaged to lose 50% of blood volume and subjected to a shock episode of 1 h. The animals were resuscitated using 25% blood volume with either 5-HMF-treated or IHP-treated erythrocytes with a 50% hematocrit level. Following resuscitation, the base excess (BE) was observed to be substantially lower compared to the baseline for the group with high P50 RBC (IHP-treated), while the mean arterial pressure (MAP) was significantly lower than the initial baseline for the group with low P50 RBC (5-HMF-treated).24 The arteriolar diameter and blood flow rate were reported to be lower in the group with high P50. Moreover, the functional capillary density was found to be considerably lower in the high P50 group than in the low P50 group at 1 and 1.5 h following resuscitation. Notably, the oxygen extraction ratio was substantially increased in the high P50 group when compared to the low P50 group, suggesting that increasing the oxygen affinity of blood using a 20 mM concentration of 5-HMF in resuscitation after hemorrhage provides enhanced microvascular function.24 This demonstrated that in conditions of limited oxygen-carrying capacity of RBCs due to hemorrhage in an organism at rest, oxygen delivery to tissues is improved through the effect of 5-HMF, presumably through its ability to increase hemoglobin affinity for oxygen.
A study by Cabrales et al. used a resuscitation protocol similar to the one used by Villela et al. and found that 5-HMF increased the oxygen affinity of hemoglobin and improved the microvascular function of fresh erythrocytes during hemorrhagic shock resuscitation.25 This ability could be applied to similar conditions where there is a need to reestablish the supply of oxygen to tissues.24, 25 It is particularly notable that the 5-HMF-induced increased oxygen affinity of hemoglobin is effective in delivering and releasing oxygen preferentially in low PO2 regions, bypassing delivery to well-oxygenated regions, thus optimizing the overall oxygen delivery process during hemorrhagic shock. This particular feature of 5-HMF makes it a prime candidate for this application and warrants further investigation.
2.10 Gout
Gout is one of the most prevalent forms of arthritis, as a result of hyperuricemia. Xanthine oxidase (XO) is a critical enzyme in the production of uric acid by catalyzing the conversion of hypoxanthine to xanthine, which is subsequently converted to uric acid.114, 115 Hyperuricemia emanates from the overproduction of uric acid or under-excretion by kidneys, and is a critical prerequisite for the development of gout, as well as a prognostic marker for adverse hypertension and a predictor of cardiac death among stroke victims.115 For over four decades, hyperuricemia and gout have been treated using Allopurinol as the only clinically available drug with xanthine oxidase inhibitory (XOI) activity, despite its well-documented renal and hepatic toxicity.116 In 2012, Lin et al. investigated the mode of action of vinegar-based 5-HMF as a potential XOI agent.26 In the study, the investigators examined the XOI activity of seven commercial vinegar samples and one laboratory-made red-koji vinegar. After showing more potent XOI activity than the other seven samples, the compounds in the laboratory-prepared vinegar were isolated and studied for structural identification and activity. 5-HMF was identified as one of the compounds in the vinegars that exhibited xanthine oxidase inhibitory (XOI) activity. Notably, 5-HMF had a dose-dependent XOI activity with an IC50 168 ug/mL. This observation was supported by Michaelis–Menten kinetics analysis that identified 5-HMF as a noncompetitive inhibitor for XO. This study provided the first scientific evidence that supported the previous observation that ingestion of vinegars led to a relief of gout discomfort.
Other researchers have widely examined the XOI activity of 5-HMF as the bioactive compound in different medicinal plants, herbs, and foods used in traditional medicine.117-119 These investigators concluded that the ability of 5-HMF to inhibit XO makes it a potential agent for use in minimizing hyperuricemia risk and relieving inflammation in gout patients.
2.11 Type 1 allergic disorders
Alizadeh studied 5-HMF for its anti-allergic effect using BALB/c mice immunized with ovalbumin (OVA).120 These immunized mice had an increased level of total serum and OVA-specific antibodies when compared to the control (p < 0.01). Mice in both the control and sensitized groups were either fed with normal chow-diet throughout the study or diets containing low and high doses of 5-HMF that provided a daily intake of 188 and 750 mg/kg of body weight during the experiments for 5 days per week. The 5-HMF-treated group significantly suppressed OVA-induced increase in serum IgE and OVA-specific IgE (p < 0.05), as well as significantly reduced interleukin-4 (IL-4) and interferon-γ (IFN-γ) in a dose-independent manner. The authors concluded that 5-HMF inhibited the upregulation of total and OVA-specific IgE through the suppression of the Th2-type immune response in immunized BALB/c mice, and suggested that 5-HMF could serve as a novel therapeutic approach for the prevention of IgE-mediated allergic diseases.120
Yamada et al. also reported the potential of 5-HMF to prevent and treat type 1 allergic disorders in a study involving the use of a hot water extract of Lycium chinense fruits (LCF) found to contain 5-HMF. The extract showed an inhibitory effect on β-hexo release from RBL-2H3 cells.27 Additionally, 5-HMF was able to suppress [Ca2+]I influx in the IgE-sensitized BSA-stimulated RBL-2H3 cells, leading to the conclusion that 5-HMF may be useful for the treatment or prevention of type I allergic diseases.
2.12 Cognitive disorders
Liu et al. studied the ability of 5-HMF to improve cognitive impairment in Aβ1–42 mouse model of Alzheimer's disease.28 5-HMF extracted from Alpinia oxyphylla Miq. was used to treat mice at doses of 15 and 150 μg/kg (ICV) for five consecutive days after ICV administration of Aβ1–42. The investigators reported significant amelioration of learning and memory impairment as evaluated by the locomotor activity, Y-maze test, and Morris water maze test. They also showed that 5-HMF significantly inhibited β-secretase activity, decreased the content of Aβ1–42 and malondialdehyde (MDA), and increased the antioxidative enzyme activities including SOD and GPx. Additionally, hippocampal slices showed that animals that were administered 5-HMF exhibited integrated and regularly arranged neurons, indicating that 5-HMF could mitigate the degree of neuronal damage. The authors concluded that 5-HMF may serve as a potential therapeutic agent for the treatment of Alzheimer's disease.
Lee et al. also evaluated the effect of 5-HMF to treat cognitive impairment induced by a muscarinic receptor antagonist, scopolamine (1 mg/kg), using the step-through task of passive avoidance, the Morris water maze task, and the Y-maze task.29 The investigators noted that a single dose of 5-HMF (5 mg/kg) by oral gavage significantly attenuated cognitive impairment induced by scopolamine in these behavioral tasks without any changes in the rats' locomotion activities. Additionally, cognitive impairment was considerably reversed after administration of a sub-effective dose of an NMDA receptor antagonist, MK-801. Moreover, a single dose of 10 mg/kg 5-HMF orally-administered in normal naïve mice improved their cognitive performance in passive avoidance tasks.29 Further, the authors reported that Western blot analysis of hippocampal tissues extracted from both hemispheres of mouse brain indicated a significant increase in the levels of extracellular signal-regulated kinases (ERK) and phosphorylated Ca2+/calmodulin-dependent protein kinase II-α (CaMKII) following a single 5-HMF (10 mg/kg) administration in male mice. The authors concluded that 5-HMF would be an effective candidate for treating cognitive disorders such as Alzheimer's disease, as it blocks the learning deficits induced by scopolamine and improves cognitive function by activating NMDA receptor signaling.29
3 CONCLUSION AND PERSPECTIVES
This review article analyzes the current research and presents peer-reviewed evidence on potential therapeutic applications of 5-HMF in treating several disease conditions. While collectively these studies vary in terms of the strength of their evidence, a subset provides reliable, intriguing findings which form a strong basis for further investigations, as 5-HMF and/or its analogs are developed for the treatment of the diseases discussed here. For example, 5-HMF showed potential for the treatment of SCD by increasing oxygen affinity and reducing polymerization of HbS and sickling of RBC, although the less-than-optimal PK properties prevented it from being developed into a clinical drug. Nonetheless, this study established the scientific proof of the mechanism of 5-HMF on sickle blood and in patients with sickle cell disease that was later validated by the FDA approval of Voxelotor, an aldehyde compound that provides the same effects as 5-HMF, albeit with greater potency and better oral bioavailability. Several other studies also show the ability of 5-HMF to improve tissue oxygenation and/or microvascular function in acute anemia, hemorrhagic shock, or hypoxic injury. In addition, for gout patients, 5-HMF has been shown to block uric acid formation through noncompetitive inhibition of xanthine oxidase in blocking catalytic action in uric acid production, thus making it a more potent and safer treatment compared to the highly toxic allopurinol. Similarly, other studies show that 5-HMF could be effective in treating type I allergic diseases by inhibiting β-hex release at the antigen-antibody and antibody-receptor binding stages. 5-HMF is also reported to possess properties that make it highly potent in the treatment of liver fibrosis by suppressing oxidative stress and scavenging free-radicals and reactive oxygen species (ROS) to attenuate liver fibrosis. Finally, this review article has collated data that show how 5-HMF can be applied in treating cognitive disorders, such as Alzheimer's disease by blocking scopolamine-induced learning deficits and improving cognitive function through activation of NMDA receptor signaling. Overall, the current research builds a case for further preclinical and clinical trials, where appropriate, to proceed with developing 5-HMF as a therapeutic candidate for several diseases. While additional testing is necessary, the data reviewed here demonstrate that the naturally occurring 5-HMF compound in some instances could be more efficacious and potentially safer than the existing conventional pharmaceutical alternatives for many diseases.
ACKNOWLEDGMENTS
This work was supported by NIH/NIMHD grant R01MD009124 (MKS) and NIH/NHLBI grants R61HL156158 and R33HL156158.
Biographies
Piyusha P. Pagare, PhD: Dr. Pagare is a Postdoctoral Fellow in the Department of Medicinal Chemistry, School of Pharmacy, at Virginia Commonwealth University. She obtained her BS and MS degrees in Chemistry from the University of Mumbai, India followed by a PhD degree from Virginia Commonwealth University in 2018 in Medicinal Chemistry. Her areas of expertise and interest are Structure-Based Drug Design, Sickle Cell Disease, Substance Use Disorder, Pain, and Cancer. Dr. Pagare has over 8 years of experience in the field of drug discovery, particularly small molecule candidates as potential sickle cell disease therapeutics. In her PhD tenure, Piyusha was instrumental in developing second-generation antisickling agents in Dr. Martin K. Safo's laboratory, some of these compounds are poised to enter phase I clinical studies. She has authored several peer-reviewed publications. Currently, Dr. Pagare's research focuses on substance use disorders and pain management, particularly toward identifying promising small molecules for opioid use disorder treatments.
Mina McGinn, BS: Ms. Mina McGinn is a Biochemistry Masters student in the Department of Radiation Oncology at Virginia Commonwealth University, Richmond, Virginia. Her research focuses on the role of exosomes in cancer progression. After completing her BS degree in 2016, Mina began research in the lab of Dr. Martin Safo at the Department of Medicinal Chemistry at VCU where she assisted with the identification of antisickling molecules for the treatment of sickle cell disease. Her contributions focused on the modification of hemoglobin structure and oxygen-binding properties in response to the binding of allosteric effectors. Her experience also concentrated on the interaction between hemoglobin and 5-HMF, as well as its further clinical applications. Mina also has 7 years of clinical background in dentistry. In 2021, she obtained her Premedical Graduate Sciences Certificate at VCU with the intent of pursuing her DDS after completing her MS degree in 2022. As part of her graduate research, Mina examines the function of exosomes in modulating cancer progression and other disease states. She focuses namely on the mechanism by which exosomes influence breast cancer tumor growth and metastasis. She aims to ultimately integrate her research and clinical experiences into her professional career in the dental field.
Mohini S. Ghatge, PhD: Dr. Mohini Ghatge is an adjunct faculty at the Institute for Structural Biology, Drug Discovery and Development, Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, Virginia. Ghatge carried out her dissertation work at the Government of India's CSIR laboratory, National Chemical Laboratory in Pune, and obtained her PhD degree in Microbiology from the University of Pune in 1993. Ghatge has been working in the field of Biotechnology for more than 35 years. Her areas of expertise and interest are in Molecular Biology, Protein Biochemistry, Enzymology, and Structural Biology. Ghatge has been working in drug discovery for the past 18 years with a particular focus on sickle cell disease. She has studied Vanillin, 5-HMF, RSR-13, and their analogs as Allosteric Effectors of Hemoglobin for their potential treatment of sickle cell disease and/or hypoxia underlying diseases. She is also involved in the study of vitamin B6 (PLP) homeostasis. Ghatge has published several peer-reviewed articles, and her work in both biotechnology and drug discovery has led to several patents.
Vibha Shekhar, BS: Ms. Vibha Shekhar is a current medical student at the Virginia Commonwealth University School of Medicine, Richmond, Virginia. She obtained her BS degree at Virginia Commonwealth University in 2020 with a major in Biology and a minor in Chemistry. Vibha will be completing her medical degree in May 2024 and seeks to pursue a career in Endocrinology. Vibha's areas of research interest are Cellular & Molecular Biology, Internal Medicine and Endocrinology, and Hematology & Oncology. She has worked under Dr. Martin Safo since 2015 at the Institute for Structural Biology, Drug Discovery, and Development, investigating the effects of chemical modulators on hemoglobin affinity for oxygen, specifically about the pathogenesis of Sickle Cell Disease.
Rana T. Alhashimi, PharmD: Ms. Rana Alhashimi is a PhD graduate student at the Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, Virginia. Rana obtained her BS degree from King Abdul-Aziz University, Saudi Arabia in 2013 in clinical pharmacy. Her PhD research is focused on Sickle Cell Disease Drug Development in Dr. Martin Safo's laboratory.
B. Daniel Pierce, PhD: Dr. Daniel Pierce is an Associate Professor in the Department of Biology at the University of Richmond, Richmond, Virginia. His research focuses on the molecular biology and biochemistry involved in the pathogenesis of Agrobacterium tumefaciens toward its plant hosts. Pierce has both intramural and extramural funding via the Jeffress Trust Award Program to develop a biochemical and biophysical understanding of Agrobacterium signal perception, create drugs to disrupt its function, and engineer bacteria using synthetic biology that stimulates plant defenses. Pierce has a variety of teaching interests, including Cancer Biology & Tumorigenesis, Cellular & Molecular Biology, Synthetic Biology, Introductory Biology, and SMART (Science Math and Research Teaching). Pierce obtained an NIH K12 IRACDA (Institutional Research and Career Development Award) for his research at Emory University with an appointment to teach at Clark Atlanta University. At Emory, he became a faculty member with ETSI, the Emory Tibet Science Initiative, and continues to teach biology to Tibetan monks exiled to India. Pierce performed his graduate work at Johns Hopkins University in the Cell, Molecular, Developmental Biology, and Biophysics program, uncovering the biophysics and biochemistry of the yeast endocytic scaffold Pan1, and obtaining his degree in 2012.
Osheiza Abdulmalik, MS DVM: Dr. Osheiza Abdulmalik is a Principal Investigator and Senior Research Associate at the Children's Hospital of Philadelphia, Pennsylvania. Research in the Abdulmalik Laboratory focuses on the development of novel therapies for hemoglobinopathies, with a particular emphasis on developing and validating novel therapeutic agents for sickle cell disease (SCD). Specifically, a major part of the lab's research focuses on the design, development, and investigation of small molecules targeting sickle hemoglobin and disrupting the polymerization that underlays the primary pathophysiology of SCD. The lab maintains ongoing, highly productive drug-development collaborations with an array of academic investigators as well as industry to investigate small molecules that can potentially benefit patients with SCD. Dr. Abdulmalik has participated in or led pioneering work in this area with Dr. Martin Safo's group at VCU, which has resulted in novel investigational candidate drugs. The lab team also investigates and optimizes non-pharmacologic therapeutic approaches for SCD and beta-thalassemia, and they study transgenes that encode stability-enhanced β-globin mRNA for β-hemoglobinopathies.
Yan Zhang, PhD: Dr. Yan Zhang is a Professor at the Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, Virginia. Dr. Zhang is also currently an Associate Member of the Institute for Structural Biology, Drug Discovery and Development, a faculty member at the Institute for Drug and Alcohol Studies, and an Associate Member of Massey Cancer Center, VCU. Zhang obtained his PhD degree from the Peking Union Medical College, Beijing, China in 1997 in organic and medicinal chemistry. Zhang's areas of interest are drug design, discovery, and development, particularly in the fields of neurological disorders, cancer, infectious diseases, and sickle cell disease. Zhang has been continuously funded as a PI by federal agencies, for example, NIH and DOD and non-profit organizations, for over 20 years, and has published over 120 peer-reviewed research articles in the related areas. Zhang has over 25 years of experience in drug design, discovery, and development, and is a peer-recognized expert in substance use disorders and pain management. For the past several years he has directed drug discovery programs to identify promising small molecules as opioid use disorder treatment agents. Dr. Zhang has also participated in the sickle cell disease drug discovery program with Dr. Safo, which has led to the discovery of several antisickling agents for the treatment of SCD. Zhang is an inventor of many drug compositions and use patents and has licensed several of these IPs to companies for further development to treat various clinical diseases.
Martin K. Safo, PhD: Dr. Martin Safo is a Professor at the Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University, Richmond, Virginia. Dr. Safo is also currently the interim director of the Institute for Structural Biology, Drug Discovery, and Development, VCU, as well as the Director of VCU Structural Biology Core. Safo obtained his PhD degree from the University of Notre Dame in 1991 in inorganic chemistry and X-ray crystallography. His areas of expertise and interest are Structural Biology, Hemoglobin Structure and Function, Sickle Cell Disease Drug Development, Vitamin B6 Metabolism, and Regulation. Safo has been continuously funded as PI by the NIH, pharma/biotech companies, and non-profit groups for almost 30 years and has over 140 peer-reviewed manuscripts. Safo is also an inventor of many drug compositions and use patents and has licensed some of these IPs to companies for further development to treat various clinical diseases. Safo has over 30 years of experience in drug discovery and is a world-renowned expert in sickle cell disease (SCD) and antisickling agents. For the past several years Safo has directed drug discovery programs to identify promising small molecule antisickling agents for the treatment of SCD. He has taken one such drug, 5-HMF through Phase II clinical studies, and poised to follow this achievement with a more promising second-generation antisickling agent of Phase I clinical study. Safo has consulted and/or has been the advisory board member for several companies in their sickle cell drug development programs.
Open Research
DATA AVAILABILITY STATEMENT
Data are freely available online through the Medicinal Research Review open access option.




