Poly (adenosine diphosphate-ribose) polymerase inhibitors in the treatment of triple-negative breast cancer with homologous repair deficiency
Abstract
Breast cancer (BC) is a highly heterogeneous disease, and the presence of germline breast cancer gene mutation (gBRCAm) is associated with a poor prognosis. Triple-negative breast cancer (TNBC) is a BC subtype, characterized by the absence of hormone and growth factor receptor expression, making therapeutic decisions difficult. Defects in the DNA damage response pathway due to mutation in breast cancer genes (BRCA 1/2) lead to homologous recombination deficiency (HRD). However, in HRD conditions, poly (adenosine diphosphate–ribose) polymerase (PARP) proteins repair DNA damage and lead to tumor cell survival. Biological understanding of HRD leads to the development of PARP inhibitors (PARPi), which trap PARP proteins and cause genomic instability and tumor cell lysis. HRD assessment can be an important biomarker in identifying gBRCAm patients with BC who could benefit from PARPi therapy. HRD can be identified by homologous recombination repair (HRR) gene-based assays, genomic-scarring assays and mutational signatures, transcription and protein expression profiles, and functional assays. However, gold standard methodologies that are robust and reliable to assess HRD are not available currently. Hence, there is a pressing need to develop accurate biomarkers identifying HRD tumors to guide targeted therapies such as PARPi in patients with BC. HRD assessment has shown fruitful outcomes in chemotherapy studies and preliminary evidence on PARPi intervention as monotherapy and combination therapy in HRD-stratified patients. Furthermore, ongoing trials are exploring the potential of PARPi in BC and clinically complex TNBC settings, where HRD testing is used as an adjunct to stratify patients based on BRCA mutations.
1 INTRODUCTION
Breast cancer (BC) is the most common global malignancy among women, accounting for one-fourth of the cancer burden, with an incidence of 2.3 million cases (11.7%).1 Hereditary susceptibility due to autosomal dominant gene mutation is a major risk factor in acquiring BC.2 BC is highly heterogeneous and is classified into various subtypes according to the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Patients with BC lacking the expression of ER, PR, and HER2 (14%–16%) are termed as triple-negative breast cancer (TNBC). TNBC is an aggressive subtype of BC, accounting for about 15%–20% of BC cases, and shows poor prognosis, high recurrence, and high mortality rates. Most of the TNBC patients develop distant metastatic recurrence which is linked to a rapidly progressing illness and early mortality (<12 months).3 Based on molecular heterogeneity, TNBC is further divided into 6 different subgroups: basal-like, mesenchymal-like, mesenchymal stem-like, luminal AR expression, immunomodulatory, and unstable type.4
The greatest risk factor for BC is the introduction of mutation in any one of the breast cancer genes (BRCA1 or BRCA2). BRCA1 and BRCA2 (BRCA1/2) tumor suppressor gene–encoding proteins that are essential for DNA damage repair, cell growth regulation, and cell division. Both proteins are known to function in homologous recombination (HR), a vital DNA repair process that uses the undamaged sister chromatid to perform high-fidelity repair of predominantly replication-associated DNA double-strand breaks (DSB). Genetic variations in essential HR genes such as BRCA1/2 lead to homologous recombination deficiency (HRD).5 Patients with TNBC mostly harbor germline BRCA1/2 mutation (gBRCAm) and are known to be deficient in HR and develop advanced or metastatic BC.6 HRD tumors are sensitive to DNA-damaging therapies such as poly (adenosine diphosphate–ribose) polymerase inhibitors (PARPi).7 Tumors with BRCA1/2 mutations are highly dependent on poly (adenosine diphosphate–ribose) polymerase (PARP) proteins, which are essential for single-strand DNA damage repair by base excision repair (BER) pathway. Hence, an interaction between 2 BRCA genes in which the mutation of either of these 2 genes alone is compatible with viability, whereas the simultaneous mutation of both genes causes death. Many novel therapies such as chemotherapy, hormonal therapy, anti-HER2 mAbs, and others for treating BC have emerged since the 1900s.
Among them, PARP inhibitors (PARPi) were developed and were the first clinically approved which showed promising activity in patients with BRCA-deficient tumors.4, 8 PARPi such as olaparib and talazoparib are Food and Drug Administration (FDA)-approved drugs for the treatment of BRCA-associated BC, and there is a growing interest in analyzing their effectiveness and safety, particularly in patients with TNBC, either as monotherapy or combination therapy. Recent evidence also shows PARPi effectivity in BRCA1/2 wild-type BC patients with HRD. Although the use of PARPi in patients with TNBC is imminent, challenges such as poor response in wild-type BRCA patients and lack of predictive biomarkers to guide PARPi therapy have to be addressed.9-11 Very few previously published reviews focused on the PARPi in TNBCs, but they focused on PARPi on HR status, PD-L1, etc.8, 11 Considering these circumstances, our review mainly focuses on BRCA mutations and HRD testing and their relevance to the clinical application of PARPi in patients with BC and TNBC.
2 PREVALENCE AND IMPACT OF BRCA MUTATION AND HRD IN BC
Germline pathogenic mutations in the BRCA1/2 genes increase the likelihood of developing several malignancies, including BC and ovarian cancer. Risk estimates show that 50%–80% of people with germline BRCA1/2 mutation may develop BC at a young age.12 BRCA1/2 mutations predispose high penetrance in hereditary carriers and are found in ~20% of germline BC. Other germline mutations such as TP53, PTEN, and STK11 are identified in <1% of BC families and are associated with cancer syndromes.13 BRCA1 mutation–associated BC was mostly diagnosed in younger women and was presented with a poor prognosis; high nuclear grade tumors; poorly differentiated, infiltrating ductal carcinomas; and stain negative for ER, PR, and HER2. BRCA2-mutated BC possesses a similar clinical prognosis in non-germline carriers and affects older women.14
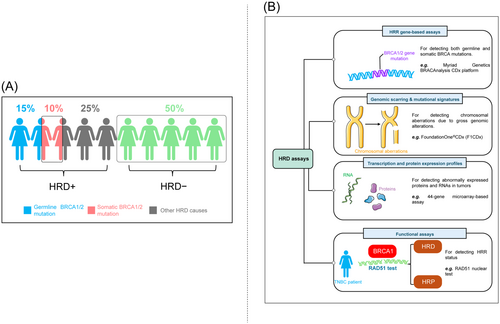
TNBC patients with mutations were generally younger at first diagnosis, with a mean age of 44 and 47 years for patients with germline BRCA1 and BRCA2 mutations, respectively, and 51 years for patients without pathogenic germline mutations.15 The prevalence rate of germline BRCA1/2 mutation in BC was 5.3%, and high in patients with TNBC (11.2%; BRCA1, 8.5% and BRCA2, 2.7%).16 An earlier study showed that 68% and 16% of patients with BC were categorized to have TNBC and BRCA1 and BRCA2 mutations, respectively.17 Mutations in BRCA1/2 lead to HRD that attenuates the DNA repair mechanism 18 and are observed in ~67% of patients with TNBC.19 However, more than 20% of patients with TNBC harbor HRD without BRCA1/2 mutations (Figure 1A).20 HRD increases the number of tumor mutations, reduces DNA repair capacity, and may induce tumorigenesis resulting in molecular lesions. In addition, mutations in other HR-related genes, such as BARD1, RAD50, RAD51, PALB2, ATM, MRN, MRE11, RPA, NBS1, CHEK2, TP53, PTEN, and BRIP1, might also lead to DNA repair deficiencies.5 As the rates of BRCA mutations and HRD are higher, consideration of these factors in clinical practice would greatly contribute to the implementation of newer targeted therapy strategies for patients with BC.
3 CURRENT SITUATION OF HRD DETECTION AND ITS RESEARCH DIRECTION IN BC
Genetic variations in the BRCA1/2 gene destabilize the HR pathway and DNA repair targeting DNA-damaging PARPi therapy. However, the effectiveness of PARPi is largely dependant on the HRD status of the patients.21 A phase III clinical evaluation of iniparib did not show positive outcomes in patients with TNBC, which could be attributed to the non-stratification of patients based on their BRCA1/2 mutation status.22 According to the National Institute for Health and Care Excellence (NICE), genetic BRCA1/2 testing is recommended when the combined BRCA1 and BRCA2 mutation carrier probability is ≥10%.23 These factors emphasize the need for a reliable and robust diagnostic tool for HRD testing. Also, the assessment of HRD serves as an important prognostic and predictive biomarker for patients on PARPi therapy. At present, HRD status is best assessed in ovarian cancer for its clinical utility in determining PARPi benefits.24 However, HRD detection methods are varied and controversial, and gold-standard methodologies are not yet available. Hence, understanding the currently employed methodologies to assess HRD is vital for its use in clinical research and practice. HRD assays can be classified into 4 categories: homologous recombination repair (HRR) gene-based assays, genomic scarring assays and mutational signatures, and functional assays (Figure 1B).
3.1 HRR gene-based assays
Sequencing HR genes to detect germline mutations was used to categorize tumors as HRD. Clinical studies suggest that germline BRCA1/2 mutation detection remains the reliable clinical biomarker of HRD detection.25 The GeparSixto trial showed that germline BRCA1/2 mutation status predicts higher pathological complete response (pCR) rates in patients with TNBC on neoadjuvant chemotherapy.26 Similarly, the NeoSTOP trial demonstrated higher pCR correlating with event-free survival (EFS) and overall survival (OS) in germline BRCA1/2 mutation patients with TNBC on platinum-based neoadjuvant chemotherapy.27 Many phase III studies (Study 19, NCT00753545; NOVA trial, NCT01847274; and OlympiAD trial, NCT02000622) have used BRCA analysis to establish germline BRCA1/2 mutation status in patients with BC and ovarian cancer.25 Of the existing BRCA-sequencing assays, the FDA has approved the sequencing-based Myriad Genetics BRACAnalysis CDx platform (Myriad Genetics) to detect deleterious germline BRCA1/2 mutation variations in patients with ovarian cancer.25
Besides germline BRCA1/2 mutation, the prevalence of somatic BRCA mutations in patients with ovarian cancer led to the FDA approval of the tissue-based FoundationFocus CDxBRCA assay (Foundation Medicine) based on the ARIEL trials, which detect both germline and somatic BRCA mutations.28 A recently developed capture-based–targeted resequencing assay, Leuven HRD assay, was compared with Myriad myChoice PLUS, and the results showed robust correlations and positive outcomes.29 However, negative predictive value, detection of variants of uncertain significance, genotype-phenotype correlations, and somatic reversion mutation leading to HR proficiency serve as important drawbacks of predicting HRD using gBRCAm testing.25 Hence, constant efforts have been made to identify genomic, transcriptomic, proteomic, and functional markers to identify HRD in tumors.
3.2 Genomic scarring assays and mutational signatures
HRD can be detected by chromosomal aberrations arising from defective repair pathways due to gross genomic alterations (also referred to as “genomic scars”). The “myChoice” HR deficiency test (Myriad Genetics Inc.) is a next-generation sequencing (NGS) assay that evaluates genomic scarring by measuring the loss of heterozygosity (LOH), large-scale transitions, and telomeric allelic imbalances. A genomic instability score (GIS) of >42 is considered positive for HRD.30 The NGS-based comprehensive genomic profiling assay “FoundationOne®CDx (F1CDx)” (Foundation Medicine) is capable of assessing 324 cancer genes, including deleterious BRCA1 and/or BRCA2 mutations, to diagnose HRD.31
In addition to genomic scarring assays, the presence of mutational signatures or patterns in the entire genome caused by mutagen exposure or genomic instability has been validated as a predictor of HRD tumors. “Signature 3” is one such mutational signature attributed to underlying HRD and detected in several cancers including BC.32 “HRDetect”, a whole genome sequencing (WGS)–based classifier, designed to detect HRD has a high sensitivity in predicting BRCA deficiency and is based on gene signatures arising due to base substitutions and rearrangements.33 Similar to HRR gene-based assays, reversion or secondary mutation restores HR functionality, but the scar would remain detectable. Hence, assays mitigating the false positivity of HRD after treatment and mutation reversal would add more clinical value.
3.3 Transcription and protein expression profiles
Unlike irreversible genetic signals, it is hypothesized that RNA and proteins fluctuate dynamically in quantity and localization based on the current HRD status. Gene expression profiling (GEP) performs real-time indication of HRD by capturing the current transcriptional state of a tumor. GEP assays may not necessarily focus on HR but analyze genes that regulate apoptosis, cell cycle entry, and DNA repair. A retrospective GEP evaluation analyzing the 93-gene chemotherapy response profile (CRP) showed an excellent response to platinum therapy in patients with ovarian cancer.25 The TAILORx trial involving HR-positive, HER2-negative, axillary node-negative invasive patients with BC who met the clinical recommendation for adjuvant chemotherapy was maintained on endocrine therapy based on a 21-gene assay (Oncotype DX Recurrence Score, Genomic Health). Adjuvant therapy showed a beneficial efficacy at 5 years in patients who had a midrange 21-gene recurrence score (OS, 98.0%; disease-free survival, 93.8%).34 The dynamicity of GEP assays was evident in a 60-gene signature assay, which classified tumors as BRCA-like and non-BRCA-like. Furthermore, GEP assays identified the evolution of platinum resistance by reading the change in the BRCAness profile using gene signatures during the therapy.35 In BC, a formalin-fixed paraffin-embedded (FFPE) compatible 44-gene microarray-based assay was developed to predict a complete pathologic response after neoadjuvant chemotherapy. A 77-gene expression profile known as BRCA1ness was developed based on a cohort of TNBC linked to a known BRCA1-like array-based comparative genomic hybridization (aCGH) profile, which helped to identify the subset of patients who are benefiting from adjuvant DSB-inducing chemotherapy.36 A similar 77-gene BRCA1ness signature developed using a centroid model was evaluated in the phase II I-SPY 2 study. Here, HER2-negative patients with BC positive for BRCA1ness receiving paclitaxel with veliparib and carboplatin had significantly higher pCR rates.37 At present, these GEP assays have not been specifically assessed for PARPi inhibition.
Similar to GEP assays, immunohistochemical analyses of tumor suppressor proteins can serve as evaluation tools for cancer treatment sensitivities. Owing to a low expression of BRCA proteins and non-BRCA DNA damage response proteins, many clinical studies on PARPi have examined ataxia-telangiectasia-mutated kinase (ATM). ATM loss has been noted in a variety of tumor types, including breast, colon, and gastric cancers.25 Reduction of ATM is linked to PARPi sensitivity in vitro, and a loss of 20% in ATM levels was noted in gastric cancer.38, 39 A correlation between ATM loss and OS improvement was demonstrated by a randomized phase II study of paclitaxel + olaparib versus paclitaxel + placebo.40 GEP and protein expression analysis is an indirect evaluation of HRD and is not included in the acceptable methodologies for HRD detection as per the European expert consensus.41 However, the focus of further research may make GEP and protein expression analysis an excellent and acceptable tool for determining prognosis and the likelihood of benefit from selected therapeutic agents such as PARPi in patients with BC.
3.4 Functional assays
Functional assays possess the ability to provide a dynamic readout of the actual HRR status by measuring the actual ability of tumors to accumulate RAD51 protein at DNA DSB.42 RAD51 is a downstream HR protein that gets employed in the sites of DNA damage to facilitate strand invasion into the sister chromatid in cooperation with the BRCA1/PALB2/BRCA2 complex. In the presence of HRD, there is a reduction or inability to form RAD51 foci. The presence of RAD51 occurs when the reversion mutation restores HRR. Limitations such as the inability of RAD51 assays to identify HRR defects, downstream of RAD51 loading onto DNA, and elicitation of RAD51 signals exogenous DNA damage have undermined its clinical utility.24 However, RAD51 can be used in the absence of exogenous factors.43 RAD51 can be assessed using DNA sequencing and immunostaining assays and is currently being investigated extensively as a biomarker to predict HRD and promote its clinical utility.44
4 STUDIES ASSESSING HRD IN PATIENTS WITH BC
GeparOLA (NCT02789332), a phase 2, randomized, open-label ongoing trial, compares paclitaxel/olaparib 100 mg twice daily (BID) with paclitaxel/carboplatin in 107 patients with HER2-negative early BC. The preliminary report showed that the rate of pCR was not significantly different between the olaparib and carboplatin arms. In the subgroup analysis, the pCR rates in the olaparib and carboplatin arms were 52.6% and 20.0%; 56.0% and 59.3% in HRD patients and HR-proficient patients, respectively.45 The phase II TBCRC 030 study evaluated HRD biomarker association with pathological response (RCB-0/1) to single-agent cisplatin or paclitaxel. HRD assay by Myriad Genetics, Inc. was performed in the NGS platform using FFPE tumor tissues. This study demonstrated that HRD is not a predictive biomarker of pathological response, as no significant association was observed between HRD score and RCB response.46 A retrospective, blinded, biomarker analysis from the tissue samples of the GeparSixto trial in patients with TNBC showed a high concordance (87%) between RAD51 and genomic HRD. Functional HRD by RAD51 was detected in 61% of the tumor patients, and the RAD51 test identified 93% tBRCA-mutated tumors and 45% non-tBRCA mutant cases. Similar pCR rates were observed between Myocet®-non-pegylated liposomal doxorubicin (PM) and PM plus carboplatin (PMCb) treatment arms (PMCb vs PM, 31% vs 39%; OR, 0.71, 0.23–2.24; p = 0.56). PMCb was beneficial to patients with RAD51-low tumors (pCR, 66% vs 33%; OR, 3.96, 1.56–10.05, p = 0.004; interaction test p = 0.02). Disease-free survival was similar when carboplatin was added in the RAD51-high (hazard ratio [HR], 0.40; log-rank p = 0.11) and RAD51-low (0.45, p = 0.11) groups.47 A similar benefit was not observed in the TNT trial conducted in a metastatic setting where no difference was observed in PFS or OS between the carboplatin and docetaxel arms when stratified according to HRD status.48
The BrighTNESS trial evaluated HRD score as a biomarker to predict response to neoadjuvant platinum-based chemotherapy in TNBC and showed that patients with HRD were more likely to achieve pCR than HR-proficient patients (OR, 13.06; 95% CI, 1.52–11.241; p = 0.0028). Among BRCA1/2 wild-type patients, HR-deficient patients were more likely to achieve a pCR (OR, 16; 95% CI, 1.65–160.41; p = 0.0041) than HR-proficient patients. Furthermore, HRD scores were highly concordant with pre- and post-therapy outcomes (Spearman correlation >99%).49 These factors indicate that HRD is an effective predictor of the pCR rates and therapy outcomes.
Studies assessed presented conflicting opinions on HRD testing. The findings clearly show that there is no robust, reliable, and accurate biomarker to identify HRD tumors that could guide PARPi treatment. The inconsistencies observed could have been caused by differences in the HRD scoring threshold used to correlate with pCR rates across studies. The TBCRC 030 and BrighTNESS trials employing a HRD scoring threshold of 33 did not correlate with favorable pCR rates in patients with TNBC, whereas the scoring threshold of ≥42 in the GeparSixto trial is associated with favorable pCR rates. Differences in outcomes based on the HRD scoring threshold suggest the need to achieve a common consensus on the HRD score to predict treatment responses in patients with TNBC. Furthermore, investigations performed have majorly predicted chemotherapy responses based on HRD, where PAPRi are only used as adjuvant therapy. Prospective clinical trials could, therefore, aid in determining whether HRD can be used as a prognostic predictor during PARPi therapy where it is used as the primary intervention in patients with BC and TNBC stratified based on HRD. In addition, limitations of HRR gene sequencing in identifying the epigenetic inactivation and restoration of HRR upon progression invite the need for additional or alternate methodologies such as functional RAD51 assay, which showed a high concordance with genomic HRD assays. Therefore, systematic validations and implementation of genomic scarring assays, functional assays, and protein expression profiles could assist in predicting the PARPi responses since PARPi activity is directly dependent on HRD positivity.
5 PARPI structure–activity RELATIONSHIP AND TREATMENT MECHANISM
5.1 Chemistry of PARP inhibitors – structure–activity relationship (Sar)
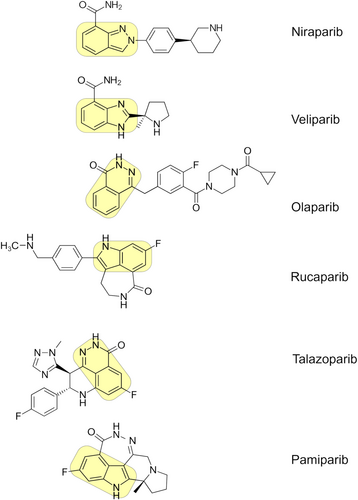
The development of PARPi has paved the way for treating various cancers including BC. Most PARPi compounds possess N heterocycle, benzene ring, halogen, and free amine (N-H) moieties.50, 51 The pharmacophore modelling of PARPi revealed the presence of nicotinamide moiety which interacts with the nicotinamide binding site of PARP enzymes. The presence of at least one free -NH group is essential which acts as a hydrogen bond donor. The improvement in the activity is more associated with the presence of an electron-rich aromatic ring or phenyl ring which is required for π-π stacking interaction. For instance, the six-membered ring showed better activity than the seven-membered ring.50-52 The carboxamide moiety in the PARPi should be restricted to either cis or trans-, which is crucial in forming hydrogen bond formation. The presence of non-cleavable bonds at the 3rd position relative to carboxamide moiety is essential for improving the conjugations. The presence of one or more heterocyclic rings containing nitrogen/fluorine/carbonyl groups is preferable for imparting a hydrophobic nature at the adenosine binding region52 (Figure 2).
5.2 Mechanism of PARPi in the treatment of HRD tumors
Hormone therapies do not help patients with TNBC since there is no receptor overexpression, thus making TNBC clinical management challenging. gBRCAm is more sensitive to DNA-damaging therapies such as PARPi therapy.53 PARPi have been postulated to kill tumor cells using synthetic lethality, wherein only tumor cells are affected and not normal cells. Hence, PARPi has evolved as an effective treatment agent for TNBC with BRCA mutation and HRD. The results of HRD in a variety of DNA lesions such as single-strand break (SSB), DSB, bulky adducts, base mismatches, insertions, deletions, and base alkylation are caused by continuous DNA damage. The repair mechanisms for DNA can vary based on the type of lesions that are present. PARP proteins are important repair regulators of SSB and repair DNA through the BER pathway. PARPi trap PARP proteins from binding to the sites of SSB, which inhibits the BER pathway thereby improving SSB to DSB conversion.54
DSB repair usually occurs through HRR pathway where BRCA1/2 protein restores DSB accurately with a high precision. BRCA1 gets employed in the sites of DSB where it promotes 5′ to 3′ resection creating 3′ overhangs, thus playing an early and prominent role in the promotion and regulation of HRR. BRCA1 modulates PALB2-dependent loading of BRCA2-RAD51 repair machinery at the sites of DSB. Then RAD51 is loaded by BRCA2 in the overhang, forming nucleoprotein filament and D loop. RAD51 then promotes strand invasion using the sister chromatid leading to error-free repair. In cases of HRD, DNA replication fork collapses due to PARP trapping by PARPi leading to the accumulation of DSB and cell death. Further, DSB repair can be performed by nonhomologous end-joining (NHEJ) repair pathway. It does not require homologous template for DSB repair, rather ligates the DNA ends directly. However, NHEJ is an error-prone pathway and leads chromosomal aberrations, cell cycle arrest, and eventually apoptosis 54 (Figure 3).

Preclinical studies have also shown PARPi efficacy in BC cells lacking BRCA mutations.55 Furthermore, niraparib treated for ovarian cancer showed progression-free survival (PFS) benefits irrespective of BRCA mutation and HRD. Similar effects cannot be replicated in non-BRCA1/2–mutated BC, highlighting the complexity in using PARPi beyond gBRCAm.56 Hence, further studies on PARPi in patients with BC stratified based on BRCA1/2 mutation status are required to extend their clinical benefit beyond BRCA.
6 PROGRESS OF PARPI IN BC WITH GBRCA MUTATION OR HRD
Over years, PARPi have gained interest among many researchers for its activity against TNBC. Several PARPi are currently being investigated in adjuvant, neo-adjuvant, and metastatic settings as monotherapy or combination therapy in BC patients with BRCA1/2 mutation and HRD (Table 1). The FDA has approved olaparib and talazoparib in 2018 as monotherapies for gBRCAm HER2-negative metastatic BC.65, 66 Many completed and on-going clinical trials (~140 clinical trials) involving different PARPi, such as niraparib, olaparib, rucaparib, talazoparib, and veliparib, for BC and TNBC have been registered.
| Study design | Clinical trial identification number | Clinical trial status | Intervention | No. of patients | ORR in % | PFS in months | OS in months | Comparator | No. of patients | ORR in % | PFS in months | OS in months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase I, nonrandomized study57 | NCT00516373 | Completed | Olaparib | 19 | 47.3 | NA | NA | None | NA | NA | NA | NA |
| Phase II, nonrandomized, multicenter, sequential cohort study58 | NCT00494234 | Completed | Olaparib 400 mg | 27 | 41 | 5.7 | NA | None | NA | NA | NA | NA |
| Olaparib 100 mg | 27 | 22 | 3.8 | NA | ||||||||
| Phase II, prospective, multicenter, nonrandomized study59 | NCT01078662 | Ongoing | Olaparib | 62a | 12.9 | 3.7 | 11 | None | NA | NA | NA | NA |
| Phase I, open-label, multicenter study60 | NCT00782574 | Completed | Olaparib continuously or intermittently with cisplatin | 42a | 71 | NA | NA | None | NA | NA | NA | NA |
| Phase I/Ib, open-label, dose-escalation study61 | NCT01445418 | Completed | Olaparib in combination with carboplatin | 28 | 22 | NA | NA | None | NA | NA | NA | NA |
| Phase III, randomized controlled, open-label, multicenter trial62 | NCT02000622 | Ongoing | Olaparib | 205 | 59.9 | 7 | 19.3 | Treatment of physician's choice (capecitabine, vinorelbine, or eribulin) | 97 | 28.8 | 4.2 | 17.1 |
| Phase Ib, multicenter, open-label, classic 3 + 3 dose-escalation clinical trial | SU2C-AACR-DT16–15 | Olaparib in combination with alpelisib | 28 | 36 | 7.2 | 21.3 | None | NA | NA | NA | NA | |
| Phase 2, open-label, single-arm study63 | NCT02657889 | Completed | Niraparib in combination with pembrolizumab | 55 | 21 | 2.3 | NA | None | NA | NA | NA | NA |
| Phase III, randomized, open-label trial64 | NCT01945775 | Completed | Talazoparib | 287 | 62.6 | 8.6 | 22.3 | Treatment of physician's choice (capecitabine, eribulin, gemcitabine, or vinorelbine) | 144 | 27.2 | 5.6 | 19.5 |
- Note: All the clinical trial data was obtained from http://clinicaltrials.gov/.
- Abbreviations: BRCA, breast cancer genes; NA, not available; NCT, National Clinical Trial number; ORR, objective response rate; OS, overall survival; PARPi, poly (adenosine diphosphate–ribose) polymerase inhibitors; PFS, progression-free survival.
- a Data of patients with breast cancer are only included; $Data of patients with breast cancer with deleterious BRCA1/2 mutations.
6.1 Olaparib
6.1.1 Olaparib as monotherapy
Olaparib, the first PARP-1 inhibitor approved by the FDA to treat primary peritoneal, ovarian or fallopian tube cancers, was developed by Kudos in collaboration with Maybridge.67 A phase I trial (NCT00516373) on olaparib demonstrated antitumor activity in patients with gBRCAm ovarian and BC and advanced solid tumors.57 Agreeable therapeutic index was observed in a phase II trial (ICEBERG, NCT00494234) in patients with confirmed BRCA mutations. It provided proof of concept for the efficacy of olaparib by showing an objective response rate (ORR) of 41% (11/27) in patients administered with olaparib 400 mg BID.58 In 2014, a multicenter, open label, noncomparative phase II clinical trial (NCT01078662) examined the efficacy of olaparib monotherapy in patients with advanced recurrent cancer with a gBRCA1/2 mutation. Among them, 60% of 62 patients with BC had BRCA1 mutation. Tumor response rate was observed in 12.9% of patients with BC, with 46.8% of patients achieving stable disease (SD) after ≥8 weeks of olaparib treatment. The median duration of response was 29 weeks, and the PFS was observed in 29% of patients at 6 months. The median OS was 11 months, and 44.7% of patients were alive at the end of 12 months.68
6.1.2 Olaparib as combination therapy
The combination of PARPi and immunotherapy has yielded promising outcomes in breast cancer patients. Olaparib is also given in combination with other cytotoxic drugs. A phase I study (NCT00782574) published in 2014 showed a 71% response rate (12/17 patients) to olaparib (50 mg, BID) plus cisplatin (60 mg/m2) combination therapy in patients with BRCA1/2 BC. However, higher doses of olaparib (100 or 200 mg BID) and cisplatin (75 mg/m2) were intolerable due to frequent grade 3 toxicities.60 In a phase I/Ib trial, women with sporadic TNBC (75% pretreated) were given olaparib in combination with carboplatin (NCT01445418). The study shows 1 complete response (CR; 69+ months) and 5/27 partial responses (19%; median: 4 months [4-7]), with an ORR of 22%.69 The FDA approval of olaparib was based on the phase III OlympiAD clinical trial. OlympiAD is a randomized, controlled, open-label, multicenter phase III trial (NCT02000622) comparing olaparib with standard therapy (capecitabine, eribulin, or vinorelbine) in HER2-negative early BC patients with gBRCAm. The trial observed median PFS (mPFS) of the olaparib arm to be ~67%, which is longer than the standard therapy arm (olaparib, 7.0 months; standard therapy, 4.2 months). The response rate was 59.9% with the olaparib arm (100 patients of 167) and 28.8% with the standard-therapy arm (19 of the 66 patients). Adverse events (AEs) rated grade 3 or higher occurred in 36.6% of patients in the olaparib arm and 50.5% in the standard-therapy arm.70 A randomized, multicenter, open-label, phase 1b, classic 3 + 3 dose-escalation clinical trial evaluated combination therapy of olaparib with the PI3K inhibitor alpelisib in epithelial ovarian cancer and BC patients with known gBRCAm status. Synergistic approach of the combination resulted in an ORR of 31.3% in gBRCAwt platinum–resistant patients and 33.3% in BRCAwt (germline and somatic) platinum–resistant patients in a setting where ORR of ~ 5% is usually expected in monotherapy of the evaluated drugs. Grade 3+ treatment-related non-hematologic and hematologic toxicities occurred in ≥10% of patients with no unexpected toxicities and no irreversible toxicities.35 In a phase I study, Olaparib was co-administered with durvalumab (anti-PD-L1 antibody) in 12 patients (2 were TNBC and 10 were ovarian cancer) which exhibited an 83% disease control rate.71
6.2 Niraparib
Niraparib was developed by Merck & Co., Inc., Rahway, NJ, USA and approved by the FDA in 2017 for ovarian and breast cancer, inhibits PARP-1 and PARP-2 and is metabolized by carboxylesterase and glucuronidation to form an inactive metabolite.72 In the TOPACIO (NCT02657889) study, the safety and efficacy of niraparib in combination with pembrolizumab was evaluated in metastatic TNBC patients. This open-label, single-arm, phase 2 study demonstrated an ORR of 27% and an mPFS of 8.3 months in patients with tBRCA mutations. Patients with BRCA wild-type tumors had a reduced ORR of 11% and an mPFS of 2.1 months. The most common grade ≥3 treatment-related AEs were anemia (18%), thrombocytopenia (15%), and fatigue (7%). Immune-related AEs were also observed in 15% of the study patients.73
6.3 Talazoparib
Talazoparib inhibits PARP catalytic activity and traps PARP-1/2 on damaged DNA, used for breast cancer and prostate cancer, developed by Lead Therapeutics and BioMarin.74 A dose-escalation phase I trial administered talazoparib either at 9 different dosage levels, ranging from 0.025 to 1.1 mg/day (arm 1), or at 1.0 mg/day (arm 2). In arm 1, higher ORR was observed in patients with BRCA 2 mutation (55%) compared with those with BRCA 1 mutation (38%). A higher clinical benefit rate (CBR) was also seen in patients with BRCA 2 mutation (91%) than those with BRCA1 mutation (50%). In arm 2 patients, an ORR of 50% was observed with a CBR of 86%. Five patients had SD for 24 weeks, and 1 patient had CR. Results from this study demonstrated the potency and tolerability of talazoparib.75 The FDA approval for talazoparib for the treatment of patients with BRCA-mutated, HER2-negative, advanced or metastatic BC was based on the EMBRACA trial (NCT01945775). It is a phase III, open-label, randomized trial compared talazoparib with physician's choice of therapy (PCT) in patients with BC with advanced disease and gBRCAm. Talazoparib was administered to 288 patients, and 144 patients were treated with PCT. The mPFS for talazoparib patients and PCT patients was 8.6 months and 5.6 months, respectively. The OS was also higher in talazoparib patients (22.3 months) than that in PCT patients (19.5 months). The ORR was significantly higher in talazoparib patients (62.6%) than that in PCT patients (27.2%).76 Apart from olaparib and talazoparib, other PARPi drugs, such as veliparib, niraparib, and rucaparib, were also evaluated as monotherapies and combination therapies, which showed promising results.
Although the results of PARPi in BC are promising, acquired resistance is conferred by various mechanism such as secondary mutation, HRR restoration, replication fork stabilization, and additionally other causes including altered cell cycle regulation, altered miRNA-622, miRNA-182 expression, altered PARP expression, and drug efflux is a cause of concern. Further challenges include dose-limiting toxicities and similar resistance mechanisms to combination drugs, which share a similar mechanism of action to that of PARPi.77-83 This necessitates further research in identifying the subset of patients with BC for the maximum benefit from PARPi combination therapy and planning treatment strategies to overcome PARPi resistance.
7 LIMITATIONS OF HRD ASSAYS
Current HRD assays have certain limitations due to tumor heterogeneity, the presence of secondary variations in HR genes, the inability to detect non-HRR gene-related PARPi resistance, inability to report specific DNA abnormalities, the quality of tumor tissue samples and others.84, 85 Multiple reasons for the inconsistency between clinical PARPi responses and HRD assay results, including the timing, quality and quantity of samples, tumor heterogeneity, reversion mutations, and PARPi resistance mechanisms that are not related to HRD.84, 85 The assays determine HRD based on genomic scars, but they are unable to reflect dynamic changes in the homologous recombination function of the cells. For instance, in the ARIEL2 study, 117 patients had both pretreatment and archival tumor biopsies used for genomic LOH assays. 34% of patients with LOH-low in the initial biopsy turned out to be LOH-high in the later biopsy, and 30% of these patients had partial responses to rucaparib. Fresh tumor samples provide more accurate results due to tumor heterogeneity and clonal evolution.86
8 FURTHER PROSPECTS OF PARP INHIBITORS IN THE TREATMENT OF BREAST CANCER WITH HRD APPROACH
Clinical trials for the elucidation of PARPi response based on HRD stratification are ongoing. Results from these trials are sought to investigate the applicability of HRD as a predictive maker for therapy decisions in patients with BC and that could add value to the modest evidence currently available. At present, the ongoing phase III BrighTNess trial, comparing veliparib plus carboplatin with carboplatin alone, may provide useful insights on the predictive value of HRD testing in patients with BC.31 This could expand the scope of HRD testing and benefit patients with BC in terms of treatment options. Several phase II clinical trials are in progress evaluating the efficacy of PARPi based on HR status. These include PARPi such as olaparib in the NOBROLA trial, rucaparib in the RUBY trial, and talazoparib in the TBB trial.87-89 The efficacy of Olaparib is currently assessed in the NOBROLA trial where Olaparib monotherapy is administered to pretreated metastatic BC patients with HR mutation nonrelated to BRCA. HRD identification is performed in the NOBROLA trial by tumor tissue analysis using FMI Lynparza HRR assay.87 The phase II RUBY trial evaluates rucaparib in patients with gBRCA wild-type metastatic BC. Early reports showed that the genome-wide assessment of LOH showed correlations with high LOH and antitumor activity. The final analysis using WGS will throw more light on HRD mutation signatures and their effects on treatment responses.88 The preliminary reports of the phase II TBB trial show antitumor activity of talazoparib in HER2-negative metastatic in patients with BC having HR pathway mutation beyond BRCA.89 In addition, another phase II trial is assessing the safety and efficacy of olaparib monotherapy and olaparib combination therapy with 2 different agents (olaparib + ceralasertib and olaparib + adavosertib) in patients with TNBC stratified based on mutation leading to HRD.90 In the phase 2 clinical study (QUADRA), patients with HRD-BRCA positive tumors sensitive were selected using the Myriad HRD assay and were treated with niraparib treatment.91 The above studies might conclude the benefit of HRD testing in PARPi treatment in patients with BC.
HRD contributes to BC development, progression, and loss of response to therapy. HRD testing provides an opportunity to identify patients with BC who might benefit from a targeted therapy such as PARPi. Studies have shown clinical activity of therapeutic agents targeting HRD, such as PARPi, in ovarian cancer and BC. However, significant challenges are observed in the precision of treatment in such cancers. Clear evidence of HRD is required for drugs such as PARPi targeting HRD to be effective. At present, there is no robust, reliable, accurate biomarker available to identify HRD tumors. Although the gene-based assay, such as Myriad HRD assay, has shown promising challenges, reversion mutation in HRR genes could render it as ineffective. In addition, 15% of the tests are not informative, and PARPi response was also observed among HRD-negative patients. Furthermore, conflicting results in pCR rates based on HRD scoring threshold were observed due to the absence of global consensus for gene-based assays. However, the majority of studies employ gene-based assays for the detection of HRD to guide PARPi therapy in patients with BC. Hence, it is important to evaluate the clinical utility of the various functional HRD assays along with gene-based assays. Thus, HRD assays can be used to maximize the accuracy in predicting PARPi response in BRCA mutant in patients with BC and TNBC.
9 CONCLUSION
Homologous recombination deficiency testing may identify the subset of breast cancer patients who are likely to benefit from PARP inhibitor therapy in the future, but its predictive potential is debatable. Although an increasing number of studies validate the applicability of homologous recombination deficiency in treatment decisions, additional biomarkers such as RAD51 are highly necessary to optimize the effective use of PARP inhibitors in breast cancer patients. In the absence of a gold standard methodology to identify homologous recombination deficiency, this should remain an experimental benefit limited to the boundaries of clinical studies until concrete outcomes from ongoing trials are available.
AUTHOR CONTRIBUTIONS
All authors substantially contributed to planning, gathering, and interpreting the information or ideas used in the paper. During the process, they substantially contributed in providing suggestions for revision or critically reviewing subsequent iterations of the manuscript and ensured that questions regarding the accuracy or integrity of any part of the work were appropriately investigated and resolved. Finally, they all reviewed and approved the final version of the paper.
ACKNOWLEDGMENTS
The authors acknowledge Swathirajan CR, PhD and Roopa Subbaiah, PhD of Indegene Pvt Ltd for providing medical writing and editorial assistance.
CONFLICT OF INTEREST STATEMENT
Nan Ma is employed by MSD China. The remaining authors declare no conflict of interest.
Biographies
Peng Yuan is the chief physician and doctoral supervisor of Cancer Hospital Chinese Academy of Medical Sciences and Peking Union Medical College. She has been working in medical oncology department for more than 25 years and is devoted to the comprehensive treatment of breast cancer. She has rich experience in chemotherapy, immunotherapy and targeted therapy for solid tumors. She has been engaged in clinical and translational research of breast cancer for a long time and presided over several pre-marketing, marketing and clinical studies of new drugs. Her research interests focus on the molecular regulation mechanism of tumor metastasis and the clinical application of artificial intelligence to accurately predict tumor metastasis. Relevant research has been published in top international journals such as JAMA and she has been invited to give oral presentation and present poster in international conferences such as ESMO BC and ASCO. Dr. Peng Yuan participated in the compilation of 15 tumor treatment guidelines, including “Chinese Guidelines for Diagnosis and Treatment of Advanced Breast Cancer”, “Guidelines and Norms for Diagnosis and Treatment of Breast Cancer of Chinese Anti-Cancer Association”, and “Manual of Clinical Oncology Internal Medicine”. She is the director of the Chinese Anti-Cancer Association and the chairman of the International Medical and Cooperation Branch. She is also a member of the Standing Committee of Breast Cancer Professional Committee, and Breast Cancer Expert Committee of Chinese Society of Clinical Oncology (CSCO).
Nan Ma academic degree is Master of Oncology and worked as a medical oncologist for 7 years. Since then, she served in AZ as a Medical Science Liaison (MSL), and currently works as a Medical Advisor (MA) in MSD China. Her main field is the treatment of breast cancer. She has many years of experience in the field of tumor treatment, and she is good at the treatment and academic support of breast cancer and lung cancer.
Binghe Xu is the academician of Chinese Academy of Engineering, Director, National Clinical Research Center for New Anticancer Drugs, Former Director, Department of Medical Oncology at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS), and Tenured Professor of Peking Union Medical College (PUMC). He received his Doctorate of Philosophy and Master of Science degree in oncology from PUMC, and his Doctorate of Medicine from Wuhan University Medical College, China. His major interests are in basic and clinical research of solid tumors, especially breast cancer, as well as clinical trials of new anticancer agents. He has participated as principal investigator (PI) for more than 100 clinical trials. Currently, he is leading PI, executive steering committee (EC) as well as steering committee (SC) members of several regional and global multicenter clinical trials. Dr. Xu has published 430 papers in peer reviewed oncology journals, this includes papers published in journals such as Nature Medicine, Lancet Oncology, Annals of Oncology, Journal of Clinical Oncology, JAMA Oncology, Cancer Cell, Cancer Research, Clinical Cancer Research, and NEJM. Dr. Xu is a faculty member of European School of Medical Oncology. He is also a panel member of the St Gallen International Consensus on the Primary Therapy of Early Breast Cancer, ESMO Clinical Practice Guideline on eBC and International Consensus Guidelines for Advanced Breast Cancer. Dr. Xu has served on the editorial boards of 20 journals including The Breast, and the International Advisory Board Member of Lancet Oncology.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review article as no new data were generated or analyzed.




