Molecular Hydrogen Therapy: Mechanisms, Delivery Methods, Preventive, and Therapeutic Application
ABSTRACT
Molecular hydrogen (H2), recognized as the smallest gas molecule, is capable of permeating cellular membranes and diffusing throughout the body. Due to its high bioavailability, H2 is considered a therapeutic gas for the treatment of various diseases. The therapeutic efficacy of hydrogen is contingent upon factors such as the administration method, duration of contact with diseased tissue, and concentration at targeted sites. H2 can be administered exogenously and is also produced endogenously within the intestinal tract. A comprehensive understanding of its delivery mechanisms and modes of action is crucial for advancing hydrogen medicine. This review highlights H₂’s mechanisms of action, summarizes its administration methods, and explores advancements in treating intestinal diseases (e.g., inflammatory bowel disease, intestinal ischemia–reperfusion, colorectal cancer). Additionally, its applications in managing other diseases are discussed. Finally, the challenges associated with its clinical application and potential solutions are explored. We propose that current delivery challenges faced by H2 can be effectively addressed through the use of nanoplatforms; furthermore, interactions between hydrogen and gut microbiota may provide insights into its mechanisms for treating intestinal diseases. Future research should explore the synergistic effects of H2 in conjunction with conventional therapies and develop personalized treatment plans to achieve precision medicine.
1 Introduction
Molecular hydrogen (H2) is a colorless, odorless, highly flammable, and poorly soluble gas at normal temperature and pressure. It is also an inert gas with reducing properties [1]. Dole et al. [2] first demonstrated the potential of H2 in treating skin cancer in 1975, marking the initial presentation of H2 as a therapeutic tool. However, this discovery did not immediately translate into a broader clinical application for H2. It was not until 2007 that Ohsawa et al. [3] published an article in Nature Medicine, illustrating the selective scavenging of hydroxyl radicals (•OH) and peroxynitrite anion (ONOO⁻ONOO-) using 2% H2 gas to treat cerebral ischemia–reperfusion (I/R) injury in rats. This breakthrough represented a significant advancement in the field of H2 medicine. Since then, numerous studies have demonstrated the protective and therapeutic effects of H2 in a variety of pathologies, including neurodegenerative diseases [4-7], cardiovascular disease [8], cancer [9, 10], dermatological conditions [11], sepsis [12], hematological diseases [13], and COVID-19 [14]. The therapeutic effects of H2 in these domains are attributed not only to its antioxidant properties but also to its anti-inflammatory, antiapoptotic, and antiallergic capabilities. An in-depth understanding of the mechanisms of action underlying H2 is critical for effectively leveraging its potential across different medical conditions.
Unlike other gaseous signaling molecules such as carbon monoxide, nitric oxide (NO), and H2 sulfide (H2S), H2 has an excellent safety profile and exerts protective effects without toxicity risks, even at high concentrations [15, 16]. Despite its high biocompatibility, clinical use of H2 is limited by its low solubility and the challenges associated with its high diffusivity [17]. Several studies have demonstrated that H2 functions endogenously as a signaling molecule [18], regulating specific molecular pathways [19]. There are two primary strategies for delivering H2 for therapeutic purposes in both animal models and human patients: exogenous administration and endogenous production. Exogenous delivery methods include H2 inhalation [20], consumption of H2-rich water (HRW) [21], injection of H2-rich saline (HRS) [22], and H2 baths [23]. Endogenous H2 production can be enhanced by the intake of substances like inulin and lactulose, which promote H2 production by intestinal H2-producing bacteria [24]. Selecting the appropriate H2 delivery method is critical and should be tailored according to the specific disease being treated.
The gut, as the largest digestive organ in the human body [25], harbors a diverse community of gut microbiota (GM), which plays a pivotal role in the maturation of the intestinal mucosal immune system and maintains a unique regional immune profile [26]. The GM predominantly resides in the lower gastrointestinal tract, specifically within the colon and rectum [27]. Over 100 trillion bacteria are present in the large intestine, approximately 70% of which are H2-producing bacteria capable of producing acetic and butyric acids via hydrogenase (an enzyme that catalyzes H2 metabolism and carbohydrate fermentation) [28]. Moreover, the bacterial hydrogenase can utilize exogenous H2 to modulate the microbial composition of the gut [29]. Consequently, GM plays a pivotal role in maintaining the health of the digestive system, and dysbiosis is linked to various digestive disorders [30, 31]. Common intestinal diseases include inflammatory bowel disease (IBD), colorectal cancer (CRC), intestinal I/R injury, irritable bowel syndrome (IBS), and intestinal obstruction. Among these ailments, IBD and CRC significantly contribute to global morbidity and mortality rates [32]. IBD is often referred to as “green cancer” due to its incurable and recurring nature [33, 34] and is also associated with an elevated susceptibility to CRC [35], age-related diseases [36], and other cancers [37]. CRC is the second leading cause of cancer-related deaths globally, often diagnosed at an advanced stage due to its asymptomatic early course, which makes it challenging to cure. Furthermore, there has been a concerning upward trend in the incidence of CRC among young people [38]. Therefore, it is imperative to explore safe and effective preventive and therapeutic strategies. Given the link between GM and intestinal diseases, H2, particularly as an endogenous gas produced by GM, presents itself as a promising therapeutic agent. To advance H2-based therapies, it is crucial to effectively translate findings from animal studies into clinical settings and promote clinical trials. Actively promoting the clinical translation of H2 by aggregating and evaluating existing clinical trial data is a key strategy for advancing H2-based therapies.
In this study, we provide a comprehensive analysis of the known biological mechanisms of action of H2 and various H2 delivery methods, summarizing their advantages and limitations. Furthermore, we review recent advancements in the application of H2 for treating intestinal diseases. We also discuss current clinical applications of H2 in managing respiratory, neurological, cardiovascular, chronic, and cancer-related diseases. Last, we highlight the challenges facing the clinical translation of H2 and propose potential solutions to overcome these barriers.
2 Mechanisms of Molecular H2
In the research on the applications of molecular H2, a growing body of evidence suggests that molecular H2 is not merely a simple antioxidant. Its biological mechanisms are intricate and multifaceted. As research progresses, molecular H2 has been found to regulate physiological processes in the body through multiple pathways, exhibiting a wide range of effects such as anti-inflammatory, antioxidant, regulation of cell apoptosis, improvement of energy metabolism, and modulation of immune function. Therefore, a systematic elucidation of the mechanisms underlying molecular H2 is of great significance for understanding its therapeutic potential and clinical applications.
2.1 Anti-Inflammatory Effect
Inflammation is a protective response of the organism to various types of stimuli, including external damage (such as infection and physical injury) and internal imbalances (such as metabolic disturbances and autoimmune reactions) [39]. The process of inflammation resolution can be divided into stages such as the clearance of stimuli, attenuation of proinflammatory signals, removal of inflammatory cells, macrophage phenotypic transformation, and tissue repair [40-42]. It is important to note that when inappropriate, excessive, or uncontrolled inflammation occurs, it can lead to a range of human diseases, and thus timely suppression of the progression of tissue inflammation and the development of new anti-inflammatory strategies are necessary. Since the Nakao team [43] first confirmed in 2008 that H2 inhalation could improve transplant-related intestinal injury by inhibiting oxidative stress, numerous studies have subsequently validated the therapeutic potential of molecular H2 in acute and chronic inflammatory diseases, marking the official entry of H2 therapy into the field of anti-inflammatory research.
Acute inflammation is the body's direct response to injury or infection, and if acute inflammation is not completely resolved, it can develop into chronic inflammation. The therapeutic effects of H2 on both types of acute inflammation have been verified. In the alcohol-induced liver injury model, H₂ improves liver tissue pathological damage by reshaping the GM homeostasis and inhibiting the activation of the liver Lipopolysaccharide/Toll-like Receptor 4/Nuclear Factor kappa-light-chain-enhancer of activated B cells (LPS/TLR4/NF-κB) pathway [44]. In a model of diabetes combined with stroke, H₂ intervention downregulates the expression levels of proinflammatory factors (IL-1β, IL-6, Tumor Necrosis Factor-alpha (TNF-α)), while activating the TLR4/NF-κB signaling pathway to achieve neuroprotective effects [45]. In an acute lung injury (ALI) model, H₂ activates the Nrf2 antioxidant pathway and reduces the concentrations of IL-1β, IL-6, and TNF-α, alleviating damage to alveolar epithelial cells [46]. In a sepsis model, H₂ regulates macrophage polarization (inhibiting the M1 phenotype/promoting the M2 phenotype) and inhibits mammalian target of rapamycin (mTOR) phosphorylation, reducing the release of inflammatory mediators such as IL-6, TNF-α, and HMGB1, while increasing the levels of anti-inflammatory factors IL-10 and Transforming Growth Factor-beta (TGF-β) [47]. These findings systematically reveal the multitarget action characteristics of H₂ in the regulation of acute inflammation.
Due to H2’s clear anti-inflammatory effects and the absence of the severe side effects associated with steroid drugs, it holds greater advantages and application potential in the treatment and prevention of chronic inflammation. It has been found that the activation of NLRP3 plays a role in many chronic inflammatory diseases, and molecular H2 can effectively inhibit the activation of NLRP3 to ameliorate endometrial cancer [8], kidney inflammation [48], septicemia [49], neuropathic pain [50], and so on. The anti-inflammatory mechanism of H₂ mainly focuses on the regulatory network of inflammatory factors (the balance of proinflammatory/anti-inflammatory factors) and the crosstalk of key signaling pathways (such as NF-κB, Nrf2, mTOR), which is the current consensus in research [51, 52]. For example, NF-κB activation facilitates NLRP3 inflammasome assembly, whereas Nrf2 induction suppresses these pathways via redox homeostasis modulation. Notably, sex-specific differences in patients may influence these mechanisms. An et al. [53] demonstrated that H2’s therapeutic efficacy is regulated by sex hormones, and exogenous estrogen supplementation enhances H2-mediated neuroprotection in male mice with intracerebral hemorrhage through inhibition of p-NF-κB and p-IKKβ expression. This finding represents a pivotal consideration for advancing personalized clinical applications of molecular H2 in inflammation-associated disorders (Figure 1).
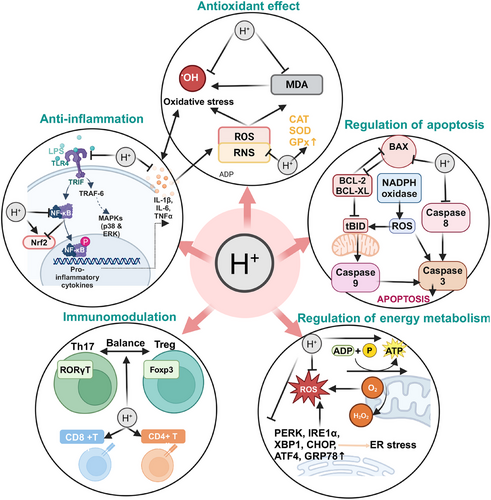
2.2 Antioxidant Effect
Recent research progress has revealed the vicious cycle mechanism between persistent oxidative stress and chronic inflammation—persistent oxidative stress is a major trigger of chronic inflammation, and the occurrence of chronic inflammation increases oxidative stress. Therefore, it is necessary to prevent persistent oxidative stress and select effective and safe antioxidants. Oxidative stress is essentially the dynamic imbalance between the generation rate of reactive oxygen species (ROS)/reactive nitrogen species and the clearance capacity of the endogenous antioxidant defense system. This leads to elevated ROS levels, and extensive interaction between free radicals and biomacromolecules, which in turn causes mitochondrial dysfunction, DNA repair mechanism damage, biomacromolecule oxidation modification, and cell programmed death cascade reactions [54]. The antioxidant effect of H₂ has been studied for 17 years. It differs from antioxidant enzymes (superoxide dismutase [SOD], catalase, and glutathione peroxidase [GPX]) and small-molecule antioxidants (vitamins C and E, flavonoids, carotenoids, melatonin, ergothioneine, etc.). H₂ has a selective antioxidant effect, meaning it specifically neutralizes the harmful free radicals •OH and ONOO⁻, but does not affect other superoxide anions and peroxides involved in normal physiological processes [3, 55, 56]. Furthermore, H₂ can activate the endogenous antioxidant system by upregulating the expression and activity of Nrf2, a transcription factor that induces the expression of various antioxidant enzymes [57-59], thereby enhancing the activity of antioxidant enzymes such as SOD, GPX, and glutathione reductase. H₂ mediates ROS regulation through Nrf2, inhibiting NF-κB/NLRP3 inflammasome activation and achieving an antioxidant–anti-inflammatory synergistic effect [60].
Based on its unique antioxidant properties, H₂ has demonstrated significant therapeutic potential in various oxidative damage models. Numerous studies have proven that inhalation of H2 gas or injection of HRS can be used to treat a range of I/R injuries and other oxidative damage diseases. For example, H2 can increase the expression of heme oxygenase-1 (HO-1) or activate the phosphatidylinositol-3-kinase (PI3K)–Akt signaling pathway to improve liver I/R injury [61, 62]; reduce myeloperoxidase (MPO) activity and IL-1β/TNF-α levels to alleviate myocardial injury [63]; inhibit the generation of malondialdehyde (MDA) to reduce valve I/R injury [64]; reduce inflammatory cell infiltration to protect skeletal muscle from I/R injury [65]; suppress oxidative stress mediated by NOX2 and angiotensin II type 1 receptor; and reduce thyroid hormone-induced myocardial hypertrophy in rats [66], reducing of lipid peroxidation to prevent ALI [67]; H2 inhalation reduces serum MDA levels and NOX-1 expression in lung tissue to prevent pulmonary veno-occlusive disease [20]; H2 alleviates ALI by reducing the levels of MDA in the serum [68], and so on. Notably, oxidative stress and inflammation exhibit bidirectional interaction characteristics in the pathological microenvironment: inflammatory cell infiltration can exacerbate ROS explosion, while oxidative damage products (such as lipid peroxides) can positively regulate proinflammatory signaling cascades. H₂ precisely scavenges toxic free radicals such as •OH, while regulating multiple signaling networks like Nrf2/NF-κB/NLRP3, achieving a synergistic blockade of the oxidative-inflammatory cascade (Figure 1).
2.3 Regulation of Apoptosis
Apoptosis is a form of programmed cell death that efficiently and systematically removes damaged cells, such as those caused by DNA damage or developmental processes [69]. Apoptosis is closely related to other forms of cell death, such as autophagy, necroptosis, and ferroptosis, but according to current literature, the regulatory effect of H₂ on cell death mainly occurs through bidirectional regulation of the apoptosis pathway. The primary mechanism involves regulating the expression of apoptosis-related proteins to inhibit apoptosis, thereby protecting cells from damage and maintaining normal tissue function. For example, H₂ upregulates the expression of the antiapoptotic factor Bcl-2 [70, 71] and downregulates the expression of proapoptotic factors Bax, Caspase-3, Caspase-8, and Caspase-12, thus reducing excessive apoptosis [72-74]. Additionally, H₂ lowers apoptosis levels by inhibiting excessive activation of PARP-1 [75]. Unlike other diseases, the antitumor effect of H₂ involves promoting apoptosis. CDK4 and CDK6 control the cell cycle transition from G1 phase to S phase, and inhibiting their expression halts tumor cell proliferation and induces apoptosis directly. Studies have demonstrated that H2 inhibits CDK4 and CDK6 to restrict lung cancer progression [76].
In the field of cancer therapy, H₂ demonstrates multidimensional proapoptotic properties. Meng et al. [77] found that H₂ can reverse immune escape in lung cancer cells by inhibiting the expression of CD47 and activating the apoptosis program. Jiang et al. [78] demonstrated that H2 promotes apoptosis by downregulating Akt phosphorylation and inhibiting the PI3K signaling pathway in non-small cell lung cancer. Chu et al. [79] found that inhalation of H2 suppresses Hypoxia-Inducible Factor 1 Alpha Subunit (HIF-1α)/NF-κB signaling pathway activation and promotes apoptosis in HeLa cells, counteracting cervical carcinogenesis. This bidirectional regulatory capability allows H₂ to protect normal tissues from excessive apoptosis (such as inflammation-induced cell death) while selectively inducing apoptosis in tumor cells. This microenvironment-dependent regulation provides a theoretical basis for the precise application of H₂ in both tissue protection and cancer therapy (Figure 1).
2.4 Regulation of Energy Metabolism
Mitochondria are the energy factories of cells, responsible for Adenosine Triphosphate (ATP) production, but also participate in important life activities such as cell differentiation, signal transduction, and apoptosis, and can regulate cell growth and the cell cycle. Mitochondrial dysfunction can lead to cardiovascular disease [80], neurodegenerative disease [81], nonalcoholic fatty liver disease (NAFLD) [82], and many other diseases. Therefore, maintaining normal mitochondrial function is crucial. Dumbuya et al.’s study [83] showed that after treating septic rats with HRS, the decline in mitochondrial membrane potential (MMP) and ATP content was improved. HRS protected mitochondrial membrane ultrastructure from damage to some extent and increased the number of mitochondria [83]. Intraperitoneal injection of HRS can prevent mitochondrial lipid peroxidation, thus preventing excessive apoptosis-induced tissue damage, protecting mitochondrial structure, increasing the antioxidant potential of mitochondria, and enhancing ATP levels in obstructive jaundice mice [84]. Dong et al.’s research [85] showed that inhaling 2% H₂ can regulate mitochondrial function and dynamics, increase MMP and ATP levels, and enhance mitochondrial activity in the treatment of sepsis-induced liver injury. Mitochondrial–endoplasmic reticulum (ER) interactions are central to cell metabolism, regulating the exchange of lipids and metabolites between organelles [85]. Cui et al. [86] found that inhalation of high concentrations of H2 enhances the expression of mitochondrial membrane proteins MFN2 and mitochondrial biogenesis-related factors (Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), Nuclear factor E2-related factor 2 (NRF2), and Mitochondrial transcription factor A (TFAM)), while decreasing dystrophin (DRP1) expression, leading to improved mitochondrial function and attenuation of sepsis-associated encephalopathy (SAE) in septic mice.
Mitochondria–ER interactions serve as central hubs of cellular metabolism, playing key roles in lipid and metabolite exchange, thereby influencing overall cell function and energy balance [87]. Molecular H2 regulates not only mitochondrial energy metabolism but also oxidative stress in the ER. Chen et al. [88] found that ER stress damages the autophagy pathway in septic mice by measuring the expression levels of ER stress-related proteins (such as PERK, IRE1α, and XBP1). H₂ alleviated inflammation and organ damage by inhibiting ER stress and activating the autophagy pathway in septic mice [88]. Sun et al. [89] found that H2-attenuated ER stress is associated with hyperoxic acute lung injury by reducing the expression of CHOP, GRP78, and XBP1. Guan et al. [73] found that H2 could downregulate the expression of CHOP, caspase-12, and GRP78, while inhibiting p38 and c-Jun N-terminal kinase (JNK) phosphorylation, and upregulating the LC3-II/I ratio in chronic indirect hypoxia-induced renal injury, suggesting that H2 reduces ER stress and activates autophagy by inhibiting oxidative stress and JNK/MAPK activation. Shen et al. [90] demonstrated that HRW prevents IBD in mice by reducing levels of p-eIF2α, ATF4, XBP1, and CHOP, key proteins in ER stress.
Summarizing current research, H2 primarily regulates energy metabolism by acting on mitochondria and the ER. Specifically, H₂ can promote ATP production by enhancing MMP and increasing the activity of mitochondrial respiratory chain complexes. Notably, the functional regulation of the mitochondrial–ER interface may be a key node for H₂’s systemic metabolic regulation, and this discovery provides new research directions for further exploring its cellular protective mechanisms (Figure 1).
2.5 Immunomodulation
The immunomodulatory effects of H₂ exhibit multidimensional characteristics, primarily enhancing immunity by protecting immune organs, reducing free radical damage and oxidative stress to immune cells, and regulating immune cell subtypes to protect the integrity of the immune system and alleviate immunosuppression. Since maternal immune dysregulation can induce preterm birth (PTB), Aoki et al. [91] used anti-CD3 ε to activate effector T cells, leading to PTB and upregulating local and systemic proinflammatory responses to explore the regulatory effects of H2 on T cells. H2 treatment inhibited several T-cell effector molecules, such as IFN-γ, IL-4, and GZMB, without affecting the expression of regulatory T (Treg) cells. It decreased IL-26 and IL-22 (Th17 cell effector cytokines), thereby reducing the rate of PTB caused by T cell activation [91]. A series of studies by Chen's team revealed the immunomodulatory effects of H₂. After inhaling H₂ for 2 weeks, patients with advanced non-small cell lung cancer showed significant improvement in T-cell exhaustion. Th cell function returned to normal ranges, and exhausted and senescent cytotoxic T cells gradually decreased to normal levels. The total number of Natural Killer T cells (NKT) and cytotoxic Natural Killer cells (NK) subgroups was higher than the pretreatment percentage. Depleted Vδ2 and Vδ1 cells significantly decreased, while cytotoxic Vδ2 cells significantly increased [92]. Additionally, the total number of γδT cells and NKT subgroups was higher than the pretreatment percentage. In thefollowing year, Chen et al. [93] conducted another study evaluating the effect of inhaling H₂ on the immune function of peripheral blood lymphocyte subgroups in healthy individuals. They unexpectedly found that inhaling high-flow (nonhigh concentration) H2 gas had an inhibitory effect on immune function in healthy humans [93]. Li et al. [94] found that HRS upregulated Tregs cells in rats with brain I/R injury and downregulated the expression of miR-21, miR-210, and NF-κB. Furthermore, Zhao et al. [95] found that HRS can protect against radiation-induced immune dysfunction by restoring the number of CD4+ T and CD8+ T cells in the spleen. Currently, research on the immunomodulatory functions of H₂ is relatively less explored compared with its anti-inflammatory and antioxidant capabilities. However, it is undeniable that H2 has a beneficial protective effect on the immune system, and further research is needed to explore the specific mechanisms of how H₂ modulates immune functions and the optimal administration methods (Figure 1).
3 Delivery Methods of Molecular H2
As research into the biological mechanisms of molecular H2 progresses, its delivery methods have become an increasingly important focus of study. Different delivery methods not only affect the bioavailability of molecular H2 but also determine its efficacy and safety in the treatment of various diseases. Common delivery methods include inhalation, oral administration of HRW, injection of HRS, promotion of endogenous H2 production, and nanomaterial-assisted delivery of molecular H2. Each delivery method has unique characteristics, and its effectiveness in treating various disease models can vary.
Inhalation of H2 gas facilitates efficient distribution throughout the body and is widely utilized as an adjunctive treatment for both acute and chronic diseases, particularly in the management of pulmonary disorders, neurological diseases, and cancer. In comparison, the consumption of HRW and HRW baths offers greater convenience, making them more suitable for daily health maintenance and the treatment of certain chronic conditions. However, these methods face challenges, such as the rapid dissipation of H2 and its limited solubility, highlighting the need for future research aimed at enhancing H2 solubility in water. The injection of HRS provides a more precise H2 delivery approach, as it rapidly increases H2 concentrations in the blood and tissues through intravenous or intraperitoneal injection, with broad applications in the treatment of various acute conditions and inflammatory diseases.
With the increasing understanding of the GM, promoting H2 production by the gut microbiome has emerged as a novel H2 delivery strategy. By modulating the metabolic activities of the GM, endogenous H2 production can be enhanced. This approach is noninvasive and sustainable, positioning it as a promising tool for disease prevention and treatment. The application of nanotechnology has further expanded the potential for H2 delivery. Due to their unique physicochemical properties, nanomaterials not only improve H2 stability but also enable targeted delivery, showing great promise, particularly in the treatment of cancer and chronic diseases. Although various H2 delivery modalities have demonstrated specific advantages across different studies, their interactions and synergistic effects still require in-depth investigation. Herein, we comprehensively summarize H2 delivery approaches and analyze the merits and limitations of each modality.
3.1 Inhalation of H2
Due to the unique physical properties of H2, which fall within the explosive range at concentrations ranging from 4 to 74%, it is essential to specify the concentration of H2 for inhalation therapy. Inhalation of 2–4% H2 has been chosen in many studies using animal models for disease treatment or clinical treatment to ensure safety [96]. After inhalation, H2 rapidly permeates all body regions through alveolar ventilation, including inaccessible areas for macromolecules. Importantly, this intervention does not significantly impact physiological parameters such as blood pressure or interfere with red blood cell oxygen-carrying capacity, thereby posing no risk of hemotoxicity even at high concentrations [16].
The inhalation of H2 through a ventilator, mask, or nasal cannula is primarily used as a therapeutic method or adjunctive measure in clinical practice [97]. Due to the high diffusivity of H2, it does not consistently act at the target site. Therefore, direct inhalation of H2 is often clinically employed as an adjunctive treatment for patients requiring long-term hospitalization, such as those with lung and organ problems, diseases of the brain and central nervous system (stroke, Alzheimer's disease [98]), and for cancer treatment and postoperative recovery. In organ transplants, inhalation of H2 has been shown to reduce intestinal and lung graft damage and prevent organ inflammation [99]. The approval of H2 inhalation therapy as an emergency treatment for patients in cardiopulmonary arrest by Japan under Advanced Medical Care B on December 1, 2016 represents a significant international advancement in the field of H2 medicine. In addition, the seventh edition of Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) issued by China National Health Commission recommends the administration of oxygen–H2 mixture (33.3% O2 and 66.6% H2) [100], propelling H2 to the forefront of current research into therapeutic medical gases. Using statistical analysis and clinical data, Zeng et al. [101] showed that H2/oxygen might be a helpful therapeutic medicinal gas to raise oxygen saturation (SpO2) and reduce hospital stays for COVID-19 patients on average (Table 1).
| Hydrogen delivery routes | Advantages and disadvantages | References |
|---|---|---|
| Inhalation of H2 | Simple and easy to implement, but risk of explosion | [96, 99-101] |
| Drinking HRW | Low cost, limited efficacy | [103-106] |
| HRW bathing | Convenient, safe, and widely used in sport | [107, 108] |
| Intraperitoneal injection of HRS | Widely used in animal models of intestinal diseases | [117-119] |
| Intravenous HRS | Rapid diffusion in the blood | [120, 122, 123] |
| Local application of HRS | Highly goal-oriented (Organ preservation fluid, eye drops, rinsing fluid) | [121, 124-127] |
| Endogenous hydrogen production | Breath test available | [128-137] |
| Nanomaterial-assisted hydrogen delivery | Targeted therapy | [138-152] |
Inhalation of H2 is clinically more feasible and straightforward compared with other methods of H2 delivery. Patients can easily self-administer H2 through inhalation, although this method may not be the most convenient. To date, no side effects have been observed with inhaled H2 therapy; nevertheless, it necessitates specialized equipment for H2 production, rendering its inhalation somewhat inconvenient. The concentration of H2 in the blood and tissues depends on the concentration of inhaled H2 and the need for continuous inhalation to maintain adequate levels and achieve therapeutic effects. A controversial issue in H2 inhalation is the choice between inhalation of a H2–oxygen (HO) mixture or pure H2, and the dose–effect relationship has not been established. Both HO mixtures and pure H2 have their advantages. Because H2 has a low molecular weight, inhalation of H2 in an HO mixture reduces airway resistance, increases oxygen dispersion, and enhances oxygen flow. At the same time, there is no risk of hypoxia when inhaling the HO mixture, which is usually obtained clinically through water electrolysis (66% H2 and 33% oxygen) [62]. Inhalation of HO carries a certain level of risk due to the high partial pressure of H2. The presence of oxygen increases the potential for combustion and explosion, especially when the H2 concentration exceeds 10%, as it can easily ignite through static electricity without detection, leading to an explosion [33]. Therefore, it is crucial to ensure absolute safety when inhaling HO, often by using H2 monitors to continuously monitor its concentration [34]. In comparison, inhalation of pure H2 poses a lower risk for humans as it does not cause explosions but carries a higher risk of oxygen deprivation (Figure 2).
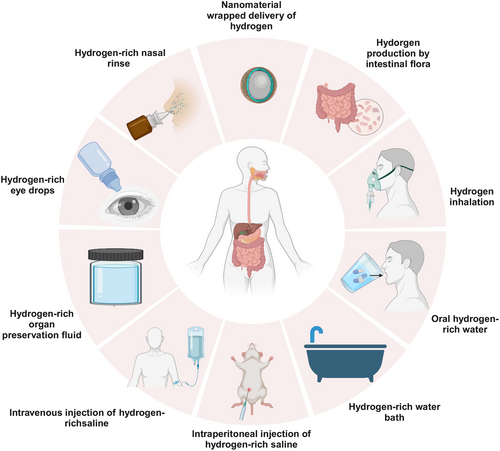
3.2 Drinking HRW and Taking Baths with HRW
HRW is a low-cost, portable, and effective way to transfer H2. It is safer and more convenient for clinical use compared with inhaled H2, as it can be consumed freely while the patient is awake without developing tolerance effects over prolonged usage. Drinking HRW exhibits distinct mechanisms of action and effects when compared with inhaling H2. Consumption of HRW is more likely to exert beneficial effects on the gastrointestinal system, as well as on the liver and brain. The potential impact on the brain may also be attributed to the upregulation of entero-brain axis communication and secondary messenger molecules [102]. The main uptake pathway for drinking HRW is the digestive tract and portal vein system. After entering the pulmonary circulation, most of the ingested H2 evaporates from the lungs, leaving a small amount for therapeutic utilization. HRW has been extensively studied in the medical field and has a wide range of applications, but most of the research is currently limited to animal testing, such as its potential for preventing liver damage and improving cognitive disorders [103, 104]. Additionally, there are suggestions that HRW may play a role in regulating endogenous H2 homeostasis and modulating GM; however, further trials are needed to explore the possible mechanisms underlying this interaction.
Recent studies have demonstrated that preworkout intake of HRW can effectively reduce lactic acid levels, thereby enhancing ventilation efficiency and exerting an antifatigue effect [105, 106]. In addition to drinking HRW, HRW baths are widely used as a delivery method for HRW. For example, the application of HRW ankle baths has been shown to reduce joint swelling on pain relief to restore mobility and balance, making it a safe and convenient way to address acute ankle sprains in the field of sports medicine [107]. Clinically, HRW baths exhibit inhibitory effects on inflammation and oxidative stress while demonstrating therapeutic benefits for conditions such as psoriasis [108]. Furthermore, through its anti-inflammatory properties, HRW baths also facilitate the healing process of diabetic foot ulcers [43] (Table 1).
- 1.
Hydrogen pack direct immersion method
This method is used to produce products such as hydrogen water cups and magnesium sticks110, which enable rapid generation of relatively high concentrations of HRW. However, this process also results in the alkalization of the water and the release of certain metal ions.
- 2.
Electrolysis of water to produce oxygen and hydrogen, the chemical reaction equation is as follows:
Currently, there are two types of electrolytic HRW in the market. One type is based on the traditional method of water electrolysis, where both electrodes are immersed in the water to produce oxygen at the anode and H2 at the cathode. This method does not affect the acidity of the water; however, it should be noted that the anode may have a strong oxidizing effect on ions present in the water, potentially leading to ozone and hypochlorite production. The other type utilizes proton permeable membrane technology, which separates the two electrodes to produce alkaline water containing H2 called "electrolytic reduced water" at the cathode and acidic oxygen-containing water at the anode111,112. Although this method of H2 production has a long history, it yields low concentrations of H2 in the resulting hydrogen-enriched water (HEW), necessitating urgent efforts to enhance its concentration. With further research and technological improvements, the consumption of HEW could provide an effective means of disease prevention and treatment in the future (Figure 2).
3.3 Injection of HRS and Topical Application
The clinical application of H2 in hospitals is highly restricted due to safety concerns. Although drinking HRW is generally safe, it is difficult to regulate the amount of H2 given because the H2 in the water tends to escape over time and some may be lost in the gastrointestinal tract. HRS provides a safer method of H2 delivery compared with direct H2 inhalation, while also providing better control of H2 concentration.
Molecular H2 is dissolved into saline at high pressure to produce HRS, a beneficial antioxidant; it has a high H2 content, weak alkalinity, and negative potential. Moreover, it has potent anti-inflammatory, antioxidative stress, and antiapoptotic properties and is safe and nontoxic [113]. HRS is typically administered to the body through intraperitoneal or intravenous injection, and these two different methods of H2 delivery elicit distinct changes in tissue H2 concentration [114, 115]. Following intraperitoneal injection of HRS, the peak concentrations of H2 in blood and various tissues (including liver, kidney, heart, spleen, pancreas, small intestine, muscle, and brain) were observed at 5 min. In contrast, after intravenous injection of HRS, maximum concentration was achieved within 1 min. Both types of injections demonstrated a dose-dependent increase in H2 concentrations within the blood as well as liver, spleen, pancreas, and brain tissue [116]. I/R injury is one of the main global causes of high morbidity, disability, and death rates; therefore, it is imperative to find an efficacious treatment strategy. For example, intraperitoneal injection of HRS has been demonstrated to reduce ALI caused by limb I/R [117] injury, attenuate renal I/R injury in rats [118], and exhibit therapeutic amelioration in a rat model of SAE through the inhibition of NLRP3/Caspase-1/TLR4 signaling pathway [119]. Additionally, intravenous administration of HRS has shown potential in mitigating intestinal I/R injury in rats [120] while immersion of lungs in HRS has proven effective in reducing pulmonary I/R injury [121]. However, frequent intraperitoneal injections of HRS pose the risk of cross-infection whereas intravenous administration carries certain dangers [122, 123] (Table 1).
HRS is also widely utilized in the treatment of ocular diseases. It can be injected into the vitreous cavity to reduce retinal excitatory damage and promote recovery of retinal function, or used as a H2-containing eye drop to protect the retina [124, 125]. In addition, a randomized, double-blind clinical trial demonstrated that HRS nasal rinses were more effective than saline rinses in improving clinical symptoms of chronic rhinitis (CR), particularly in patients with. This highlights the potential effectiveness of HRS in the clinical management of CR patients [126]. Currently, most studies on HRS as an injectable have been conducted in animal trials, and further exploration is needed to promote its clinical application. However, HRS can also be used topically as an organ preservation solution, eye drops, and rinse solution for disease treatment. The common method for producing HRS is to dissolve H2 in saline at high pressure (0.4–0.6 MPa) for 2 h to achieve a supersaturation level (>0.6 mmol/L) [127]. Saturated HRS is sterilized using gamma radiation and kept in sealed aluminum bags at 4°C and atmospheric pressure. Freshly HRS is prepared weekly to maintain a constant concentration. Gas chromatography is used to confirm the H2 content of saline using the technique outlined by Ohsawa et al. [3], which is widely used for determining H2 content in saline (Figure 2).
3.4 Endogenous H2 Production
Intestinal gases produced by GM include methane (CH4), H2, H2S, and carbon dioxide. Among these gases, H2 constitutes up to 74% of the total gases produced by GM. For instance, Escherichia coli (E. coli) in the intestine can produce H2 through its hydrogenase enzyme [128]. However, a significant portion of this H2 is used by other bacteria, while a minor fraction is absorbed by the intestinal mucosa and subsequently excreted via pulmonary respiration and defecation. Consequently, the overall H2 production of the GM is determined by both its production and consumption. Differences in GM among individuals contribute to variations in H2 production, and researchers have conducted GM transplantation experiments across different animals to observe alterations in H2 production and differences in disease resistance [129]. However, the amount of H2 generated by GM can be easily disturbed by external factors, and the intake of various nondigestible sugars such as high straight-chain amylose corn starch, pectin, oligofructose, and inulin in mammals can stimulate increased H2 production by GM [130, 131]. It has been shown that H2 produced by GM exhibits close associations with certain diseases; for example, H2 can promote intestinal motility and thus serve as a potential treatment for constipation, while a deficiency of H2 may be implicated in the development of Parkinson's disease (PD) [132, 133] (Table 1).
Due to its endogenous nature, H2 is being increasingly explored by researchers for breath testing (BT) to achieve rapid diagnosis of clinical gastrointestinal diseases. BT is an effective and noninvasive diagnostic tool for many gastrointestinal disorders [134]. The H2 breath test (H2BT) works on the basis that part of the gas produced by the fermentation of colonic bacteria diffuses into the abdominal venous circulation and travels to the lungs, where it is easily measured while breathing. Therefore, the H2BT has been employed for various purposes: (a) evaluating carbohydrate malabsorption of different absorbable sugars such as lactose and fructose in the small intestine [135], (b) measuring the time interval between ingestion of nonabsorbable carbohydrates (e.g., lactose) and their interaction with bacteria in the cecum and colon (oral-cecum passage time) [136], and (c) frequently utilizing carbohydrates like glucose or lactulose for H2 exhalation to identify small GM overgrowth in IBS (SIBO) [137] (Figure 2).
3.5 Nanomaterial-Assisted H2 Delivery
All of these delivery methods, whether exogenous or endogenous H2 production, face a common challenge: the accurate delivery of H2 to the targeted diseased area. The therapeutic effect of H2 would be greatly improved if it could be targeted at the site of disease and released continuously, as well as stored efficiently. Nanomaterials have garnered considerable attention from researchers due to their unique physicochemical properties. Although the clinical translation of nanotechnology remains a significant challenge, it provides a promising platform for the advancement of H2 medicine. This has led to the emergence of the concept of nanohydrogen medicine, resulting in the development of numerous nanodelivered H2 materials (Table 1).
3.5.1 Nanobubble H2 Water
H2, being insoluble in water and highly diffusible with greater buoyancy, poses challenges for the production, preparation, and preservation of highly concentrated HRW. Nanobubbles exhibit excellent stability and permeability in liquids, enabling the physical dissolution of H2 through nanobubbles to produce nanobubble H2 water (NBW). This process allows for maximum dissolution of H2 in the water while retaining it for longer periods [138]. Japanese researchers utilized the 2,2′-bipyridyl method to measure the reducing activity of NBW and confirmed its superior antioxidant activity compared with HRW at equivalent H2 concentration [139]. It has been demonstrated that the fine H2 nanobubbles are highly stable in water, and there are H2 nanobubbles that remain in HRW even after long periods of storage, thereby reducing the rate at which H2 escapes from water [140] (Figure 2 and Table 2).
| Hydrogen-carrying nanomaterials | Characteristics of action | Diseases | References |
|---|---|---|---|
| Treatment of diseases of the locomotor system | |||
| CBN@GelDA hydrogel | Promotion of H2 generation and reduction of ROS production | OA | [141] |
| Mg@PLGA MPs | Can be stored in situ at the injection site | OA | [142] |
| AB@HPDA | Continuous release of hydrogen | Intervertebral disc degeneration | [143] |
| Treatment of diseases of the nervous system | |||
| TSIIA/PNS/AB-loaded CCC@mPP NPs | Exploiting the synergistic effects of multiple drugs | Sensorineural hearing loss | [144] |
| Treatment of diseases of the endocrine system | |||
| Bacillus-Chlorella gel patch | Extension of hydrogen delivery time | Diabetic wounds | [145] |
| Intelligent microneedle patch-MgH2 | Promotion of H2 generation and reduction of ROS production | Diabetic wounds | [146] |
| A dual-action liquid metal dressing | Dual action of antibiotic delivery and hydrogen generation for antibacterial and anti-inflammatory purposes | Diabetic wounds | [147] |
| Treatment of diseases of the endocrine system | |||
| C3N4@Gel | Rapid penetration into the biofilm to achieve an antimicrobial effect | Diabetic wounds | [148] |
| Treatment of diseases of the circulatory system | |||
| TN-PdHs | Target-oriented effect | Atherosclerosis | [149] |
| PdH nanoparticles | It exerts local capture and storage of H2 passing through the liver and rapidly catalyzes the hydrogenation of •OH to H2O | Nonalcoholic steatohepatitis | [150] |
| H2-PFOB nanoemulsions | Ultra-high biosafety | Myocardial ischemia–reperfusion | [151] |
| AB@hMSN@PEG | Releases ultra-high doses of H2 in the gut and effectively prolongs the duration of action | Metabolic dysfunction-associated fatty liver disease | [152] |
- Abbreviations: AB@hMSN@PEG, a novel H2 will be more nanocapsule by encapsulating ammonia borane into hollow mesoporous silica nanoparticles; AB@HPDA, ammonia borane-loaded hollow polydopamine; C3N4@Gel, C3N4 nanosheets loading hydrogel; CBN@GelDA hydrogel, an injectable calcium boride nanosheets loaded hydrogel platform; Mg@PLGA MPs, comprising magnesium-containing poly (lactic acid)-poly (glycolic acid) particles; OA, osteoarthritis; perfluorooctyl bromide nanoemulsions (PFOB NEs) were synthesized and then hydrogenated by hydrogen absorption; TN-PdHs, tetrapod needle-like Pd nanocrystals for H2 adsorption.
3.5.2 Nanomaterials as Carriers of H2 for the Treatment of Motor and Neurological Disorders
Diseases of the locomotor system are characterized by their insidious onset, slow progression, prolonged duration, and challenging eradication. Therefore, this type of disease is often treated with a combination of drugs, and several studies have demonstrated that the combination of H2 and nanoparticles performs well in the treatment of locomotor system diseases. Zhang et al. [141] developed an injectable hydrogel platform loaded with calcium boride nanosheets (CBN@GelDA hydrogel), which serves as a highly efficient loaded and sustainably releasable H2 precursor for use in osteoarthritis (OA) therapy. This platform effectively scavenges ROS, diminishes the expression of pertinent inflammatory cytokines, and suppresses M1 macrophages while inducing the M2 phenotype. These actions ultimately lower chondrocyte death and aid in breaking the vicious cycle of OA progression [141]. Wan et al. [142] proposed a local drug delivery system comprising magnesium-containing poly (lactic acid)–poly (glycolic acid) particles (Mg@PLGA MPs) to deliver high concentrations of therapeutic gaseous H2 to inflamed tissues. In a mouse model, Mg@PLGA MPs were intramuscularly injected near the knee joints of OA, serving as an in situ reservoir that continuously released a concentration of gaseous H2 above the therapeutic threshold. This approach effectively reduced tissue inflammation, prevented cartilage destruction, and halted the progression of OA lesions [142]. Wang et al. [143] developed ammonia borane-loaded hollow polydopamine (AB@HPDA) to achieve prolonged H2 release for the treatment of intervertebral disc degeneration (Table 2).
Previous studies have shown positive results when using H2 for the treatment of neurological disorders. Xiao et al. [144] constructed Tanshinone IIA /Panax notoginseng saponins/Ammonia borane /Carboxymethyl chitosan/calcium carbonate-chitosan NPs/Monomethoxy poly(ethylene glycol)-PLGA NPs (TSIIA/PNS/AB-loaded CCC@mPP nanoparticles) that can exert a synergistic polypharmacy effect, indirectly or directly promoting the scavenging of excessive intracellular ROS, reducing the release of inflammatory cytokines, and enhancing the efficacy of traditional Chinese medicine complexes and H2 donors in treating sensorineural hearing loss (Table 2).
3.5.3 Nanomaterials as Carriers of H2 for the Treatment of Diabetic Wounds
Diabetic wounds are one of the most serious complications of diabetes, posing a significant threat to the lives and health of diabetic patients. However, there are currently limited clinical treatments available for this disease. Molecular H2 therapy may be a promising approach to address this issue. Bacillus-Chlorella (Bac-Chl) gel patch developed by Chen et al. [145] achieves continuous H2 production for 60 h to promote chronic diabetic wound healing in vivo by delivering H2 to reduce oxidative stress and inflammation. Wang et al. [146] devised an intelligent microneedle patch-MgH2 capable of prolonged release of MgH2 to generate more H2, which effectively reduces ROS production and promotes diabetic wound healing. Bi et al. [147] developed a dual-action liquid metal dressing with antibiotic delivery and H2 generation for antimicrobial and anti-inflammatory purposes, providing a gentle method of H2 therapy and drug delivery while treating chronic diabetic wounds. Xu et al. [148] proposed the concept of sonocatalytic H2/hole-combined therapy for antibiofilm and infected diabetic wound healing, developing a piezoelectric C3N4 nanosheets loading hydrogel (C3N4@Gel) that produces H2 capable of rapidly penetrating biofilms, which is not achievable with conventional antimicrobial agents. This approach demonstrates both antimicrobial activity and promotion of diabetic wound healing through H2 release [148]. However, most studies on H2 nanomedicine in diabetic wounds have primarily focused on animal experiments, with fewer investigations into the underlying mechanisms and limited clinical evidence to support these findings; thus, further comprehensive research is needed in the future (Table 2).
3.5.4 Nanomaterials as Carriers of H2 for the Treatment of Cardiovascular and Liver Diseases
The therapy of liver and cardiovascular illnesses has made substantial use of H2. However, the majority of research that has already been done focuses mostly on how H2 helps to reduce oxidative damage, inflammation, and apoptosis. There is an urgent need to surpass the current international research levels by exploring novel approaches. Nanohydrogen medicine may offer a new direction. Hu et al. [149] utilized TN-PdHs for targeted management of atherosclerosis, while Tao et al. [150] intravenously injected PdH nanoparticles into mild and moderate nonalcoholic steatohepatitis (NASH) model mice, followed by daily inhalation of 4% H2 for 3 h. The experimental results demonstrated that after the intravenous injection, Pd nanoparticles could be specifically accumulated in the liver and play a dual role as a H2 captor and ·OH filter. This enhanced the effectiveness of H2 treatment in preventing and treating NASH by enabling them to quickly catalyze the hydrogenation of ·OH to H2O and locally capture and store H2 that was entering the liver during daily H2 inhalation [150]. Nie et al. [151] developed H2-PFOB nanoemulsions (NEs) with high H2-carrying capacity, which effectively facilitate targeted penetration into the ischemic myocardium and exhibit excellent antioxidant and anti-inflammatory properties against myocardial I/R injury with a good biosafety profile. Jin et al. [152] combined H2 with nanomaterials to construct H2 nanocapsules AB@hMSN@PEG by encapsulating ammonia borane into hollow mesoporous silica nanoparticles, enabling the release of ultra-high doses of H2 in the intestines. This novel strategy successfully extended the duration of action, consequently reducing the early stages of obesity, diabetes mellitus, and fatty liver disease in mice that are caused by genetic mutations and diet-induced metabolic dysfunction without generating any tissue toxicity. Furthermore, it increased the abundance of Akkermansia muciniphila in the intestinal tract [152]. These findings provide further evidence that H2 can exert ameliorative and therapeutic effects on diseases through modulation of the liver–gut axis, highlighting GM remodeling as a potential mechanism for H2 treatment of metabolic dysfunction (Table 2).
3.5.5 Nanomaterials Combine with H2 for Targeted Tumor Therapy
The combination of nanotechnology and H2 therapy can enhance the targeted delivery of H2 to tumors, where H2 is produced in response to endogenous or external stimuli, thereby maximizing its bioavailability over time. Nanomaterials have three significant advantages: minimal invasiveness, implantability within the body, and rapid biochemical reactivity. Various nanocatalysts such as palladium hydride nanocrystals, PdH-Metal-Organic Framework (MOF), Mg-based galvanic cell, SnS1.68-WO2.41 nanocatalysts, a kind of biocompatible carboxymethyl cellulose-coated/stabilized Fe (Fe@CMC) nanoparticle with photoacoustic imaging (PAI), as well as small-sized nanoparticles (20–100 nm) like Fe and Au–TiO2 heterojunction nanoplatforms (AuTiO2@ZnS), have been utilized for tumor-targeted transport in H2 therapy [153-155].
In addition to nanomaterials for H2 delivery, H2 therapy can serve as a complementary approach to conventional treatments by the energy-modulating effects of H2 to enhance efficacy and reduce toxicity. For instance, continuous administration of H2 during cryopreservation has been shown to improve the viability of allogenic osteochondral tissue [156]. Furthermore, novel oral acid-controlled release H2 MgB2@Polyvinylpyrrolidone (PVP) pills have been developed for combined use with injectable adriamycin chemotherapy drugs, aiming at achieving enhanced effectiveness and reduced toxicity in gastric cancer chemotherapy [157, 158]. The biocompatible micromotor platform Mg/PLGA/CHI, developed by Song et al. [159], generates H2 during its action to eliminate excess ROS in HepG2 cells and significantly enhances the diffusion of chemotherapeutic drugs (Table 2). In summary, the efficacy of H2 delivery via nanomaterials primarily relies on (a) the effective encapsulation of H2, (b) the successful delivery of H2 to the target area of the body, and (c) the successful release of H2.
Each mode of H2 delivery has its advantages, and selecting the appropriate mode for a specific disease is critical. While current research primarily focuses on exogenous H2, it is essential to explore safe and effective methods to enhance endogenous H2 production by GM for disease prevention and treatment. If certain ingredients can be identified as both safe and capable of augmenting endogenous H2 production, they may play a positive role in future disease prevention and treatment strategies. The combination of exogenous and endogenous H2 may yield unexpected effects in treating certain diseases due to variations in absorption pathways within the body.
4 Preventive and Therapeutic Applications for Intestinal Diseases
According to a summary of published articles, H2 has been found to invariably involve the gut flora in the treatment of different systemic diseases, for example, inhalation of H2 modulates the gut flora to ameliorate acute alcoholic liver injury [160], l-Arabinose elicits gut-derived H2 production and ameliorates metabolic syndrome in C57BL/6J mice on a high-fat diet (HFD) [161]. Molecular H2 therapy ameliorates metabolic disturbances and inhibits inflammation in mice with SAE by increasing beneficial bacteria and inhibiting harmful bacteria through actions on the GM, and so on [162]. Therefore, we believe that the interaction between H2 and GM should not be ignored, and as H2 can act on intestinal flora to improve other diseases, then H2 directly treating intestinal diseases may bring better results.
Although H2 can be delivered in various ways, the current applications of H2 in the treatment of intestinal diseases are almost all exogenous, with only one case of endogenous production of H2 by intestinal flora for the treatment of intestinal diseases. According to statistics, the delivery mode of H2 varies among different intestinal diseases. The shortest duration of action is 7 days, and the longest is 63 days in the animal model of IBD. For the injection of HRS and the inhalation of H2 in the treatment of intestinal I/R injuries, and the inhalation of H2 every day or the consumption of HRW in the treatment of CRC. Two studies achieved targeted treatment of intestinal injuries using nanomaterials encapsulating H2. In this section, we summarize the effects and mechanisms of H2 in the treatment of intestinal diseases, mainly simple bowel injury, IBD, intestinal I/R injury, and CRC.
4.1 IBD and Intestinal Injury
IBD, which typically encompasses Crohn's disease and Ulcerative colitis (UC), is characterized by a dysregulated autoimmune response to intestinal ecological dysregulation, triggered by exposure to various irritating environmental factors [163]. The main therapeutic goal for patients with IBD is to reduce inflammation and alleviate symptoms such as abdominal pain and bowel changes. Endoscopy combined with biopsy represents the most efficacious approach for establishing a diagnosis and managing the disease [164]. Current drug treatments for IBD include conventional and biological therapies, with conventional treatments involving anti-inflammatory drugs, immunosuppressants, antibiotics, and probiotics; while biological therapies primarily consist of various anti-TNF-α drugs along with numerous other novel biological agents (e.g., JNK inhibitors) [165]. Due to its high biosafety profile, H2 has been investigated as a potential treatment option for IBD and intestinal injuries. Increased intestinal inflammation, LPS production, infection risk, and decreased short-chain fatty acid (SCFA) content are the main features of IBD pathogenesis. According to our summary of the mechanism of action of H2 for the treatment of IBD, it is primarily used to inhibit the production of ROS, reduce the levels of proinflammatory factors, inhibit the activation of relevant inflammatory signaling pathways, and promote the growth of intestinal probiotic bacteria and the metabolites of intestinal flora (SCFAs) through the oral intake of HRW or the injection of HRS, to achieve the goal of treating IBD (Table 3).
| Diseases | Models | Route and protocol | Mechanisms involved | References |
|---|---|---|---|---|
| Enteropathy | IND-induced enteropathy in mice | HRW, oral; Duration: 5 days | ROS↓ Production of SCFA↑ | [168] |
| Radiation-induced intestinal damage | Whole-body radiotherapy mice |
HRS (10 mL/kg BW), intraperitoneally; Duration: single injection |
ROS↓ Mitochondrial apoptosis pathways↓ |
[171] |
| Constipation | Constipation modeling by oral administration of loperamide to Sprague–Dawley rats |
HRW, oral; Duration: 14 days |
SIRT1/Nrf2/ HO-1↑ ROS↓ |
[172] |
| IBD | The rat model of IBD induced by LPS | HRW, oral; Duration: 7 months |
Nrf-2 signaling pathway↑ NF-κB signaling pathway↓ |
[169] |
| IBD | A rat model of IBD induced by colorectal administration of TNBS |
HRW, oral; Duration: 14 days |
MPO, IL-1β, IL-6, TNF-α, ROS↓ | [170] |
| IBD | IBD was induced by feeding DSS |
HRW, oral; Duration: 7 days |
IL-1β, IL-12, TNF-α↓ | [174] |
| IBD | A rat model of IBD induced by colorectal administration of TNBS |
HRS (0.2 mL/10 g), peritoneal injection/oral Lactulose (0.1, 0.15, 0.2 mL/10 g); Duration: Twice a day for 7 days |
HMGB1/RAGE/NF-κB↓, AMPK/mTOR and Nrf2/HO-1 ↑ |
[175] |
| IBD | IBD was induced by feeding DSS |
HRS (5 mL/kg BW) peritoneal injection; Duration: Twice a day for 7 days |
ER stress↓, HO-1↑ |
[90] |
| IBD | IBD was induced by feeding DSS |
HRW, oral; Duration: 14 days |
Total thiol, SOD, and CAT↑ | [176] |
| IBD | IBD was induced by feeding DSS |
Oral MgH2@EC@ES; Duration: 8 days |
ATP↑ | [177] |
| IBD | IBD was induced by feeding DSS |
Oral SiH NPs (20 mg/kg); Duration: 8 days |
ROS↓, Diversity of GM↑ | [179] |
| UC | DSS-induced chronic ulcerative colitis mice |
HRW (0.8 ppm), oral; Duration: 63 days |
TNF-α and Harmful GM↓, GSH↑ |
[181] |
| IBD | IBD was induced by feeding DSS to the mice | HRS (3.0 ppm) 0.1 mL each intraperitoneal injection; Duration: 17 days |
The epithelial expression of Nos2↓, intestinal-SCFA-producing bacteria↑, Production of SCFA↑ |
[182] |
| Intestinal I/R injury | A rat intestinal intussusception (II) model |
HRS (5 mL/kg BW), intraperitoneally; Duration: single injection |
TNF-α, MDA↓, SOD↑ | [188] |
| Intestinal I/R injury | The rat model of intestinal I/R injury |
HRS (5 mL/kg BW), intraperitoneally; Duration: Two injections |
MPO, MDA, IL-6 and TNF-α↓ | [191] |
| Intestinal I/R injury | The rat model of intestinal I/R injury |
HRGS (2 mL) injected by oral route, abdominal puncture, and the dorsal vein of the penis; Duration: single injection |
MPO, MDA, IL-6 and TNF-α↓ | [192] |
| Intestinal I/R injury | The rat model of intestinal I/R injury |
Inhalation of 2% H2; Duration: 3 h |
CCL2, IL-1 β, IL-6, and TNF-α↓ | [43] |
| Intestinal I/R injury | The rat model of intestinal I/R injury |
HRS (5 mL/kg) intravenous injection; Duration: single injection |
MPO, MDA, and NF-κB↓ | [195] |
| Intestinal I/R injury | The rat model of intestinal I/R injury | HRS intravenous injection (concentration: ≥0.6 mmol/L, ≥0.6 ppm) 10 or 20 mL/kg; Duration: single injection | NF-κB/NLRP3 pathway↓ | [120] |
| Intestinal I/R injury | The mice model of intestinal I/R injury |
HRS (3 µmol/kg) intraperitoneal injection; Duration: one injection per day for 5 days |
miR-199a-3p↓, IGF1/PI3K/Akt/mTOR↑ |
[199] |
| CRC | Stage IV CRC cancer patients |
Inhalation of H2 (flow rate: 1.67 L/min; hydrogen purity: 99.99%); Duration: 3 h a day for 3 months |
PD‑1+ CD8+↓, Mitochondria↑, PD‑1− CD8+↑ |
[206] |
| CRC | CRC cell lines (ROK/SW480/HCT116) and xenograft mouse models |
Inhalation of 66% H2 (66% H2 and 33% O2); Duration: 2 h a day for 21 days |
AKT/SCD1↓ | [213] |
| CRC | A colorectal cancer xenograft model |
HRW, oral and oral gavage (200 µL daily); Duration: Lasts 14 days |
MDA↓, CAT, and SOD↑ | [214] |
- Abbreviations: CRC, colorectal cancer; ER, Endoplasmic reticulum; IBD, inflammatory bowel disease; IND, indomethacin; MgH2@EC@ES, a new core–shell structure of intestine-targeted controlled hydrogen-releasing MgH2 microcapsules by encapsulating MgH2 microparticles with ethyl cellulose, Eudragit S100 and Span-83; SCFA, short-chain fatty acids; TNBS, trinitrobenzene sulfonic acid; UC, ulcerative colitis.
4.1.1 H2 Improves Intestinal Damage by Reducing ROS
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely utilized analgesics and are known to cause gastrointestinal damage, with a greater impact on the small intestine than the stomach. The prevention and treatment of NSAID enteropathy pose significant challenges, as there are currently no viable measures available to prevent initial damage caused by uncoupling of mitochondrial oxidative phosphorylation. Therefore, interventions should primarily focus on downstream inflammatory processes [166]. Considering the potential involvement of ROS in the pathogenesis of this condition, antioxidants hold promise for effectively preventing and treating NSAID-induced enteropathy [167]. Akita et al. [168] discovered that oral HRW inhibited the production of cytokines, MPO, mucosal permeability, and ROS in the small intestinal mucosa induced by indomethacin, an NSAID drug. Additionally, HRW altered the metabolism of intestinal microorganisms and promoted the production of more favorable SCFA [168]. Jin et al. [169] found that HRW significantly activated the Nrf-2 signaling pathway, suppressed ROS expression, attenuated NF-κB signaling pathway activation, and exhibited a protective effect against chronic intestinal inflammation induced by lipopolysaccharide in rats (Figure 3). Hu et al. [170] showed that Electrolyzed Hydrogen Water (ERW) could control colonic inflammation and relieve gastroparesis by inhibiting overproduction of ROS and blocking the communication between oxidative stress and inflammation (Figure 3). Qiu et al. [171] showed that HRS treatment reduced intestinal damage and improved intestinal function in whole-body radiation exposed mice by inhibiting oxidative stress-induced injury and systemic inflammation, suppressing intracellular ROS production and blocking the mitochondrial apoptosis pathway (Figure 3). Chen et al. [172] demonstrated that HRW can reduce ROS production and alleviate intestinal oxidative stress by acting on the SIRT1/Nrf2/HO-1 signalling pathway, thereby alleviating constipation. The above studies have demonstrated that despite the diverse delivery methods of H2, all of them effectively ameliorated intestinal damage by reducing ROS production (Table 3).
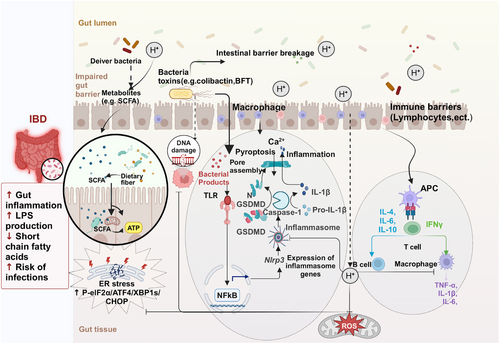
4.1.2 H2 Improves Intestinal Damage by Inhibiting ER Stress
The ER stress occurring in the intestinal epithelial cells (IEC) contributes to the pathogenesis of IBD, and targeting ER stress represents a crucial approach to improving IBD [173]. According to Chen et al. [83], H2 reduced inflammation and organ damage in septic mice via reducing ER stress through the autophagic route. The DSS colitis model's speed, simplicity, repeatability, and controllability make it a popular choice in IBD research. Kajiya et al. [174] demonstrated that H2 significantly inhibited DSS-mediated colonic tissue destruction with macrophage infiltration to prevent DSS-induced colitis in mice. The AMPK/mTOR, HMGB1/RAGE/NF-κB, and Nrf2/HO-1 pathways have been elucidated as the molecular mechanisms involved in the dynamic balance between colonic injury [175]. Shen et al. [90] observed a significant reduction in the protein expression of p-eIF2α, ATF4, XBP1s, and CHOP after intraperitoneal injection of HRS into a DSS-induced IBD mouse model, indicating that HRS can inhibit ER stress (Figure 3). Furthermore, treatment with HRS resulted in a significant upregulation of HO-1 expression, and the use of ZnPP reversed the protective effect of HRS [90] (Figure 3). LeBaron et al. [176] used DSS-induced IBD mice as a model to investigate the therapeutic effects of the colitis drugs sulfasalazine and HRW alone and in combination, showing that sulfasalazine and HRW alone significantly decreased inflammation (high-sensitive C-reactive protein) and restored redox balance (total thiol, SOD, and CAT). There was a trend for the combination treatment to be more effective than either HRW or sulfasalazine alone. Furthermore, HRW tended to be as effective as, and often more effective than, sulfasalazine [176]. Liu et al. [177] developed for the first time a novel gut-targeted controlled-release H2 MgH2 microcapsule, MgH2@EC@ES, and evaluated the therapeutic efficacy of the microcapsule in a mouse model of ulcerative colitis, which showed that the microcapsule could dose dependently repair the IBD damage caused by DSS and repair the impaired mitochondrial energy metabolism function in colitis and that a high dose of MgH2@EC@ES was more effective than the first-line drug 5-aminosalicylic acid, however, the authors did not evaluate the effect of the combination of the two in the article [177] (Table 3).
4.1.3 H2 Improves Intestinal Damage by Regulating GM and Increasing SCFA Production
Xue et al. [44] demonstrated that H2 altered the composition of the GM, leading to an increase in the relative abundance of Mycobacterium anisopliae and Mycobacterium thickum, while concurrently inhibiting the LPS/TLR 4/NF-κB inflammatory pathway, thereby preventing HFD-induced NAFLD. In a mice model of alcoholic liver disease, Liu et al. [160] found that H2 inhalation reduced hepatic oxidative stress, inflammation, and steatosis while also ameliorating liver injury. Moreover, H2 inhalation improved the GM by increasing the abundance of Trichospiraceae Clostridium, enhanced the integrity of the intestinal barrier, and mechanistically blocked the activation of the LPS/TLR 4/NF-κB pathway in the liver [160]. Wang et al. [178] showed that H2 significantly altered the GM composition and ameliorated methamphetamine (METH)-induced psychiatric disorders in METH abusers. These findings suggest that H2 is likely to have an ameliorative and therapeutic effect on disease by acting on GM.
Wei et al. [179] employed orally administered silicon H2 nanoparticles (SiH NPs) for targeted scavenging of ROS at inflammatory sites, thereby alleviating symptoms of IBD and restoring GM diversity by enhancing the abundance of beneficial bacteria. Takahashi et al. [180] found that treatment with H2 increased Lactinobactor and decreased Akkermansia, Gracilibacter, and Marvinbryantia, thereby ameliorating the effects of a HFD, which led to dysbiosis and small intestinal damage in senescence-accelerated mice. Song et al. [181] discovered that giving HRW to mice with a chronic UC model created by DSS improved histopathological changes, raised Glutathione (GSH) concentration, and lowered TNF-α levels. Additionally, HRW inhibited the overgrowth of pathogenic or conditionally pathogenic bacteria (Enterococcus faecalis, Clostridium perfringens, and Bacteroides fragilis) in DSS-induced chronic UC. These results suggest that consuming HRW can modulate gut bacterial dysbiosis during the progression of chronic UC [181]. Meanwhile, Ge et al. [182] discovered that the administration of HRW activates the growth of SCFA-producing bacteria and increases the production of SCFAs. This, in turn, activates the intracellular butyrate-sensor peroxisome proliferator-activated receptor γ signaling, leading to a reduction in epithelial expression of Nos2. Consequently, it promotes the restoration of an anaerobic environment in the colon and improves the DSS-induced acute colitis model in mice [182]. Akita et al. [168] performed fecal microbiota transplantation using cecum contents obtained from HRW-drinking mice and subsequently analyzed the SCFAs content in the cecum contents. The findings revealed that HRW exhibited not only a direct antioxidant effect but also an indirect anti-inflammatory effect by augmenting the SCFAs content [168] (Figure 3).
Competitive interactions among natural GM are critical for maintaining colony stability. Probiotics or microbial transplants can modulate the intestinal microenvironment, facilitating the development of beneficial bacteria to reduce the proliferation of harmful bacteria, ultimately leading to the formation of a new intestinal immune homeostasis. Molecular H2 has demonstrated its impact on GM in both IBD and intestinal injury treatments, promoting the enrichment of beneficial bacteria and subsequently enhancing SCFA production to alleviate IBD and intestinal injury (Table 3).
4.2 Intestinal I/R Injury
H2 has been extensively investigated for its potential to mitigating I/R injury in various organs. For instance, Li et al. [94] demonstrated that HRS exerts a neuroprotective effect against whole brain I/R by upregulating Treg cells and downregulating the expression of miR-21, miR-210, and NF-κB. Liu et al. [183] showed that HRS reduces apoptosis induced by skin I/R and improves flap survival modulation of the Bax/Bcl-2 ratio and inhibition of the activated Apoptosis Signal-Regulating Kinase 1/JNK pathway. Lu et al. [184] revealed that HRS attenuates hepatic I/R injury by inhibiting ER stress-induced apoptosis. Zhang et al. [185] protected rats from liver I/R injury by activating the NF-κB signaling pathway after 1 h of H2 (2%) inhalation before liver transplantation. Nie et al. [186] identify inhibition of oxidative stress and NLRP3-mediated apoptosis as crucial mechanisms by which H2 attenuates myocardial I/R injury. Zhai et al. [187] demonstrated that oral administration of lactulose promotes bacterial H2 production in the gastrointestinal tract, leading to activation of Nrf2 expression and subsequent reduction of brain I/R in rats. These data suggest that both HRS and H2 may possess therapeutic potential for mitigating organ I/R injury through their anti-inflammatory, antioxidant, apoptosis inhibition, and mediation of various signaling pathways.
The intestine is one of the organs most sensitive to I/R injury, which refers to the exacerbation of intestinal damage following the restoration of blood flow after ischemia [188]. Reduced contractile activity, increased microvascular permeability, and mucosal barrier failure are the hallmarks of intestinal I/R injury. These changes can ultimately lead to systemic inflammatory response syndrome, as well as multiorgan dysfunction and failure [189]. At present, limited research has been conducted on the therapeutic effect of H2 in treating intestinal I/R injury, and the underlying mechanisms are summarized below (Table 3).
4.2.1 Through Inhibition of the NF-κB/NLRP 3 Pathway, H2 Reduces Intestinal I/R-Induced Coagulation Problems and Inflammation
Organ and tissue damage are not the only things caused by intestinal I/R injury; it also throws off the balance of coagulation. Under physiological conditions, the coagulation system, anticoagulation system, and fibrinolytic system cooperate to maintain a dynamic balance [190]. MPO serves as an inflammatory biomarker, while TNF-α and IL-6 are classical proinflammatory factors during the inflammatory phase. Wu et al. [188] demonstrated that administration of HRW significantly reduces serum TNF-α levels and mitigates intestinal mucosal injury in I/R rats (Figure 4). Eryilmaz et al. [191] observed that although the administration of HRS does not significantly alter the levels of MPO, MDA, IL-6, and TNF-α in intestinal I/R rats, it results in reduced tissue damage and apoptosis. In contrast, in another study by Shigeta et al. [192], intraluminal injection of H2-rich glucose saline (HRGS) was found to be effective in inhibiting MDA production and thus attenuating intestinal I/R injury in rats. Protease-activated receptor 1 on the surface of monocytes is activated by tissue factor (TF) expression upregulation, whereas TNF-α initiates the cytokine cascade and encourages monocytes to express TF, creating a harmful cycle of inflammatory cascade and coagulation system activation [193]. One of the main mechanisms tying immunity and inflammation together is NF-κB, which is particularly important for controlling proinflammatory proteins and taking part in the transcription of TF genes [194]. TNF-α, IL-6, and IL-1 β are typical stimulatory signaling molecules of the NF-κB pathway. Buchholz et al. [43] found that inhalation of 2% H2 attenuated I/R-induced upregulation of the inflammatory mediators CCL2, IL-1β, IL-6, and TNF-α during small intestinal transplantation in rats. Mao et al. [195] found that administration of HRS suppresses the expression of proinflammatory cytokines IL-1β and TNF-α, as well as inhibits the activation of NF-κB in intestinal I/R mice, thereby attenuating the damage caused by intestinal I/R (Figure 4). Yang et al. [120] reported that in a rat model of intestinal I/R injury, elevated levels of TF, p-NF-κB p65, NLRP3, Caspase-1, and inflammatory factors were observed. Intestinal I/R causes coagulation dysfunction and activates NF-κB/NLRP3 signaling pathway in rats. However, treatment with HRS attenuates the inflammatory response and upregulates TF expression to improve coagulation disorders and alleviate intestinal injury induced by I/R [120] (Figure 4).
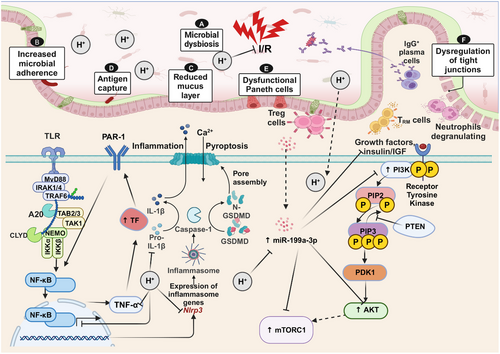
Oxidative stress plays an important role in intestinal I/R injury and HRS has been shown to protect the intestine from I/R injury by inhibiting lipid oxidation, mitigating intestinal inflammation, reducing oxidative stress, and inhibiting apoptosis [43, 188, 196]. Further research is necessary to determine the exact mechanism and signaling pathway that underlie H2’s protective effects in intestinal I/R injury (Table 3).
4.2.2 H2 Downregulates the Expression of miR-199a-3p to Activate IGF-1/PI3K/mTOR Pathway and Reduce Intestinal I/R Injury
MicroRNAs regulate 30–80% of the human genome and serve as crucial biomarkers in the pathogenesis of human diseases, including intestinal I/R. In rats suffering from myocardial I/R injury (MI/RI), Zhang et al. [197] demonstrated that miR-98-5p targets TLR4, which inhibits TLR4 and activates the PI3K/Akt signaling pathway, protecting MI/RI. Yao et al. discovered a significant upregulation of miR-199a-3p following intestinal I/R injury, which was found to target insulin-like growth factor-1 (IGF-1), mTOR, and PI3K regulatory subunit 1 (PIK3r1) that encodes a key PI3K part of the gene. In other words, miR-199a-3p targets the IGF-1/PI3K/Akt/mTOR pathway involved in cell growth and division [198, 199]. The protein expression levels of these targeted molecules were generally reduced after intestinal I/R injury as evidenced by decreased expression of IGF-1, PI3K, Akt, and mTOR. This suggests that miR-199a-3p may inhibit the prosurvival pathway. However, HRS pretreatment (Before intestinal I/R surgery in mice, intraperitoneal injection of HRS (3 µmol/kg) was administered for 5 days in a row.) significantly increased the protein levels of these four members and exhibited a protective effect similar to that observed with miR-199a-3p inhibitor treatment. In conclusion, HRS treatment may protect IECs from I/R injury by downregulating the expression of miR-199a-3p and activating the IGF-1/PIK3r1/mTOR-related cell survival pathway [199] (Figure 4 and Table 3).
4.3 Colorectal Cancer
Cancer is a significant global public health issue, and CRC ranks as the second leading cause of cancer-related mortality [200]. Current therapeutic approaches for CRC include surgery, radiotherapy, chemotherapy, and targeted therapy [201]. While these anticancer treatments have demonstrated efficacy in enhancing the prognosis of patients with CRC, a significant number of patients encounter unfavorable side effects following surgery and chemotherapy. Growth factors, PI3K/AKT/mTOR, and MAPK are examples of signaling pathways that are crucial to the pathophysiology of CRC [202]. Research has indicated that H2 inhibits tumor cell activity, proliferation, invasion, and migration through various molecular mechanisms, in a manner that depends on both dose and time. Additionally, it has been shown to mitigate nephrotoxicity in patients undergoing radiotherapy and chemotherapy [203]. According to Wang et al. [76], H2 dramatically slows tumor growth in vivo and, in a concentration-dependent manner, decreases cell proliferation and induces apoptosis in vitro. Furthermore, H2 lowers lung tissue levels of ROS, TNF-α, IL-1 β, IL-8, and IL-13 and elevates the SOD expression. Further evidence of the suppression of SMC3 expression in A549 and H1975 cells was provided by immunohistochemical labeling [76]. Yang et al. [9] demonstrated that administration of HRW in a xenograft mouse model results in a significant reduction in both the volume and weight of endometrial tumors, thereby inhibiting the growth of endometrial cancer through ROS/NLRP 3/caspase-1/GSDMD-mediated apoptosis. As a result, H2 therapy has drawn more attention and is now acknowledged as a potentially effective cancer treatment strategy.
4.3.1 H2 Improves Prognosis by Restoring Depleted CD8+ T Cells in Patients with CRC Cancer
High infiltration of CD8+T cells in CRC indicates a favorable prognosis and positive response to immunotherapy [204]. However, prolonged interactions between T cells and tumors impair T-cell competence, while PGC-1α inactivation leads to mitochondrial dysfunction, thereby compromising the immune activity of CD8+ T cells. It has been shown that H2 can activate PGC-1α to restore mitochondrial function and rescue depleted CD8+T cells [205]. Programmed cell death 1 (PD-1) is considered a marker of T-cell depletion and is associated with poor prognosis in various cancers. In a clinical study conducted by Akagi and Baba [206], involving 55 patients diagnosed with stage IV CRC, it was observed that inhalation of H2 for 3 h/day and concurrent chemotherapy in the hospital resulted in a reduction in the abundance of exhausted terminal PD-1+CD8+ T cells. This depletion of CD8+ T cells may be attributed to the activation of mitochondria through PGC-1α, leading to an increase in the population of active terminal PD-1−CD8+ T cells and ultimately improving patient prognosis [206] (Figure 5 and Table 3).
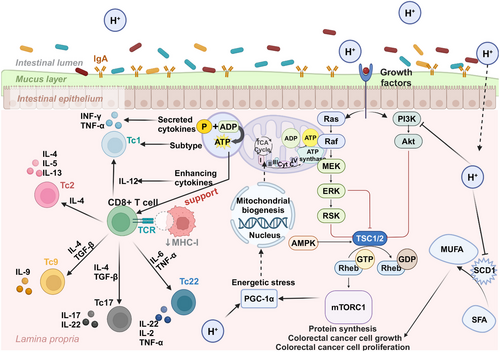
4.3.2 H2 Inhibits CRC Cell Proliferation by Suppressing the AKT/SCD1 Pathway
Stearoyl-CoA desaturase 1 (SCD 1) serves as the rate-limiting enzyme in the synthesis of monounsaturated fatty acids and oleic acid from saturated fatty acids and stearic acid [207]. All malignancies share the trait of cell proliferation, which is dependent on fatty acids for the creation of signaling molecules and cell membranes [208]. Given its high expression levels in various cancer types and pivotal role in regulating fatty acid metabolism to facilitate cancer cell growth, SCD 1 is regarded as a promising therapeutic target for cancer treatment [209]. In addition, SCD1 has been identified as a marker for CSCs in CRC [210], and the SCD 1 inhibitors effectively attenuate the stemness properties of CSCs [211]. Protein kinase B, also referred to as Akt, is a 57-kDa serine/threonine kinase that functions as a signaling molecule for cell growth and development. By transferring signals from upstream regulatory proteins like PI3K to several downstream effectors like glycogen synthase kinase 3 β (GSK3 β), which in turn interacts with other compensatory signaling pathways, the Akt pathway functions as an effective mediator [212]. The PI3K/AKT pathway controls a variety of cellular processes, including autophagy, apoptosis, and proliferation. The prevalence of human diseases resulting from dysregulated Akt signaling has prompted the active development of small molecule inhibitors targeting PI3K and Akt.
The phosphorylation of AKT and the elevation of pAKT levels are commonly observed in most malignancies. In their study, Zhang et al. [213] investigated the impact of H2 on SCD1 and PI 3K/AKT signaling pathways in CRC cell lines (RKO, SW480, and HCT116), revealing a significant downregulation of SCD1 in H2-treated CRC cells. Interestingly, H2 exhibits distinct effects on different CRC cells; it enhances PI3K expression in RKO cells while diminishing it in SW480 and HCT116 cells. Moreover, H2 decreases the expression of both PI3K and p-AKT specifically in SW480 and HCT116 cells. Therefore, H2 demonstrates its potential to inhibit the PI3K/Akt pathway for reducing proliferation in CRC cells, and this reduction in AKT phosphorylation may induce inhibition of downstream signaling [213]. AKT activator SC79 was used by Zhang et al. [213] to treat RKO, SW480, and HCT116 cells. This led to a dose-dependent upregulation of pAKT expression. Treatment of all CRC cells with SC79 reverses the inhibitory effect of H2 on cell proliferation and significantly upregulated SCD1 and pAKT expression in a dose-dependent manner. These findings suggest that H2 inhibits CRC cell survival by reducing pAKT levels and targets the AKT/SCD1 pathway to inhibit tumor growth both in vitro and in vivo [213] (Figure 5). Asgharzadeh et al. [214] treated a CRC mouse model with H2 water and 5-fluorouracil individually, while also setting up a combination treatment group to explore the effects. The results showed that H2 water alone significantly improved detected antioxidant markers (SOD and CAT) and reduced MDA levels. However, the combination of H2 water and 5-fluorouracil significantly attenuated MDA levels more effectively than 5-fluorouracil alone [214]. This also highlights the potential of H2 therapy as an adjunct to cancer treatment (Table 3).
5 Preventive and Therapeutic Applications for Other Diseases
Molecular H2 assumes a critical function not only in the prevention and management of gastrointestinal disorders but also in demonstrating therapeutic advantages across a multitude of physiological systems within the human organism. This highlights the multifaceted therapeutic attributes of H2 and its prospective role as a notable medical intervention. Comprehensive investigations have clarified the mechanisms that underlie the therapeutic effects of H2, revealing its capacity to neutralize deleterious free radicals, modulate intracellular signaling pathways, and promote cellular viability. Recent research indicates that the therapeutic ramifications of H2 have been thoroughly examined across an array of pathological conditions, encompassing respiratory ailments, disorders of the central nervous system, cardiovascular abnormalities, hepatic and renal pathologies, as well as cancer-related diseases.
5.1 Lung Diseases
Lung diseases represent a diverse group of conditions that impact the respiratory system, characterized by distinct etiologies, clinical manifestations, and treatment strategies. These conditions often involve intricate interactions between environmental factors and comorbidities, presenting substantial challenges for accurate diagnosis and effective management. In recent years, H2 has gained attention as a promising therapeutic intervention, exhibiting significant potential in ameliorating various lung diseases and providing novel insights into their management. Lung injuries comprise a wide array of pathologies resulting from a multitude of etiological factors, including but not limited to mechanical trauma, chemical exposure, and lifestyle-related influences. A comprehensive comprehension of these pulmonary injuries is imperative for accurate diagnosis and effective therapeutic interventions. Studies have shown that H2 possesses notable therapeutic potential in mitigating various types of lung injuries. In a hyperoxia-induced lung injury model, mice exposed to a high-oxygen (98%) environment were treated with 2% H2 gas inhalation, which activated the Nrf2 pathway and protected the lungs from oxidative stress-induced damage [215]. Similarly, Nrf2 is a key mediator in the therapeutic effects of H2 gas on sepsis-induced lung injury. This was demonstrated by Yang et al. [216], who compared the responses of wild-type and Nrf2-deficient mice. Inhalation of 2% H2 gas for 1 h significantly elevated the levels of SOD, CAT, and HO-1 while reducing HMGB1 levels in wild-type mice. It is noteworthy that these therapeutic effects were not observed in Nrf2-deficient mice, thereby emphasizing the essential role of Nrf2 in mediating the therapeutic properties of H2 gas [216]. The therapeutic effectiveness of H2 gas in treating sepsis-induced lung damage in mice has been thoroughly demonstrated; nevertheless, its preventive capabilities require additional experimental investigation. Wang et al. [217] proposed an innovative approach by integrating H2 gas with engineered microalgae, providing a promising avenue for the prevention of sepsis-associated lung injury.
ALI is a severe and life-threatening clinical condition. Numerous studies have confirmed that H2 alleviates lung injury through various mechanisms. Nrf2 serves as a critical target in H2 gas therapy for both hyperoxia-induced lung injury and sepsis-related lung injury. Similarly, in a seawater-induced ALI model, Nrf2 also plays a vital role. Inhalation of 2% H2 gas for 2 h significantly improved partial pressure of oxygen, reduced inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as the activities of MDA and MPO, while upregulating the expression of Nrf2 and HO-1 [46]. Li et al. [218] performed an extensive study on the impact of H2 gas on LPS-induced ALI and found that H2 gas significantly improved survival rates, enhanced lung function, reduced weight loss, and diminished the levels of proinflammatory cytokines such as IL-6, TNF-α, and IL-1β in mice. Furthermore, H2 gas offered protection against lung damage by influencing AMPK activity and decreasing the expression of Drp1 and Caspase-3 [218]. Yin et al. [219] conducted both in vivo experiments using an LPS-induced ALI mouse model and in vitro experiments with RAW264.7 macrophages. The results showed that H2 gas inhalation increased the 72-h survival rate of mice to 80%, reduced lung injury, and suppressed the release of inflammatory cytokines. The in vitro experiments further demonstrated that H2 gas inhibited the expression of ROS, NO, TNF-α, IL-6, and IL-1β in macrophages, while also reducing TLR4 expression and NF-κB activation [219]. Fu et al. [113], employing an LPS-induced ALI rat model and human pulmonary microvascular endothelial cells, demonstrated that HRS mitigates ALI by downregulating the mTOR/TFEB signaling pathway.
Chronic obstructive pulmonary disease (COPD), the third leading cause of mortality globally, is marked by a high prevalence and significant healthcare burden. Smoking and exposure to toxic particulates represent the primary risk factors. Current therapeutic strategies encompass smoking cessation, inhalation therapy, and pulmonary rehabilitation [220]. Nonetheless, H2 gas has emerged as a promising therapeutic option for the management of COPD. Animal studies suggest that H2 gas inhalation may serve as an effective strategy for managing COPD. In a mouse model of cigarette smoke solution-induced COPD-like injury, inhalation of 42% H2 gas for 75 min twice daily over 9 weeks resulted in a 100% survival rate, compared with 80% in the untreated group. Histopathological evaluations indicated that H2 gas treatment reduced emphysema-like changes and pulmonary inflammation [221]. Su et al. [222] developed a COPD rat model using cigarette smoke and LPS, observing that a 2-h daily inhalation of H2 gas significantly enhanced lung function and decreased inflammatory cytokine levels. The therapeutic effects were attributed to the suppression of M1 macrophage polarization and the promotion of M2 macrophage polarization, effectively mitigating pulmonary inflammation. In addition, in a COPD mouse model, inhalation of a gas mixture containing 42% H2, 21% oxygen, and 37% nitrogen significantly reduced emphysema, collagen deposition, and goblet cell hyperplasia, while inhibiting the activation of ERK1/2 and NF-κB signaling pathways [222].
Molecular H2 has shown multiple therapeutic advantages in treating pulmonary diseases, including its ability to directly repair lung tissue damage and provide comprehensive protective effects by regulating molecular signaling pathways. Its ability to modulate oxidative stress and inflammatory responses in ALI, along with its role in promoting tissue repair and maintaining immune balance in COPD, underscores its potential to influence disease progression at the level of pathological mechanisms.
5.2 Central Nervous System Diseases
Molecular H2 has been extensively studied for its potential neuroprotective effects in various neurological disorders, with its therapeutic efficacy primarily attributed to its strong antioxidant, anti-inflammatory, and antiapoptotic properties. These mechanisms help slow the progression of neural damage. H2 gas has shown significant benefits in neurodegenerative diseases, acute neurological conditions, cognitive impairments, mood disorders, and neuropathic pain. Oxidative stress plays a pivotal role in the pathogenesis of neurodegenerative diseases. It not only triggers lipid peroxidation and DNA damage but also promotes neuroinflammation, further aggravating neuronal injury—key features of various neurodegenerative diseases. Molecular H2 mitigates neuronal damage through its anti-inflammatory and antioxidant properties, while also regulating cellular signaling pathways to enhance neuronal integrity. Due to its high biosafety, H2 is regarded as a promising strategy for slowing the progression of neurodegenerative diseases and serving as a long-term therapeutic approach [223]. For example, Hou et al. [104] studied the effects of HRS on cognitive impairment in APP/PS1 mice and found that, although HRW could not clear Aβ, it significantly improved the cognitive abilities of the mice, with this improvement exhibiting gender dependence. In contrast, Zhang et al. [224], using PdH nanoparticles in 3×Tg-AD mice, reported that H2 gas significantly inhibited the overexpression of APP, BACE1, and sAP, thereby reducing Aβ production. This intervention effectively halted the progression of AD, alleviating cognitive impairment, synaptic deficits, and neuronal death [224]. Moreover, Lin et al. [225] conducted a 7-month treatment with HRW in 3×Tg-AD mice and demonstrated that it prevented synaptic loss and neuronal death, reduced amyloid plaques, hyperphosphorylated tau protein, and neurofibrillary tangles. Additionally, HRW improved disturbances in brain energy metabolism and dysbiosis of GM while mitigating inflammatory responses [225]. The interaction between the gut and brain axis is believed to play a critical role in alleviating neuroinflammation, with the regulation of GM by HRW considered a key mechanism underlying its neuroprotective effects.
In the treatment of acute neurological disorders, rapid diagnosis, and timely intervention are essential. Studies have demonstrated that H2 therapy provides significant neuroprotective effects in models of acute neurological conditions, including cerebral I/R injury, subarachnoid hemorrhage (SAH), and traumatic brain injury (TBI). For instance, in a cerebral I/R injury model, Lee et al. [226] reported that drinking HRW reduced Bax and TNF-α levels in the hippocampus of mice, decreased ROS levels, and improved anxiety and memory performance. Moreover, intraperitoneal injection of HRS showed notable efficacy by promoting HO-1 expression, increasing SOD levels, and reducing MDA levels, thereby mitigating oxidative stress-induced brain damage [227]. In an SAH model, exposure of mice to a 3% H2 environment activated Nrf2, upregulated GpX4 expression, and inhibited TLR4 activation, significantly alleviating neurological damage [228]. Similarly, intraperitoneal injection of HRS effectively suppressed the NF-κB pathway and the formation of the NLRP3 inflammasome [50], highlighting the multitarget regulatory effects of H2 in providing neuroprotection against SAH. Moreover, Wang et al. [229] demonstrated using a microvascular endothelial cell model that H2 gas activates the PI3K/AKT/GSK3β signaling pathway, thereby enhancing cell survival and providing new insights into the treatment of TBI. In the context of neuropathic pain, molecular H2 also shown promising therapeutic effects. A 2022 study reported that HRW alleviated inflammatory pain in mice and simultaneously improved associated depressive and anxiety-related behaviors [230]. Additionally, Lian et al. [231] found that in mice with chemotherapy-induced neuropathic pain caused by oxaliplatin, drinking HRW significantly reduced inflammation by inhibiting the LPS–TLR4 pathway and decreasing the expression of TNF-α and IL-6. Furthermore, HRW consumption improved GM composition by increasing the abundance of beneficial bacteria such as Lachnospiraceae, Lactobacillaceae, and Ruminococcaceae, while promoting the production of SCFA. These changes alleviated neuropathic pain through the gut–brain axis [231]. Overall, H2, by regulating immune responses, protecting neurons, and modulating the gut–brain axis, exhibits multifaceted therapeutic effects and holds significant promise as a potential treatment for central nervous system diseases.
5.3 Cardiovascular System Diseases
Cardiovascular diseases have always been a major factor contributing to global morbidity and mortality, necessitating a comprehensive management strategy that includes drug therapy, lifestyle changes, and innovative treatment approaches. However, existing traditional treatment strategies urgently need innovative breakthroughs. Molecular H2, as a novel bioactive molecule, has significant therapeutic potential for various cardiovascular conditions, including hypertension, atherosclerosis, myocardial I/R injury, and myocardial infarction.
Hypertension, as a chronic condition, is a critical risk factor for the development of various cardiovascular diseases and, if left uncontrolled, can lead to severe health complications. A study demonstrated that intermittent hypoxia rats inhaling 3% H2 gas for 2 h daily over 35 days showed significantly reduced levels of 8-hydroxy-2-deoxyguanosine and increased SOD levels. These findings indicate that H2 gas alleviates oxidative stress, improves vascular dysfunction, and effectively lowers blood pressure without noticeable side effects [232]. Furthermore, in a rat model of hypertension induced by nephrectomy, inhalation of 1.3% H2 gas successfully controlled blood pressure by reducing sympathetic nervous system activity and enhancing parasympathetic nervous system activity [233]. Atherosclerosis is a disease characterized by persistent inflammation of the arterial walls, with its progression being heavily influenced by inflammatory responses. To overcome the limitations of current therapeutic approaches, researchers have developed nanomaterial-assisted H2 delivery systems, which have significantly enhanced antioxidant and anti-inflammatory efficacy. In ApoE−/− mice models, intraperitoneal injection of HRS was shown to inhibit the expression of Lectin-like Oxidized Low-Density Lipoprotein Receptor 1 (LOX-1) and the activation of NF-κB, effectively preventing the onset of atherosclerosis [234]. While existing medications can alleviate inflammation and reduce lesions, their limited efficacy and stability hinder broader application. To address these challenges, Xu and colleagues [235] developed a Palladium Hydride-Tellurium (PDH-Te) nanozyme that not only exhibits potent ROS-scavenging abilities but also continuously releases H2 gas to suppress proinflammatory cytokine release, thereby significantly improving atherosclerosis. Additionally, H2 has been shown to regulate hypercholesterolemia, a key risk factor for atherosclerosis. For instance, intravenous injection of H2 nanobubble solution slowed disease progression with good safety and no adverse effects on animal survival rates [236].
In a myocardial ischemia model, intraperitoneal injection of 10 mL/kg HRS prior to reperfusion significantly reduced oxidative stress by modulating the total oxidative status and total antioxidant status, thereby minimizing myocardial cell damage and degeneration [237]. Another study showed that perfusion of H2-rich Krebs-Ringer solution (H2 concentration: 0.6 mmol/L, pH: 7.3) in rat hearts activated the JAK2–STAT3 signaling pathway while downregulating the p-STAT1/STAT1 pathway, thus reducing myocardial apoptosis [238]. Additionally, HRS mitigated I/R injury by promoting mitochondrial autophagy via the PINK1/Parkin pathway to eliminate damaged mitochondria [239]. Nie et al. [151] developed H2-PFOB NEs with a high H2-loading capacity that deeply penetrated ischemic myocardium, significantly enhancing antioxidant and anti-inflammatory effects and demonstrating superior therapeutic efficacy. When combined with low-intensity focused ultrasound-induced H2 release, the therapeutic effects of H2-PFOB NEs were further enhanced [151].
In cardiac arrest (CA) and cardiopulmonary resuscitation models, H2 gas has also demonstrated protective effects on cardiac tissue by reducing injury markers and inflammation. Gong et al. [240] reported that inhalation of 2% H2 gas significantly reduced levels of myocardial injury markers, such as creatine kinase-MB and cardiac troponin-T, while protecting myocardial tissue from further damage by inhibiting autophagy. Furthermore, in a myocardial infarction model, daily inhalation of 2% H2 gas for 3 h over 28 days effectively suppressed the activation of the NLRP3 inflammasome, reduced cardiac fibrosis, and improved cardiac function [241]. These studies suggest that H2 therapy may serve as a viable adjunct to conventional treatments, offering new avenues for myocardial protection and recovery. The integration of nanomaterial-assisted H2 delivery technologies has demonstrated significant potential to enhance therapeutic efficacy and offers promising prospects for achieving precision treatment of cardiovascular diseases.
5.4 Liver and Kidney Diseases
The liver and kidneys, as vital organs responsible for metabolism and detoxification, are crucial for maintaining overall health. Liver and kidney diseases are often interrelated, frequently cooccurring due to shared pathophysiological mechanisms and common risk factors. Therefore, preserving the health of these organs and identifying associated risk factors at an early stage are essential for safeguarding overall well-being. NAFLD not only affects the liver but may also have systemic impacts, including on the kidneys and other organs. Studies have demonstrated that administering 0.6 mmol of HRS to NAFLD rats improves insulin sensitivity, enhances glucose tolerance, reduces liver enzyme and lipid levels, and achieves therapeutic effects by inhibiting TNF-α and IL-1β in the liver while upregulating PPARα and PPARγ expression [242]. Furthermore, Liu et al. [243] found that the therapeutic effects of H2 gas on NAFLD are dose dependent, with 4% H2 outperforming 67% H2 in reducing liver enzyme levels Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) and lipid accumulation. In another study, Xue et al. [44] demonstrated that inhalation of 4% H2 in an NAFLD rat model significantly lowered plasma LPS levels, inhibited the LPS/TLR4/NF-κB signaling pathway to reduce liver inflammation, and enhanced intestinal barrier function by upregulating Zo-1 and occludin expression. It also positively regulated the GM, increasing the Bacteroidetes/Firmicutes ratio, thereby highlighting the crucial role of the gut–liver axis in H2’s therapeutic effects on NAFLD [44]. H2 has also shown potential in mitigating chemotherapy-induced liver injury. For instance, Yang et al. [244] observed that CRC patients receiving mFOLFOX6 chemotherapy exhibited stable liver function tests (ALT, AST, Alkaline Phosphatase (ALP)) after drinking HRW, indicating its hepatoprotective effects. Similarly, Gao et al. [245] demonstrated that injecting HRS in rats effectively reduced ALT and AST levels caused by doxorubicin, decreased ROS and MDA production, and regulated the Bax/Bcl-2 ratio to alleviate inflammation and apoptosis. Li et al. [246] also proposed the potential protective effects of H2 against cisplatin-induced side effects, although further research is needed to validate this hypothesis.
Chronic kidney disease (CKD) is a progressive disease whose major contributing factors include diabetes and hypertension. Studies suggest that H2 not only holds therapeutic potential for managing diabetes and hypertension but also effectively aids in the prevention and treatment of CKD. For instance, in CKD rats, drinking HRW significantly reduced plasma Monocyte Chemoattractant Protein-1 (MCP-1) levels [247]. In a mouse model of kidney injury induced by a high-oxalate diet, HRW consumption markedly improved serum creatinine, blood urea nitrogen, and kidney injury markers such as kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), while reducing proinflammatory cytokines and oxidative stress markers. HRW has been shown to mitigate renal injury by inhibiting key signaling pathways, including PI3K/AKT, NF-κB, and TGF-β [48]. Meanwhile, H2 has also demonstrated potential in the prevention of CKD [248]. In glycerol-induced acute kidney injury models, inhalation of both 4 and 67% H2 gas demonstrated enhanced antioxidant, anti-inflammatory, and antiapoptotic effects. Although no clear dose-response relationship was observed, higher concentrations of H2 gas (67%) produced more pronounced improvements in kidney histology and morphology compared with lower concentrations (4%) [249]. Similarly, in I/R injury models, HRS treatment significantly reduced renal fibrosis and preserved kidney function [250]. Another study comparing short-term and long-term HRS injections revealed that intraperitoneal HRS increased the Bcl-2/Bax ratio and inhibited the expression of apoptotic proteins such as caspase-3, caspase-8, and caspase-9, but long-term HRS treatment was more effective in promoting renal recovery than short-term treatment [251], as it sustained the suppression of inflammation and apoptosis. These findings underscore the dose-dependent nature of H2 therapy in liver and kidney diseases. Moreover, prolonged treatment may further enhance therapeutic outcomes, providing theoretical support for the application of H2 gas in metabolic diseases.
5.5 Cancer
Cancer encompasses a group of complex diseases characterized by uncontrolled cell growth and proliferation, with diverse manifestations and underlying mechanisms. While traditional approaches such as surgery, chemotherapy, and radiotherapy remain the cornerstone of cancer treatment—particularly effective for early-stage cancers—their limitations have prompted researchers to explore innovative therapies. As a result, there has been a growing interest in developing novel adjunctive therapies with high safety profiles and minimal side effects. Advances in targeted therapy, immunotherapy, gene therapy, nanomedicine, and stem cell therapy have opened new avenues for cancer treatment. However, managing side effects continues to pose a significant challenge. Molecular H2, with its excellent safety profile and broad therapeutic potential, presents a promising complementary approach to cancer treatment. Preliminary progress has already been achieved in its application to various types of cancer.
As noted earlier, the therapeutic effects of H2 were initially identified through hyperbaric H2 therapy for treating skin cancer [2]. Since then, its therapeutic efficacy has been validated in various cancers, including CRC, lung cancer, gastric cancer, and breast cancer. For example, Zhang et al. [252] reported that H2 gas increased apoptosis in A549 cells while reducing the expression of XIAP and BIRC3 proteins in studies on A549 cells and their nude mouse models. Furthermore, inhalation of 60% H2 gas significantly reduced tumor volume in experimental mice [252]. Meng et al. [77] extended these findings by investigating the effects of H2 gas on A549 and H1975 lung cancer cells. They discovered that H2 gas significantly enhanced apoptosis by inhibiting CD47 expression and suppressed cell growth, invasion, and migration [77]. These findings further reinforce the potential role of H2 therapy in lung cancer treatment, particularly in modulating apoptosis-related pathways.
In gastric cancer research, Zhu et al. [10] found that H2 gas downregulated the expression of lncRNA MALAT1 and EZH2 while upregulating miR-124-3p in Human Gastric Cancer Cell Line MGC-803 (MGC-803), Human Gastric Cancer Cell Line BGC-823 (BGC-823) gastric cancer cell lines and gastric cancer mouse models, thereby significantly inhibiting tumor growth and reducing cancer cell proliferation and migration. The potential of combination therapies has also been validated. For instance, combining platinum nanocolloid (Pt-nc) with H2 gas effectively inhibited the growth of human promyelocytic leukemia HL60 cells and NUGC-4 cells derived from human gastric adenocarcinoma [253]. Chu et al. [79] demonstrated that H2 gas exerts selective effects on HeLa cervical cancer cells and HaCaT keratinocytes. In a HeLa xenograft mouse model, continuous H2 gas inhalation significantly reduced tumor growth. Cell-based experiments revealed that H2 gas selectively increased apoptosis in HeLa tumor cells while having minimal impact on nontumor cells such as HaCaT keratinocytes [79]. This selective action highlights the potential of H2 gas as a safer option for cancer therapy.
Additionally, as a metabolite produced by GM, H2 gas plays an important role in maintaining tumor resistance. Current studies have demonstrated that H2 therapy is effective in treating various cancers with no significant side effects. Moreover, recent research has explored the potential for combining H2 therapy with conventional treatments such as chemotherapy and radiotherapy, demonstrating improved efficacy and reduced side effects [254]. However, despite its promising potential as an adjunctive cancer therapy, H2 gas alone may not achieve sufficient therapeutic effects. At this stage, H2 therapy should not replace traditional treatments such as chemotherapy or radiotherapy. Future research should focus on the combined application of H2 gas with other emerging therapies to fully harness its potential.
6 Clinical Applications of Molecular H2
With the successful demonstration of molecular H2’s therapeutic and preventive effects on various diseases in animal models, the clinical application of H2 has also gained importance, with several studies in recent years showing that H2 has demonstrated good therapeutic efficacy and safety in clinical settings. In this article, we reviewed the molecular mechanisms through which H2 alleviates intestinal diseases in animal models and discussed its impact on other diseases mediated via the GM [225]. Here, we will briefly summarize the clinical applications of H2 in treating gastrointestinal diseases and provide an overview of clinical trials involving its use in respiratory, neurological, cardiovascular, chronic diseases, and cancer.
6.1 Clinical Applications of Molecular H2 in the Treatment of Gastrointestinal Diseases or Through Modulation of GM
It should be acknowledged that there are currently few clinical trials of H2 for the treatment of intestinal disorders, but it is worth noting that the measurement of exhaled H2 and CH4 gases following the ingestion of readily metabolizable carbohydrates in humans has emerged as an important noninvasive breath test to aid in the diagnosis of SIBO [255, 256]. This is the most widespread clinical application of H2 to assist in the treatment of intestinal disorders. Furthermore, noninvasive detection of head and neck squamous cell carcinoma [257], gestational diabetes mellitus [258], heart failure [259], and autism (ASD) [260], based on the ratio of exhaled H2 to CH4, has also shown potential, because SIBO is associated with the development of some diseases, and alterations in the composition of the GM of the patient result in changes in the amount of exhaled H2 gas. Additionally, there are several clinical uses of H2 in the treatment of other diseases that have been demonstrated to work by acting on the GM, such as Xie et al. [261] who found that H2 could alter the composition of the GM in people with opioid addiction and thus act on the gut–brain axis to improve the symptoms of depression and anxiety in people addicted to opioids. In a randomized double-blind controlled clinical study, Liang et al. [262] found that HRS could improve metabolism by modulating GM in patients with impaired fasting glucose (Table 4).
| Diseases | Route | Number of patients and protocol | Effects | Limitations | Trial phase | Date posted | Clinical trial reference |
|---|---|---|---|---|---|---|---|
| Molecular hydrogen to treat gastrointestinal diseases or act on gut microbiota | |||||||
| CRC | Hydrogen inhalation | 55, Duration 3 h/day/3 months | PD‑1+ CD8+↓, Mitochondria↑, PD‑1- CD8+↑ | Adherence issues | Phase II | January 2019 | [206] |
| IBS | Oral lactulose | 263, Drinking a combined lactulose nutrient drink every 15 min for 4h | Lactulose hydrogen breath test | Noncontrolled study | Phase II | September 2022 | [256] |
| HNSCC | Produced in the intestines | 135, Breath samples taken after 6 h of fasting | H2BT | / | Phase II | August 2020 | [257] |
| GDM | Produced in the intestines | 320, fasting for 12 h | H2BT | Single-center study | Phase II | June 2021 | [258] |
| HF | Oral lactulose | 102, Drinking a combined lactulose nutrient drink | Lactulose hydrogen breath test | Noncontrolled study | Phase II | October 2018 | [259] |
| ASD | Produced in the intestines | 1550, Exhaled gases were collected both after a 12-h fast and following glucose intake | H2BT | / | Phase III | August 2017 | [260] |
| Opioid addiction | Hydrogen inhalation | 108, Duration 2 h/day/3 months | Regulates gut microbiota | No stratification | Phase II | August 2024 | [261] |
| IFG | HRW, oral | 73, Drink 1000 mL of HRW per day for 8 weeks | Regulates gut microbiota | No stratification | Phase II | June 2023 | [262] |
| Molecular hydrogen therapy for respiratory diseases | |||||||
| Asthma and COPD | Inhalation of 2.4% H2 | 20, 2.4% hydrogen containing steam mixed gas was inhaled once for 45 min | Inhibits IL-4, IL-6, and IL-1β | Small sample size | Phase I | May 2020 | [263] |
| AECOPD | Inhalation of hydrogen–oxygen mixture | 108, hydrogen–oxygen mixture therapy (v/v = 2:1), flow rate 3.0 L/min, 6–8 h/d. | Improvement in symptoms such as dyspnea, cough, and sputum production in patients | No stratification | Phase II | May 2021 | NCT04000451 |
| COVID | Inhalation of hydrogen–oxygen mixture | 88, hydrogen–oxygen mixture therapy (v/v = 2:1), flow rate 3.0 L/min; 6–8 h/d | Increased SpO2 | No stratification | Phase I | June 2023 | NCT04378712 |
| Tracheal Stenosis | Inhalation of hydrogen–oxygen mixture | 35, Air, H₂–O₂, and O₂ inhalation was administered in four consecutive breathing steps: air for 15 min, H₂–O₂ (6 L/min, H₂:O₂ = 2:1) for 15 min, oxygen (3 L/min) for 15 min, and H₂–O₂ for 120 min | Reduce the inspiratory effort | No stratification | Phase I | September 2018 | NCT04378712 |
| Long-COVID | HRW, oral | 32, 500 mL HRW, twice a day for14 days | Effective strategies to reduce fatigue and improve cardiorespiratory endurance, musculoskeletal function, and sleep quality | Small sample size | Phase I | May 2024 | [266] |
| Molecular hydrogen therapy for neurological diseases | |||||||
| PD | Inhalation of hydrogen–oxygen mixture | 20, Participants inhaled 6.5 (0.1) vol% H2 in 2 L/min of mixed air for 16 weeks, twice a day for 1 h each session | No beneficial effects were found, but safety was assured | Small sample size | Phase I | July 2021 | UMIN000039217 |
| Cerebral infarction | Injections of HRS | 34, HRS intravenously for 7 days | Reduced patient MRI index | Small sample size | Phase I | June 2011 | [268] |
| Acute cerebral ischemia | Injections of HRS | 38, Intravenous HRS at 200 mL/h twice daily (every 12 h) | No beneficial effects were found, but safety was assured. | Open-label study | Phase I | June 2013 | [269] |
| SAH | Injections of HRS | 450, Intravenous hydrogen-rich fluid infusion (200 mL) twice daily for 14 days | Results not yet published | No stratification | Phase II | September 2014 | UMIN000014696 |
| Cerebral infarction | Inhalation of 3% H2 | 50, Inhalation of 3% H2 (1 h twice daily) for 7 days | Improve NIHSS score | Limited follow-up duration | Phase I | November 2017 | [271] |
| AD | Inhalation of 3% H2 | 19, Inhalation of 3% H2 (1 h twice daily) for 6 months | Improvement of neuronal integrity in the hippocampus | Small sample size | Phase I | March 2023 | [272] |
| Molecular hydrogen therapy for cardiovascular system diseases | |||||||
| PCAS | Inhalation of 2% H2 | 5, Inhalation of 2% H2 for 18 h | No adverse effects identified | Small sample size | Phase I | July 2016 | [273] |
| Patients undergoing CPB | Inhalation of 1.5–2% H2 | 24, Inhalation of 1.5–2% H2 throughout procedure | Increased ATP and reduced oxidative stress | Small sample size | Phase I | April 2023 | [274] |
| Potential metabolic syndrome | HRW, oral | 20, of HRW for 10 weeks (0.9–1.0 L/day) | Decreased LDL-C and apoB levels and increased SOD activity | Small sample size | Phase I | July 2013 | [275] |
| Cardiogenic OHCA | Inhalation of 2% H2 | 72, Inhalation of 2% H2 for 18 h | Combined TTM improves neurological prognosis after cardiogenic OHCA | No stratification | Phase II | August 2024 | jRCTs031180352 |
| Molecular hydrogen therapy for chronic diseases | |||||||
| T2DM | HRW, oral | 36, Drinking 900 mL/d of HRW for 8 weeks | Reduces LDL and cholesterol | Noncontrolled study | Phase I | March 2008 | [277] |
| Dry eye disease | Hydrogen production capsule | 10, 10 capsules of SUPER H2 | Increased tear secretion | Small sample size | Phase I | March 2021 | UMIN000037169 |
| NAFLD | Inhalation of 66% H2 | 43, inhalation hydrogen–oxygen (v/v = 2:1) mixture therapy (3 L/min) for 1 h/d | Lowering blood lipids and reducing inflammation | Noncontrolled study | Phase I | June 2022 | ChiCTR-IIR-16009114 |
| NAFLD | HRW, oral | 12, Per day of HRW for 28 days | Decreased liver fat content and a reduction in serum AST levels | Small sample size | Phase I | April 2019 | NCT03625362 |
| Visceral fat and skin spots | Hydrogen-rich water bath | 4, The subjects underwent a daily 10-min warm (41°C) water bath with dissolved hydrogen (300–310 µg/L, < 10 µg/L for normal water) for 1–6 months | Reduces LDL and cholesterol, and reduces skin blemishes | Small sample size | Phase I | April 2019 | [280] |
| Psoriasis and Parapsoriasis en plaques | Hydrogen-rich water bath | 47, Hydrogen-water bathing was administered through the skin by immersing the whole body in hydrogen water twice a week, with a 3-day interval | Reduction in the area of damaged skin and relief of itching | Noncontrolled study | Phase I | May 2018 | ChiCTR-ONC-17013055 |
| Molecular hydrogen therapy for chronic diseases | |||||||
| Psoriasis | Drop (1 ppm H2-saline); inhalation of 3% H2; drinking of HRW | 3, Three methods were used to administer H2 | Individual patients had reduced levels of IL-6 and IL-17 | Small sample size | Phase I | August 2015 | [96] |
| Inflammatory | HRW bath with nano-sized bubbles | 15, Daily bathing for up to 2–25 months | Reduces inflammation in patients and improves antioxidant capacity in healthy individuals | Small sample size | Phase I | July 2022 | [282] |
| Inflammatory | Oral solid hydrogen capsules | 30, Low dose group: Medium dose group: High dose group (1:3:6 capsule per day) for 28 days | Anti-inflammatory and antioxidant | Small sample size | Phase I | September 2024 | [283] |
| Overweight | HRW, oral | 5, One liter of HRW day | Attenuate glutamate- and GABA-dependent hypothalamic appetite stimulation, leading to hunger suppression and weight loss | Small sample size | Phase I | February 2023 | NCT06722326 |
| Molecular hydrogen therapy for cancer | |||||||
| Stage III and IV cancer patients | Inhalation of 66% H2 | 82, Inhalation hydrogen–oxygen (v/v = 2:1) mixture therapy (flow rate of 3000 mL/min) for inhalation time >3 h per day for at least >3 months | Treating different cancers has different effects, but can improve the quality of life of cancer patients | / | Phase I | July 2019 | [284] |
| Non-small cell lung cancer | Inhalation of 66% H2 | 20, Inhalation of a mixture of hydrogen (66.7%) and oxygen (33.3%) at a flow rate of 3 L/min for 4 h per day for 2 weeks | Decreased number of depleted and senescent cytotoxic T cells and increased killer Vδ1 cells | Small sample size | Phase I | October 2020 | NCT03818347 |
| Non-small cell lung cancer | Inhalation of 66% H2 | 58, Patients treated with hydrogen were divided into four groups: H2-only, H2 + chemotherapy, H2 + targeted therapy, and H2 + immunotherapy groups | Controlling cancer progression to reduce drug therapy side effects | Single-center study | Phase I | April 2020 | NCT03818347 |
| Bone marrow damage in cancer patients | Inhalation of 3% H2 | 23, Inhalation of 3% H2 (flow rate of 4 L/min for 30 min/day) | Improves bone marrow damage in patients by increasing RBC and PLT counts | Small sample size | Phase I | July 2021 | UMIN000035864 |
- a This includes an overview of the diseases under investigation, methods of administration, the number of patients, treatment protocols, outcomes, limitations, stages of experimentation, the date of the initial trial submission, and clinical trial references (Numbers correspond to references in this article, and other numbers represent US Clinical Trial Numbers, EU Clinical Trial Numbers, Japanese Clinical Trial Numbers, and Chinese Clinical Trial Numbers.).
- Abbreviations: AD, Alzheimer's disease; ASD, autism spectrum disorders; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; GDM, gestational diabetes mellitus; HF, heart failure; HNSCC, head and neck squamous cell carcinoma; IBS, irritable bowel syndrome; IFG, impaired fasting glucose; NAFLD, nonalcoholic fatty liver disease; OHCA, out-of-hospital cardiac arrest; PCAS, post-CABG angina syndrome; PD, Parkinson's disease; SAH, subarachnoid hemorrhage; T2DM, type 2 diabetes mellitus.
6.2 Clinical Applications of Molecular H2 in Respiratory Diseases
H2 is particularly effective in treating respiratory diseases, as it is primarily administered by inhalation, and clinical studies have demonstrated the potential of H2 for diseases such as asthma, COPD, and COVID-19. Wang et al. [263] found that a single 45-min inhalation of 2.4% H2-containing steam mixed gas reduced airway inflammation in both asthmatics and COPD patients in a prospective study of 10 asthmatics and COPD patients. Niu et al. [264] assessed glycolytic and mitochondrial oxidative phosphorylation activity in asthmatics and a mouse model of allergic airway inflammation, showing that H2 increased ATP production as well as the activity of mitochondrial respiratory chain complexes I and III. Zheng et al. [265] conducted a prospective clinical trial to evaluate whether HO mixtures are superior to oxygen in improving symptoms in patients with acute exacerbation of COPD (AECOPD). The results showed a significant reduction in cough assessment test scores in the H2/oxygen group, with no adverse events reported, indicating that H2/oxygen therapy was more effective and safe compared with standard oxygen therapy [265]. Similarly, Zeng et al. [101] treated 33 patients with H2/oxygen therapy and found that it improved dyspnea and disease progression in COVID-19 patients, resulting in higher SpO2 levels and shorter hospital stays. Tan et al. [266] conducted a randomized, single-blind, placebo-controlled trial with 32 participants and found that although H2-enriched water did not improve dyspnea in patients with long-COVID compared with acute COVID-19, it alleviated fatigue and improved cardiorespiratory endurance, musculoskeletal function, and sleep quality (Table 4).
6.3 Clinical Applications of Molecular H2 in Neurological Diseases
Animal experiments have demonstrated that H2 plays a beneficial role in treating neurological disorders, while human trials suggest its potential therapeutic value for neurodegenerative conditions. Yoritaka et al. [267] conducted a randomized, double-blind, placebo-controlled pilot study to investigate the ameliorative effects of inhaled H2 on PD, but their findings indicated that although inhalation of H2 was safe, it had no significant beneficial effect on PD patients. Although molecular H2 demonstrates limited therapeutic efficacy in PD, its antioxidant effects and mitochondrial function modulation in acute brain injury and neurodegenerative diseases remain scientifically noteworthy. Ono et al. [268] recruited 34 patients with cerebral infarction due to atherosclerotic disease, dividing them into two groups: one with 26 patients receiving edaravone alone, and the other with eight patients receiving a combination of edaravone and HRS. Surprisingly, the combination group showed a more pronounced improvement in MRI markers [268]. Nagata et al. [269] recruited 38 patients hospitalized for acute ischemic stroke in an open-label, prospective, nonrandomized study to assess the safety of intravenous H2-enriched glucose-electrolyte solution combined with t-PA. The results indicated that the therapy was relatively safe for patients with acute cerebral infarction, though the study lacked a separate H2-only group due to insufficient patient numbers, and one patient experienced diarrhea, which could not be definitively linked to the treatment [269]. In a 2014 publication by Takeuchi et al. [270], they announced that they had initiated a double-blind randomized controlled trial (RCT) in a large study population, enrolling 450 patients with high levels of SAH, in order to evaluate the efficacy of H2 therapy in the treatment of early brain injury, delayed cerebral ischemia and vasospasm, but unfortunately, the results of this clinical study have not been published so far. Following the discovery of the synergistic effects of H2 with other therapies, Ono et al. [271] later recruited 50 patients with acute cerebral infarction to study the effects of H2 alone. The patients were divided into two groups, one receiving H2 treatment and the other receiving gas without H2, and the results showed that H2 treatment stabilized vital signs and improved patients' ability to perform daily activities, with no adverse reactions observed. No adverse effects were found during the experiment, and safety was guaranteed [271]. In 2023, Ono et al. [272] published another article demonstrating that inhalation of 3% H2 alone for 1 h for 6 months provided symptomatic relief and improvement in patients with AD (Table 4).
6.4 Clinical Applications of Molecular H2 in Cardiovascular System Diseases
At present, the ameliorative effect of H2 on cardiovascular disease in humans still needs to be confirmed by large-scale study populations. However, some clinical studies have already demonstrated the high safety and feasibility of H2 in treating cardiovascular disease. The first human pilot study of H2 for post-CA syndrome was conducted in 2016, showing that inhalation of H2 led to a favorable neurological prognosis. However, the study was limited by a small sample size and the short duration of H2 inhalation due to the capacity limitations of the gas cylinders [273]. In another study, 24 patients undergoing cardiopulmonary bypass (CPB) surgery were randomized into two groups: 12 patients received inhaled H2 at a concentration of 1.5–2.0%, while the control group did not receive H2, and the results showed that H2-treated patients had improved erythrocyte function and reduced oxidative stress [274]. Song et al. [275] found beneficial lipid-lowering effects of H2 in animal studies, and thus they included 20 patients with potential metabolic syndrome to investigate the ameliorative effects of H2-enriched water in humans, showing that supplementation with H2-enriched water appeared to reduce serum low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (apoB) levels, and to improve the function of dyslipidemia-impaired high-density lipoprotein cholesterol, reducing oxidative stress. This means that H2 may play a beneficial role in the prevention of potential metabolic syndrome [275]. In a recent posthoc analysis of a RCT, Tamura et al. [276] found that the combination of H2 inhalation and Targeted Temperature Management (TTM) (TTM32–TTM34) improved neurological prognosis after cardiogenic out-of-hospital cardiac arrest (OHCA) compared with TTM only (TTM32–TTM34) at 32–34°C. However, the limitations of this study also need to be taken into account; this was a posthoc analysis with sample size, and the results of this study could be used as a reference to design a larger sample size to be explored in subsequent studies (Table 4).
6.5 Clinical Applications of Molecular H2 in Chronic Diseases
As early as 2008, 1 year after the anti-inflammatory and antioxidant effects of H2 were first identified, a randomized, double-blind, placebo-controlled crossover trial involving 30 patients with type 2 diabetes mellitus (T2DM) demonstrated the beneficial effects of HRW consumption in preventing T2DM and insulin resistance [277]. Another small exploratory study revealed the protective effect of H2 on the lacrimal gland. This study, which involved both mice and 10 human subjects, showed that H2-producing supplements significantly increased the concentration of exhaled H2 and improved tear stability and symptoms of dry eye [278]. Tao et al. [279] conducted a 13-week clinical trial of H2/oxygen inhalation in 43 subjects with NAFLD. Their findings showed that H2/oxygen inhalation improved blood lipid profiles and liver enzyme levels, with significant improvements in hepatic fat content in moderate-to-severe cases as detected by ultrasound and CT scanning [279]. In another study, Asada et al. [280] selected two men and two women to investigate the effects of HRW on oxidative stress-related skin issues and lipid metabolism markers, as well as to determine whether dissolved H2 remained effective after boiling. The results indicated that HRW baths improved visceral fat and skin spots and that dissolved H2 showed resistance to high temperatures [280]. Zhu et al. [281] found that HRW baths had a therapeutic effect on psoriasis, with patients receiving HRW baths experiencing a reduction in the size of their psoriasis and a reduction in itching. In another clinical study on psoriasis treatment, H2 was administered via HRS injection, HRW ingestion, and inhalation of 3% H2. The results suggested a reduction in IL-6 and IL-17 levels in individual patients following H2 treatment [96]. Nanobubble HRW baths provide better solubility of H2 in water than HRW baths alone and reduce H2 escape to a certain extent. Tanaka et al. [282] found that nanobubble HRW baths not only alleviated the inflammatory symptoms and skin appearance of patients with connective tissue diseases but also improved the serum antioxidant capacity of healthy patients. In 2022, a clinical study demonstrated the anti-inflammatory and antioxidant effects of orally administered solid H2 capsules in individuals with chronic inflammation, highlighting the potential long-term health benefits of H2 [283] (Table 4).
6.6 Clinical Applications of Molecular H2 in Cancer
Recent years have witnessed groundbreaking advancements in the application research of H₂ within cancer therapeutics. Chen et al. [284] conducted a prospective follow-up study involving 82 patients with stage III and IV cancers receiving H2 inhalation therapy. They found that H2 inhalation improved the quality of life and helped control cancer progression, with effects varying across different cancers. The best outcomes were observed in lung cancer patients, whereas pancreatic and gynecological cancers showed the least improvement [284]. Chen et al. [92] included 20 patients with non-small cell lung cancer to study the ameliorative effects of H2 and found that the superior therapeutic effect of H2 in lung cancer patients was mainly through the improvement of immune senescence in lymphocyte populations. Beyond its direct antitumor effects, molecular H2’s capacity to mitigate treatment-related adverse effects provides a strategic advantage for its integration into comprehensive cancer care protocols. In another clinical trial the following year, Chen et al. [254] recruited 58 adult patients to investigate the effects of H2 inhalation alone versus H2 in combination with chemotherapy, targeted therapy, and immunotherapy, respectively, for the treatment of advanced non-small-cell lung cancer. Sixteen months of follow-up found that progression-free survival in the control group was lower than that in the H2 inhalation group alone, and significantly lower than that in the other three combination therapy groups. In this study, not only the therapeutic effect of H2 alone but also the synergistic effect of H2 in combination with other therapies was highlighted [254]. In a retrospective observational study, Hirano et al. [285] found that H2 inhalation showed promising results in cancer treatment and also ameliorated bone marrow damage induced by intensity modulated radiation therapy (IMRT) in cancer patients, without compromising the antitumor effects of IMRT, thereby underscoring its potential in adjuvant therapy. These findings provide multilayered evidence for the integration of H2 in comprehensive cancer management. The clinical value of molecular H2 has become increasingly prominent across multiple systemic diseases mentioned above. Future research necessitates sustained breakthroughs in three dimensions: in-depth exploration of mechanisms (e.g., epigenetic regulation), optimization of intervention strategies (e.g., precision delivery systems), and interdisciplinary integration (e.g., H2 medicine with artificial intelligence (Table 4).
7 Conclusions and Prospects
Molecular H2 has gained widespread attention in the medical field, with studies showing its multiple properties, including anti-inflammatory, selective antioxidant, antiapoptotic, energy metabolism regulation, and immune modulation, making it widely used in the treatment of various diseases. However, the mechanisms and therapeutic efficacy of molecular H2 in different patients still need further investigation. Molecular H2 targets core pathways such as NF-κB, MAPK, and the NLRP3 inflammasome to synergistically regulate and suppress inflammation. However, research by An et al. [53] found that estrogen can enhance H2’s inhibitory effect on p-NF-κB, suggesting that gender differences may influence the anti-inflammatory effects of molecular H2. The antioxidant effect of molecular H2 is mainly achieved by selectively scavenging toxic free radicals and activating endogenous antioxidant systems. Elderly patients are more sensitive to H2 therapy due to mitochondrial dysfunction and a decreased ability to eliminate free radicals, which may result in the accumulation of chronic inflammation and oxidative stress. This also explains the significant intervention effects of molecular H2 in treating age-related diseases, such as atherosclerosis and Alzheimer's disease. Through the Nrf2 pathway, molecular H2 induces the expression of antioxidant enzymes (such as SOD, CAT, and GPx), enhancing cell tolerance to oxidative stress. Therefore, the polymorphisms of genes such as Nrf2 may affect the efficacy of molecular H2. In the future, genetic testing could be used to identify H2-sensitive genetic markers to optimize treatment plans for specific populations. Although the mechanisms of molecular H2 have been somewhat confirmed, its protein and gene-level targets remain unclear. Yu et al.’s research team [286], through proteomics and genomics analysis, identified 199 proteins that are considered highly relevant to the mechanism by which H2 improves gut barrier function in sepsis patients. Additionally, Ohsawa et al. [287] found through metabolomics analysis that transient H2 exposure can inhibit energy metabolism-related pathways (such as the reduction of glutathione levels) and trigger protective stress mechanisms. Chen et al. [288], based on UPLC–QTOF/MS metabolomics, revealed the regulatory effects of H2 on the metabolic pathways in mice with ischemic stroke. These studies suggest that, in the future, multiomics joint analysis can be used to explore the interaction between molecular H2 and biological systems, uncover new targets, and enable precision therapy. While potential therapeutic mechanisms still need further exploration, existing research evidence indicates that H2 therapy has become an efficient tool for treating various diseases. To date, there has been only one reported case of diarrhea, which may be an isolated incident. However, whether H2 therapy is truly perfect and whether there are undetectable potential adverse reactions still needs further verification.
H2 exerts its influence on the treatment of intestinal diseases through different mechanisms. For instance, it regulates the production of SCFAs mediated by GM to ameliorate intestinal inflammation, suppresses inflammatory and apoptosis signaling pathways to inhibit colitis progression, and modulates immunity for effective management of colon cancer. However, despite this significant progress, there remains a relative scarcity of research on the molecular mechanisms underlying H2 as a signaling molecule for treating intestinal diseases, most of the current research on H2 for the treatment of intestinal diseases focuses on basic research on the use of H2 therapy alone, with only a few cases exploring the effects of H2 therapy in combination with existing drugs for the treatment of intestinal diseases. According to the current combined therapeutic effect of H2 and clinical drugs, the safety of H2 in the treatment of intestinal diseases can be ensured and it shows potentiation, but the mechanism of H2 in the treatment of intestinal diseases is still to be studied in depth. It is also worth noting the close relationship between the intestinal flora and H2. As we have said before, many diseases are associated with changes in the GM. In the previous section, we summarized the fact that many diseases are associated with changes in the GM and that treatment of the disease is often followed by a restoration of the diversity of the GM. In recent years, the “gut–brain axis,” “gut–liver axis,” and “gut–kidney axis” have attracted significant attention, particularly the “gut–brain axis.” Changes in the diversity of the GM are accompanied by neurodevelopmental changes throughout the human lifespan, with many bi-directional communication pathways between the GM and the brain, including immune-regulatory responses, neuronal innervation, and microbial metabolite signaling. The intestinal flora is symbiotic with H2-producing and H2-consuming bacteria, and it is known that exogenous H2 produces changes in the composition and diversity of GM, but the duration of such changes is unknown, and whether the balance of H2-producing and H2-consuming bacteria undergoes new changes when exogenous H2 is discontinued needs to be studied in greater depth. In conclusion, the close relationship between GM and H2 should not be ignored, and the modulation of GM composition and function by H2 and the modulation of GM metabolites by H2 require in-depth study, which will help to discover new therapeutic targets and mechanisms of action of H2.
H2 has shown good therapeutic effects through different delivery modes. However, the clinical utilization of H2 presents several challenges. Although there are many means of H2 delivery in animal experiments, the delivery modes in clinical applications are relatively homogeneous, and more clinical trials are needed for validation, especially for the clinical promotion of nanomaterial-assisted delivery of H2. There are still some drawbacks to be addressed with the several modes of H2 delivery commonly used in clinical practice. First, managing the equipment for preparing, storing, transferring, and distributing inhaled H2 requires meticulous care to prevent potential explosions. Although the current clinical H2 concentration is between 2 and 4%, which is generally safe from explosion, safety considerations and cost make the clinical use of higher concentrations difficult. Some studies indicate that the efficacy of H2 may be dose dependent, with higher concentrations possibly providing better treatment outcomes for certain conditions. Second, oral administration of HRW lacks targeted delivery to specific lesions. Last, issues related to the timing, method, and dosage of HRW injections need resolution.
In addition to the challenges of drug delivery methods, the clinical promotion of molecular H2 also faces several key issues: (1) Limited clinical trials: Most clinical trials on H2 are small, single-center studies, lacking large-scale, multicenter, RCTs to verify long-term efficacy. Additionally, there are significant variations in the doses and concentrations of molecular H2 used (e.g., inhaled H2 concentrations range from 2 to 66%), and treatment protocols have not been adjusted based on disease stages (such as early/late-stage cancer) or comorbidities (such as diabetic nephropathy). Future research needs to explore the timing, dose-response relationship, and how H2 can be combined with other therapies. This requires larger sample sizes and longer follow-up periods for validation. (2) Safety concerns with combination therapies: The safety of combining H2 with other medical gases, as well as how different populations accept and adjust H2 concentrations, requires further study. Currently, H2 is mainly combined with oxygen, and only a few studies have investigated using H2 with other gases (e.g., nitrogen–oxygen–H2 mixtures). The impact of H2 on specific diseases, particularly those related to genetic background or sex hormones, also needs further validation. (3) Adjunctive therapeutic effects: More research is needed on the adjunctive therapeutic effects of H2. Some studies have shown that the high biocompatibility of molecular H2 enhances the effects of existing therapies without interference, particularly in reducing the side effects of chemotherapy and radiotherapy. However, more animal and clinical trials are necessary to verify these findings. (4) Precision H2 therapy for different populations: Despite H2’s broad intervention effects, its efficacy is influenced by factors such as age, gender, and genetic background. However, clinical studies often lack patient stratification, and personalized treatment remains a significant challenge. (5) Standardized regulatory framework: A standardized regulatory framework for H2 clinical trials and long-term safety assessments is needed. The appropriate concentrations of H2 for patient inhalation, drinking HRW, or injecting HRS should be clearly defined. The therapeutic effects of high-concentration H2 should be explored within a reasonable range. Additionally, distinctions between chronic and acute diseases should be made, and differentiated regulatory indicators should be established.
In recent years, nanomedicine has developed rapidly, and the efficacy of nanomaterials in addressing H2 gas diffusion and targeted therapy has been well established. Numerous studies have demonstrated that H2 gas encapsulated in nanomaterials exhibits significant therapeutic effects in treating various diseases. However, its application in the treatment of gastrointestinal diseases remains limited. While nanomaterials can help reduce H2 gas leakage and assist in targeted H2 therapy, their long-term safety, potential for abnormal immune responses, and biodegradability still require further validation in preclinical and clinical trials. Future efforts should focus on the development of nontoxic, biodegradable nanomaterials to aid H2 delivery. H2 medicine is an emerging scientific field that has made remarkable progress in recent years. Further elucidation of its underlying mechanisms will provide valuable insights for clinical applications. In the future, H2 medicine and nanohydrogen medical systems are expected to significantly improve, offering new opportunities for the diagnosis, treatment, and prevention of human diseases.
Author Contributions
Xiaohong Pan, Qianqian Lu, and Yuming Zhou contributed to reviewing, editing, and supervising the manuscript. Jiayi Jin was involved in writing the first draft as well as creating all the illustrations and tables. Lijun Yue, Maoru Du, Feng Geng, and Xue Gao were responsible for reviewing the literature. All authors have read and approved the final manuscript.
Acknowledgments
This work was supported by the Yantai Region and College Integration Development Project (2021XDRHXMPT28) and the Shandong Provincial Natural Science Foundation (ZR2022MC121). All illustrations in this article were created by BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Statement
The authors have nothing to report.
Open Research
Data Availability Statement
The authors have nothing to report.




