Innovative pathological network-based multitarget approaches for Alzheimer's disease treatment
Abstract
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease and is a major health threat globally. Its prevalence is forecasted to exponentially increase during the next 30 years due to the global aging population. Currently, approved drugs are merely symptomatic, being ineffective in delaying or blocking the relentless disease advance. Intensive AD research describes this disease as a highly complex multifactorial disease. Disclosure of novel pathological pathways and their interconnections has had a major impact on medicinal chemistry drug development for AD over the last two decades. The complex network of pathological events involved in the onset of the disease has prompted the development of multitarget drugs. These chemical entities combine pharmacological activities toward two or more drug targets of interest. These multitarget-directed ligands are proposed to modify different nodes in the pathological network aiming to delay or even stop disease progression. Here, we review the multitarget drug development strategy for AD during the last decade.
Abbreviations
-
- 5-HT
-
- 5-Hydroxytryptamine, serotonin
-
- 6-OHDA
-
- 6-Hydroxydopamine
-
- ACh
-
- acetylcholine
-
- AChE
-
- acetylcholinesterase
-
- AChEI
-
- acetylcholinesterase inhibitor
-
- AChR
-
- acetylcholine receptor
-
- AD
-
- Alzheimer's disease
-
- ADMET
-
- absorption, distribution, metabolism, excretion, and toxicology
-
- APP
-
- amyloid precursor protein
-
- ATP
-
- adenosine triphosphate
-
- Aβ
-
- amyloid-β
-
- BACE-1
-
- β-site amyloid cleaving enzyme 1
-
- BBB
-
- blood–brain barrier
-
- BIM
-
- hydroxyphenylbenzimidazole
-
- BuChE
-
- butyrylcholinesterase
-
- cAMP
-
- cyclic adenosine monophosphate
-
- CAS
-
- catalytic active site
-
- CD
-
- concentration required to double the specific luciferase reporter activity
-
- CDK
-
- cyclin-dependent kinase
-
- cGMP
-
- cyclic guanosine monophosphate
-
- CHIP
-
- C-terminus of Hsc70-interacting protein
-
- CNS
-
- central nervous system
-
- CSF
-
- cerebrospinal fluid
-
- DARPP-32
-
- dopamine and 3′,5′-cyclic adenosine monophosphate-regulated neuronal phosphoprotein 32
-
- DCFDA
-
- 2′,7′-dichlorofluorescin diacetate
-
- DHP
-
- dihydropyridine
-
- DMT
-
- disease modifying therapy
-
- DPPH
-
- 2,2-Diphenyl-1-picrylhydrazyl
-
- EC50
-
- half maximal effective concentration
-
- eeAChE
-
- Electrophorus electricus acetylcholinesterase
-
- EpRE
-
- electrophile response element
-
- eqAChE
-
- Equine serum acetylcholinesterase
-
- eqBuChE
-
- Equine serum butyrylcholinesterase
-
- ERAD
-
- endoplasmic-reticulum-associated protein degradation
-
- ERK
-
- extracellular signal-regulated kinase
-
- FAD
-
- flavin adenine dinucleotide
-
- Fbx2
-
- neuron-specific F-box protein
-
- FDA
-
- Food and Drug Administration of United States GABA, γ-aminobutyric acid
-
- GADPH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- GAP43
-
- growth associated protein 43
-
- GCLm
-
- glutamate-cysteine ligase regulatory subunit
-
- GFAP
-
- glial fibrillary acidic protein
-
- GPCR
-
- G-protein-coupling receptor
-
- GSK-3
-
- glycogen synthase kinase-3
-
- H3R
-
- histamine 3 receptor
-
- hAChE
-
- Human acetylcholinesterase
-
- HAT
-
- histone acetyltransferase
-
- hBACE-1
-
- Human β-site amyloid cleaving enzyme 1
-
- hBuChE
-
- Human butyrylcholinesterase
-
- HDAC
-
- histone deacetylase
-
- hERG
-
- human ether-a-go-go-related gene
-
- hMAO-A
-
- Human mono-amine oxidase A
-
- hMAO-B
-
- Human mono-amine oxidase B
-
- HO-1
-
- heme oxygenase-1
-
- IC50
-
- half maximal inhibitory concentration
-
- IL
-
- interleukin
-
- iNOS
-
- inducible nitric oxide synthase
-
- JC-1
-
- 5,5,6,6′-tetrachloro-1,1′,3,3-tetraethylbenzimidazoylcarbocyanine iodide
-
- Ki
-
- affinity constant
-
- LOAD
-
- late-onset Alzheimer's disease
-
- LPS
-
- lipopolysaccharide
-
- LRRK2
-
- leucine rich repeat kinase 2
-
- mAChR
-
- muscarinic acetylcholine receptor
-
- MAO
-
- mono-amine oxidase
-
- MAP
-
- microtubule-associated protein
-
- MAPK
-
- mitogen-activated protein kinase
-
- MRC1
-
- mannose receptor C-type 1
-
- MTDL
-
- multitarget directed ligand
-
- nAChR
-
- nicotinic acetylcholine receptor
-
- NDD
-
- neurodegenerative disease
-
- NFT
-
- neurofibrillary tangle
-
- NOR
-
- novel object recognition
-
- NOS2
-
- nitric oxidase synthase 2
-
- NOX
-
- NADPH oxidase
-
- NQO1
-
- NAD(P)H dehydrogenase quinone 1
-
- NRF2
-
- nuclear factor (erythroid-derived 2)-like 2 transcription factor
-
- N-myc
-
- N-myc proto-oncogene protein
-
- OA
-
- okadaic acid
-
- ORAC
-
- oxygen radical absorbance capacity
-
- OS
-
- oxidative stress
-
- PAS
-
- peripheral anionic site
-
- PD
-
- Parkinson's disease
-
- PDE
-
- phosphodiesterase
-
- PKA
-
- protein kinase A
-
- PP
-
- phosphoprotein phosphatase
-
- PP1
-
- protein phosphatase 1
-
- PP2A
-
- protein phosphatase 2A
-
- PS1 and PS2
-
- presenilin 1 and 2
-
- rAChE
-
- rat acetylcholinesterase
-
- rBuChE
-
- rat butyrylcholinesterase
-
- RNS
-
- reactive nitrogen species
-
- ROS
-
- reactive oxygen species
-
- SAR
-
- structure–activity relationship
-
- SCF
-
- Skp1/Cullin/F-box
-
- SERT
-
- serotonin transporter
-
- ThT
-
- Thioflavin T
-
- TNF-α
-
- tumor necrosis factor α
-
- Trolox
-
- (±)−6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid
-
- T.e.
-
- Trolox equivalent
-
- UBB
-
- UPS-associated ubiquitin B gene
-
- UCh-L1
-
- ubiquitin C-terminal hydrolase
-
- UPS
-
- ubiquitin-proteasome system
-
- VGCC
-
- voltage-gated calcium channel
-
- WT
-
- wildtype
-
- β-TrCP
-
- β-Transducing repeat containing proteins
1 INTRODUCTION
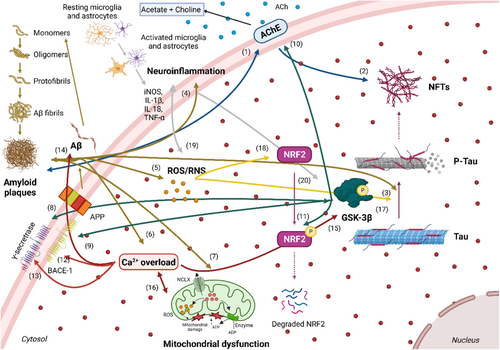
Alzheimer's disease (AD) is an age-associated neurodegenerative disorder, characterized by memory loss, retardation of thinking and reasoning, progressive cognitive impairment, and changes in personality and behaviors.1 AD is the most frequent cause of dementia, accounting for 60%–80% of all cases.2 In 2022, AD affected over 55 million people around the world being the seventh leading cause of mortality and morbidity, a figure that will increase to 152 million by 2050,3 with an estimation of 10 million new cases each year and a projected healthcare expenditure of around US $1.1 trillion in the United States.4 AD incidence increases dramatically with age, being 5% of people aged 65–74 years, 13% of people aged 75–84 years, and 33% of people aged 85 years or older.5 Although several genetic predisposition factors have been described, only 5% of cases are familiar or early-onset, while 95% of the cases are sporadic or late-onset (LOAD). To date, the causes driving LOAD onset and development remain elusive; however, different pathological pathways have been implicated, including aberrant protein aggregation, mitochondrial dysfunction, OS, calcium ion (Ca2+) dyshomeostasis, chronic neuroinflammation, and autophagy failure, among others.
The progressive increase in LOAD cases, together with the fact that there is still no effective treatment, highlights the urgent need to find effective drugs.
1.1 Alzheimer's disease physiopathology
The main histological hallmarks in AD include a remarkable neuronal loss, mainly from the cholinergic system, the presence of extracellular amyloid-β peptide (Aβ) deposits that conform senile plaques, and the intracellular accumulation of hyperphosphorylated microtubule-associated tau protein that constitute the intracellular neurofibrillary tangles (NFTs).6 Cholinergic dysfunction in AD is associated with a large decrease of the neurotransmitter acetylcholine (ACh), decreased levels of nicotinic and muscarinic ACh receptors (nAChRs, mAChRs, respectively), and increased activity of ACh-esterase (AChE) and butyryl-choline esterase (BuChE).
Senile plaques are mainly composed of Aβ peptide aggregates adopting a β-sheet conformation, which derives from the amyloid precursor protein (APP).7 In aged subjects or under pathological conditions, APP is cleaved by β-secretase, also known as β-site amyloid cleaving enzyme 1 (BACE-1), at the N-terminal releasing the large soluble peptide APP-β (sAPPβ) and a 99-residue-C-terminal fragment (C99). Then, the C99 peptide is further processed by γ-secretase at several positions, generating mainly the 40- and 42-aminoacid-long peptides, known as Aβ1-40 and Aβ1-42 (amyloidogenic pathway). Aβ1-40 is the most common; however, Aβ1-42 is the main component of Aβ plaques, being more toxic.8 γ-secretase cleavage also releases the APP intracellular domain (AICD) that promotes neuronal death.9 Therefore, an imbalance between secretase activity or the presence of mutated APP forms accelerates the toxic Aβ production.10 Aβ1-42, being more hydrophobic than Aβ1-40, is prone to form oligomers and to aggregate, first to form insoluble Aβ fibrils and oligomers and then, in bulkier aggregates named senile plaques.11
Intracellular NFTs are mainly composed of hyperphosphorylated tau protein, a highly soluble microtubule-associated protein (MAP), which promotes neuronal microtubule stabilization to form the cytoskeleton. Tau plays an important role in synaptic plasticity and it is thought to play a key role in learning and memory processes.12 Tau function and affinity for microtubules depend on its phosphorylation status; under pathological conditions, tau protein can be hyperphosphorylated inducing tau aggregation and the formation of insoluble paired helical filaments (PHF).13 Insoluble NFTs damage cytoplasmic functions and interfere with axonal transport, which eventually leads to cell death, neurotoxicity, and neurodegeneration.14 Serine-threonine kinases as Mitogen-Activated Protein Kinases (MAPK), Extracellular Signal-Regulated Kinases (ERK), Protein Kinase A (PKA), Cyclin-dependent kinase 5 (CDK5), or glycogen synthase kinase − 3β GSK-3β can phosphorylate tau, promoting its separation from the microtubules; among them, GSK-3β the most relevant.15 GSK-3β is overactivated and overexpressed in the brain of AD patients being implicated in AD pathology by several mechanisms.16 In this line, current knowledge indicates that AD progression is strongly associated with tau pathology rather than Aβ accumulation.14
Oxidative stress (OS) is one of the biochemical alterations involved in AD pathogenesis and progression.17 The brain is highly susceptible to OS due to its high energy demand, high oxygen consumption, and abundance of easily peroxidable lipids.18 During aging, this susceptibility is even higher due to energy deprivation, resulting in chronic OS. OS is induced by an imbalance in the redox state, involving an excess of reactive oxygen species (ROS) and reactive nitrogen species (RNS), antioxidant system dysfunction, and a low regenerative capacity.19 Mitochondrial oxidative phosphorylation and electronic transport chain (ETC) for ATP generation are the major source of free radicals like hydrogen peroxide (H2O2), hydroxyl radical (OH·), and superoxide radical (O2-·). Although ROS plays a physiological role in cell signaling, its production must be tightly controlled since an abnormal increase in ROS production leads to irreparable cellular damage. Oxidative damage affects lipids, proteins, nucleic acids, sugars, and essential molecules for the structural and functional integrity of neurons.20 This imbalance between pro-oxidant and antioxidant defenses causes brain tissue damage, neuronal death, and neurodegeneration.21 In fact, increased levels of peroxided biomolecules have been reported during AD progression in postmortem AD brains.22
Calcium (Ca2+) dyshomeostasis has also been widely described as a pathological pathway related to AD development.23 Intracellular Ca2+ signals regulate important processes of neuronal physiology, including cellular growth and differentiation, action potentials, synaptic plasticity, learning, and memory.24 Ca2+ influx into neurons is tightly coordinated and controlled by a complex network formed by a vast array of Ca2+ ion channels, pumps, and exchangers, together with G-protein-coupled receptors (GPCRs) and Ca2+ binding proteins, a network described as the “neuronal Ca2+ signaling toolkit.”24 Ca2+ imbalance disrupts normal neuronal functions and eventually leads to AD.25 However, age-related Ca2+ homeostasis dysregulation results in cytosolic Ca2+ ([Ca2+]c) overload, which contributes to synaptic dysfunction, facilitates Aβ peptides generation through Ca2+-mediated BACE-1 activity,26 promotes NFTs formation, and contributes to excitotoxic and/or apoptotic death of neurons.27
Neuroinflammation is also a pathological process closely related to AD development. Moreover, compelling research points to low-grade chronic neuroinflammation developed during decades before disease onset as a potential disease trigger. Central nervous system (CNS) possesses its own immune system, shaped by glial cells (microglia and astrocytes), although, in some cases, the peripheral immune cells can provide additional protection to the CNS. Inflammation plays a key role in protection against infections and it is important for tissue repair. However, low-grade chronic inflammation can be detrimental. In AD, microglial cells are predominantly found surrounding amyloid plaques in an effort to eliminate them.28 The neuroinflammatory process is mainly mediated by glial cells, which are activated by increased OS levels and immunogens acting on pattern recognition receptors (PRRs) like Toll-like receptors (TLRs).29 Glial activation promotes ROS and RNS release, induction of pro-inflammatory enzymes like inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and NADPH oxidase (NOX), and pro-inflammatory cytokines, such as inducible nitric oxide synthase (IL-1β), IL-6, IL-18, and TNF-α (Figure 1). In contrast, anti-inflammatory cytokines production, such as IL-4, IL-10, and IL-13, represents an alternative route to resolve the inflammatory process. This system.
Considering aberrant protein accumulation observed in AD, the ubiquitin-proteasome system (UPS) plays a major role in AD.30 This system processes aberrant proteins and regulates many biological processes by removing unfolded, misfolded, and aberrant toxic proteins in normal conditions.31 However, UPS activity is decreased in the cortex, hippocampus, parahippocampal gyrus, superior and middle temporal gyri, and inferior parietal lobe of AD brains and is also affected by normal aging.30, 32 UPS dysfunction slows or stops protein degradation and is associated with the main AD pathological hallmarks, including Aβ accumulation, plaque formation, tau hyperphosphorylation, cytotoxicity, and cell death.31 Interestingly, decreased proteasome activity does not result in decreased proteasome expression, suggesting that proteasome inhibition in AD might be a posttranslational modification.32 Once proteasome is inhibited, ubiquitinated, misfolded, aggregated, and oxidized proteins accumulate, initiating a pathological feedback loop that causes proteasome inhibition.33 Ubiquitin and ubiquitinated proteins have been detected in oligomeric Aβ plaques and NFTs PHF,34 and a mutation in the UPS-associated ubiquitin B gene (UBB), namely UBB+1, accumulates in AD leading to neuronal degeneration and spatial memory impairment.30, 35
1.2 Interconnected pathological pathways in AD onset and progression
Based on the complexity and connectivity of the different pathological pathways involved in AD onset and progression (Figure 1), it has been described as a multifactorial disease.36 Regarding the cholinergic system, AChE is related to both senile plaques and NFTs. The AChE peripheral anionic site (PAS) acts as a seeding site for small Aβ oligomers, accelerating their aggregation,37 resulting in Aβ deposition and senile plaque formation. Additionally, AChE-Aβ complexes exhibit increased neurotoxic capacity than free Aβ.38 Moreover, AChE also accumulates in NFTs.39
As previously described, Aβ peptides promote tau hyperphosphorylation leading to NFTs formation,40 dysregulate intracellular Ca2+ concentration,41 OS,42 and mitochondrial dysfunction.43 Aβ activates CDK5, GSK-3β, and c-Jun N-terminal kinase (JNK),44 kinases involved in tau hyperphosphorylation.45 Furthermore, GSK-3β modulates APP cleavage by increasing BACE-1 expression,46, 47 which leads to enhanced Aβ production.
Considering Ca2+ homeostasis dysregulation, Aβ mediates [Ca2+]c overload by several mechanisms.48 Aβ is able to form Ca2+-permeable pores in the plasma membrane, allowing Ca2+ entry, ROS generation, increased OS levels, and subsequent mitochondrial dysfunction, leading to cell death.49 Ca2+ entry through l-type voltage-gated calcium ion channels (l-VGCCs) also promotes Aβ oligomerization50 and activates CDK5.51 CDK5 activates BACE-1 increasing its proteolytic activity and subsequent aberrant Aβ production.52 Moreover, Aβ can directly increase OS state at the mitochondrial level through protein oxidation and lipid membrane peroxidation,53 favoring Aβ accumulation54 and ROS formation.55
Bearing in mind the neuroinflammatory process, Aβ fibrils are able to bind to TLR4 activating the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway and also activating glial cells, leading to the release of pro-inflammatory and neurotoxic cytokines.56, 57 These mechanisms are interrelated, demonstrating the multifactorial AD character that ultimately leads to neurodegeneration and neuronal death.
Regarding the importance of GSK-3β in AD, this kinase is closely related to both main AD hallmarks, Aβ senile plaques, and NFTs. As previously described, it is the main kinase responsible for tau hyperphosphorylation and increased BACE-1 expression, leading to NFTs and Aβ formation and accumulation, respectively.46 Once aggregated, Aβ promotes tau hyperphosphorylation through GSK-3β activation.44 Additionally, GSK-3β promotes cholinergic activity imbalance increasing synaptic AChE expression during apoptosis.58 Concerning OS, GSK-3β and the nuclear factor erythroid 2–related factor 2 (NRF2) activities are negatively correlated since GSK-3β downregulates NRF2,59, 60 increasing OS levels. In this line, OS also accelerates tau hyperphosphorylation through activation of GSK-3β and CDK5,61 and/or inactivation of protein phosphatase 2A (PP2A).62 Ca2+ overload activates calpain, which, in turn, activates CDK5 and cleaves the mitochondrial sodium-calcium exchanger (NCLX), affecting both GSK-3β and PP2A activities, resulting in tau hyperphosphorylation.63 Additionally, Ca2+ entry into the cytosol also activates Ca2+/calmodulin-dependent protein kinase II (CAMKII), which is able to phosphorylate tau.64 Moreover, increased intracellular Ca2+ levels induce OS elevation and mitochondrial dysfunction, then, OS induces cytosolic membrane damage, promoting Ca2+ channel activation and pump inhibition, which, finally leads to a higher [Ca2+]c overload. Therefore, the general increase in cytosolic Ca2+ levels severely limits normal cellular function, leading to apoptosis.65 Considering the neuroinflammatory process, GSK-3β overactivation observed in AD activates glial cells by promoting the activation of NF-κB and inactivation of the cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) pathways to modulate the expression of pro-inflammatory and anti-inflammatory cytokines.66, 67
In summary, excessive ROS and RNS production is involved in the aggregation and deposition of Aβ peptides through the oxidation of critical amino acids leading to their aggregation.68 Once formed, Aβ peptides increase ROS, mitochondrial failure, and [Ca2+]c imbalance, leading to exacerbated OS levels through cytochrome C oxidase inhibition.69 OS also accelerates tau hyperphosphorylation through GSK-3β and CDK5 activation.61 As mentioned, reactive species activate Ca2+ channels and inhibit pumps, leading to membrane damage and Ca2+ influx. OS also activates calpain and CAMKII.70 Calpain activation affects both GSK-3β and PP2A activities, leading to tau hyperphosphorylation63 and oxidizes plasma membrane Ca2+-ATPases triggering Ca2+ overload. On the other hand, activated CAMKII phosphorylates tau that finally increases [Ca2+]c.64, 71 Regarding neuroinflammation, OS activates glial cells leading to the inflammatory response and pro-inflammatory cytokines release.69 Taken together, all these mechanisms initiate different pathological feedback loops that spread neuronal damage.
Considering UPS failure in AD30 and Aβ production, UPS has been demonstrated to behave as α-, β-, and γ-secreases in in vitro studies.34 Proteasome inhibitors enhance APPα and Aβ40 production in cells expressing wildtype PS1/2, while Aβ42 formation is exacerbated in cells expressing mutated PS1/2. Aβ accumulation in extracellular senile plaques causes an additional UPS inhibition.72 Therefore, proteasome activators would enhance PS degradation reducing the intracellular concentration of these proteins.
Moreover, the neuron-specific F-box protein (Fbx2), a member of the Skp1/Cullin/F-box (SCF)-Fbx2-E3 ligase complex, contributes to neuron-specific proteins clearance by promoting their ubiquitination and proteasomal degradation via the endoplasmic-reticulum-associated protein degradation (ERAD) pathway.73 Specifically, Fbx2 recognizes and binds to BACE-1, increasing its ubiquitination and degradation.74 On the other hand, the ubiquitin C-terminal hydrolase (Uch-L1) stabilizes ubiquitin monomers to be tagged onto proteins destined to be degraded by the proteasome. Uch-L1 levels are decreased in postmortem AD patients' brains and in AD transgenic mouse models, contributing to AD pathogenesis through ubiquitinated Aβ and tau accumulation.1 In this context, Aβ formation could be reduced by activating Fbx2 and promoting BACE-1 degradation and/or by activating Uch-L1.35
Regarding tau phosphorylation, tau conformational and aggregative states determine its degradation mechanism. In this line, full-length tau is degraded by the proteasome while truncated tau form is degraded by autophagy.75 In the early stages, monomeric tau is recognized by the E3 ligase, resulting in subsequent targeting to the proteasome and further degradation. However, tau oligomers and PHF-tau aggregates bind and inhibit the proteasome in AD brains. Proteasome inhibition blocks soluble tau protein degradation, increases insoluble tau levels, and promotes protein misfolding and subsequent NFT formation.72, 76 C-terminus of Hsc70-interacting protein (CHIP), a co-chaperone E3 ligase, specifically ubiquitinates p-tau promoting its proteasomal degradation.77 However, CHIP levels are decreased in AD78 and the accumulation of ubiquitinated tau prevents ubiquitin recycling, thereby inhibiting ubiquitination.77 In fact, Thomas et al. demonstrated that ubiquitination was inhibited by p-tau, and reducing p-tau recovered the ubiquitination levels.79
Finally, in AD, the UBB+1 mutation compromises UPS function and accumulates in Aβ plaques and NFTs.30 The functionality of cellular processes correlates with the UBB+1 expression levels. At low levels, the UBB+1 protein is an efficient substrate for the proteasome, being polyubiquitinated and degraded by itself. Low UBB+1 reduces ROS toxicity, protects against autophagy dysfunction, and delays apoptotic and necrotic cell death, among others.80 However, at high levels, UBB+1 protein acts as a potent and specific proteasome inhibitor, which can accumulate and impair proteasome capacity.72 Additionally, high UBB+1 affects ubiquitin signaling, induces protein aggregation, impairs mitochondria function, and increases ROS toxicity and cell death.80 The dual substrate/inhibitor profile of UBB+1 makes it an endogenous marker for proteasome inhibition in some neurodegenerative diseases. All these interactions support that UPS plays an important role in AD pathology. Therefore, UPS is considered a potential target for AD treatment.
1.3 Pathological network approach for drug design: Multitarget drugs toward AD complexity
As AD knowledge advances, new pathological pathways are depicted prompting the formulation of novel hypotheses to integrate them, including the cholinergic hypothesis, Ca2+ hypothesis, innate immunity hypothesis, mitochondrial hypothesis, neuroinflammation hypothesis, OS hypothesis, and excitotoxicity hypothesis. However, important AD physiopathological aspects remain unclear.
The multifactorial nature of AD suggests the existence of an underlying complex network that comprises genetics, enzyme activities, receptor expression, protein interactions, alteration of metal concentrations, cell cycle survival disruption, ion homeostasis dysregulation, and protein misfolding, among others. AD multifactorial character promoted the hypothesis of the need to target more than one pathological pathway simultaneously to develop an effective therapy by using “Multi-Target Directed Ligands” (MTDLs).81, 82 This strategy proposes that acting on several targets simultaneously could increase the potential effectiveness of novel drugs against AD. This strategy is based on the design of single chemical entities able to interact with several pharmacological targets of relevance for AD, minimizing adverse effects and improving pharmacokinetic and ADMET profiles. In the AD field, MTDLs must hit targets located upstream in the neurotoxic cascades to achieve maximum efficiency in stopping or delaying neurodegeneration for a disease-modifying effect.
In this review, we will summarize the latest approaches to basic developments that will bring the next generation of multitarget AD drugs in the coming years.
2 RECENT ADVANCES IN THE MULTITARGET DRUG DEVELOPMENT
2.1 GSK-3β-based multitarget drugs
As introduced, GSK-3β is a ubiquitously expressed serine-threonine kinase that plays a central role in the regulation of many biological pathways from glycogen metabolism to gene transcription.15 In the CNS, the GSK-3β isoform is the most abundant and its expression levels are known to increase with age.83 GSK-3β function can be regulated by phosphorylation and dephosphorylation on different sites: its activity is positively regulated by auto-phosphorylation at Tyr216, while phosphorylation at Ser9 leads to its inhibition.84 GSK-3β is overactivated and overexpressed in AD brains and it is implicated in AD pathology by distinct mechanisms.16 There are a considerable number of amino-acid residues at tau protein susceptible to GSK-3β phosphorylation, mainly at Ser199 Ser396 and Ser413.85 Tau hyperphosphorylation leads to loss of axonal transport function and neuronal integrity. Once initiated, the tau aggregation cascade is self-replicating and NFTs continue to accumulate, leading to neuronal death.86
GSK-3β is involved in Aβ formation and accumulation in the AD brain by modulating APP cleavage.87 APP and PS1, one of the catalytic components of the γ-secretase complex, have been identified as GSK-3β substrates.86, 88 This finding suggests that GSK-3β overactivity causes higher Aβ production and its subsequent deposition in the AD brain.89 GSK-3β involvement in Aβ-plaque formation has been also associated with BACE-1 overactivation. Higher BACE-1 expression and activity detected in the brains of AD patients is consistent with increased Aβ levels.90 Several in vitro and in vivo studies have established that GSK-3β inhibition leads to decreased Aβ production and restores hyperphosphorylation of tau, thus, a high number of drug discovery programs against tau pathology are focused on the development of GSK-3β inhibitors.91, 92 Additionally, GSK-3β inhibition has been linked to memory consolidation, neurogenesis, synaptic plasticity, long-term potentiation, and neuroinflammation reduction.93 These findings support the hypothesis that GSK-3β can be considered as a therapeutic target for the treatment of AD.
2.1.1 GSK-3β and BACE-1 inhibition
Based on the above-mentioned findings, GSK-3β and BACE-1 have emerged as ideal candidates for the multitarget approach based on the tau and amyloid cascade, respectively. Therefore, simultaneous GSK-3β and BACE-1 inhibition represents an innovative breakthrough for AD treatment.
Prati et al.94 reported novel hybrid compounds combining a guanidine moiety and a cyclic amide group as both BACE-1 and GSK-3β inhibitors. The fluorinated derivative (1, Figure 2) showed an IC50 of 18.03 µM to inhibit BACE-1 activity and an IC50 of 14.67 µM to inhibit GSK-3β activity in vitro. This compound exhibited an anti-inflammatory profile able to reduce iNOS expression induced by lipopolysaccharide (LPS) in primary rat glial cells, without any toxicity in glial or neuronal cells. Interestingly, it showed neurogenic properties. Regarding its pharmacokinetic profile, it had good oral bioavailability (66%) and a good blood–brain barrier (BBB) permeability. In an effort to optimize 1 properties, the triazinone core was modified expanding its aromatic substitution pattern, the 3,4-dihydro-triazinone carbonyl oxygen was replaced by sulfur to access 3,4-dihydro-triazinthiones and the inclusion of different N-alkyl or N-aryl groups at the exocyclic C6-amino group.95 The N-propyl derivative (2, Figure 2) was the most potent GSK-3β inhibitor (IC50 = 4.34 µM), while it exhibited moderate BACE-1 inhibitory potency (IC50 = 36.83 µM). Compound 2 showed anti-inflammatory properties reducing nitrite production and iNOS expression in primary rat glial cells. Interestingly, compound 2 kept its neurogenic properties at 10 µM, being able to differentiate neuronal stem cells into mature neurons.
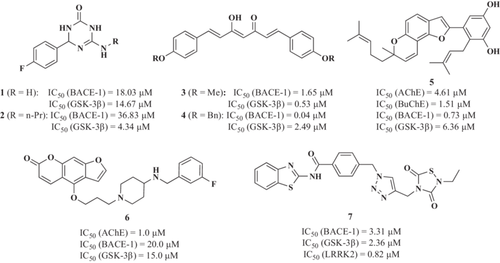
Taking a different approach to identifying dual BACE-1/GSK-3β inhibitors, Di Martino et al.96 reported a novel MTDL based on curcumin.97 Regarding BACE-1 inhibition, novel derivatives showed improved inhibition compared with curcumin-β-keto-enol tautomer with IC50 values ranging from nanomolar to low micromolar (IC50 between 40 and 2.69 µM) and increased GSK-3β inhibitory potency (IC50 values from 0.53 to 16.99 µM). Derivatives 3 and 4 (Figure 2) were the most potent BACE-1 and GSK-3β inhibitors, with an IC50 to inhibit BACE-1 of 1.65 and 0.04 µM, and IC50 toward GSK-3β of 0.53 and 2.49 µM, respectively. Although compounds 3 and 4 showed uncertain capability to cross the BBB, they did not show any cytotoxic effects in the T67 cell line. Moreover, compound 4 increased the expression of NAD(P)H dehydrogenase quinone 1 (NQO1), a phase II antioxidant enzyme that catalyzes the reduction of quinones to hydroquinones by scavenging superoxide molecules.98
Paudel et al.99 described a triple target, with compound 5 being able to inhibit cholinesterases, BACE-1, and GSK-3β. Arylbenzofuran (5, Figure 2) was able to inhibit AChE, BuChE, BACE-1, and GSK-3β with IC50 values of 4.61, 1.51, 0.73, and 6.36 µM, respectively. Derivative 5 also inhibited Aβ1-42 self-induced and Aβ1-40 AChE-induced aggregation in a concentration-dependent manner being more potent than reference drugs curcumin and donepezil. Similarly, in 2021, Wang et al. described a notopterol derivative with furacoumarin as a scaffold also with triple AChE/BACE-1/GSK3β inhibitory activity, having an IC50 of 1.0, 20.0, and 15.0 μM, respectively. This compound (6) also showed good BBB penetrability, suitable bioavailability, and oral safety. Interestingly, it improved memory and learning in an AD in vivo model induced by Aβ.100
Similarly, Nozal et al.101 reported a novel 1,2,3-triazole derivative designed to inhibit BACE-1, GSK-3β, and LRRK2 (leucine-rich repeat kinase 2). LRRK2 plays a potential role in AD as it resides within a region on chromosome 12, being linked to LOAD cases,102 and it is a key modulator of neuroinflammation, as LRRK2 inhibition attenuates this process and cytotoxicity in in vivo AD models.103 Derivative 7 (Figure 2) showed an IC50 value of 3.31 µM to inhibit BACE-1 activity and IC50 values of 2.36 and 0.82 µM to inhibit both GSK-3β and LRRK2, respectively. Derivative 7 also exhibited an interesting neuroprotective profile against the tau phosphorylation cellular toxicity model at 10 µM and the reduction of Aβ-induced neurotoxicity, showing a significant decrease in Aβ1-42 levels in extracellular fluids at the same concentration. Further studies may be performed to determine the BBB penetration profile of this molecule.
2.1.2 GSK-3β and tau aggregation inhibition
Tau cascade is recognized as a promising target for drug development for AD treatment.104 In this context, recent studies described novel MTDLs able to inhibit both tau aggregation and GSK-3β, a mixture of activities that could, potentially, reduce hyperphosphorylated tau inside neurons. Following this hypothesis, Gandini et al.105 reported the design, synthesis, and biological evaluation of novel 2,4-thiazolidinedione derivatives. The N-heterocyclic derivatives, with the indole (8, Figure 3) and the 5-methoxy-N-methylindol derivative (9, Figure 3), inhibited GSK-3β at micromolar range (IC50 values of 4.93 and 0.89 µM, respectively). Interestingly, both compounds were able to inhibit 60% and 80% tau aggregation at 10 µM, respectively, measured by Thioflavin T (ThT) fluorescence assay using the tau-derived hexapeptide Ac-VQIYK-NH2 (AcPHF6). Moreover, they were able to cross the BBB by passive permeation. Both derivatives were able to improve cell viability in the okadaic acid-induced tau hyperphosphorylation cellular toxicity model at 10 µM. Additionally, derivative 9 also displayed inhibition of both the truncated 18K-tau fragment and full-length tau aggregation, as demonstrated by a heparin-induced tau assembly assay in which the fibrillization of the truncated 18K-tau fragment or the full-length 2N4R tau was monitored by ThT fluorescence.
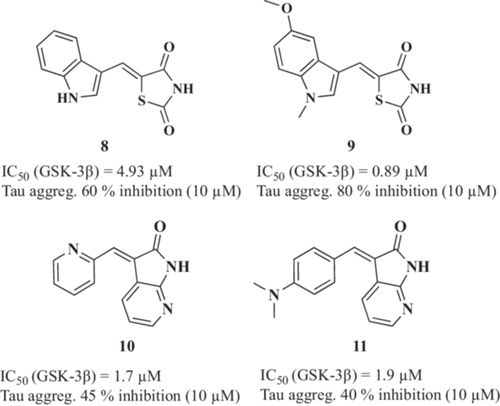
Thereafter, Ali et al.106 reported novel dual GSK-3β/tau aggregation inhibitors based on 3-arylidene-indolin-2-one structure. Compounds 10 and 11 (Figure 3) were identified as potent GSK-3β inhibitors (IC50 values of 1.7 and 1.9 µM, respectively) with a dose-dependent tau 301LP anti-aggregation effect in HeLa cells level (45% tau aggregation reduction for compound 10 and 40% for compound 11, both at a 10 µM concentration). Additionally, the cytotoxic effect of the compounds was assessed in K562, U251, HCT-116, A375, and PBMC cell lines for 72 h with the lowest LD50 values of 12.8 µM (HCT-116) and 4.7 µM (K562) for compounds 10 and 11, respectively.
2.1.3 GSK-3β and Aβ aggregation inhibition
SAR502250 (12, Figure 4) is a potent GSK-3β inhibitor (IC50 = 12 nM)107 evaluated in neuroprotection and neuropsychiatric AD models by Griebel et al.108 This compound was able to attenuate tau hyperphosphorylation in the spinal cord and the cortex of P301L human tau transgenic mice. Moreover, it prevented neuronal death of primary hippocampal neurons cultures treated with Aβ neurotoxic fragment Aβ25–35. Considering behavioral performance, SAR502250 improved cognitive deficit of aged transgenic APP(SW)/Tau(VLW) mice or adult mice after infusion of Aβ25–35.
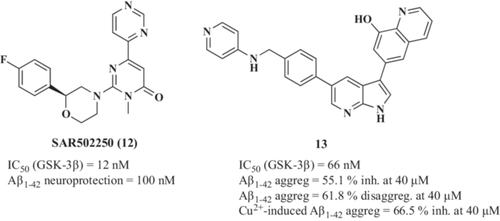
In 2020, Shi et al.109 described novel 1H-pyrrolo[2,3-b]pyridine family of compounds designed as MTDLs being GSK-3β inhibitors and metal chelators. Lead compound 6-(5-(4-((pyridine-4-ylamino)methyl)phenyl)−1H-pyrrolo[2,3-b]pyridine-3-yl)quinoline-8-ol (13, Figure 4) showed high potency to inhibit GSK-3β (IC50 = 66 nM). GSK-3β also plays a central role in the regulation of the Wnt/β-catenin signaling pathway.110 In the resting state, GSK-3β phosphorylates β-catenin, triggering its proteasomal degradation maintaining it at a very low concentration in the cytosol.111 Therefore, GSK-3β inhibition can lead to stabilized β-catenin, which translocates to the nucleus and activates the transcription of its target genes.112 Compound 13 increased β-catenin abundance in a dose-dependent manner (β-catenin/glyceraldehydes 3-phosphate dehydrogenase (GAPDH) ratio increased from 0.30 of the control to 0.46, 0.57, 0.83 at 5, 10, and 20 µM, respectively) and the inactive form of GSK-3β phosphorylated at Ser9 (pGSK-3β/GAPDH ratio increased from 0.35 of the control to 0.53 at 20 µM) in SH-SY5Y cells. Additionally, compound 13 decreased Ser396 phosphorylated tau levels in a concentration-dependent manner when cells were treated with 20 µM of Aβ25-35 (pTau/GAPDH ratio decreased from 0.83 of the control to 0.33 at 20 µM). Interestingly, compound 13 demonstrated Fe2+, Zn2+, Cu2+, and Al3+ chelation capacity at 20 µM. Finally, 13 inhibited Aβ1-42 aggregation (55.1% inhibition at 40 µM) and reduced the Aβ1-42 aggregation (61.8% reduction at 40 µM) being more potent than reference drug curcumin. Considering its chelation capacity, 13 inhibited the Cu2+-induced Aβ1-42 aggregation by 66.5% at 40 µM, being more potent than the reference compound clioquinol. Interestingly, 13 increased the mRNA expression of neurogenesis markers (growth-associated protein 43 (GAP43), N-myc proto-oncogene protein (N-myc), and microtubule-associated protein 2 (MAP-2)) and promoted neurite outgrowth at 10 µM in SH-SY5Y cells.
2.1.4 GSK-3β and CDK5 inhibition
CDK5 is a proline-directed serine-threonine kinase, which participates in the regulation of neuronal movements, such as neuronal migration and differentiation, regulation of synaptic plasticity, neurotransmitter release, and memory consolidation. However, under pathological conditions, CDK5 is activated113 promoting aberrant APP, tau protein hyperphosphorylation, and neurofilaments, thus accelerating AD development. CDK5 deregulation contributes to Aβ production, formation of NFTs, synaptic damage, mitochondrial dysfunction, and neuronal apoptosis.114 CDK5 and GSK-3β deregulation has been implicated in abnormal tau hyperphosphorylation in AD.115 The ATP binding site of CDK5 shares a high homology with the corresponding ATP site of GSK-3β, thus a lack of selectivity over this kinase may be desirable; in this context, compounds that inhibit both enzymes may provide increased efficacy.116
Holzer et al.117 reported novel dual CDK5/GSK-3β inhibitors based on the benzofuropyridine scaffold. Previous findings showed that the benzofuropyridine moiety was able to inhibit cyclin-dependent kinase 1 (CDK1).118 Successfully, the 3-ethoxy-4-phenylbenzofuro[2,3-b]pyridine-6-ol derivative (14, Figure 5) exhibited interesting triple inhibitory capacity with IC50 values of 0.17, 0.08, and 0.46 µM for CDK1, GSK-3β and CDK5, respectively. Moreover, this compound reduced tau phosphorylation by 61% at 8 µM and inhibited tau aggregation by 65% at 10 µM.
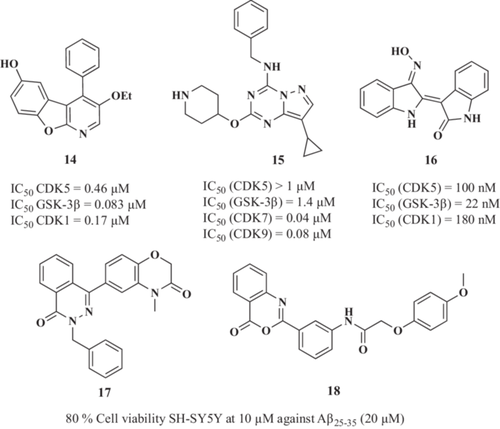
Reinhardt et al.119 showed that CDK5 knockdown was not sufficient to afford protection, indicating that the combination of GSK-3β and CDK5 inhibition is required to protect human iPSC-derived motoneurons against inflammatory-like conditions. Using a phenotypic screening, Reinhardt et al.119 identified a pyrazolo[1,5-a][1,3,5]triazine derivative (15, Figure 5) as an active hit. Compound 15 inhibited both GSK-3β (IC50 = 1.4 µM) and moderately inhibited CDK5 (20% at 1 µM) and protected neurons in vivo in zebrafish models of motoneuron degeneration and AD being a preliminary hit compound for optimization.
Another line of research has been directed to indirubins, extensively studied as potential dual CDK5 and GSK-3β inhibitors. Leclerc et al.120 reported the synthesis and pharmacological evaluation of a series of indole derivatives and dimers. Novel derivatives showed potent CDKs (IC50 = 50–100 nM) and GSK-3β (IC50 = 5–50 nM) inhibitory capacity. Authors demonstrated that indirubins inhibit GSK-3β by binding at the ATP binding site, similar to their binding to CDKs. From this chemical family, indirubin-3'-monoxime derivative 16 (Figure 5) inhibited human recombinant tau hyperphosphorylation in a dose-dependent manner with an IC50 value of 100 nM. Moreover, 16 also reduced dopamine and 3’,5’-cyclic adenosine monophosphate-regulated neuronal phosphoprotein 32 (DARPP-32) phosphorylation by CDK5 in striatum area brain slices at 10 µM.
To find a novel chemical core with this combination of targets, Xie et al.121 performed a virtual screening toward GSK-3β and selected compounds with high binding scores that were thereafter docked toward CDK5. Compounds 17 and 18 (Figure 5) showed the highest energy binding capacities being selected as potential dual GSK-3β and CDK5 inhibitors, although they were not experimentally tested. In vitro evaluation of hit compounds demonstrated their neuroprotective capacity against Aβ25-35-induced toxicity in human neuroblastoma cells, reducing cell death by 14% and 17%, respectively, at 10 µM.
2.1.5 GSK-3β inhibition and NRF2 induction
As described, OS is one of the most prominent biochemical alterations in AD.17 There is evidence of neurotoxic effects of hyperphosphorylated tau, Aβ aggregates, and OS, and more importantly, there is an intense crosstalk between these factors creating a pathological feedback loop, intensifying neuronal damage and accelerating cognitive decline.42
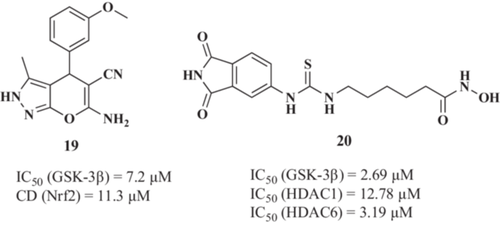
Cells exposed to increased ROS activate multiple signaling pathways to counteract or mitigate this aggression. Among them, the NRF2/electrophile response element (EpRE) transcriptional pathway is considered the most important antioxidant response, promoting the expression of numerous antioxidant and anti-inflammatory enzymes.122 As mentioned above, GSK-3β constitutes an NRF2 negative regulation mechanism, which phosphorylates NRF2 creating a recognition site for β-Transducing repeat-containing protein (β-TrCP). β-TrCP leads to Cullin-1/Rbx1-mediated NRF2 ubiquitination and its subsequent degradation.59 Thus, GSK-3β promotes NRF2 cytosolic localization, inhibiting its transcriptional activity and blocking its antioxidant and cytoprotective functions. Considering these insights, Gameiro et al.123 reported the first compound targeting GSK-3β and NRF2 simultaneously as a novel approach for AD therapy. The chosen 6-amino-4-(3-methoxyphenyl)−3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (19, Figure 6) was able to inhibit GSK-3β with an IC50 value of 7.2 µM and to induce NRF2 with a CD (concentration required to double the specific luciferase reporter activity) value of 11.3 µM. Additionally, 19 showed neuroprotective properties at 1 µM against the okadaic acid-induced tau hyperphosphorylation and the rotenone/oligomycin A-induced OS model124 with protection percentages of 56.2% and 55.3%, respectively. Interestingly, compound 19 showed anti-inflammatory activity being able to reduce nitrite and ROS production in primary glial cell cultures challenged with LPS (IC50 nitrite reduction = 12.9 µM; IC50 ROS reduction = 18.1 µM). ROS reduction upon LPS treatment was related to a decrease of NADPH oxidase (NOX) activity in a concentration-dependent manner. Moreover, further studies demonstrated that compound 19 was able to reduce TNF-α release induced by LPS by 33% at 10 µM, by 50% at 30 µM and iNOS expression at 30 µM to almost basal levels in primary glial cultures.
2.1.6 GSK-3β and HDAC inhibition
Recently, epigenetic modifications have been considered a promising strategy for AD treatment.125 The role of histone acetylation in rescuing learning and memory impairment has promoted its regulation through histone deacetylases (HDACs) as a novel AD treatment strategy.124, 126 Histones are building blocks of the nucleosome, the chromatin fundamental unit. Histone acetylation plays an important role in regulating chromatin condensation and gene transcription. Thus, histone acetylation activates transcription while histone deacetylation suppresses gene transcription. These acetylation and deacetylation processes are catalyzed by histone transferases (HATs) and HDACs, respectively, in a balanced state; however, in AD, this homeostasis is disturbed.127 Therefore, HDAC inhibitors have gained increasing interest as potential AD drugs.128
GSK-3β contributes to the regulation of histone modifications involved in epigenetics. GSK-3β directly phosphorylates histone 1.5, histone 3,129 HDAC3 130 and HDAC4.131 In this line, in hippocampal neurons, GSK-3β phosphorylates HDAC6 enhancing its activity.132, 133 GSK-3β also modulates post-translational modifications of histones by regulating several HATs.134 Furthermore, combined GSK-3β and HDACs inhibition induces synergistic neuroprotective effects compared with single target inhibition, with potentially improved therapeutic selectivity.135, 136
De Simone et al.137 reported the first GSK-3β and HDAC dual inhibitor for AD treatment. Authors described a new hybrid family that combines hydroxamic acid, an HDAC inhibitor that chelates the Zn2+ ion located at HDACs active site, and the maleimide-like scaffold moiety, which is known to competitively bind to the GSK-3β ATP binding site. The 6-(3-(1,3-dioxoisoindolin-5-yl)thioureido-N-hydroxyhexanamide derivative 20 (Figure 6) showed IC50 values of 12.78 and 3.19 µM toward HDAC1 and HDAC6, respectively, and it was able to inhibit GSK-3β with an IC50 of 2.69 µM. Interestingly, compound 20 induced H3 acetylation and blocked Cu2+-induced tau hyperphosphorylation. Derivative 20 also showed neuroprotective properties toward OS-induced neuronal death in SH-SY5Y cells treated with H2O2 at 0.1 µM by restoring cell viability to almost control values and restored p53 levels to control levels at 10 µM. Considering its neuroprotective character, compound 20 completely reversed neurotoxicity induced by 6-hydroxydopamine (6-OHDA) in primary differentiated cerebellar granule neurons at 5 µM and promoted neurogenesis at 10 µM. Moreover, the immunomodulatory capacity of compound 20 was demonstrated in primary microglial cultures being able to reduce neuroinflammation by decreasing the expression of nitric oxidase synthase 2 (NOS2) and mannose Receptor C-type 1 (MRC1) in cells treated with LPS and compound 20 at 5 µM. Finally, compound 20 showed no toxicity in in vivo wildtype zebrafish model. Compound 20 also demonstrated effectiveness in vivo in a model of cyclin-dependent kinase-like 5 gene (CDKl5) deficiency,138 being able to exert a neuroprotective effect following 15-day intraperitoneal treatment (50 mg/kg), evidenced by an increase in hippocampus neuronal survival, a restoration of neuronal dendritic spines number and mature state and a decrease in hippocampus microglia enlarged body size, related to a state of activation. Thus, although not evaluated in an AD model, compound 20 presents an adequate profile to exert a therapeutic effect in the brain in vivo.
2.1.7 GSK-3β and DYRK1A inhibition
The dual-specificity tyrosine phosphorylation-regulated kinase-1A (DYRK1A) is a protein kinase that belongs to the Dyrk family. DYRK1A can phosphorylate tau on several Ser and Thr residues. Indeed, it is responsible for tau phosphorylation at epitopes that prime tau for further phosphorylation by GSK-3β.139 Additionally, DYRK1A activity has been related to an enhancement of the APP amyloidogenic processing via phosphorylation increasing Aβ production.140 In good agreement, DYRK1A activity is increased in AD brains and its inhibition led to cognitive improvement in the APP/PS1/Tau 3xTg-AD mice model by reducing tau hyperphosphorylation and Aβ levels.140 Thus, DYRK1A inhibition in combination with GSK-3β can reduce tau hyperphosphorylation more effectively, preventing NFT formation while simultaneously reducing Aβ pathology.140
In this line, Liu et al.141 reported a new series of GSK-3β and DYRK1A inhibitors based on the β−carboline structure of the alkaloid harmine. This work led to the identification of compound ZDWX-25 (21, Figure 7) as a potent dual GSK-3β and DYRK1A inhibitor (IC50 = 71 nM, IC50 = 103 nM, respectively) with good BBB permeability (PAMPA assay). Additionally, ZDWX-25 treatment (1 µM) reduced tau and DYRK1A hyperphosphorylation (Ptau-Ser396, PDYRK1A-Tyr321/273) and NFT formation while inhibiting GSK-3β and DYRK1A in okadaic acid-injured SH-SY5Y cells. Interestingly, ZDWX-25 also showed a protective effect in vivo in the APP/PS1/Tau 3xTg-AD mice model.142 Treatment with ZDWX-25 for 2 months at 15 mg/kg reduced tau hyperphosphorylation and aggregation in the hippocampus and temporal cortex, reducing the cognitive impairment associated with the model.
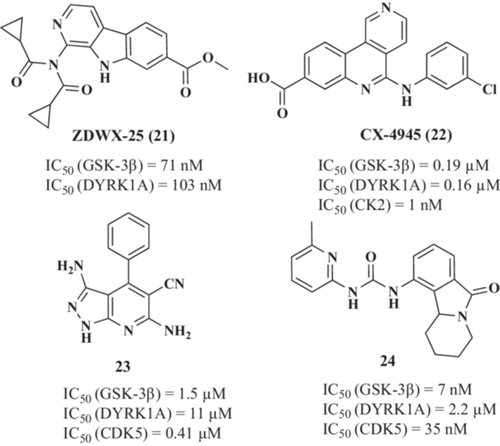
In a drug repurposing strategy, Grygier et al.143 reported that CX-4945 (22, Figure 7) (also known as silmitasertib), a clinically approved casein kinase 2 (CK2) inhibitor for cancer treatment,144 is also a dual GSK-3β (IC50 = 0.19 µM) and DYRK1A (IC50 = 0.16 µM) inhibitor. CX-4945 reduced tau hyperphosphorylation exerting a protective effect in in vitro and in in vivo models based on DYRK1A overexpression.145 Additionally, CX-4945 showed an anti-inflammatory effect in the astrocytoma cell line U373 cells and human primary astrocytes stimulated with IL-1β or TNF-α.146 In IL-1β stimulated astrocytes, CX-4945 treatment (10 µM) reduced IL-6 and MCP-1 production (55% and 75% reduction, respectively). Similarly, in TNF-α-stimulated astrocytes, a reduction in IL-6 and MCP-1 (50% and 60%, respectively) levels was observed following the same treatment. Interestingly, these anti-inflammatory properties were not reproduced by the CK2 inhibitor 4,5,6,7-tetrabromo-1H-benzotriazole, suggesting that its GSK-3β and/or DYRK1A inhibitory capacity might be related to this activity.
Other compounds based on 3,6-diamino-1H-pyrazolo[3,4-b]pyridine structure (23, Figure 7)147 or tetrahydropyridine isoindolone (valmerin) structure (24, Figure 7)148, 149 have been reported as multitarget GSK-3β, DYRK1A, and CDK5 inhibitors. Among valmerin derivatives, several compounds showed a strong inhibitory effect on the three kinases, with IC50 values in the submicromolar range for GSK-3β and CDK5 and in the low micromolar range for DYRK1A. Among them, compound 24 exhibited most potent multitarget inhibition (IC50 GSK-3β = 7 nM; IC50 CDK5 = 35 nM; IC50 DYRK1A = 2.2 µM). However, the potential therapeutic effect of these compounds in AD models has not been assessed yet. Indeed, a cytotoxic effect in the submicromolar range was found for several of the valmerin derivatives in a panel of 6 cell lines: Huh7 (liver), Caco2 (colon), MDA-MB231 (breast), HCT-116 (colon), PC3 (prostate), and NCIH727 (lung).
2.1.8 Dual GSK3β and AChE inhibitors
Additionally to previously described involvement of AChE and GSK-3β in AD pathology, both enzymes are cross-linked under pathological conditions. It was initially described an increased expression of AChE synaptic isoform during apoptosis activation.150 Later it was demonstrated that GSK-3β mediates the increased expression of synaptic AChE during apoptosis.58 As many cells undergo apoptosis due to the presence of senile plaques and NFTs of AD brains, the combination of AChE and GSK-3β as therapeutic targets for the development of MTDLs for AD treatment has been recently investigated.
Another example are thee tacrine-pyridothiazole derivatives described by Jiang et al.151 Their tacrine moiety was included to bind at the AChE CAS, and the pyridoxathiazole fragment, was inclued to interact at the GSK-3β ATP-binding site. Among these hybrids, the 2-(2-(cyclopropanecarboxamido)pyridine-4-yl)−4-methoxy-N-(3-((1,2,3,4-tetrahydroacridin-9-yl)amino)propyl)thiazole-5-carboxamide derivative (25, Figure 8) showed a nanomolar inhibition of both hAChE (IC50 = 6.4 nM) and GSK-3β (IC50 = 66 nM). Authors proposed that the pyridoxathiazole moiety is able to bind at the AChE PAS to generate a dual binding ligand with potential implications in Aβ aggregation. In this regard, compound 25 inhibited 46% Aβ self-aggregation at 20 µM. Moreover, compound 25 significantly reduced tau hyperphosphorylation in mouse neuroblastoma N2A cells. In vivo studies confirmed that compound 25 significantly ameliorated cognitive decline induced by scopolamine in ICR mice and showed reduced hepatotoxicity compared with tacrine.
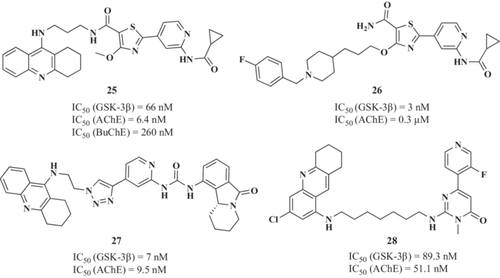
In the same line, Jiang et al.152 have recently reported a novel hit compound (26, Figure 8) obtained by fusing the pharmacophores of pyridothiazole with benzylpiperidine scaffold. It showed IC50 values of 0.3 μM for hAChE and 3 nM for hGSK-3β. Furthermore, it has a good iinhibitory activity of Aβ1-42 self-aggregation with a ~25% of reduction at 25 μM, which leads to anti-inflammatory and neuroprotective properties in an in vitro model of OS neuronal death. It also exhibited good BBB permeability (PAMPA assay).
In 2019, Oukoloff et al. designed a new series of tacrine-valmerin hybrids as potential AChE/GSK-3β inhibitors, including a 1,2,3-triazole moiety as linker.153 As a result, hit compound 27 (Figure 8) showed the most promising in vitro activities, inhibiting both human AChE and GSK3β at the nanomolar range (9.5 and 7 nM, respectively). Moreover, in the neuroblastoma cell line SH-SY5Y it exhibited low cytotoxicity and a good ability to penetrate the BBB in tne MDCK-MDR1 cell line without interacting with efflux pumps such as P-gp.
Yao et al.154 reported novel tacrine-pyrimidone hybrids as dual AChE/GSK-3β inhibitors for AD treatment. The 2-((7-((3-chloro-5,6,7,8-tetrahydroacridin-1-yl)amino)heptyl)amino)−6-(3-fluoropyridin-4-yl)−3-methylpyrimidin-4(3H)-one derivative (28, Figure 8) was selected as lead candidate showing high potency to inhibit both AChE and GSK-3β in the nanomolar range (IC50 = 51.1 and 89.3 nM for AChE and GSK-3 β, respectively). Derivative 28 confirmed its ability to inhibit GSK-3β in vitro by reducing ptau levels at both Ser86 and Ser396 sites at 0.5 and 5 µM, respectively, and protecting neuronal cells from cytotoxic glyceraldehyde in SH-SY5Y derived neuron models at the same concentrations. Moreover, derivative 28 was able to maintain the axon length of neurons in the presence of this toxic, preventing neurite shortening in differentiated SH-SY5Y cells. Behavioral studies in the AD scopolamine-related mouse model showed the ability of 28 to ameliorate the memory impairment caused by scopolamine, achieving the therapeutic effects of tacrine at the same administered dosage (15 mg/kg).
2.2 MTDLs with calcium-modulating activity
As mentioned above, neuronal Ca2+ homeostasis dysregulation results in [Ca2+]c overload contributing to synaptic dysfunction, Aβ peptide generation, NFT formation,26, 155 and finally, neuronal death.25 Therefore, the use of VGCC blockers to stabilize neuronal Ca2+ dysregulation could represent an interesting strategy for AD prevention and treatment.156-159
2.2.1 GSK-3β inhibition and calcium channel blockade
Based on these evidence, Bisi et al.160 described the first dual VGCCs and GSK-3β modulators for AD treatment. Authors designed a new maleimide-anthracene-based derivative with potential therapeutic activity in AD. Among described derivatives, the N-heptyl-12,14-dioxo-9,10-[3,4]epipyrroloanthracene-9(10H)-carboxamide derivative (29, Figure 9) exhibited interesting GSK-3β inhibition capacity (IC50 = 3.12 µM) and it was the most potent VGCC blocker reducing Ca2+ entry elicited by K+ 70 mM-evoked depolarization in SH-SY5Y cells with an IC50 value of 9 µM. Following these results, the authors reported a hit-to-lead optimization program focused on reducing molecular weight by simplifying the complex polycyclic structure.161 However, although some of the new derivatives maintained a multitarget profile, they experienced a reduction in potency as exemplified by compound 30 (Figure 9), (GSK-3β IC50 = 52.5 µM; Ca2+ entry blockade IC50 = 12.0 µM).
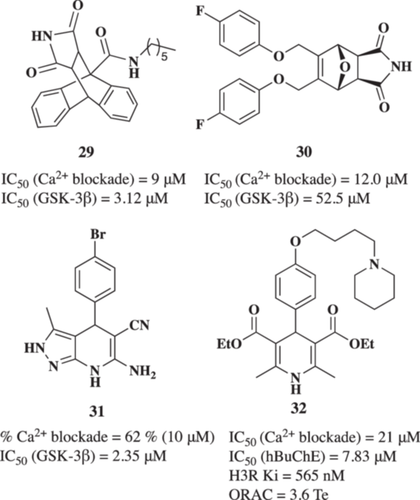
Recently, Michalska et al.162 reported a novel series of 1,4-dihydropyridine derivatives as dual VGCC antagonists and GSK-3β inhibitors. Among these compounds, the 6-amino-4-(4-bromophenyl)-3-methyl-4,7-dihydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile derivative (31, Figure 9) exhibited interesting GSK-3β inhibition capacity (IC50 = 2.35 µM) and it was able to reduce the VGCC response to 62% at 10 µM. Derivative 31 also demonstrated an interesting antioxidant activity (ORAC = 0.96 Trolox equivalents, T.e.), potent anti-inflammatory capacity able to reduce nitrite production in BV2 microglial cells (EC50 = 4.50 µM), good neuroprotective profile in in vitro models of tau hyperphosphorylation, OS, and [Ca2+]c overload at 1 µM with survival percentages of 86.7%, 59.7%, and 69.4%, respectively. Interestingly, derivative 31 reduced cell death induced by tau hyperphosphorylation in acute treatment of hippocampal slices treated with okadaic acid by reducing ROS production.
2.2.2 Calcium channel blockade and H3R antagonism
Histamine is an endogenous amine that acts as a neurotransmitter in the CNS. The histaminergic system is composed of histamine and its receptors, playing an important role in maintaining brain homeostasis function.163 There are four histamine receptors (H1R-H4R) involved in inflammation in the periphery, gastric acid secretion, dilatation of capillaries, and muscle contraction. H3R activation inhibits neurotransmitter release; thus, the development of H3R antagonists was proposed as a therapeutic strategy to increase, correct, and regulate histamine levels and other neurotransmitters, such as ACh, serotonin, dopamine, and norepinephrine.164 Additionally, the H3R-mediated histaminergic autoinhibitory effect depends on Ca2+ release from intracellular stores, however, it is impaired in depolarized cells.165 Depolarization of histaminergic neurons increases [Ca2+]c through VGCCs.166
Based on these considerations, Malek et al.167 reported the design, synthesis, and pharmacological evaluation of novel diethyl 2,6-dimethyl-4-(4-(2-(amine-1-yl)alkoxy)phenyl-1,4-dihydropyridine-3,5-dicarboxilates as MTDLs for AD treatment. Its design considered the connection of the selective l-VGCC blocker 1,4-dihydropyridine (DHP) scaffold and the cycloalkylamine moiety present in the H3R antagonist Pitolisant. After pharmacological evaluation of all designed derivatives, the diethyl 2,6-dimethyl-4-(4-((5-(piperidin-1-yl)pentyl)oxy)phenyl)-1,4-dihydropyridine-3,5-dicarboxylate derivative 32 (Figure 9) was identified as a moderate VGCC blocker (IC50 = 21 µM), with potent affinity toward H3R (Ki = 565 nM), selective hBuChE inhibition (IC50 = 7.83 µM) and antioxidant properties (ORAC = 3.6 Te). The multitarget activity of compound 32 resulted in an interesting neuroprotective profile. 32 increased cell viability in the rotenone/oligomycin A-induced OS cell model at 1 and 3 µM (64.8% and 49.9% protection, respectively). It also protected cells in the okadaic acid-induced tau hyperphosphorylation cell model (41.8% and 20.9% protection, respectively). Furthermore, compound 32 restored cognitive impairment induced by LPS in vivo, demonstrating its potential as a multitarget drug for AD treatment.
2.2.3 Calcium channel blockade and AChE inhibition
Zhang et al.168 described a new 1,4-dihydro-naphthyridine derivative SCR-1693 (33 × 52, Figure 18), ethyl 5-amino-4-(2-chlorophenyl)-2,7,7-trimethyl-1,4,6,7,8,9-hexahydrobenzo[b][1,8]-naphthyridine-3-carboxylate compound 33 × 52 as a selective, reversible, and noncompetitive AChE inhibitor (IC50 = 0.68 μM) based on previously developed MTDLs reported.169, 170 It also inhibited in a dose-dependent manner KCl-evoked intracellular Ca2+ increase in SH-SY5Y cells (IC50 = 28.6 μM) and possessed high efficiency in decreasing Aβ25–35-induced SH-SY5Y cell death at a dose of 0.02–2.5 μM. Finally, in a mouse model of impairment in learning and memory induced by icv-Aβ25–35 injection, 33 ×52 at 1 mg/kg improved this impairment in several tests (Morris water maze and Y-maze).
A particularly relevant approach was undertaken by Arce et al.171 to develop a novel MTDL family based on donepezil scaffold and l-glutamic acid as linkers for different substituents. Lead compound 34 showed interesting and selective AChE inhibitory capacity with an IC50 of 0.53 μM and the capacity to reduce OS. However, its VDCC blockade capacity was not initially designed and this activity was subsequently described. Interestingly, compound 34 blocked Ca2+ entry to the cytosol of chromaffin cells after 70 mM K+ stimuli with an IC50 of 5.3 μM.172 Further evaluation demonstrated a specific l-VDCC blockade by this compound. To complement its multitarget profile, compound 34 elicits antioxidant effect and this combination resulted in a potent neuroprotective profile against OS with good BBB penetration. This profile prompted its in vivo evaluation in a cerebral stroke model in which compound 34 reduced the volume of the infarct by reducing OS and pro-inflammatory markers173 (Figure 10).
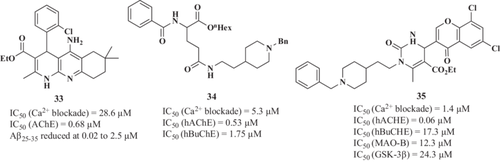
In 2021, Ismaili et al.174 reported 35 (Figure 18) as an MTDL with hAChE (IC50 = 0.06 μM), hBuChE (IC50 = 17.3 μM), MAO-B (IC50 = 12.3 μM), GSK-3β (IC50 = 24.3 μM), and tau (75.3% at 50 μM) and Aβ1-42 aggregation (66% at 50 μM) inhibitory activities and as calcium channel antagonist (IC50 = 1.4 μM). This compound, named ethyl 1-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methyl-2-oxo-4-(4-oxo-4H-chromen-3-yl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate, possesses a selected benzylpiperidine motif of donepezil and chromone and has also proved to be BBB permeable and effective in reducing scopolamine-induced cognitive deficits in mice (10 mg/kg intraperitoneally).
2.3 PDE inhibitors multitarget combinations
Phosphodiesterases (PDEs) are an enzyme family (PDE1-PDE11) responsible for the hydrolysis and degradation of the two intracellular second messengers, cAMP and the cyclic guanosine monophosphate (cGMP). Both messengers play a major role in signal transduction through the brain and the periphery.175 Among these subtypes, PDE4, PDE7, and PDE8 specifically hydrolyze cAMP, whereas PDE5, PDE6, and PDE9 specifically hydrolyze cGMP. The remaining PDE isoforms are able to process both cGMP and cAMP. PDEs are highly expressed in the human brain and their inhibition is proposed to modulate the neurodegenerative processes by increasing the concentrations of cAMP and cGMP in brain tissue.175, 176 Moreover, specific PDE inhibitors have been shown to improve memory performance in different in vivo models of AD.177
2.3.1 PDEs and AChE inhibition
Considering the positive results obtained with PDE inhibitors, novel MTDLs were designed based on tadalafil (36, Figure 11) (a PDE5A inhibitor approved for the treatment of pulmonary hypertension) aiming to integrate dual inhibitory activity toward AChE and PDE5.178 The first-generation of AChE-PDE5 dual inhibitors were synthesized by replacing the substituent group at the N-atom of piperazine-2,5-dione with morpholine-4-ethyl, 1-benzylpyrrolidine-3-yl, N,N-dimethylaminoethyl, N-methyl-N-benzylaminoethyl, and substituted or unsubstituted benzylpiperidine moieties. These compounds showed improved BBB permeability and AChE inhibitory activities compared with tadalafil, maintaining their PDE5 inhibitory capacity. Representative derivative 37 (Figure 11) exhibited significant and selective inhibitory capacity against both targets (AChE IC50 = 0.032 µM and PDE5A IC50 = 1.53 µM). 37 citrate derivative relieve scopolamine-induced learning and memory deficits in in vivo, demonstrating that 37 treated animals exhibited shorter escape latency and less frequent errors than scopolamine group at a dosage of 10 mg/kg, being comparable with donepezil.
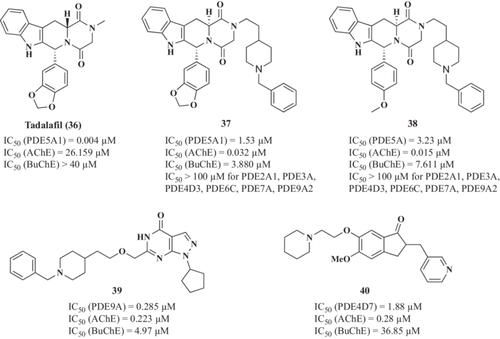
Unfortunately, 37 was nearly insoluble in water (water solubility of citrate = 250 µg/mL), which may limit its further development due to poor oral bioavailability. Herein, Ni et al.179 developed alternative tadalafil derivatives to obtain more potent dual-target PDE5A/AChE inhibitors with improved water solubility. Among new compounds, (6 R,12aS)-2-(2-(1-benzylpiperidin-4-yl)ethyl)-6-(4-methoxyphenyl)-2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione (38, Figure 11) exhibited good selective dual-target AChE/PDE5 inhibition and good BBB permeability. Furthermore, its citrate form possessed improved water solubility to 420 µg/mL, and a good capacity to improve cognitive impairment induced by scopolamine in the in vivo model, inhibiting cortical AChE activities and enhancing CREB phosphorylation ex vivo.
Recent examples of MTDLs directed toward these targets are the novel pyrazolopyrimidone-donepezil hybrids reported by Hu et al.180 Their design fused the pyrazolopyrimidinone subunit of previously reported PDE9A inhibitor C33 and benzyl piperidine subunit of donepezil using different linkers. Among them, compound 6-((2-(1-benzylpiperidin-4-yl)ethoxy)methyl)1-cyclopentyl-1,5-dihydro-4H-pyrazolo[2,4-d]pyrimidin-4-one (39, Figure 11) exhibited excellent and balanced dual-target AChE and PDE9A inhibitory activities (IC50 = 0.285 and 0.223 µM, respectively), low neurotoxicity, and the ability to cross the BBB. The in vivo studies of 39 revealed that it ameliorates learning deficits induced by scopolamine and improves cognitive and spatial memory in the Aβ25−35-induced cognitive deficit mice in the Morris water maze test.
Liu et al.181 study focused on the development of potential AD treatments by exploring dual inhibitors of PDE4 and AChE using dihydro-1H-inden-1-ones. Among the array of compounds evaluated, compound 40 (Figure 11), marked by the introduction of a 2-(piperidin-1-yl)ethoxy group at the 6-position of the indanone ring, demonstrated remarkable inhibitory activities and selectivity against both AChE and PDE4D. Compound 40 exhibited strong AChE inhibition with an IC50 value of 0.28 μM and PDE4 inhibition (IC50 = 1.88 μM). Furthermore, it displayed notable anti-neuroinflammatory properties, outperforming both donepezil and combination therapy (donepezil + rolipram). Compound 40 revealed low neurotoxicity and exhibited a significant neuroprotective effect against Aβ25-35-induced cell death.
2.3.2 PDEs and HDAC inhibition
Simultaneous PDEs and HDACs inhibition has recently been validated as a potentially novel therapeutic approach for AD treatment.182 Rabal et al.183 reported the first dual PDE5 and HDAC inhibitors designed as a combination of sildenafil, a PDE5 inhibitor, and the pharmacophoric features of HDAC inhibitors. PDE5 is distributed in the hippocampus, cortex, and cerebellum and is upregulated in the brains of AD patients compared with age-matched healthy control subjects.184 Consequently, cGMP levels are significantly decreased in the cerebrospinal fluid of AD patients.185 Thus, PDE5 inhibition could increase cGMP levels,186 and GSK-3β inhibition could decrease phosphorylated tau levels,186, 187 enhancing synaptic plasticity and cognitive function, as previously demonstrated in different AD animal models.188 After a deep lead optimization strategy, authors selected the 3-[[4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6H-pyrazolo[4,3-d]-pyrimidin-5-yl)phenyl]methyl]cyclobutenecarbhydroxamic acid 41 (Figure 12) as lead compound. Derivative 41 showed a highly potent and balanced PDE5 (IC50 = 60 nM) and HDAC6 (IC50 = 91 nM) inhibition demonstrating, also, good selectivity as it showed lower inhibitory activity against class I HDACs (HDAC1, HDAC2, and HDAC3, IC50 = 310, 490, and 322 nM, respectively). Selectivity toward HDAC6 is associated with a safer profile as strong inhibition of class I HDACs is associated with toxic effects. Compound 41 showed low cytotoxicity in the THLE-2 hepatic cell line and in primary neuronal cultures (LC50 = 7.2 µM and LC50 = 17.7 µM, respectively) and an improved ability to cross the BBB. This multitarget profile prompted an in vivo evaluation in the Tg2576 AD model. Chronic treatment of Tg2576 mice during 4 weeks with compound 23 (40 mg/kg, intraperitoneal, daily) decreased Aβ42 levels, APP, and hyperphosphorylated tau to almost 50% compared with vehicle-treated animals. More importantly, it elicited a positive effect on spatial memory and reversed cognitive impairment. Tau hyperphosphorylation reduction was related to a significant increase of GSK-3β inactive form levels. Additionally, compound 41 could restore memory through the cGMP/CREB pathway activation that plays an important role in synaptic plasticity.189 Additionally, it rescued the impaired long-term potentiation evident in hippocampal slices from APP/PS1 mice.
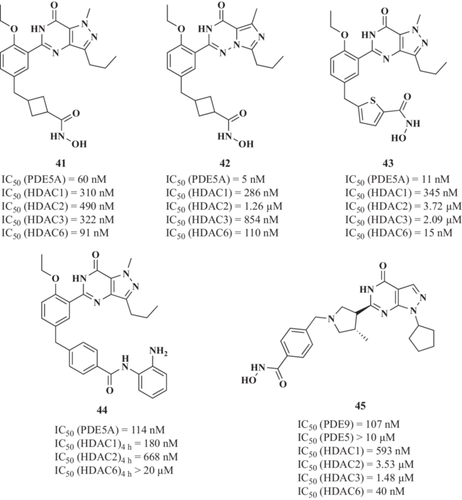
To improve the biochemical and ADMET properties of compound 41, a new series of derivatives were designed by replacing the sildenafil moiety with different PDE5 inhibitors, such as vardenafil and tadalafil,190 or by including different HDAC6-selective inhibitors, such as tubastatin A and nexturastat A.191 Vardenafil-derivative 42 (Figure 12) displayed a potent HDAC6 affinity (IC50 = 110 nM), moderate class I HDACs inhibitory profile (IC50 against HDAC1 and HDAC2 of 286 and 1260 nM, respectively), and good PDE5 inhibitory activity (IC50 = 5 nM).190 On the other hand, the sildenafil-based analog 43 (Figure 12) also showed a potent HDAC6 affinity (IC50 = 15 nM), moderate class I HDACs inhibitory profile (IC50 against HDAC1, HDAC3, and HDAC2 of 345, 2090, and 3720 nM, respectively), good PDE5 inhibitory activity (IC50 = 11 nM), and reduced the levels of the AD-related markers hAPP and tau hyperphosphorylation in Tg2576 neurons.191
Continuing with the optimization process, chemical series of compound 41 were developed and evaluated in in vivo models of AD.192 The sildenafil- derivative 44 (Figure 12) showed potent PDE5A inhibition (IC50 = 114 nM) and the highest HDAC1 and HDAC2 inhibition in the mid-nanomolar range (IC50 = 180 and 668 nM, respectively). Compound 44 also showed an adequate ADMET profile and in vivo target engagement (histone acetylation and cAMP/cGMP response element-binding phosphorylation) in the CNS. Thus, 44 was assayed in a mouse model of AD (Tg2576); however, it failed to produce significant improvement in memory after 2 weeks of treatment (20 mg/kg).
Recently, a novel series of dual PDE9 and HDAC inhibitors have been reported.193 These compounds were designed using selective PDE9 inhibitors (PF-04447943 and PF-04449613) as well as privileged chemical structures, bearing zinc binding groups (hydroxamic acids and ortho-amino anilides) to target HDAC proteins. The PF-04447943-based hydroxamic acid analog 45 (Figure 12), bearing an N1-cyclopentyl group, was the most potent HDAC6 inhibitor, (IC50 = 40 nM), and it exhibited potent PDE9 inhibitory capacity (IC50 = 107 nM). Moreover, it exhibited a good in vitro ADMET profile and BBB permeability to advance through in vivo efficacy models. Chronic treatment of aged Tg2576 mice with 45 reversed the AD phenotype in an effective manner.194 In fact, Tg2576 mice treatment with 45 during 4 weeks (40 mg/kg, intraperitoneally) promoted a significantly higher response in the fear conditioning test (60.9% freezing) compared with vehicle-treated animals (36.9%). In the Morris water maze test, the latency to find the platform was prolonged in Tg2576 mice relative to the WT mice, whereas the escape latency was shorter when these Tg2576 mice received 45, although globally no significant differences were found between groups. Aβ42 levels in parieto-temporal cortical extracts showed a significant decrease in Tg2576 mice treated with 45 (7.70 pg Aβ/µg of protein) relative to those that received the vehicle alone (17.8 pg Aβ/µg of protein). These results showed that 45 ameliorates memory impairment and diminished AD pathological markers although its effect was lost after a wash-out period of 4 weeks.
2.3.3 Metal ions chelation and PDE inhibition
Unbalanced metal ion levels are a well-established pathological AD feature.195-197 Thus, metal−Aβ interactions modulation and metal distribution in the brain could represent an interesting therapeutic strategy to both inhibit Aβ aggregation and reduce the OS status.198 Aβ can complex transition metal ions such as Fe3+ and Cu2+ through histidine residues.199 Binding to these metals favors peptide aggregation and its pro-oxidant effects, generating ROS via the Fenton reaction.55 This interaction causes the reduction of the ions from Fe3+ to Fe2+ producing H2O2 as a by-product. Then, the Fenton reaction takes place where Fe2+ reduces H2O2 recovering Fe3+ and releasing OH- and OH·, highly reactive species with a very short half-life.200 Abnormally high concentrations of both metals Fe3+ and Cu2+ are accumulated in Aβ plaques.201 Additionally, it has been suggested that OS promotes the amyloidogenic pathway of APP processing, thus, initiating a positive feedback loop in which oxidative damage promotes Aβ misfolding and misfolded Aβ promotes oxidative damage.202
Su et al.203 reported a new series of multifunctional agents that combine PF-04447943 (PDE9 inhibitor) and clioquinol as bio-metal chelator. Among them, derivative 46 (Figure 13) exhibited an excellent and selective inhibitory activity toward PDE9 (IC50 = 34 nM), the ability to chelate bio-metals at 20 µM and the subsequent formation of 46-metal ion (II) complex, together with antioxidant properties (ORAC = 4.47 Te). Compound 46 demonstrated a good capacity to inhibit Cu2+-induced Aβ1-42 aggregation (64.7% reduction) and disaggregation of Cu2+-induced Aβ1-42 fibrils (64.6% reduction at 50 µM).
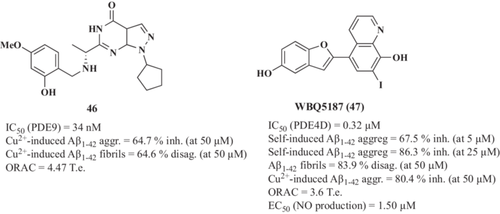
In this line, Wang et al.204 used clioquinol and moracin M as scaffolds to design new multi-target hybrids for AD. Clioquinol moiety was included aiming to chelate bio-metals whereas moracin M-related group was included to integrate PDE4D inhibitory capacity. Compound WBQ5187 (47, Figure 13) was selected as lead compound showing good PDE4D inhibitory potency (IC50 = 0.32 µM), appropriate bio-metal chelating capacity, which demonstrated the interaction of 47 with Cu2+, Zn2+, Fe2+, and Fe3+ at 50 µM, antioxidant capacity (ORAC = 3.6 Te) and potent anti-inflammatory properties (EC50 = 1.50 µM to reduce NO production in LPS-stimulated BV2 cells). Considering the interconnection of metal ions and Aβ, compound 47 inhibited self-induced Aβ1-42 aggregation (67.5% and 86.3% inhibition at 5 and 25 µM, respectively), disassembled self-induced Aβ aggregates (83.9% disaggregation of Aβ1-42 fibrils at 50 µM), and modulated Cu2+-induced Aβ aggregation (80.4% inhibition at 50 µM) being more efficient than clioquinol and moracin M. Interestingly, compound 47 showed significant interactions with Aβ1-42 peptide through Arg5, His6, His14, Gln15, and Phe20 residues as demonstrated by NMR. Finally, compound 47 showed a potent neuroprotective profile in vivo; it protected hippocampal neurons from necrosis and demonstrated improvements in cognitive abilities and conventional reference spatial memory compared with those animals treated with donepezil and clioquinol, or untreated rats.
2.4 Metal ions chelation multitarget combinations
2.4.1 Metal ions chelation and MAO inhibition
Mono-amine oxidase enzymes (MAO-A and MAO-B) catalyze oxidative deamination of mono-amines producing H2O2 and ROS as secondary products, thus, increasing oxidative injury and promoting the toxic environment characteristic of neurodegeneration.205, 206 Monoamine neurotransmitters regulated by MAOs, such as dopamine, norepinephrine, and serotonin (5-HT), are crucial for memory and cognition.207 MAO inhibitors were proposed to improve monoaminergic neurotransmission and decrease ROS formation and OS, thus exerting antioxidant and neuroprotective properties and, consequently, to induce cognitive improvement.208 A prominent example is rasagiline (48, Figure 13), a potent and selective MAO-B irreversible inhibitor approved in 2005 for Parkinson's disease (PD) treatment, which has entered Phase II clinical trials for AD treatment.209
Based on these premises, Mi et al.210 developed a novel class of multitarget hybrids by linking coumarin (MAO-B inhibitor) and hydroxypyridinone (Fe3+ chelator) using a cleaver approach based on click chemistry. Obtained derivatives displayed excellent Fe3+ chelating activity and moderate to good MAO-B inhibitory activity. Compound 49 (Figure 14) exhibited the most potent MAO-B inhibitory capacity with an IC50 value of 0.68 µM and a Fe3+ affinity of pFe3+ = 19.8.
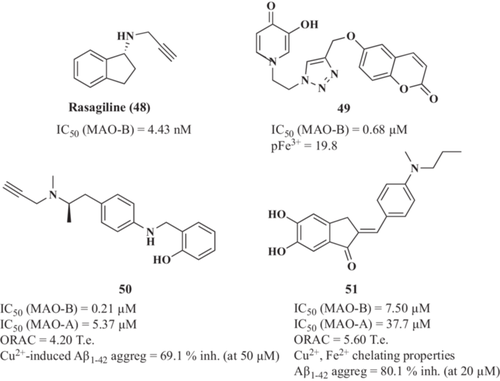
Using a similar approach, Xie et al.211 reported a family of selegiline-clioquinol hybrids. Selegiline (MAO-B inhibitor) was also approved for PD treatment.212 The (R)-2-(((4-(2-(methyl(prop-2-yn-1-yl)amino)propyl)phenyl)amino)methyl)phenol (50, Figure 14) was selected as the lead compound being a potent MAO-B inhibitor (IC50 = 0.21 µM) and effective bio-metal chelator of Cu2+, Fe2+, and Zn2+. Compound 50 final structure also integrates good antioxidant activity (ORAC = 4.20 Te) and the ability to inhibit Cu2+-induced Aβ1-42 aggregation (69.1% at 50 µM).
Complementing this family, benzylideneindanone derivatives showed multifunctional activity as potential anti-AD drugs due to their ability to inhibit MAO-B activity, to block self-induced Aβ aggregation, their antioxidant, and bio-metal chelator capacity.213 Compound 51 (Figure 14) had the greatest ability to inhibit Aβ1-42 aggregation (80.1% at 20 µM) and to inhibit MAO-B (IC50 = 7.5 µM). Moreover, it chelated bio-metals such as Cu2+ and Fe2+, with a stoichiometry of the 51-Cu2+ complex of 1:1, inhibit Cu2+-induced Aβ1-42 aggregation noticeably through chelating Cu2+, and disassembling the well-structured Aβ fibrils at 50 µM.
2.4.2 Metal ions chelation, Aβ aggregation inhibition, and H3R antagonism
The AD pathological network is highly complex and the crosslinks among its components lead to the proposal of novel trifunctional multitarget hybrids by Sheng et al.214 Their rational design included an aminopropoxyphenyl moiety as H3R antagonist and the 3-hydroxy-4-pyridinone subunit due to its chelating properties with high affinity for Cu2+, Zn2+ and Fe2+,215 as well as its potential radical scavenging. The new 1-phenyl-3-hydroxy-4-pyridinone derivatives share similar structural features with SFK-64346, a well-known Aβ aggregation inhibitor that contains an aminopropyloxy-phenyl-nezofuran moiety. Among them, compound 3-hydroxy-2-methyl-1-(4-(3-(pyrrolidine-1-yl)propoxy)phenyl)-pyridin-4(1H)-one (52, Figure 15) showed excellent selective H3R antagonism (IC50 = 0.32 nM), free radical scavenging capacity (ORAC = 1.54 T.e.), potent metal chelating activities of Cu2+ and Fe2+, with a stoichiometry of the 52-Cu2+ complex of 2:1, confirming that the 3-hydroxy-4-pyridinone moiety is essential for the metal chelating activity. 52 demonstrated self-induced Aβ1−42 anti-aggregation capacity (80.1% inhibition at 20 µM and IC50 value for Aβ1−42 aggregation of 2.85 µM) and Cu2+-induced Aβ1−42 anti-aggregation ability (57.9% disaggregation at 50 µM).
2.4.3 Metal ions chelation and tau aggregation inhibition
Kim et al.216 reported the effects of metal ions on Aβ and tau aggregation. Zn2+, Cu2+, Fe3+, Mg2+, Mn2+, Pb2+, Cd2+, Hg2+, and Al3+ promote tau hyperphosphorylation and induce tau aggregation, whereas Fe2+ and Li+ reduce tau hyperphosphorylation.
Focusing on the tau hypothesis, Silva et al.217 reported the evaluation of natural polyphenols and synthetic derivatives as potential tau aggregation inhibitors with metal chelating properties. The structure–activity relationship (SAR) study demonstrated that the nitrocatechol scaffold is required for a significant tau anti-aggregating activity. α-cyanocarboxamide derivative 53 (Figure 15) was one of the most potent inhibitors of tau aggregation, measured as an aggregation of the tau-derived peptide AcPHF6 (90.5% inhibition at 50 µM). Its potent anti-aggregating activity was explained by molecular docking studies, suggesting that the cyano group formed two hydrogen bond interactions with NH3+ of two Lys side chains (distance <2.8 Å) and the amide carbonyl was involved in a hydrogen bonding interaction with Lys (distance = 1.85 Å). The N-benzyl ring established a π-cation interaction with NH3+ of Lys (distance = 4.82 Å). These additional interactions support the increased tau-aggregation inhibitory activity of 53. The presence of a less flexible amide bond increases conformational stability in the steric zipper β-sheet model of tau hexapeptide 306VQIVYK311. Compound 53 also exhibited significant Cu2+ chelation capacity.
2.5 AChE inhibition combined with novel targets
AChE colocalizes with the Aβ peptide deposits and plays a key role in the Aβ-aggregation process in which stable AChE-Aβ complexes are formed, accelerating Aβ aggregation. AChE PAS serves as a nucleation center for Aβ aggregates accelerating their formation and propagation.37 AChE-PAS is the seeding location for small molecular weight oligomers,38 inducing conformational and biochemical changes of Aβ in solution and accelerating its fibrinogenesis. AChE-Aβ complexes are formed when AChE interacts with growing amyloid fibrils218 and, after aggregation, they exhibit considerably increased neurotoxicity compared with free Aβ.219 Aβ peptides formation and aggregation finally results in Aβ deposition in plaques, an important hallmark of AD pathogenesis. Thus, renewed interest has emerged in the development of AChE PAS binding ligands since it would exert a double beneficial action, AChE inhibition to increase ACh levels at the synaptic cleft and Aβ anti-aggregation profiles. Thus, compelling literature reports numerous examples of MTDLs combining AChE inhibition with other therapeutic targets, here, we have selected relevant examples.
2.5.1 AChE and BACE-1 inhibition
As mentioned above, under pathological conditions, BACE-1 cleaves APP, followed by the γ-secretase complex, causing the generation of Aβ peptides that tend to oligomerize and accumulate as Aβ deposits, contributing to Aβ fibril formation. Aβ assembly progresses oligomers and senile plaques, causing neurotoxicity and inducing neuronal cell death.14 Furthermore, AChE is able to catalyze these conformational changes accelerating the aggregation process. Both enzymes, BACE-1 and AChE are linked by the production and aggregation process. Thus, the development of new molecules targeting both enzymes represents an interesting approach to designing new agents for AD treatment.
Zha et al.220 reported a new family of benzofuran hybrids to obtain activity against these selected targets. The N1-(benzofuran-2-ylmethyl)-N7-(1,2,3,4-tetrahydroacridin-9-yl)-heptane-1,7-diamine derivative (54, Figure 16) revealed a nanomolar potency to inhibit cholinesterase activity (IC50 = 0.86 nM and 2.18 nM toward hAChE and hBuChE, respectively) and micromolar capacity to inhibit BACE-1 (IC50 = 1.35 µM). Moreover, it inhibited self- and AChE-induced Aβ aggregation and presented lower hepatotoxicity than tacrine. In vivo studies demonstrated better behavioral test performance and cognitive improvement in scopolamine-treated ICR mice compared with vehicle-treated animals.
Compelling research evidence has demonstrated that donepezil analogs have a double bond on the indanone moiety that can act as BACE-1 inhibitors due to their structural rigidity.221 Therefore, numerous donepezil analogs were synthesized by alkylation or benzylation of the indanone central core,221, 222 or by the connection of this scaffold with other subunits, such as phthalimide or pyrimidine.223, 224 Regarding the structural modifications, compounds reported by Green et al.222 were able to inhibit AChE and BuChE. Interestingly, the p-bromobenzyl analog (55, Figure 16) also inhibited BACE-1 with an IC50 value of 3.4 µM.
Costanzo et al.221 obtained donepezil-like compounds using a greener version of the synthetic procedure previously reported for methoxy-substituted donepezil precursors by aldol condensation. The bis-methoxy-derivative (56, Figure 16) showed selective and potent AChE inhibitory capacity at the nanomolar range (IC50 AChE 58 nM and IC50 BuChE = 4.74 µM) and inhibited BACE-1 with an IC50value lower than 1 µM, IC50 = 0.697 µM).
In this line, donepezil analogs developed by Gabr et al.225 represented by lead compound 57 (Figure 16) showed potent AChE and BACE-1 inhibitory capacity (AChE IC50 = 4.11 nM and BACE-1 IC50 = 18.3 nM), metal chelating properties, low toxicity on SH-SY5Y neuroblastoma cell line, and good BBB permeability.
The connection of donepezil pharmacophore benzylpiperidine with the pyrimidine-2,4-diamine scaffold yielded a new MTDL core possessing multifunctional activity against cholinesterase enzymes, BACE-1 and Aβ aggregation inhibitory capacity.224 Biological assays revealed that the nature of the substituent on the C4 benzylamine group affected the biological profile. The N2-(1-benzylpiperidin-4-yl)-N4-(3,4-dimethoxybenzyl)pyrimidine-2,4-diamine candidate 58 (Figure 16) inhibited AChE (IC50 = 9.90 µM), BuChE (IC50 = 11.40 µM), and BACE-1 (34% inhibition at 10 µM). Target combination led to the ability to inhibit self- and AChE-induced Aβ aggregation (17.4% and 59.3% at 100 µM, respectively). It also demonstrated low toxicity in the SH-SY5Y neuroblastoma cell line (81% of cell viability at 40 µM).
Belluti et al.226 used the fluorinated benzophenone scaffold as a central core of a new small library of 3-fluoro-4-hydroxy analogs for AD. The benzophenone is a privileged scaffold, a versatile pharmacophore nucleus, which could be structurally modified to provide analogs to reach different therapeutic targets.227 The in vitro screening allowed the authors to identify fluoro-4-hydroxyphenyl-4-[4-(2-hydroxyethyl)piperidin-1-ylmethyl]phenylmethanone (59, Figure 16) as the lead candidate with dual AChE (IC50 = 2.52 µM) and BACE-1 (IC50 = 2.32 µM) inhibitory profile along with antioxidant properties (compound 59 was able to inhibit 30% of ROS formation elicited by the organic peroxide t-BuOOH at 100 µM and Aβ25-35 peptides at 5 µM in the OS cell model at concentrations of 3 and 5 µM of 59). The presence of the hydroxy group at the aromatic ring could explain the ability of the compound to counteract ROS formation at the neuronal level.
Viayna et al.228 reported the synthesis of rhein-huprine hybrids as disease-modifying AD potential drugs. The in vitro screening of this new family of compounds showed potent inhibitory activities against hAChE, hBuChE, and BACE-1, along with the ability to act as dual Aβ42 and tau anti-aggregating agents. Furthermore, they showed good BBB permeability. (60, Figure 16), selected as a lead candidate, (IC50 values of 2.39, 513, and 80 nM toward hAChE, hBuChE, and BACE-1, respectively), revealed an interesting protective profile against the Aβ-induced synaptic dysfunction, preventing the loss of synaptic proteins. Consequently, compound 60 demonstrated a positive effect to improve the long-term potentiation in an ex vivo study using brain slices of C57bl6 mice. In vivo studies in APP-PS1 transgenic mice treated by intraperitoneal injection for 4 weeks with compound 60 (2 mg/kg) reduced soluble Aβ and increased mature APP levels.
Kumar-Waiker et al.229 have recently reported a series of N-benzylpiperidine hybrids of 5-phenyl-1,3,4-oxadiazol-2-thiol, being 61 (Figure 16) the lead compound showing high hAChE (IC50 = 0.076 μM), hBuChE (IC50 = 1.204 μM), and hBACE-1 (IC50 = 0.230 μM) inhibitory capacity. Moreover, it showed a significant Aβ aggregates reduction. Its interest prompted its in vivo evaluation in which it exhibited significant learning and memory behavior restoration in the scopolamine- and Aβ-induced mouse AD models, respectively. In these in vivo models, authors analyzed different parameters in ex vivo hippocampal slices in which compound 61 showed reduced levels of AChE, malondialdehyde, and NO, and decreased levels of TNF-α and IL-6 mRNA, among other properties.
Fares et al.230 reported new tacrine-chromene derivatives with a MTDL profile. Derivative 62 (Figure 16) showed hAChE (IC50 = 0.44 μM), hBuChE (IC50 = 0.08 μM), BACE-1 (IC50 = 0.38 μM), and MAO-B (IC50 = 5.15 μM) inhibitory capacity, a tetra-model combination not reported before. 62 showed low cytotoxicity in SH-SY5Y and HepG2 cell lines and good predicted BBB permeability. Besides, they had the ability to bind to PAS, which allowed them to decrease Aβ aggregation (59% at 10 μM), antioxidant activity, and metal-chelating properties toward Fe2+, Zn2+, and Cu2+.
Finally, Digiacomo et al.231 described a novel series of tacrine derivates by combining caffeic, ferulic, and lipoic acids with tacrine, which exhibited relevant antioxidant and both AChE and BACE1 inhibitor capacities. Among these compounds, 63 showed an IC50 of 0.04 μM and 1.03 μM for AChE and BACE1, respectively. Furthermore, it also had BACE1 inhibitory activity (47% at 10 μM) and displayed an appreciable neuroprotective effect at 3 μM against glutamate-induced neuronal death in HT22 neuronal cells.
2.5.2 AChE inhibition and serotonin receptor antagonism
The serotonergic system is implicated with the cholinergic system in the cognitive function of neurodegenerative diseases.232 Serotonin (5-HT) receptors 5-HT1A, 5-HT4, 5-HT6, and 5-HT7 are associated with learning and memory. Serotonergic neurons also accumulate NFTs and a decreased number of these neurons are reported in AD brains.233 In addition, Aβ deposits in the projection sites of these neurons lead to axonal degeneration and, finally, to neuronal loss.231 Moreover, the 5-HT4 and 5-HT6 receptors can modulate the activity of several secretases, thereby affecting APP processing.234 Thus, partial 5-HT4 receptor agonists such as donecopride could be used to treat the cognitive symptoms of AD acting as a functional agonist when low receptor numbers are present, minimizing the potential receptor desensitization.235, 236 The 5-HT6 receptor is expressed primarily in the brain cortex and the hippocampus, and it modulates primarily γ-aminobutyric acid (GABA) and glutamate levels, facilitating the secondary release of other neurotransmitters like ACh.237 Thus, 5-HT6 receptor antagonists would be beneficial to ameliorate AD symptoms. 5-HT6 receptor and cholinesterase have been combined as targets of novel MTDLs recently reported.236, 238, 239 Representative examples of these series of compounds are depicted in Figure 17.
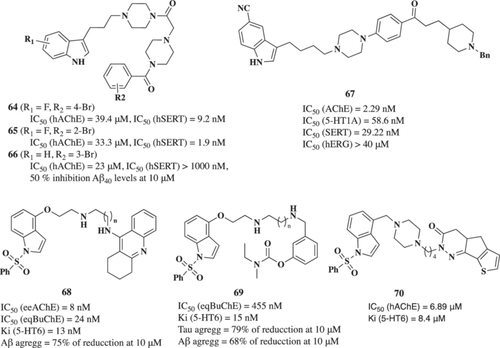
In 2020, Rodríguez-Lavado et al.240 reported a new series of indole-benzamide derivatives as MTDL for the serotonin transporter (SERT) and AChE, due to their affinity on the serotonin systems.241 Novel derivatives showed IC50 values in the micromolar range toward human AChE (hAChE) (from 3.4 µM to higher than 50 µM). Interestingly, the 5-fluorine substitution at the indole moiety resulted in nanomolar affinity in SERT, derivatives 64 and 65 (Figure 17) the most potent with IC50 values of 9.2 nM and 1.9 nM, respectively. Additionally, the 1-(4-(3-(1H-indol-3-yl)propyl)piperazin-1-yl)-2-(4-(3-bromobenzoyl)piperazin-1-yl)ethan-1-one derivative (66, Figure 17) exhibited significant anti-Aβ activity, inhibiting 50% of Aβ40 levels in N2A mouse neuroblastoma cells at 10 µM.
Li et al.242 in 2021 reported compound 67 (Figure 17) as a hybrid derivative of the antidepressant vilazodone (5-HT1A receptor partial agonist and serotonin transporter inhibitor) and donepezil aimed to treat comorbidity of AD and depression. This compound exhibited strong triple-target bioactivities in vitro of AChE, 5-HT1A, and SERT with IC50 values of 2.29, 58.6, and 29.22 nM, respectively. Compound 67 also showed acceptable brain distribution, ameliorating depressive symptoms and cognitive dysfunction (Y maze assay and tail suspension test) in the scopolamine mouse model.
Wichur et al.243 have also recently reported two novel compounds (68 and 69, Figure 17) with both 5-HT6R antagonism (Ki = 13 nM and Ki = 15 nM respectively) and cholinesterase inhibition. Compound 68 is a tacrine-based ligand with balanced activities toward eeAChE (IC50 = 8 nM) and eqBuChE (IC50 = 24 nM). Interestingly, it reduced Aβ aggregation by 75% at 10 µM. At the same concentration, it did not show toxicity on HepG2 cells; however, it showed low BBB permeability according to the PAMPA assay. On the other hand, 69 contains rivastigmine-derived phenyl N-ethyl-N-methylcarbamate fragment, being able to inhibit eqBuChE (IC50 = 455 nM) and both tau and Aβ aggregation properties (79% and 68% at 10 µM, respectively). Furthermore, 69 did not influence CYP isoenzymes 3A4 and 2D6 activity at those concentrations and it is BBB membrane-permeable in PAMPA assay.
Finally, Asproni et al.244 reported a novel compound (70, Figure 17) that featured a tricyclic scaffold, namely thieno[3,2-h]cinnolinone, thienocyclopentapyridazinone and thienocycloheptapyridazinone, connected through alkyl chains with hAChE inhibitory capacity (IC50 = 6.89 µM) and serotonin 5-HT6 receptor subtype interaction (Ki = 8.4 nM).
2.5.3 AChE inhibition and NRF2 induction
Exacerbated OS is considered one of the main pathological pathways related to NDD development including AD. OS is closely connected to the apparition of senile plaques, as previously discussed, besides, aberrant proteins increase the formation of ROS accelerating the advance of the disease. The NRF2/EpRE transcriptional pathway is considered the most important intrinsic cell defense mechanism under neuropathological conditions. It reduces OS and inflammation by promoting the expression of numerous antioxidant and anti-inflammatory enzymes.245
Based on these considerations, Benchekroun et al.246 designed a trifunctional hybrid based on ferulic acid, a hydroxycinnamic acid with antioxidant and neuroprotective properties, melatonin, an endogenous neurohormone with potent antioxidant and neuroprotective capacity, and tacrine as AChE inhibitor. The novel ferulic acid-tacrine-melatonin hybrid 71 (Figure 18) was selected as the lead compound with the best pharmacological profile. Compound 71 inhibits cholinesterase activity with IC50 values of 1.29 µM toward hAChE and 234 nM toward hBuChE; it significantly activates the NRF2 transcriptional pathway in AREc32 cells with a CD value of 6.31 µM. This combination of targets resulted in an interesting neuroprotective profile against Aβ1-40 and Aβ1-42 toxicity, H2O2, and rotenone/oligomycin A-induced OS cell models (70.6%, 57.4%, 37.6%, and 33.7% protection, respectively, at 1 µM). To complete its evaluation, 71 showed a potent antioxidant effect in the ORAC test (ORAC = 9.11 Te), BBB permeability, and low toxicity in HepG2 cells.
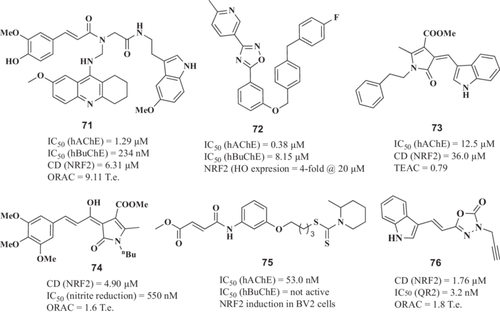
Fusing the benzylpiperidine motif from the AChE inhibitor donepezil and the 1,2,4-oxadiazole core a from an NRF2 activator previously reported,247 Wang et al.248 have recently reported the hybrid compound 72 (Figure 18) with great AChE inhibitory (eeAChE IC50 = 0.07 μM; hAChE IC50 = 0.38 μM) and a significant NRF2 induction, since it upregulates HO-1, NQO1, and GCLm (downstream proteins) and favors its translocation to the nuclei. 72 also exhibited neuroprotective functions in vitro against the H2O2 insult (above 50% of cell survival at 10 μM) and it also reduced Aβ1-42 aggregation. Interestingly, it also reduced ROS levels and pro-inflammatory cytokines liberated after LPS insult in BV2 cells. In the scopolamine AD mouse model, this compound was also effective (10 mg/kg) in increasing the arrival times and simplifying the tracks in the Morris water maze, improving mice cognition and alleviating inflammation via oral administration (10 mg/kg).
An interesting approach was reported by Cores et al.249 describing a new class of MTDL based on bisaventhramide, a natural product with excellent antioxidant capacity. From all derivatives, compound 73 (Figure 18) showed AChE inhibitory capacity (IC50 = 12 μM) and Nrf2 induction activity (CD = 36 μM) being able to activate the phase II antioxidant response-related enzyme expression. Moreover, it showed a lack of cytotoxicity in neuronal and hepatic cellular and antioxidant capacity. The target combination included in derivatives 73 resulted in an interesting neuroprotective profile in two different AD models, OS, and tau hyperphosphorylation. Although further in vivo evaluation has not been performed yet, its profile positions compound 73 as a candidate for future lead optimization and in vivo evaluation.
In this line, a new class of MTDL hybrids was reported as curcumin-piperlongumine derivatives. This design was proposed to combine the NRF2 induction capacity of curcumin and the antioxidant and anti-inflammatory properties of the natural alkaloid peperlongumine. Compound 74 (Figure 18) was selected as the lead candidate due to its balanced combination of activities, although in this case the AChE activity was not evaluated. Compound 74 structural modifications compared with 73 resulted in an increased NRF2 induction capacity (CD = 4.9 μM) and improved antioxidant capacity (ORAC = 1.6 T. e. and DPPH IC50 = 14.2 μM). Moreover, compound 74 showed low toxicity in cellular models and the ability to Tau protein aggregation in the PHF6 aggregation model. Regarding its anti-inflammatory properties, it showed a concentration-dependent reduction of nitrite production induced by LPS in the BV2 microglial cell line showing an IC50 of 550 nM. It also showed potent neuroprotective activity in the OS and tau hyperphosphorylation toxicity models of AD complementing its pharmacological profile.
Guo et al.250 described compound 75 as a dimethyl fumarate-tranilast modified dithiocarbamate hybrid with improved AChE inhibitory activity (IC50 = 0.053 μM) and potent Nrf2 activation at 7 μM confirmed with Western blot. The total Nrf2 amount was upregulated in a time-dependent manner, being at 4 h the maximum level of nuclear Nrf2 in BV2-2 microglia cells. This property also led to an increase in Nrf2-dependent antioxidant enzymes, including HO-1, NQO1, and GPX4, that showed the biggest upregulation observed at 10 μM. Moreover, compound 75 showed an interesting neuroprotective profile against H2O2-induced damage. In the LPS inflammatory model, 75 decreased ROS accumulation and NO production, and this activity was associated with a reduction in iNOS and COX-2 levels. It also significantly blocked TNF-α and IL-6 production. Besides, 75 was also predicted to cross the BBB in the PAMPA assay (Pe 17.95 × 10−6 cm/s). Finally, 75 was tested (30 mg/kg) in the scopolamine mice model, which reversed the step-down latency and number of errors.
Recently, Herrera-Arozamena et al.251 described a novel series of indole- and naphthalene-oxadiazolones, among which N-propargyl-oxadiazolone 76 outstood as a hit compound. It displayed remarkable selectivity to quinone reductase 2 (QR2) ligand (Ki = 3.2 nM), a potent NRF2 induction in the AREc32 cell line (CD = 1.76 μM) and the ability to promote cell differentiation into neurons, as it greatly increased the expression of both TuJ-1 and MAP-2 (two well-known neuronal markers). It is also predicted to be BBB permeable as demonstrated by the PAMPA assay and had antioxidant properties in ORAC assay (1.8 T.e.)
2.5.4 AChE and MAO inhibition
The two isoforms of MAO (MAO-A and MAO-B) produce peroxidases, which cause OS and the depletion of neurotransmitters; inhibiting these enzymes allows for the accumulation of depleted neurotransmitters and decreases the formation of oxidative-free radicals, which eventually confers neuroprotection.252
One of the first examples of this MTDL combination, Ladostigil (77, Figure 19), was designed considering the connection of MAO enzymes and OS.252 It combined the carbamate moiety of rivastigmine and the indole-amino moiety of the MAO-B inhibitor rasagiline to target both MAO-B and cholinesterases in the brain. This compound was intensively evaluated as being the most representative MTDL, which has completed a Phase II clinical trial for the treatment of mild cognitive impairment and AD-type dementia (NCT01354691). Preclinical studies showed that Ladostigil 77 improves memory in both scopolamine-induced and streptozocin-induced cognitive impairment rat models. Further experimental data revealed that Ladostigil is able to regulate APP processing, exert neuroprotective properties, and reduce microglial activation in addition to its MAO and cholinesterase inhibitory properties (IC50 > 100, 37.1, 31.80 and 1.98 µM toward MAO-A, MAO-B, AChE, and BuChE, respectively).

Another example of a dual AChE and MAO-B inhibitor is the pyrimidinylthiourea family. Lead derivative 1-ethyl-3-(6-(4-((methyl(prop-2-yn-1-yl)amino)methyl)-1H-imidazol-1-yl)pyrimidin-4-yl)thiourea (78, Figure 19) developed by Li et al.253 displayed good and balanced inhibitory activities against AChE (IC50 = 0.324 μM) and MAO-B (IC50 = 1.427 μM). Molecular docking studies showed that the pyrimidinylthiourea moiety binds AChE CAS and the propargylamine moiety directly interacts with MAO-B flavin adenine dinucleotide (FAD). Moreover, compound 78 showed antioxidant ability (ORAC = 1.126 T.e.), and specific Cu2+-chelating ability producing selective 78-Cu2+ complexes with the thiourea fragment as responsible for the metal chelating action. Additionally, derivative 78 showed significant inhibitory activity against Cu2+-induced Aβ1-42 aggregation, which is related to its neuroprotective properties in rat primary cortical neurons challenged with Cu2+-induced Aβ neurotoxicity. Furthermore, 78 showed low cytotoxicity and appropriate BBB permeability in vitro. Furthermore, the 78-hydrochloride derivative (30 mg/kg) significantly improved memory and cognitive function in the scopolamine-induced cognitive impairment in a mouse model, showing shorter escape latency and less frequent errors in the channel water maze.
Reis et al.254 reported a novel multitarget derivative as a dual AChE-MAO-B inhibitor. Its design included a chromone core functionalized with a phenylcarboxamide moiety and an acrylate subunit containing a tertiary amine to target MAO-B. From the small chemical library obtained, derivative 2-(dimethylamino)ethyl (E)-3-(4-oxo-3-(phenylcarbamoyl)-4H-chromen-6-yl)acrylate (79, Figure 19) was identified as the most promising multitarget lead. Compound 79 is a selective hMAO-B inhibitor (IC50 = 0.63 µM) with cholinesterase inhibitory activity in the low micromolar range. Compound 79 demonstrated a wider safety window, with no significant decrease in cellular viability in SH-SY5Y and HepG2 cell lines.
Nadeem et al.255 evaluated the indole core containing 2-arylidine derivatives of thiazolopyrimidine as dual AChE/MAO-B inhibitors. Successfully, these new derivatives (80 and 81, Figure 18) exhibited interesting double inhibitory capacity with IC50 values of 10.36, 0.042, and 0.069 µM for AChE, and 0.13, 0.33, and 0.14 µM for MAO-B, respectively. Moreover, all the compounds showed BBB penetration and were nonneurotoxic in the neuroblastoma SH-SY5Y cell line at 40 µM.
Li et al.256 reported compound 82 (Figure 19) as a dual AChE-MAO-B inhibitor (IC50 = 0.58 and 0.41 μM, respectively). This compound is a novel chromanone–1-benzyl-1,2,3,6-tetrahydropyridin hybrid that exhibited low neurotoxicity, showed interesting neuroprotection against mitochondrial dysfunction and oxidation (DCF-DA fluorometric assay, JC-1 fluorescent assay), and displayed improvements in cognitive behaviors and spatial memory (Morris water maze) in a scopolamine-induced AD mouse model (intraperitoneal 10 mg/kg).
Rullo et al.257 have also recently reported 84 (Figure 19) as a dual AChE-MAO-B inhibitor, a coumarin-based compound with a CH2OH/CHF2 bioisosteric replacement. This compound displayed outstanding in vitro activities (IC50 = 550 nM and 8.2 nM toward AChE and MAO-B, respectively). Moreover, it showed high solubility and brain-permeant capacity, good oral bioavailability, and negligible cytotoxic effects in SH-SY5Y and HepG2 cell lines.
Finally, Lu et al.258 described in 2013 a novel series of resveratrol derivates, with compound 85 as the lead compound of the study. Compound 85 inhibited AChE, MAO-A, and MAO-B (IC50 = 6.27, 7.08, and 14.1 μM, respectively). This compound exhibited a potent inhibitory activity of both self-induced Aβ (82% disaggregation at 25 µM, (IC50 = 6.51 µM) and Cu2+-induced Aβ1-42 aggregation (94.23% inhibition at 50 µM) and a potent scavenger capacity (ORAC = 4.72 T.e.). It also has the ability to chelate biometals, such as Cu2+, Fe2+, Fe3+, and Zn2+. Besides, it significantly reduced ROS levels in SH-SY5Y at the DCFH-DA assay at 2.5 μM, and based on measured permeability in the PAMPA assay, it was also able to cross the BBB.
2.5.5 AChE inhibition and H3R antagonism
An MTDL strategy involving AChE and H3R may exert a synergistic effect on upregulating synaptic levels of ACh, which is a potential approach for the treatment of AD. Previous in vivo studies showed that H3R activation reduced ACh release in the rat frontoparietal cortex and hippocampus.259
One of the first examples of this type of combination was a novel series of quinoxaline derivatives developed by Huang et al.260 Compound 86 (Figure 20) showed potent inhibitory activities against AChE, H3R, and BACE-1. Its structure was designed to combine the 2-amino-3,4-dihydroquinazoline core of a previously reported BACE-1 inhibitor as the core ring moiety for H3R antagonism and a benzyl pyrrolidine fragment of the AChE inhibitor BYYT-25 to function as the basic center for H3R. After molecular docking optimization, new quinoxaline derivatives were synthesized and biologically evaluated. N-(3-aminoquinoxalin-2-yl)-4-(piperidin-1-ylmethyl)benzamide 86 showed the most promising profile with potent activity toward H3R, AChE, and BACE-1 targets (H3R antagonism, IC50 = 280.0 nM; H3R inverse agonism, IC50 = 189 nM; AChE, IC50 = 483 nM; BACE 1, 46.64% inhibitory rate at 20 µg/mL) and high selectivity for H3R over H1R, H2R, or H4R.
In this line, Bajda et al.261 described a novel multifunctional compound from a nonimidazole H3R ligand library applying docking-based virtual screening studies to select the hits that were able to bind both CAS and PAS of AChE and BuChE. The most promising derivatives combined the flavone moiety via a six-carbon atom linker with a heterocyclic moiety, including asazepane, piperidine, or 3-methylpiperidine. These compounds showed the highest inhibitory activities toward cholinesterases and well-balanced potencies against H3R. Two derivatives were chosen as good lead structures for further optimization and development of novel multitarget anti-AD agents. Derivative 2-(4-(6-(azepan-1-yl)hexyloxy)phenyl)-6-methyl-4H-chromen-4-one hydrogen oxalate (87, Figure 20) (IC50 = 0.46 µM for AChE; IC50 = 0.44 µM for BuChE; Ki = 159.8 nM for H3R) and its analog 6-methyl-2-(4-(6-(piperidin-1-yl)hexyloxy)phenyl)-4H-chromen-4-one hydrogen oxalate (88, Figure 20) (IC50 = 0.36 µM for AChE; IC50 = 0.76 µM for BuChE; Ki = 228.2 nM for H3R).
Combining AChEIs with H3R antagonism in one structure, Łażewska et al.262 have reported novel acetyl- and propionyl-phenoxy-pentyl(-hexyl) derivatives 89 (Figure 20) as promising compounds to dually inhibit cholinesterases and antagonize H3Rs. Compound 89 (1-(4-((5-(azepan-1-yl)pentyl)oxy)phenyl)propan-1-one) has a high affinity for human H3Rs with a Ki of 30 nM with high capacity to inhibit hAChE and hBuChE (IC50 = 3.60 and 0.55 µM, respectively). Furthermore, it also exhibited low toxicity in HepG2 and SH-SY5Y cells at 50 µM.
2.5.6 AChE inhibition and metal ions chelation
Chaves et al.263 designed novel MTDLs combining the metal chelating group hydroxyphenylbenzimidazole (BIM) to the benzylpiperidine or benzylpiperazine subunit of donepezil intending to combine AChE inhibition, Aβ aggregation, and bio-metal dysregulation modulation. Successfully, obtained derivatives were able to chelate Cu2+ and Zn2+ through their bidentate BIM moiety. Compound 90 (Figure 21), selected as the lead compound, exhibited an IC50 value of 1.87 µM toward AChE, displayed the highest reduction of Aβ-induced cell toxicity, and reduced 43.7% and 66.0% self- and Cu-induced Aβ1-42, respectively.
A series of hybrid compounds, including the bis-hydroxy-triazole tridentate, nontoxic, and highly safe Fe2+ chelator, deferasirox, and tacrine, were reported by Wang et al.264 as MTDLs against AD. Novel hybrids combined the metal chelation ability of deferasirox and the AChE inhibitory properties of tacrine. Successfully, in vitro studies showed that novel compounds exhibited good multifunctional activities, inhibiting AChE and chelating metal ions. In particular, compound 4-(3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl)-N-(8-((1,2,3,4-tetrahydroacridin-9-yl)amino)octyl)benzamide 91 (Figure 21) was able to chelate both Fe3+ and Cu2+ ions and displayed moderate inhibitory activity against AChE (IC50 = 13.7 μM). Further evaluation demonstrated an interesting radical scavenging capacity (56% RNS reduction measured by DPPH assay), low cytotoxicity, and good neuroprotective activity against the toxicity induced by H2O2 in PC12 cells.
A novel series of tacrine–hydroxyphenyl benzimidazole hybrid molecules were synthesized and evaluated by Hiremathad et al.,265 for their multitarget activity. Due to their structure, the tacrine moiety was expected to provide AChE inhibition, while the hydroxyphenyl benzimidazole moiety should exhibit metal chelation. Among the series, several molecules were proposed as lead compounds; 92 (Figure 21) showed the highest AChE inhibition (IC50 = 6.3 nM) and the most effective against the neurotoxicity induced by the Fe/Asc (500 μM/5 mM, respectively) in SH-SY5Y; 93 (Figure 21) with the highest self- and Cu-induced Aβ aggregation inhibition (74.6% and 77.3%) thanks to its metal chelating capacity; and 94 (Figure 21), which exhibited the best antioxidant (radical scavenging) capacity (EC50 = 564 μM in the DPPH assay).
Fancellu et al.266 developed a strategy for repositioning the well-known AChE inhibitor tacrine by coupling it to benzofuran derivatives. Out of this series, compound 95 (Figure 21) outstood for its good activity for AChE inhibition (IC50 = 0.13 μM), Aβ self-aggregation inhibition (57.8%), metal (Cu2+ and Fe2+) chelating ability, and neuroprotective effects (at 20 µM) in Aβ42- and L-ascorbic Acid (AscH(-))/ferrous sulfate-induced toxicity assay in SH-SY5Y cells.
Finally, Jalili-Baleh et al.267 synthesized and evaluated a series of coumarin-lipoic acid conjugate as anti-AD agents, being 96 (Figure 21), the lead compound of the series with potent AChE (IC50 = 16.4 μM) and Aβ self- and AChE-induced aggregation (51.2% and 47.4%, at 100 μM, respectively) inhibitory activities. This compound had also inhibitory effects on intracellular ROS formation, selective bio-metal chelation (Cu2+ and Fe2+), and significantly protected PC12 cells against H2O2-induced death (64% at 10 μM) and Aβ42-induced cytotoxicity (64.7% at 10 μM).
2.6 Aberrant protein elimination and processing stimulation
2.6.1 UPS and autophagy activation
As previously described, the UPS system is affected in AD contributing to aberrant protein aggregates accumulation including Aβ and hyperphosphorylated tau.268 Thus, UPS activation has been proposed as a potential target for AD treatment facilitating protein aggregates clearance. In this line, several attempts to find small molecules with the ability to activate and/or accelerate the UPS system have reported different types of molecules able to induce its activity.269 In 2017, a concise search of UPS activators revealed different types of active small compounds, including p38 MAPK inhibitors (PD169316, 97, Figure 22) and MK2 inhibitors (98, Figure 22).269
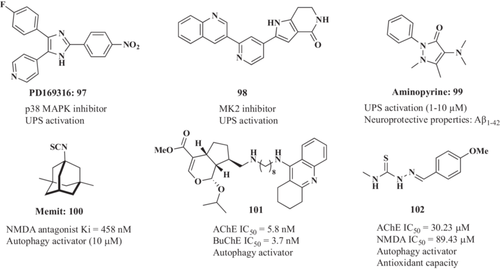
In this line, Santoro269 et al., recently reported a novel class of UPS activators from pyrazolone core. The authors tested a small chemical library from which aminopyrine (99, Figure 22) demonstrated UPS activation capacity in vitro and in neuronal cells. It was further demonstrated that aminopyrine stimulates the ChT-L activity of the 20 s proteasome by binding to the CPα rings. Interestingly, this compound showed a neuroprotective effect in vitro against Aβ42-induced cytotoxicity.
In this sense, there are a few groups whose multitarget strategies have been also focused on autophagy, reporting several compounds with promising results. This is the case of compound 100, described in 2019 by Sestito et al.,270 as a novel NMDAR antagonist (Ki = 458 nM) equivalent of memantine with hydrogen sulfide-releasing abilities (a gaseous transmitter with dual roles as a neuromodulator and smooth muscle relaxant). AD neuropathology is marked by a sharp decrease in H2S generation in the CNS. Thus, an exogenous substance that produces H2S also promotes neuroprotection against oxidative damage, preventing inflammation, encouraging cell proliferation, and maintaining the mitochondrial function, eventually providing defense against damage caused by Aβ to cells. On functional assays, 100 exhibits neuroprotective and ROS scavenging properties in vitro at 10 μM in a model of inflammation induced by LPS and TNF-α in H9-derived human neural stem cells. Besides, in another experiment with H9 and rat microglia cells, 10 μM of 100 reduced amyloid oligomer formation when incubated with Aβ1-42. Autophagy induction capacity was also demonstrated in U-87MG cells, a human glioblastoma cell line, which has autophagy impairment due to elevated mTOR. In this sense, treatment with 10 μM of 100 significantly altered the expression of common autophagy markers, including LC3II, p62, mTOR, and Akt, according to western blot studies. Finally, in the same cell line, 24 h treatment with 10 μM of 100 did not exert cytotoxicity as demonstrated in the MTT assay. In this line, a similar pro-drug conjugate approach was reported by García-Viñuales et al.271 in which silybin B was conjugated to a trehalose moiety to generate an interesting anti-Aβ aggregation derivative with neuroprotective properties.
In 2021, Lin et al. described compound 101 as a tacrine/genipin derivate.272 Based on the hypothesis that autophagy has been shown to directly impact the formation of Aβ plaque by influencing the secretion of Aβ into the extracellular space, they decided to combine the AChE inhibitor activity of tacrine with the autophagy inductor genipin, more specifically with one of its derivates: CHR21, resulting in 101. In this study, a functional assay was performed on SH-SY5Y exposed to cytotoxic 35 mM glutamate, which led to cell death through different mechanisms: programmed cell apoptosis, upregulation of protein levels, such as AChE, i-NOS, and formation of A oligomers of high molecular weight, while downregulated the ratios of LC3-II/LC3-I and phospo-ULK/ULK (autophagy-related markers). In this sense, treatment with 1.25 µM 101 corrected all of these glutamate-induced dysfunctions: it inhibited glutamate, increased AChE (IC50 = 5.8 nM) and BuChE (IC50 = 3.7 nM) levels, decreased NO and iNOS levels, reduced proapoptotic proteins p53 and Bax2 expression, and upregulated autophagy-related markers. 101 was also able to cross the BBB in the PAMPA assay.
More recently, Varma et al. reported in 2023 a novel 4-methoxybenzaldehyde thiosemicarbazone derivate, 102, assed for cholinesterase inhibitory activity (IC50 of 30.23 µM), NMDA antagonism (IC50 of 89.4 µM) Compound 102 showed, also, anti-inflammatory properties, antioxidant potential and autophagy induction capacity. In the functional assays, BV-2 cells served as a model of nitrite production when activated by LPS, 102 significantly decreased NO levels (IC50 = 89.9 µM). For the assessment of autophagy, SH-SY5Y cells were transfected to express the transgene mCherry-GFP-LC3 (a protein that monitors the formation of autophagosomes and autolysosomes), reporting that treatment with 5 µM of 102 significantly increased puncta fluorescence (an indicator of upregulated autophagy flux). Finally, for the study of the antioxidant activity, treatment with 25 µM of 102 significantly reduced ascorbate consumption.
2.6.2 Aβ and tau aggregation inhibitors
An alternative approach to facilitate aberrant protein clearance is the inhibition of aberrant protein aggregation. In this line, a plethora of compounds has been designed with the ability to interact with Aβ or p-Tau oligomers avoiding or imitating their aggregation capacity. Considering the critical role of Aβ aggregates in the pathogenesis of AD,273 their pharmacological targeting has been considered a promising approach for the treatment of the disease. Indeed, the recent satisfactory results obtained with anti-Aβ monoclonal antibodies in phase III clinical trials for AD, able to reduce the Aβ burden leading to cognition improvement, confirm their therapeutic potential. In this line, compounds able to reduce Aβ aggregation could provide similar protective effects. In a similar fashion, tau aggregates are also deeply involved in AD pathogenesis. Thus, compounds able to simultaneously inhibit the aggregation of Aβ and tau, therefore acting on two of the main pathogenic pathways, could provide a more efficient treatment for AD.
Taniguchi et al.274 evaluated the anti-aggregation properties of several classes of compounds and found various phenothiazines and polyphenols, including the well-known methylene blue, with strong anti-Aβ and anti-tau aggregation activities. Based on this work, Fuse and coworkers275 developed a new series of thiophene-based compounds with a dual anti-aggregation effect. Among them, derivative 103 (Figure 23) was the most potent, exhibiting submicromolar IC50 values for inhibition of the aggregation of both pathogenic proteins (IC50 = 0.29 μM for Aβ1-42 aggregation and IC50 = 0.30 μM for tau aggregation). A series of AD-oriented multitarget 1-benzylamino-2-hydroxyalkyl derivatives was reported in 2018 by Panek et al.276 and then further expanded.277 Novel compounds showed different pharmacological profiles based on their activities as eeAChE, hBuChE, hBACE1, Aβ1-42 aggregation, and tau aggregation inhibitors. Derivative 104 showed an overall balanced activity as an inhibitor of Aβ1-42 aggregation (IC50 = 3.09 μM), tau aggregation (69% at 10 μM), hBuChE (IC50 = 5.74 μM), and hBACE1 (IC50 = 41.60 μM). Additionally, the authors showed through kinetic studies with Aβ1-40 that compound 104 could act at several steps of Aβ aggregation, as it could avoid aggregation via interaction with soluble Aβ1-40 while also avoiding fibers elongation and exhibiting disaggregating properties.
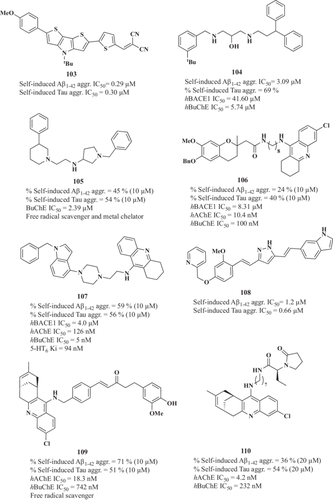
In 2020, Wichur et al.278 developed a series of 1-benzylpyrrolidine-3-amine multitarget compounds that combined Aβ1-42 and tau anti-aggregation properties with additional activities. Among them, compound 105 was selected as the hit compound based on its overall pharmacological profile. It showed potent inhibition of Aβ1-42 (45% at 10 μM) and tau (54% at 10 μM), eqBuChE inhibition (IC50 = 2.39 μM), free radical scavenging properties, and metal chelating capacity.
Pérez-Areales et al.279 developed a novel series of derivatives by merging 7-methoxy-2,2-dimethylchroman-6-ol and 6-chlorotacrine scaffolds, which showed an interesting AD-oriented pharmacological profile. Novel compounds were Aβ1-42 and tau aggregation inhibitors as well as AChE, BuChE, and BACE1 inhibitors while showing good BBB permeability according to the PAMPA assay. The authors evaluated compound 106, which showed an overall balanced pharmacological profile (Figure 23), in the double-transgenic APP/PS1 mice. Dairy intraperitoneal 10 mg/kg doses of the compounds for 4 weeks led to a reduction of Bace1 gene expression, an increase in GPX1 and NRF2 protein levels, and Hmox1 gene expression. Additionally, compound 106 reduced sAPPβ protein levels and increased Adam10 gene expression. 106 treatment led to positive tendencies in improving cognition and reduction of amyloid burden and OS, although most of the observed effects did not reach statistical significance.
Similarly, Szalaj et al.280 reported a series of novel multitarget compounds that included inhibition of Aβ and tau aggregation in their multifaceted profile. Compound 107 showed interesting inhibition of Aβ1-42 and tau aggregation in cells (59% and 56% inhibition at 10 μM, respectively) with additional activities as inhibition of hBACE1 (IC50 = 4 μM), hAChE (IC50 = 26 nM), and eqBuChE (IC50 = 5 nM) and antagonism of the human 5-HT6 receptor (Ki = 94 nM).
Curcumin is one of the better-known compounds with dual Aβ and tau anti-aggregation effects (IC50 Aβ1-42 aggregation = 5.4 μM and IC50 tau aggregation = 3.0 μM).281 Based on its structure, Narlawar and coworkers282 generated a library of curcumin-derived isoxazoles and pyrazoles, which showed tau aggregation inhibition properties and high affinity for Aβ1-42 aggregates. Later, Okuda et al.281 developed novel curcumin derivatives with improved potency against both targets. Among them, compound 108 (PE859) was selected as a hit compound. 108 exhibited a slightly superior inhibition of Aβ1-42 and tau aggregation (IC50 = 1.2 and 0.66 μM, respectively) than curcumin. Additionally, compound 108 showed a good pharmacokinetic profile, being able to reach the CNS following oral administration. 108 therapeutic effect was evaluated in JNPL3 human tau P301L transgenic mice.283 Treatment with 108 at a 40 mg/kg/day dose for 6 months was able to reduce sarkosyl-insoluble tau levels in the spinal cord of the mice, leading to an improvement of the characteristic motor dysfunction of the mice, measured via tail hanging and rotarod tests. Encouraged by these results, the authors also evaluated compound 108 in the senescence-accelerated mouse prone 8 (SAMP8) mice model.284 108 was orally administered at 1 and 3 mg/kg/day doses to 2-month SAMP8 mice for 9 weeks. Treatment with 108 was able to improve mice's cognitive function in the Y-maze test at both doses, although no statistical differences with the control group were observed in the Morris water maze and rotarod tests. Nevertheless, 108 was able to reduce insoluble Aβ1-40 and insoluble tau levels, with no effect on the levels of their soluble forms.
Pérez-Areales and coworkers285 also reported another family of compounds based on huprine structure, the shogaol-huprine hybrids, which showed Aβ1-42 and tau anti-aggregating activities. The most potent compound with this dual activity among the reported derivatives was compound 109, which was able to reduce Aβ1-42 and tau aggregation at 10 μM in cells (71% and 51% inhibition, respectively). 109 also displayed inhibition of hAChE (IC50 = 18.3 nM), hBuChE (IC50 = 742 nM), free radical scavenging effects (10.5 and 8.8 Trolox equivalents in ABTS and DPPH assays), and BBB permeability in the PAMPA assay.
Sola et al.286 also reported a family of multitarget compounds based on huprine and levetiracetam structures with moderate dual Aβ1-42 and tau anti-aggregation properties. Compound 110, which inhibited Aβ1-42 (36% inhibition at 20 μM) and tau (54% inhibition at 20 μM) aggregation, hAChE (IC50 = 4.2 nM), and hBuChE (IC50 = 232 nM), showed target-engagement in C57BL6J mice following intraperitoneal 5 mg/kg administration, showing a 43% inhibition of brain AChE activity. Additionally, the potential therapeutic effect of 110 was evaluated in the APP/PS1 mice AD model. Daily intraperitoneal administration of 110 at 5 mg/kg dose for 4 weeks to 5 months in APP/PS1 mice was able to reduce the spontaneous epileptic seizures and, also, to improve their cognition in the two-object recognition test. At the molecular level, 110 treatment reduced the cortical Aβ burden of the APP/PS1 mice, while no statistically significant reduction was observed for the soluble Aβ1-42 and Aβ1-40 levels. Additionally, 110 treatment led to a reduction of the GFAP-positive astrocytes and Iba1-positive microglial cells surrounding Aβ plaques.
3 CONCLUSIONS
The MTDL design strategy is considered an appropriate pharmacological approach to develop new drug candidates aimed at the treatment of disorders that involve complex pathological mechanisms such as NDDs, particularly AD. AD pathogenesis is composed of a plethora of different interconnected mechanisms directed to increase Aβ peptide and hyperphosphorylated tau levels, loss of neurons and synapses, metal ion accumulation, neuroinflammation, neurotransmitter and calcium homeostasis dysregulation, mitochondrial dysfunction, OS, proteostasis dysregulation, autophagy failure, and other abnormalities in brain tissue. Besides the recently FDA-approved monoclonal antibody against Aβ (Lecanemab), the drugs mainly used in clinics to treat AD patients (donepezil, rivastigmine, galantamine, and memantine) are basically symptomatic treatments with no ability to stop or delay the progression of the disease. Therefore, the multitarget approach is currently considered the best potential hypothesis to develop an effective treatment for AD. During the last decade, efforts have been employed in the design and synthesis of a wide variety of potential multi-acting anti-AD drugs, and many new significantly active and promising chemical entities have been discovered. Here, we review several groups of MTDLs, obtained via combination of structurally active pharmacophores interacting with different targets. Despite none of these MTDL drug candidates have reached the clinical phase of development, we hope that in the coming years, Medicinal Chemists will discover new small molecules, which will be able to effectively modify the course of this neurodegenerative disorder.
ACKNOWLEDGMENTS
The authors are grateful to financial support from the Spanish Ministry of Health (Instituto de Salud Carlos III) (grant PI17/001700 and PI20/00433), Spanish Ministry of Science (grant PID2021-123481OB-I00, and Comunidad de Madrid (grant P2022/BMD-7230-CAM-22) to RL. P. M. expresses thanks to UAM for the FPI fellowship. The authors acknowledge the support by the European COST Action CA20121: Bench to bedside transition for pharmacological regulation of NRF2 in noncommunicable diseases (BenBedPhar). Webpage: https://benbedphar.org/about-benbedphar/, and would also like to thank “Fundación Teófilo Hernando” for its continued support. The authors acknowledge the support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies
Paloma Mayo completed her BSc in Chemistry at the Universidad Complutense de Madrid, her MSc in Organic Chemistry at the Universidad Complutense de Madrid, and her PhD in Organic Chemistry at the Universidad Autónoma de Madrid. She has shown great interest in various fields, such as organic chemistry, medicinal chemistry, and pharmacology. Her research work is focused on the development of novel multitarget-directed ligands combining GSK-3β and AChE inhibition and L-VGCC blockade with complementary activities for the treatment of Alzheimer's disease.
Jorge Pascual is PhD and Postdoctoral Researcher at the Medicinal Chemistry Institute (CSIC, Spain). His scientific interest is aimed to discover novel therapeutics for the management of demyelinating diseases, currently with great interest in the Nrf2-ARE pathway along with multi-targeting strategies as main approaches for the treatment of neurodegenerative pathologies, such as Alzheimer's, Parkinson's, and Multiple sclerosis.
Enrique Crisman completed his BSc in Biotechnology at the University of Oviedo and his MSc in Drug Discovery at the University San Pablo CEU, University Complutense of Madrid, and University of Alcalá de Henares. Currently, he is enrolled in the Medicinal Chemistry PhD program at the University Complutense of Madrid. His research work is focused on the development of new multitarget-directed ligands combining NRF2 induction with complementary activities for the treatment of Alzheimer's disease.
Cristina Domínguez completed her BSc in Medicinal and Biological Chemistry at the Manchester Metropolitan University and her MSc in Pharmacological Research at the Autonomous University of Madrid. She has carried out her research for her master's thesis at the Medicinal Chemistry Institute (CSIC, Spain). Her research primarily focuses on employing multi-targeting strategies to tackle the complexities of Alzheimer's disease, with a specific interest in the NRF2-ARE pathway.
Manuela G. López is MD PhD and full professor of Pharmacology at the School of Medicine at the Autonomous University of Madrid (UAM). Her group has a particular interest in the identification of new potential therapeutic targets to develop innovative and disease-modifying therapies for neurodegenerative diseases, with a special focus on modulating neuroinflammation (microglia-astrocyte interaction), oxidative stress, and autophagy. In collaboration with different medicinal chemists, she has actively participated in the development of different multitarget compounds for Alzheimer's and other neurodegenerative diseases. She has published over 200 scientific articles and has an h index of 55. She is a co-inventor of 12 patents related to neuroprotective compounds for neurodegenerative diseases and stroke.
Rafael León is PhD and Full Scientist at the Medicinal Chemistry Institute (CSIC, Spain). His research interest is focused on the development of innovative multitarget drugs for chronic complex diseases. Activity combinations are designed using the NRF2-ARE phase II antioxidant response as the main target combined with specific pathological pathways of different diseases, including Alzheimer's, Parkinson's, and Multiple sclerosis.
Open Research
DATA AVAILABILITY STATEMENT
Data availability is not applicable since no new data was generated in this study.




