Reassessment of the genetic basis of natural rifampin resistance in the genus Rickettsia
Julien Amoros and Noor Fattar contributed equally.
Graphical Abstract
Abstract
Rickettsia, a genus of obligate intracellular bacteria, includes species that cause significant human diseases. This study challenges previous claims that the Leucine-973 residue in the RNA polymerase beta subunit is the primary determinant of rifampin resistance in Rickettsia. We investigated a previously untested Rickettsia species, R. lusitaniae, from the Transitional group and found it susceptible to rifampin, despite possessing the Leu-973 residue. Interestingly, we observed the conservation of this residue in several rifampin-susceptible species across most Rickettsia phylogenetic groups. Comparative genomics revealed potential alternative resistance mechanisms, including additional amino acid variants that could hinder rifampin binding and genes that could facilitate rifampin detoxification through efflux pumps. Importantly, the evolutionary history of Rickettsia genomes indicates that the emergence of natural rifampin resistance is phylogenetically constrained within the genus, originating from ancient genetic features shared among a unique set of closely related Rickettsia species. Phylogenetic patterns appear to be the most reliable predictors of natural rifampin resistance, which is confined to a distinct monophyletic subclade known as Massiliae. The distinctive features of the RNA polymerase beta subunit in certain untested Rickettsia species suggest that R. raoultii, R. amblyommatis, R. gravesii, and R. kotlanii may also be naturally rifampin-resistant species.
1 INTRODUCTION
Members of the Rickettsia genus are obligate intracellular bacteria that infect eukaryotes including humans and other vertebrates, arthropods but also protozoa, algae, and plants (Gillespie & Salje, 2023; Perlman et al., 2006; Weinert et al., 2009; Weinert, 2015). There are over 30 currently recognized Rickettsia species, classified into at least 10 distinct phylogenetic groups (Binetruy et al., 2020; Davison et al., 2022; Gillespie & Salje, 2023; Perlman et al., 2006; Weinert et al., 2009, 2015; Weinert, 2015). The most extensively studied species within this genus are those pathogenic causing human diseases, such as R. prowazekii, the causative agent of epidemic typhus, and R. rickettsii, responsible for Rocky Mountain spotted fever (Gillespie & Salje, 2023; Perlman et al., 2006; Weinert, 2015).
Rifampin (also termed rifampicin) is one commonly prescribed antibiotic for treating bacterial infections (Goldstein, 2014; Tupin et al., 2010), although it is not considered as a first-line treatment for rickettsial infections in humans (Blanton, 2019). Rifampin is a broad-spectrum antibiotic inhibiting bacterial RNA polymerase, thereby disrupting RNA synthesis and impeding bacterial protein production (Goldstein, 2014; Koch et al., 2014; Tupin et al., 2010). Rifampin is effective against certain Rickettsia species from diverse groups (Table 1). While limited data are available for the Transitional, Helvetica, Canadensis, and Bellii groups, rifampin resistance was more extensively investigated for species of the Spotted Fever and Typhus groups (Drancourt & Raoult, 1999; Rolain & Raoult, 2005; Rolain et al., 1998). Indeed, members of the Typhus group, such as R. prowazekii and R. typhi, are naturally sensitive to rifampin, as well as most species of the Spotted Fever group (Table 1). However, rifampin susceptibility is not a universal feature within the Spotted Fever group, as experimental studies have identified at least four naturally rifampin-resistant species in this group: R. massiliae, R. rhipicephali, R. montanensis, and R. aeschlimanii (Eremeeva et al., 2006; Rolain et al., 1998, 2002).
| Rickettsia group | Species | Strain | GenBank accession number | Susceptibility to rifampin | Reference |
|---|---|---|---|---|---|
| Spotted Fever Group | Rickettsia aeschlimannii | MC16 | GCA_001051325a | R | Rolain et al. (1998) |
| Rickettsia africae | ESF-5 | GCA_000023005a | S | Rolain et al. (1998); Strand et al. (2017) | |
| Rickettsia conorii | A-167 | GCA_000261325a | S | Rolain et al. (1998) | |
| Malish 7 | GCA_000007025a | S | Kim et al. (2019); Rolain et al. (1998) | ||
| Malish 7 - RIF. resistant | SRR8404402b | R* | Kim et al. (2019) | ||
| ISTT CDC1 | GCA_000263815a | S | Rolain et al. (1998) | ||
| Moroccan | AF076435c | S | Rolain et al. (1998) | ||
| Rickettsia honei | RB | GCA_000263055a | S | Rolain et al. (1998) | |
| Rickettsia japonica | YH | GCA_000283595a | S | Rolain et al. (1998) | |
| Rickettsia massiliae | MTU1 | AF076433c | R | Rolain et al. (1998) | |
| AZT80 | GCA_000283855a | R | Eremeeva et al. (2006) | ||
| Bar-29 | AF076436c | R | Rolain et al. (1998) | ||
| Rickettsia montanensis | VR-611 | n.a._ | R | Rolain et al. (1998) | |
| Rickettsia parkeri | Maculatum 20 | n.a.__ | S | Rolain et al. (1998) | |
| Rickettsia rhipicephali | 3-7-female6-CWPP | GCA_000284075a | R | Rolain et al. (1998) | |
| Rickettsia rickettsii | R | GCA_000284075a | S | Rolain et al. (1998) | |
| Rickettsia sibirica subsp. sibirica | 246 | GCA_000166935a | S | Rolain et al. (1998) | |
| Rickettsia sibirica subsp. mongolitimonae | HA-91 | GCA_000247625a | S | Rolain et al. (1998) | |
| Rickettsia slovaca | 13-B | GCA_000237845a | S | Rolain et al. (1998) | |
| Typhus | Rickettsia prowazekii | BreinI | GCA_000367405a | S | Miyamura et al. (1989); Rolain et al. (1998) |
| Madrid E | GCA_000195735a | S | Rachek et al. (1998) | ||
| Erifr1 | n.a.__ | R* | Rachek et al. (1998) | ||
| Rickettsia typhi | Wilmington | GCA_000008045a | S | Rolain et al. (1998, 2002) | |
| Ethiopian - RIF. resistant | n.a.__ | R* | Troyer et al. (1998) | ||
| Transitional | Rickettsia akari | MK | n.a.__ | S | Rolain et al. (1998) |
| Rickettsia australis | Phillips | GCA_000273745a | S | Rolain et al. (1998) | |
| Rickettsia felis | URRWXCal2 | GCA_000012145a | S | Rolain et al. (2002) | |
| Helvetica | Rickettsia helvetica | C6P9 | n.a.__ | S | Rolain et al. (1998) |
| Canadensis | Rickettsia canadensis | 2678 | n.a.__ | S | Rolain et al. (1998) |
| Bellii | Rickettsia bellii | 369L42-1 | n.a.__ | S | Rolain et al. (1998) |
- Note. R* indicates laboratory-selected resistance through artificial mutagenesis or selection. GenBank accession numbers of rpoB gene sequences are indicated if available:
- a assembled genome sequences.
- b unassembled reads.
- c complete rpoB sequences; n.a., non-available.
In bacteria, the most common mechanism underlying rifampin resistance involves missense mutations within the rpoB gene, which encodes the RNA polymerase β subunit (Goldstein, 2014; Koch et al., 2014; Tupin et al., 2010). In most bacterial genera, rifampin-resistant clinical isolates typically harbor mutations that map to the center of the rpoB gene sequence in three clusters (I, II and III), at positions 500–700 corresponding to the enzyme's active center (Goldstein, 2014; Koch et al., 2014; Tupin et al., 2010). The majority of these mutations are located within a small region in cluster I dubbed the Rifampin Resistance Determining Region (RRDR). These mutations adversely impact the rifampin binding site, resulting in decreased affinity for the antibiotic (Goldstein, 2014; Koch et al., 2014; Tupin et al., 2010). Additionally, in the opportunistic pathogen Nocardia farcinica, the rifampin resistance mechanism also involves a rpoB paralog gene, which encodes a rifampin-refractory β subunit (Ishikawa et al., 2006). In a few pathogenic bacteria, other alternative resistance mechanisms include rifampin inactivation by specific enzymes (Hoshino et al., 2009; Liu et al., 2018; Spanogiannopoulos et al., 2014; Stogios et al., 2016; Tribuddharat & Fennewald, 1999) or excretion by efflux systems, whereby bacteria pump out the antibiotics to the external environment using transporter proteins (Chandrasekaran & Lalithakumari, 1998; Hui et al., 1977; Louw et al., 2009).
In Rickettsia, rifampin resistance mechanisms have exclusively been associated with residue changes in the RNA polymerase β subunit, resulting from missense mutations in the rpoB gene (Drancourt & Raoult, 1999; Kim et al., 2019; Rachek et al., 1998; Troyer et al., 1998). Indeed, resistance associated with rpoB mutations has been artificially selected in the laboratory in three species that are primarily susceptible to rifampin, R. conorii, R. typhi and R. prowazekii (Kim et al., 2019; Rachek et al., 1998; Troyer et al., 1998) (Table 1). For naturally rifampin-resistant species, a previous genetic investigation concluded that a single point rpoB mutation resulting in a phenylalanine-to-leucine change at position 973 (Phe-973→Leu-973) is the mechanism driving natural rifampin resistance (Drancourt & Raoult, 1999). However, this assertion was based on observations of Rickettsia species within the Spotted Fever group exclusively, and no further investigation has been conducted into other groups.
In this study, we investigate natural rifampin resistance patterns within the Rickettsia genus. We first assessed rifampin resistance in a previously untested Rickettsia species of the Transitional group, R. lusitaniae, for which no culture is currently available. To this aim, laboratory-reared Ornithodoros moubata ticks naturally infected by the R. lusitaniae R-Om strain (Duron et al., 2017, 2018) were subjected to rifampin treatment, and then Rickettsia density was monitored using specific qPCR assays. Subsequently, we compared the complete rpoB gene sequences of R. lusitaniae R-Om with sequences of other Rickettsia species previously characterized as susceptible or resistant to rifampin and extended this analysis to include most other Rickettsia groups. We further explored available Rickettsia genomes for potential alternative resistance mechanisms, and retrace the evolutionary emergence of natural rifampin resistance in the genus. As a whole, our observations refute the prevailing notion that the residue Leu-973 is the key driver of natural rifampin resistance in Rickettsia species.
2 EXPERIMENTAL PROCEDURES
2.1 Ticks, housing conditions and antibiotic treatment
Ticks were from a laboratory colony of O. moubata sensu stricto (Neuchâtel strain), which was established from field specimens collected in Southern Africa (Duron et al., 2018). Around two-thirds of specimens of this laboratory colony are naturally infected with the R. lusitaniae R-Om strain, which exhibits 100% nucleotide identity with the gltA gene sequence of the R. lusitaniae type strain (Duron et al., 2018) primarily identified in the tick Ornithodoros erraticus (Milhano et al., 2014).
Ticks were maintained in the laboratory at 26°C with 80–90% relative humidity under complete darkness (Buysse et al., 2021; Duron et al., 2018). A blood meal made of heparinized cow blood was offered to ticks every 7 weeks using an artificial feeding system. Ticks were allowed to feed on blood through a parafilm membrane using a specific apparatus including: (i) A tick chamber closed on top by a nylon cloth to avoid tick escape and closed below by the parafilm membrane, (ii) a blood chamber containing a magnet, and (iii) a hot magnetic steering device to mix and warm blood at 38°C. After feeding, each batch of ticks was kept in separate plastic containers until the next feeding.
To test for the antibiotic resistance pattern of R. lusitaniae R-Om, a rifampin solution was added to the blood meal at a final concentration of 10 mg/ml (Duron et al., 2018). Twenty randomly sampled ticks were fed with rifampin-treated blood, while 20 other ticks were fed with nontreated blood as a control. The ticks obtained their initial blood meal at nymphal stage 1, followed by another blood meal at nymphal stage 2 (7 weeks later). They were then kept until molting at nymphal stage 3, at which point they were analyzed to check the quantity of Rickettsia. No additional rifampin-treated blood can be provided afterwards because, following two rifampin-treated blood meals, most of the treated ticks ceased feeding. This was due to the antibiotic's elimination of their obligate nutritional endosymbiont, a Francisella-like endosymbiont, required for their normal growth through B vitamin provisioning (Duron et al., 2018).
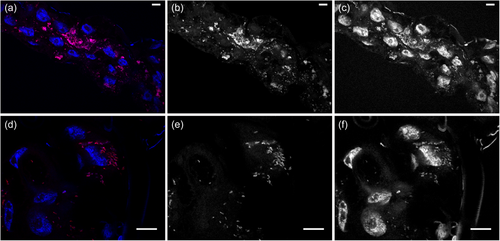
2.2 Fluorescence in situ hybridization and imaging
Visualization of R. lusitaniae was conducted through fluorescence in-situ hybridization (FISH) assays following a protocol modified from Manz et al. (Manz et al., 1992). We focused on Malpighian tubes of ticks since these organs typically host a high density of intracellular bacteria (Buysse et al., 2019; Duron & Gottlieb, 2020). Malpighian tubules of adult O. moubata ticks were dissected and further fixed in 4% paraformaldehyde-PBS-0.1% Triton X-100, for 1 h at room temperature. Thereafter, Malpighian tubules were washed twice in PBS for 15 min and stored at 4°C in 1:1 (v/v) ethanol-PBS solution. The organs were dehydrated in 50%, 80% and 100% ethanol for 3 min each, then immersed in 50 µl of the following hybridization buffer: 900 mM NaCl, 35% formamide, 20 mM Tris-HCl (pH 8), 0.01% SDS, 5 µL of the probe Rick_R2 (30 ng/µl, [CY3]-TTTCTGCAAGTAACGTCATTATC, Eurofins). The probe is specific for Rickettsia spp. and was designed for this study. The organs were then hybridized for 1 h 30 min to 3 h maximum at 46°C, then washed for 20 min at 48°C in 200 µl of a washing buffer containing: 20 mM Tris (pH 8), 70 mM NaCl, 5 mM EDTA (pH 8) and 0.01% SDS 10%. Subsequently, the organs were gently rinsed in bi-distilled water in a Petri dish placed on a glass slide, dried, and embedded in CitiDAPI (DAPI 10 mg.ml−1; Citifluor AF1 antifading, Citifluor, England). Negative controls were obtained by incubating organs without Rickettsia-specific probes and by checking for tissue autofluorescence.
Confocal images were acquired on an Olympus confocal laser-scanning microscope (Olympus IX81) and FV3000 2.0 software (Olympus), installed on an inverted microscope IX-83 (Olympus, Tokyo, Japan). Multiple fluorescence images were acquired sequentially with a 60x objective (UplanXAPO, water immersion, 1.42 NA; Olympus) or a 100x objective (UPLAPO, oil immersion, 1.5 NA; Olympus). Fluorescence was excited with the Helium-Neon laser line (543 nm, for Cy3) and a blue diode (405 nm, for DAPI) and the emitted fluorescence was detected through spectral detection channels between 430–470 and 570–670 nm, respectively.
2.3 Quantitative PCR assay
We developed a real-time quantitative PCR assay (qPCR) to quantify the density of R. lusitaniae R-Om strain in ticks. DNA was extracted from the tick's whole body using the DNeasy blood and tissue kit following the manufacturer's instructions (QIAGEN). qPCR was performed with a Light Cycler 480 (Roche) using the SYBR Green Master Mix. Two qPCRs were performed for each tick: one was specific for the Rickettsia R-Om gltA gene, and the other was specific for the O. moubata OmAct2 gene (Table A1 in Appendix 1). Since both genes are present in a single copy per haploid genome of the tick and the bacterium, the ratio between gltA and OmAct2 concentrations provides the number of Rickettsia R-Om genomes relative to the number of O. moubata genomes, thus correcting for the quality of DNA template. Each DNA template was analyzed in triplicate for gltA and OmAct2 quantifications. Standard curves were plotted using dilutions of a pEX-A2 vector (Eurofins) containing one copy of each of the gltA and OmAct2 gene fragments.
2.4 Statistical analyses
All statistical analyses were carried out using R (https://www.r-project.org). We tested for the effect of rifampin treatment on R. lusitaniae through quantitative analyses. To determine if rifampin modifies the R. lusitaniae density within each infected tick, qPCR results of the control and treated groups were analyzed using a Wilcoxon-Mann-Whitney test.
2.5 Analysis of rpoB gene sequences
Neither genomic nor complete rpoB gene sequences of R. lusitaniae were available in public databases. Thus, we reexamined the raw reads from a prior metagenomics investigation of O. moubata Neuchâtel laboratory colony, specifically targeting the Francisella-like endosymbiont also present in this tick species (Duron et al., 2018). The ticks used for the rifampin test were sourced from the same laboratory cohort, collected during the same period, and reared in identical conditions to those used for the metagenomics sequencing. Raw reads were mapped against the complete rpoB gene sequence of R. felis (GenBank CP000053, locus tag RF_1146) with BWA-MEM (Li, 2013) and then retrieved to reconstruct the gene sequence using Megahit (v1.2.9) (Li et al., 2015).
We further compared the complete rpoB gene sequence of R. lusitaniae R-Om strain obtained in this study with the complete rpoB gene sequences of Rickettsia spp. either susceptible (n = 16) or naturally resistant (n = 5) to rifampin available in GenBank (Table 1). Alignments of nucleotides and amino acids were performed using ClustalO (Sievers et al., 2011). The Unipro UGENE software (Okonechnikov et al., 2012) was used to visualize mutations throughout the rpoB gene sequences, and the BioEdit software (Hall, 1999) to identify residues with analogous functions in proteins. The resistance clusters I, II, and III were identified along the Rickettsia rpoB gene sequences through alignment with the rpoB sequence of E. coli strain NCM3722 (GenBank CP011495; clusters I, II, and III at positions 507–533, 563–572, and 678, respectively). The final alignments of the rpoB gene sequences were then used to identify mutations specifically associated with natural rifampin resistance in the genus Rickettsia. Consensus sequence logos were created from aligned rpoB gene sequences of rifampin-resistant and susceptible Rickettsia species and strains (Gagniuc, 2021). We also examined all Rickettsia genomes for the presence of rpoB paralogs using tBLASTn (Altschul et al., 1990).
We further expanded the analysis of rpoB gene sequences to a global set of representative Rickettsia species and strains with genomic sequences available on public databases, including strains whose rifampin resistance phenotype is unknown (Table A2 in Appendix 1). The whole genomes of the Rickettsia species and strains were then used for phylogenomics. The Rickettsia genomes were processed with a standardized genome annotation approach using Prokka (Seemann, 2014), and single-copy orthologs (SCOs) were next identified using OrthoFinder (v2.3.11) (Emms & Kelly, 2019). SCOs were individually aligned with MAFFT (v7.450) (Katoh & Standley, 2013) and ambiguous positions were removed using trimAl (v1.2rev59) (Capella-Gutiérrez et al., 2009) before individual alignments concatenation using Amas 1.0 (Borowiec, 2016). The best substitution models were determined using modeltest (v0.1.5) (Darriba et al., 2020) and maximum likelihood (ML) trees were computed using RAxML (v8.2.9) (Stamatakis, 2014) with 1,000 bootstrap replicates.
| Gene (locus taga) | Putative gene product | Length (amino acids) |
|---|---|---|
| Genes present in all Rickettsia genomes but harboring residues specific to rifampin-resistant species | ||
| rpoB (MCC_01550a) | β subunit of RNA polymerase | 1373 |
| rpoA (MCC_06070a) | α subunit of RNA polymerase | 340 |
| rpoC (MCC_01555a) | β‘ subunit of RNA polymerase | 1372 |
| rpoD (MCC_00410a) | σ subunit of RNA polymerase | 634 |
| rpoZ (MCC_05500a) | ω subunit of RNA polymerase | 127 |
| YajC (MCC_05555a) | Subunit of the Sec membrane complex | 141 |
| TolC (MCC_02285a) | Outer membrane porin | 453 |
| MsbA1 (efrA) (MCC_03565a) | Subunit of a multidrug efflux ABC transporter | 459 |
| Genes only present in rifampin-resistant Rickettsia species (without orthologs in susceptible species) | ||
| Rrhi37F6_00877b | Hypothetical protein | 192 |
| Rrhi37F6_00879b | Hypothetical protein | 62 |
| Rrhi37F6_01547b (CopG) | Helix-turn-helix protein | 76 |
| Rrhi37F6_01549b | Predicted transcriptional regulators containing the CopG/Arc/MetJ DNA-binding domain and a metal-binding domain | 59 |
| Rrhi37F6_00531b (ompA) | Outer membrane protein (truncated) | 97 |
| Rrhi37F6_01548b (ParA) | Plasmid stability protein | 213 |
| Rrhi37F6_00259b | Hypothetical protein | 68 |
| Rrhi37F6_00257b | IS982 family transposase (pseudogenized) | 65 |
| Rrhi37F6_01535b | Transposase (pseudogenized) | 37 |
| Rrhi37F6_01536b | Guanosine polyphosphate pyrophosphohydrolase/synthetase | 937 |
| Rrhi37F6_01542b | IS3 family transposase (pseudogenized) | 55 |
- a Locus tags in the genome of Rickettsia rhipicephali strain 3-7-female6-CWPP (GenBank CP003342.1).
- b Putative additional genes without orthologs in rifampin-susceptible Rickettsia species, identified in this study, in the genome of R. rhipicephali strain 3-7-female6-CWPP. Details are presented in Table A3 and Figure A1 in Appendix 1.
The 3D structures of the beta subunit of two rifampin-sensitive species (R. conorii A-167 and R. rickettsii R) and two rifampin-resistant (R. rhipicephali 3-7-female-6-CWPP and R. aeschlimannii MC16) were modeled using Colab AlphaFold2 (Jumper et al., 2021; Mirdita et al., 2022). PrankWeb 3 (https://prankweb.cz/) was then used to predict potential ligand-binding sites for each subunit (Jakubec et al., 2022). The predicted sites were compared to the rifampin/beta subunit attachment site from the Mycobacterium tuberculosis crystal structure (accession 5UHB in the RCSB Protein Data Bank; https://www.rcsb.org/) to identify the hypothetical rifampin-binding sites on the beta subunits of R. conorii A-167, R. rickettsii R, R. rhipicephali 3-7-female-6-CWPP and R. aeschlimannii MC16.
2.6 Alternative rifampin resistance mechanisms in Rickettsia genomes
We further investigated the whole genomes of Rickettsia for potential alternative mechanisms associated with natural rifampin resistance that are not dependent on rpoB. We compared the whole genomic content of naturally rifampin-resistant (n = 3) and -susceptible (n = 15) Rickettsia species (Table A2 in Appendix 1) to identify orthogroups specific to the rifampin-resistant Rickettsia species. The list of orthogroups specific to naturally rifampin-resistant Rickettsia species were then obtained using Orthofinder (v2.3.11) (Emms & Kelly, 2019) and Roary (Page et al., 2015). To control for false positives, we used tBLASTn to verify that orthogroups putatively associated with natural rifampin resistance were indeed absent in rifampin-susceptible species.
We used tBLASTn to check whole genomes of naturally rifampin-resistant (n = 3) or susceptible Rickettsia species (n = 15) for the presence of genes known to encode enzymes inactivating rifampin and efflux systems pumping out rifampin described in rifampin-resistant bacteria (Brandis et al., 2012; Comas et al., 2011; Song et al., 2014). In addition, we used the Resistance Gene Identifier (RGI) tool of the Comprehensive Antibiotic Resistance Database (CARD) (Jia et al., 2017). The RGI tool was parametrized to search for genes associated with rifamycin resistance (the class of antibiotics including rifampin) and to generate a list of putative rifamycin resistance genes in Rickettsia genomes.
3 RESULTS
3.1 Rickettsia lusitaniae R-Om is susceptible to rifampin
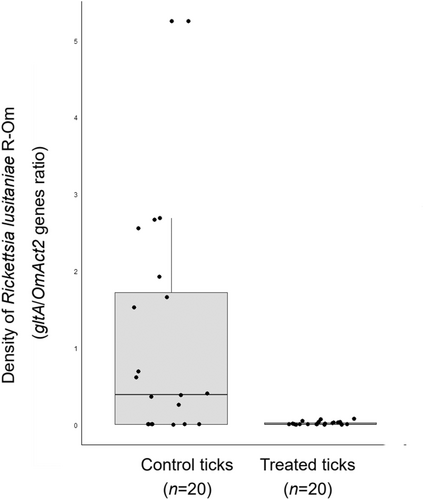
FISH confirmed the presence of R. lusitaniae R-Om in O. moubata and revealed a high concentration of Rickettsia in Malpighian tubules (Figure 1a–f). Real-time qPCR also showed that R. lusitaniae R-Om is present at high concentration in most ticks fed on untreated blood (control ticks) (Figure 2a,b). A bimodal distribution is observed for the controls, with seven out of 20 ticks having an estimated R. lusitaniae R-Om concentration close to 0. This infection pattern was expected since not all O. moubata specimens in this lab colony are infected by R. lusitaniae R-Om (Duron et al., 2018). However, the density of R. lusitaniae R-Om was 58x lower in the rifampin-treated group (average of gltA/OmAct2 ratios ± SE: 0.018 ± 0.020, n = 20) than in the control group (mean ± SE: 1.048 ± 1.386, n = 20) (Wilcoxon-Mann-Whitney test, two-sided, p = 0.002). This result indicates that R. lusitaniae R-Om is susceptible to rifampin.
3.2 Inaccuracy of the rpoB Phe-973→Leu-973 mutation for rifampin resistance
Alignments of rpoB gene sequences showed that the mutation Phe-973→Leu-973 primarily associated with natural rifampin resistance do not explain alone the pattern observed in Rickettsia species and strains. As expected, the residue Leu-973 is present in naturally rifampin-resistant Rickettsia species and strains and absent in susceptible species and strains of the Spotted Fever group (Figure 3a–c). However, the residue Leu-973 is also present in all rifampin-susceptible Rickettsia species belonging to the other Rickettsia groups (Figures 3b,c). Indeed, analysis of raw reads obtained from the O. moubata metagenome allowed us to reconstruct the complete rpoB gene sequence of the R. lusitaniae R-Om strain (length: 4123 nucleotides; 1373 amino acids). While the R. lusitaniae R-Om strain is susceptible to rifampin, the residue Leu-973 is present in its rpoB gene sequence. Similarly, the residue Leu-973 is consistently present in other rifampin-susceptible Rickettsia species of the Transitional group (R. australis, R. felis) and in rifampin-susceptible Rickettsia species of the Typhus group (R. typhi, and R. prowazekii) (Figures 3b,c). As a result, the residue Leu-973 is not specific to naturally rifampin-resistant Rickettsia species.
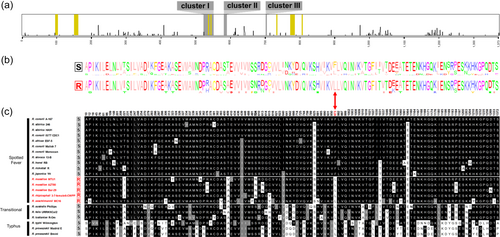
Further comparisons with the rpoB gene sequences of additional Rickettsia strains and species for which the rifampin resistance profile is unknown showed that the residue Leu-973 is conserved across all Rickettsia groups (Figure 4a–c). Only the rifampin-susceptible species belonging to the Spotted Fever group do not harbor the residue Leu-973, but instead harbor Phe-973. Examination of Rickettsia genomes confirmed that rpoB is a single-copy gene, with no paralog present in the genus. Phylogenomic analyses revealed that the residue Leu-973 is ancestral to the Rickettsia genus. The residue Phe-973 is rather a derived trait, which has only evolved in a subclade of the Spotted Fever group from the ancestral residue Leu-973 (Figure 4a–c). In this context, the nomenclature Leu-973→Phe-973 is thus more appropriate.
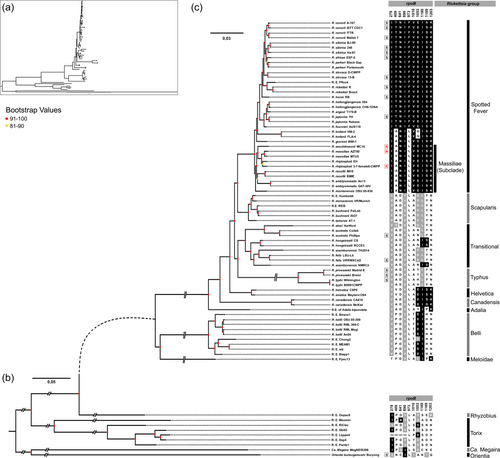
Phylogenomic analyses also indicated that all naturally rifampin-resistant species cluster in a monophyletic subclade, termed Massiliae, of the Spotted Fever group (Figure 4c). Remarkably, the Massiliae subclade also includes a number of Rickettsia strains and species, for which the rifampin resistance pattern is unknown: R. massiliae (strain MTU5), R. rhipicephali (EH), R. montanensis (OSU 85-930), R. raoultii (BIME, IM16), and R. amblyommatis (An13, GAT-30V).
3.3 Distinct sets of mutations in rpoB are associated with naturally rifampin-resistant Rickettsia
The rifampin-resistant clusters I, II, and III exhibited a low amino acid polymorphism in the genus Rickettsia, with only one amino acid variant identified in cluster I in RRDR (Ser-524→Asn-524, Figure 3a). The residue Asn-524 is associated with some naturally rifampin-resistant species, but not all: R. aeschlimani strain MC16 harbors the residue Ser-524, which is shared with all the susceptible species, suggesting that residue Asn-524 is not involved in natural rifampin resistance. Outside of the three cluster regions, there is no single specific residue in the rpoB sequences associated with naturally rifampin-resistant species (Figures 3b,c). Notably, we identified a set of 10 residues (Ile-279, Ala/Val-409, Asn-641, Ile-890, Leu-973, Val-1010, Glu-1053, Ile-1180, Ser-1189, and Lys-1203) which are shared by naturally rifampin-resistant species, but also with part of susceptible species (Figure 5a). However, pairing specific residues at positions 409 or 973 with specific residues at positions 279, 641, 890, 1010, 1053, 1180, 1189, or 1203 fits the rifampin resistance pattern (Figures 5a,b). Indeed, the pairing of Leu-973 and Ser-1189 is specific to rifampin-resistant Rickettsia and is never found in other Rickettsia species and strains. A total of 16 potential pairings exist, each matching the resistance pattern (Figures 5a,b). None of these residues is located within the hypothetical rifampin binding site (Figure 5d–g). Although there are a few subtle variations between Rickettsia species, no noticeable differences are observed between the hypothetical rifampin binding sites of resistant and sensitive Rickettsia species (Figure 5d–g). However, eight of these 10 residues (Ile-279, Ala/Val-409, Asn-641, Ile-890, Val-1010, Glu-1053, Ile-1180, and Lys-1203) have larger or similar side chains compared to the residues present in rifampin-susceptible species. Only two residues (Leu-973 and Ser-1189) have smaller or similar side chains to those observed in rifampin-susceptible species. Hence, the RNA polymerase β subunit of naturally rifampin-resistant species tends to contain a higher proportion of residues with larger side chains than those of susceptible species. The steric hindrance may potentially reduce its susceptibility to binding with rifampin.
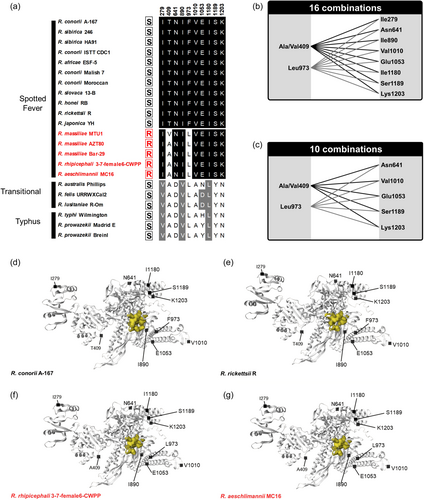
Furthermore, the number of residue pairs putatively associated with resistance patterns can be reduced by considering exclusively non-analogous residues (i.e., amino acids with different chemical or functional properties) differing between rifampin-resistant and susceptible species. This approach led to the exclusion of residues 279, 890, and 1180 (Figure 5c). Indeed, at each of these three positions, residues in resistant and susceptible species belong to the aliphatic hydrophobic group and share similar chemical properties. Hence, this could limit the number of residue pairing putatively associated with rifampin resistance to 10, all of which were absent in susceptible species (Figure 5c).
All the residue combinations putatively associated with natural rifampin resistance are also present in members of the Massiliae subclade. This also includes species and strains for which resistance pattern has never been tested: R. massiliae (strain MUT5), R. rhipicephali (EH), R. montanensis (OSU 85-930), R. raoultii (BIME, IM16), and R. amblyommatis (An13, GAT-30V) (Figure 4c). In addition, two species of the Spotted Fever Group, R. gravesii (BWI-1) and R. kotlanii (HM-2, FLA-4), harbor the same (or almost) residue combinations, although they do not belong to the Massiliae subclade (Figure 4c). No other species share these combinations of residues.
3.4 Putative other mechanisms of rifampin resistance
Examination of Rickettsia genomes reveals the presence of 18 additional genes potentially involved in natural rifampin resistance (Table 2, Table A3 and Figure A1 in Appendix 1). These candidate genes include eight genes potentially associated with rifampin metabolism and harboring specific mutation polymorphisms, and 11 other genes present in naturally resistant Rickettsia species, but absent in susceptible species. The Rickettsia genomes contain no homologs for genes encoding enzymes involved in rifampin inactivation present in N. farcinica (Hoshino et al., 2009; Liu et al., 2018), Pseudomonas aeruginosa (Tribuddharat & Fennewald, 1999) and Listeria monocytogenes (Spanogiannopoulos et al., 2014; Stogios et al., 2016).
Five candidate genes are involved with rpoB in the formation of the RNA polymerase complex: rpoA (α subunits), rpoC (β’ subunit), rpoD (σ subunit), and rpoZ (ω subunit) genes (Table 2). Three of them (rpoA, rpoC and rpoD) harbor at least one residue specific to rifampin-resistant strains (Figure A1 in Appendix 1). Although these RNA polymerase subunits are not the binding site of rifampin, they are physically organized all around the β subunit. Three other candidate genes are involved in antibiotic efflux pump systems, consistently present in all Rickettsia genomes, and also harbor residue specific to rifampin-resistant strains: YajC (encoding a subunit of the Sec membrane complex), TolC (a porin of outer membrane), and MsbA1 (a subunit of a multidrug efflux ABC transporter) (Table 2, Figure A1 in Appendix 1).
All the 11 other candidate genes have been identified through pangenomic analyses and found specifically present in naturally rifampin-resistant Rickettsia species and absent in susceptible species (Table 2, Table A3 in Appendix 1). These 11 candidate genes are present either in the main chromosome (n = 5) or in plasmids (n = 6) of naturally rifampin-resistant Rickettsia species. However, none of these 11 genes could be associated with antibiotic resistance. One gene is homolog to ParA, which encodes a plasmid stability protein driving the isolation and allocation of plasmids into daughter cells during cell division (Ebersbach & Gerdes, 2005). Another gene is homolog to CopG, which encodes a DNA-binding protein involved in the control of plasmid copy number (Gomis-Ruth, 1998). The nine other candidate genes encode for short truncated protein fragments: One for a 97 amino acid fragment of the surface antigen encoded by the ompA gene, three for pseudogenized transposases, and five for short hypothetical proteins (59–92 amino acids) of unknown functions (Table 2, Table A3 in Appendix 1).
4 DISCUSSION
Our analysis of current genetic data expands our understanding of the mechanisms and evolution of rifampin resistance within the Rickettsia genus. Indeed, we characterize R. lusitaniae as a species susceptible to rifampin although its rpoB gene sequence contains the residue Leu-973. We further observe that the residue Leu-973 is conserved in all Rickettsia groups, being present in all rifampin-susceptible species except those of the Spotted Fever group. Consequently, the rpoB residue Leu-973 solely cannot be further used to diagnose natural rifampin resistance for Rickettsia species and strains. Alternative resistance mechanisms thus exist, which could involve either mutations reducing access to the rifampin binding site through alterations in the structure of the RNA polymerase β subunit or related subunits, or genes detoxifying rifampin through efflux pumps. There is no genomic evidence suggesting that Rickettsia can inactivate rifampin by a specific enzymatic activity. Crucially, the observed resistance pattern across Rickettsia groups shows that natural rifampin resistance is restricted to a unique monophyletic subclade, and potentially to a few other related species, within the Spotted Fever group. This pattern reveals that the emergence of natural rifampin resistance was driven by a major phylogenetic constraint, resulting from ancient genomic features shared by a unique set of closely related Rickettsia species, rather than being a consequence of recent selection due to exposure to rifampin. Such a phylogenetic constraint implies that the mutations specific to rifampin-resistant Rickettsia species may be conserved due to shared evolutionary history rather than being directly related to rifampin resistance. Consequently, this phylogenetic constraint obscures the true genetic factors responsible for rifampin resistance in the genus Rickettsia.
The rifampin resistance mechanism of Rickettsia is distinct from mechanisms observed in most other resistant bacteria. While an accumulation of missense mutations in RRDR is typically observed in rifampin-resistant bacteria (Forrest & Tamura, 2010; Goldstein, 2014), this pattern is not observed in RRDR of naturally resistant Rickettsia species. Polymorphism of residues exists in other rpoB regions, and some residues, if combined two-by-two, perfectly match the natural rifampin resistance phenotype. These mutations induce no substantial structural changes in the RNA polymerase β subunit, but they most often code for amino acids with larger side chains than those found in susceptible Rickettsia species. This can result in crowding at the binding site, thereby inhibiting the rifampin molecules from binding to the RNA polymerase. Similarly, the α, β’, and σ subunits, all assembled in close proximity to the β subunit in the RNA polymerase, harbor missense mutations specific to naturally rifampin-resistant species. These peripheral structural changes can also prevent or limit rifampin access to the binding site on the RNA polymerase β subunit and then confer resistance to Rickettsia species, as suggested in a few other bacteria (Brandis et al., 2012; Comas et al., 2011; Liu et al., 2018; Song et al., 2014). However, while changes in the β subunit typically result in high-level rifampin resistance, alterations in other RNA polymerase subunits lead to smaller, yet still substantial, reductions in susceptibility to rifampin (Brandis et al., 2012).
Efflux pumps are present in both rifampin-resistant and susceptible Rickettsia species, but three of their key genes, YajC, TolC, and MsbA1, harbor residues specific to naturally resistant species. The YajC and TolC genes are involved in the regulation of the main multidrug efflux machinery, imparting resistance to broad-spectrum antibiotics in bacteria (Du et al., 2015; Gill & Garcia, 2011; Jia et al., 2022; Okusu et al., 1996; Ramos et al., 2014). MsbA1 is a drug transporter gene that forms part of the inner membrane ATP-binding cassette (ABC) transporter, which can extrude antibiotics from the cell and induce resistance (Alexander et al., 2018; Díez-Aguilar et al., 2021; Jia et al., 2022; Reuter et al., 2003; Woebking et al., 2008). In addition, analysis of the pangenome of naturally rifampin-resistant Rickettsia species led to the identification of a specific duplication of the porin ompA gene. In bacteria, ompA plays a crucial role in regulating cellular permeability and can be associated with efflux systems in the inner membrane to facilitate the extrusion of antibiotics (Choi & Lee, 2019; Nie et al., 2020). However, the second copy of the ompA gene in naturally rifampin-resistant Rickettsia species is truncated and may be nonfunctional, hindering any definitive conclusions. Additional analyses are required to validate the role of these alternative mechanisms of natural rifampin resistance in Rickettsia species.
Analysis of Rickettsia phylogeny reveals that the distribution of natural rifampin resistance is not random across species and suffers from a major phylogenetic constraint. Rifampin-susceptible species are scattered along the phylogeny, belonging to different groups, suggesting that rifampin-susceptibility is an ancestral trait in the genus Rickettsia. Furthermore, the extended genome-wide analysis also revealed that some other Rickettsia species of the Spotted Fever group, non-tested for rifampin resistance, shared key genetic features with species known to be naturally rifampicin-resistant. These non-tested species include R. amblyommatis and R. raoultii, which also belong to the Massiliae subclade, suggesting that they may be other naturally rifampin-resistant species. In addition, two other non-tested species, R. gravesii and R. kotlanii, shared similar genetic features with species known to be naturally rifampicin-resistant, including key residues in their RNA polymerase β subunit, suggesting that they could be other resistant species. However, neither R. gravesii nor R. kotlanii belong to the Massiliae subclade, although they belong to the Spotted Fever group. All susceptible species in the Spotted fever group cluster in a distinct monophyletic subclade nested among the resistant or putative resistant species, suggesting that they have a unique evolutionary origin. The emergence of rifampin resistance is found at the root of the Spotted Fever group, as indicated by the phylogenetic partition of resistant (or putative resistant) versus susceptible strains. Additionally, resistance has possibly reverted to susceptibility during the subsequent diversification of Spotted Fever species. Previous phylogenetic investigations have estimated the origin of the Spotted Fever group to be 25 million years ago (Weinert et al., 2009; Weinert, 2015), providing unequivocal evidence that the emergence of natural rifampin resistance predates the use of antibiotics by humans.
To conclude, our study challenges previous assumptions regarding natural rifampin resistance in Rickettsia. Currently, the most reliable predictor of natural rifampin resistance is based on phylogenetic pattern: This phenotype is inherently linked with the Massiliae subclade, although it is likely that other closely related species, such as R. gravesii and R. kotlanii, are also resistant. These observations emphasize the importance of ongoing surveillance and research to understand how rifampin interacts with rickettsial targets and how Rickettsia can evolve antibiotic resistance. This is particularly crucial considering that new Rickettsia strains, species and groups are described each year, frequently without information on their antibiotic resistance status (Binetruy et al., 2020; Buysse & Duron, 2020; Hajduskova et al., 2016; Lacroux et al., 2023; Weinert et al., 2009).
AUTHOR CONTRIBUTIONS
Julien Amoros: Data curation; Formal analysis; Visualization; Writing—original draft; Methodology; Investigation; Writing—review and editing. Noor Fattar: Data curation; Formal analysis; Visualization; Writing—original draft; Methodology; Investigation; Writing—review and editing. Marie Buysse: Data curation; Formal analysis; Visualization; Writing - original draft; Methodology; Investigation; Writing— review and editing. Meriem Louni: Data curation; Formal analysis; Visualization; Writing—original draft; Methodology; Investigation; Writing—review and editing. Joanne Bertaux: Formal analysis; Visualization; Writing—original draft; Methodology; Investigation; Writing—review and editing; Data curation. Didier Bouchon: Conceptualization; Writing—original draft; Writing—review and editing; Project administration. Olivier Duron: Conceptualization; Writing—original draft; Writing—review and editing; Project administration; Supervision; Funding acquisition; Resources; Investigation; Validation.
ACKNOWLEDGMENTS
The authors are grateful to Ella Marcy, Frédéric Landmann, Maxime Duhayon and Laurence Vial for her precious advice. The authors also acknowledge the ISO 9001-certified IRD i-Trop HPC (South Green Platform) at IRD Montpellier for providing HPC resources that have contributed to the research results reported within this paper (www.bioinfo.ird.fr; www.southgreen.fr). This work was funded by French Agence Nationale de la Recherche (ANR, France, ref. ANR-21-CE02-0002, Laboratoire d'Excellence CEBA, ref. ANR-10-LABX-25-01 and LabEx CeMEB, ref. ANR-10-LABX-04-01), the University of Montpellier (KIM RIVE [Key Initiative Montpellier: Risks and Vectors] and MUSE [Montpellier University of Excellence]) and the Région Occitanie [Key challenge RIVOC].
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
Tick feeding and manipulation were performed in a Biosafety Level 2 insectarium (Baillarguet insectarium platform; doi: 10.18167/infrastructure/00001) according to the regulations established by the Ethical and Animal Welfare Committee of the institution where the experiments were conducted (CIRAD, Montpellier, France), complying with the European legislation. The Baillarguet insectarium platform is a member of the National Infrastructure EMERG'IN and of the Vectopole Sud network (http://www.vectopole-sud.fr/) led by the joint units Intertryp (IRD, Cirad) and ASTRE (Cirad, INRAE). During the experiment, blood was taken from cows sheltered in the CIRAD animal facility according to a protocol approved under number APAFIS#1445-2015081217184829v2 by the French Ministry of Research.
APPENDIX 1
| Gene | Gene product | Target organism | Primers | Sequence (5′-3′) |
|---|---|---|---|---|
| OmAct2 | Actin 2 | Ornithodoros moubata | Omou_actqF2 | CGGTATTGCCGACCGTATGC |
| Omou_actqR1 | CTCCCTGTCCACCTTCCAGC | |||
| gltA | Citrate synthase | Rickettsia lusitaniae R-Om | Rick-gltA-F-Nw | GCTATTTGCAGGAGTGGGTTG |
| Rick-gltA-R-Nw | TCCGTTTAGGTTRATGGGCTT |
| Rickettsia group | Species | Strain | GenBank | Sequence available | Susceptibility to rifampin (cf. Table 1) |
|---|---|---|---|---|---|
| Spotted Fever Group | R. aeschlimannii | MC16 | GCA_001051325 | Complete genome | R |
| R. africae | ESF-5 | GCA_000023005 | Complete genome | S | |
| R. amblyommatis | An13 | GCA_002078335 | Complete genome | U | |
| GAT-30V | GCA_000284055 | Complete genome | U | ||
| R. argasii | T170-B | GCA_000965185 | Complete genome | U | |
| R. conorii | A-167 | GCA_000261325 | Complete genome | S | |
| Malish7 | GCA_000007025 | Complete genome | S | ||
| Moroccan | AF076435 | rpoB gene sequence | S | ||
| ISTT CDC1 | GCA_000263815 | Complete genome | S | ||
| ITTR | GCA_000257435 | Complete genome | U | ||
| R. fournieri | AUS118 | GCA_900243065 | Complete genome | U | |
| R. gravesi | BWI-1 | GCA_000485845 | Complete genome | U | |
| R. heilongjiangensis | 054 | GCA_000221205 | Complete genome | U | |
| CH8-1DNA | GCA_009731545 | Complete genome | U | ||
| R. honei | RB | GCA_000263055 | Complete genome | S | |
| R. japonica | YH | GCA_000283595 | Complete genome | S | |
| Nakase | GCA_002357175 | Complete genome | U | ||
| R. kotlanii | HM-2 | GCA_030295365 | Complete genome | U | |
| FLA-4 | GCA_030295345 | Complete genome | U | ||
| R. massiliae | AZT80 | GCA_000283855 | Complete genome | R | |
| Bar-29 | AF076436 | rpoB gene sequence | R | ||
| MTU1 | AF076433 | rpoB gene sequence | R | ||
| MTU5 | GCA_000016625 | Complete genome | U | ||
| R. montanensis | OSU 85-930 | GCA_000284175 | Complete genome | U | |
| R. parkeri | Black Gap | GCA_018610945 | Complete genome | U | |
| Portsmouth | GCA_000284195 | Complete genome | U | ||
| R. raoultii | IM16 | GCA_001975185 | Complete genome | U | |
| BIME | GCA_023674445 | Complete genome | U | ||
| R. rhipicephali | 3-7-female6-CWPP | GCA_000284075 | Complete genome | R | |
| EH | GCA_026309315 | Complete genome | U | ||
| R. rickettsii | Brazil | GCA_000283955 | Complete genome | U | |
| R | GCA_000831525 | Complete genome | S | ||
| R. siberica subsp. sibirica | 246 | GCA_000166935 | Complete genome | S | |
| BJ-90 | GCA_000246715 | Complete genome | U | ||
| R. siberica subsp. mongolitimonae | HA-91 | GCA_000247625 | Complete genome | S | |
| R. slovaca | 13-B | GCA_000237845 | Complete genome | S | |
| D-CWPP | GCA_000252365 | Complete genome | U | ||
| Rickettsia endosymbiont of Proechinophthirus fluctus | Pfluc4 | GCA_020404545 | Complete genome | U | |
| Scapularis | R. buchnerii | ISO7 | GCA_000696365 | Complete genome | U |
| PalLab | GCA_026723685 | Complete genome | U | ||
| R. monacensis | IrR/Munich | GCA_000499665 | Complete genome | U | |
| R. tamurae | AT-1 | GCA_000751075 | Complete genome | U | |
| Rickettsia endosymbiont of Ixodes pacificus | Humboldt | GCA_000965155 | Complete genome | U | |
| Rickettsia endosymbiont of Ixodes scapularis | REIS | GCA_000160735 | Complete genome | U | |
| Transitional | R. akari | Hartford | GCA_000018205 | Complete genome | U |
| R. asembonensis | TH2014 | GCA_019249195 | Complete genome | U | |
| NMRCii | GCA_000828125 | Complete genome | U | ||
| R. australis | Cutlak | GCA_000284155 | Complete genome | U | |
| Phillips | GCA_000273745 | Complete genome | S | ||
| R. felis | LSU-Lb | GCA_000804505 | Complete genome | U | |
| URRWXCal2 | GCA_000012145 | Complete genome | S | ||
| R. hoogstraalii | CS | GCA_026309295 | Complete genome | U | |
| RCCE3 | GCA_000964845 | Complete genome | U | ||
| R. lusitaniae | R-Om | This study | rpoB gene sequence | S | |
| Typhus | R. prowazekii | BreinI | GCA_000367405 | Complete genome | S |
| Madrid E | GCA_000195735 | Complete genome | S | ||
| R. typhi | B9991CWPP | GCA_000277305 | Complete genome | U | |
| Wilmington | GCA_000008045 | Complete genome | S | ||
| Helvetica | R. asiatica | Maytaro1284 | GCA_007989425 | Complete genome | U |
| R. helvetica | C9P9 | GCA_000255355 | Complete genome | U | |
| Canadensis | R. canadensis | CA410 | GCA_000283915 | Complete genome | U |
| McKiel | GCA_000014345 | Complete genome | U | ||
| Adalia | Rickettsia endosymbiont of Adalia bipunctata | - | - | Complete genome | U |
| Bellii | R. bellii | An04 | GCA_002078315 | Complete genome | U |
| OSU 85-389 | GCA_000018245 | Complete genome | U | ||
| RML 369-C | GCA_000012385 | Complete genome | U | ||
| RML Mogi | GCA_000965045 | Complete genome | U | ||
| Rickettsia endosymbiont of Bembidion lapponicum | Blapp1 | GCA_020404495 | Complete genome | U | |
| Rickettsia endosymbiont of Bembidion nr. | Btrans1 | GCA_020404375 | Complete genome | U | |
| Rickettsia endosymbiont of Columbicola hoogstraali | Choog2 | GCA_020404365 | Complete genome | U | |
| Rickettsia endosymbiont of Bemisia tabaci | MEAM1 | GCA_002285905 | Complete genome | U | |
| wb | GCA_001707925 | Complete genome | U | ||
| Meloidae | Rickettsia endosymbiont of Pyrocoelia pectoralis | Ppec13 | GCA_020404425 | Complete genome | U |
| Rhyzobius | Rickettsia endosymbiont of Oxypoda opaca | Oopac6 | GCA_020881235 | Complete genome | U |
| Torix | Rickettsia endosymbiont of Gnoriste bilineata | Gbili3 | GCA_020881275 | Complete genome | U |
| Rickettsia endosymbiont of Labidopullus appendiculatus | Lappe4 | GCA_020881075 | Complete genome | U | |
| Rickettsia endosymbiont of Pseudomimeciton antennatum | Pante1 | GCA_020881195 | Complete genome | U | |
| Rickettsia endosymbiont of Cimex lectularius | RiClec | GCA_020410805 | Complete genome | U | |
| Rickettsia endosymbiont of Bryobia graminum | Moomin | GCA_020881085 | Complete genome | U | |
| Rickettsia endosymbiont of Sericostoma sp. | Ssp4 | GCA_020404565 | Complete genome | U | |
| Candidatus Megeira (Outgroup) | Candidatus Megaira endosymbiont of Mesostigma viride | NEIS296 | GCA_020410825 | Complete genome | U |
| Orientia (Outgroup) | Orientia tsutsugamushi | Ikeda | GCA_000010205 | Complete genome | S |
- Abbreviations: R, naturally resistant species and strains; S, susceptible; U, unknown.
| Putative genea | Length (amino acids) | Gene localization | Position |
|---|---|---|---|
| Rrhi37F6_00877 | 192 | Chromosome (CP003319) | 785,232 to 785,811 |
| Chromosome (CP003342) | 756,448 to 757,025 | ||
| Chromosome (CCER01000014) | 149,448 to 150,023 | ||
| Rrhi37F6_00879 | 62 | Chromosome (CP003319) | 784,432 to 784,599 |
| Chromosome (CP003342) | 757,390 to 757,570 | ||
| Chromosome (CCER01000014) | 150,656 to 150,841 | ||
| Rrhi37F6_01547 (CopG) | 76 | Plasmid (CP003320) | 1090 to 1317 |
| Plasmid (CP003343) | 13,034 to 13,261 | ||
| Plasmid (CCER01000015) | 13,131 to 13,358 | ||
| Rrhi37F6_01549 | 59 | Plasmid (CP003320) | 51 to 227 |
| Plasmid (CP003343) | 14,124 to 14,300 | ||
| Plasmid (CCER01000015) | 14,233 to 14,409 | ||
| Rrhi37F6_00531 (ompA) | 97 | Chromosome (CP003319) | 1,086,010 to 1,086,225 |
| Chromosome (CP003342) | 454,648 to 454,923 | ||
| Chromosome (CCER01000011) | 199,573 to 199,863 | ||
| Rrhi37F6_01548 (ParA) | 213 | Plasmid (CP003320) | 437 to 1075 |
| Plasmid (CP003343) | 13,276 to 13,914 | ||
| Plasmid (CCER01000015) | 13,373 to 14,011 | ||
| Rrhi37F6_00259 | 68 | Chromosome (CP003319) | 300,377 to 300,562 (1); 575,565 to 575,750 (2); 829,521 to 829,706 (3); 1,153,860 to 1,154,045 (4) |
| Chromosome (CP003342) | 210,128 to 210,316 (1); 386,792 to 386,980 (2); 712,238 to 712,426 (3); 980,467 to 980,655 (4); 1,259,577 to 1,259,765 (5) | ||
| Chromosome (CCER01000003) | 4707 to 4910 (1); 74,738 to 74,932 (2) | ||
| Chromosome (CCER01000007) | 196,482 to 196,676 (3) | ||
| Rrhi37F6_00257 | 65 | Chromosome (CP003319) | 301,032 to 301,208 (1); 576,220 to 576,411 (2); 895,439 to 895,630 (3); 1,153,199 to 1,153,390 (4) |
| Chromosome (CP003342) | 209,465 to 209,659 (1); 387,449 to 387,643 (2); 644,881 to 645,072 (3); 979,804 to 979,998 (4); 1,258,914 to 1,259,108 (5) | ||
| Chromosome (CCER01000003) | 74,080 to 74,274 (1) | ||
| Chromosome (CCER01000007) | 195,824 to 196,018 (2) | ||
| Rrhi37F6_01535 | 37 | Plasmid (CP003320) | 12,201 to 12,311 |
| Plasmid (CP003343) | 1971 to 2081 | ||
| Plasmid (CCER01000015) | 2104 to 2214 | ||
| Rrhi37F6_01536 | 937 | Plasmid (CP003320) | 9041 to 12,007 |
| Plasmid (CP003343) | 3384 to 5240 | ||
| Plasmid (CCER01000015) | 2404 to 5214 | ||
| Rrhi37F6_01542 | 55 | Plasmid (CP003320) | 4970 to 5147 |
| Plasmid (CP003343) | 9530 to 9706 | ||
| Plasmid (CCER01000015) | 10,787 to 10,951 |
- Note. Accession numbers refer to genomes of R. massiliae strain AZT80 (CP003319), R. rhipicephali strain 3-7-female6-CWPP (CP003342), and R. aeschlimannii (CCER01000003-15).
- a provisional names.

Open Research
DATA AVAILABILITY STATEMENT
The full rpoB nucleotide sequence for the R. lusitaniae R-Om strain can be found in GenBank, with the accession number PP399358. The data set for the qPCR quantification of Rickettsia in both rifampin-treated and untreated ticks, along with the command lines used for statistical and phylogenomic analysis, are accessible on GitHub: https://github.com/julien01A/Amoros-Fattar-2024-Rifampin