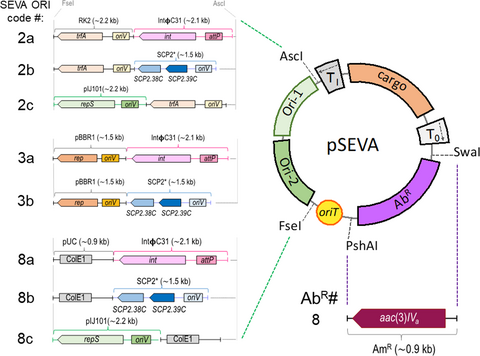
Multifunctional SEVA shuttle vectors for actinomycetes and Gram-negative bacteria
Graphical Abstract
A set of 23 new shuttle plasmid vectors for use in actinomycetes has been developed using the modular SEVA architecture. The figure shows a trimodular structure of these new conjugative shuttle vectors for actinomycetes and E. coli, based on the pSEVA backbone, showing the replication module, the antibiotic selection region, and the cargo sector.
Abstract
Actinomycetales, such as the genus Streptomyces, are well-known cell factories employed to produce a wide variety of secondary metabolites for industrial use. However, not only is the genetic engineering of Streptomyces more complicated and tedious than other model laboratory species, such as Escherichia coli, there is also a considerable lack of genetic tools, hindering its adoption as a common chassis for synthetic biology. In this work, 23 novel shuttle vectors are presented that follow the canonical SEVA (Standard European Vector Architecture) common architecture with the goal of increasing the genetic toolbox repertoire for Streptomyces and other actinomycetes. The ORI module of these plasmids is composed of the combination of two origins of replication, one for Gram-negative bacteria and the other for Streptomyces, a Gram-positive bacteria. Origins of replication have been included in the collection for integrative, low-copy number, and medium-to-high-copy number vectors for Streptomyces. Also, a new selection marker has been developed that confers resistance to apramycin. The functionality of these plasmids was tested via the heterologous expression of GFP and the heterologous production of the plant flavonoid apigenin in Streptomyces albus J1074, with successful results in both cases, therefore expanding the current repertoire of genetic manipulation tools in Streptomyces species.
1 INTRODUCTION
Traditionally, Escherichia coli (a Gram-negative species with a medium G + C content) is the bacterium of choice not only as a model organism but also as a common host to express heterologous DNA. As such, it is a preferred chassis for synthetic biology. This is, in part, due to the fact that it is a well-known and easy-to-manipulate bacterium with a number of genetic tools available. However, it also has some significant drawbacks that hamper its selection as a universal bacterial chassis. For one, E. coli lacks some protein modifications, such as glycosylation, and in some cases, incorrect protein folding causes insolubility of expressed protein products. Moreover, E. coli does not have the capacity to synthesize a broad range of secondary metabolites (including many commercial drugs) due to either the absence of specific precursor metabolites or due to problems with the codon usage of the genes of biosynthetic pathways when they are heterologously expressed in this laboratory microorganism (Anné, Maldonado, Van Impe, Van Mellaert, & Bernaerts, 2012; Silva-Rocha et al., 2013).
In contrast, Streptomyces is a Gram-positive, aerobic actinomycete with high C + G content and a mycelial life cycle, which includes a sporulation phase. It is a suitable host for the controlled production of many secondary metabolites useful in industrial and biomedical applications, such as antibacterials, antifungals, antitumorals, and immunosuppressants (Arakawa, 2018; Barajas, Blake-Hedges, Bailey, Curran, & Keasling, 2017; Bunet et al., 2014; Čihák et al., 2017; Kashiwagi, Ogino, & Kondo, 2017).
There are various vectors in the literature that allow researchers to work with and to express imported genes or gene clusters in the genus Streptomyces. These plasmids can be divided into three groups: (a) high-copy number plasmids, (b) low-copy number plasmids, and (c) integrative vectors. High-copy number plasmids are useful for cloning DNA or for achieving elevated expression levels of heterologous DNA. Among these, the most attractive are the ones derived from the well-known pIJ101 plasmid (pWHM3 or pIAGO) (Fernández et al., 1998; Kendall & Cohen, 1988; Vara, Lewandowska-Skarbek, Wang, Donadio, & Hutchinson, 1989). The pIJ101 replicon contains up to 300 copies, and it also has a wide host range of replication within the Streptomyces genus (Kieser, Chater, Bibb, Buttner, & Hopwood, 2000). Low-copy number vectors are of interest as they reduce the metabolic burden of high-copy plasmids. These plasmids are useful in maintaining a small dosage of the encoded proteins or RNAs, which in some cases may cause a toxic effect if overexpressed, or complementing mutant strains by providing the wild-type allele in trans. The most commonly used low-copy vectors are derived from SCP2*, a 31 kb circular native plasmid from S. coelicolor A3(2) (Haug et al., 2003; Larson & Hershberger, 1986). These contain 1–5 copies per genome (Kieser et al., 2000). Finally, integrative vectors allow for stable, single copy insertions of the cloned DNA into the chromosome. Different integrative vectors utilize different integration sites present in the chromosome of Streptomyces, such as ΦC31 or ΦBT1 derivatives, as well as mini-circle vectors (Gonzalez-Quiñonez et al., 2016; Heil, Cheng, & Charles, 2012; Motamedi, Shafiee, & Cai, 1995; Smith, Burns, Wilson, & Gregory, 1999).
These plasmids have been extensively used. Nevertheless, like E. coli, they have their drawbacks, such as large size, unknown sequences (in some cases), the limited number of restriction sites for cloning, and lack of standardization. In order to confront these deficiencies, a few years ago the Standard European Vector Architecture (SEVA) database (http://seva.cnb.csic.es) was created to both facilitate the entry of a field of fixed format to the repertoire of genetic tools for bacteria and also to build a plasmid repository which allows users to choose the optimal vector for deployment of complex prokaryotic phenotypes in Gram-negative bacteria, such as Escherichia or Pseudomonas. In the SEVA platform, every DNA portion is minimized to the shortest, but still functional, sequence and is edited to remove a number of restriction sites (Anné et al., 2012). In short, SEVA plasmids are composed of three modules: (a) an antibiotic marker, (b) the origin of replication (ORI), and (c) the cargo segment. These modules are flanked by uncommon restriction sites to facilitate their exchange to create different plasmid combinations (Martínez-García, Aparicio, Goñi-Moreno, Fraile, & de Lorenzo, 2015; Martínez-García et al., 2019; Silva-Rocha et al., 2013).
In this work, three types of Streptomyces replicons have been standardized and formatted following the explicit rules of the SEVA-DB. These three replicons of Streptomyces—one integrative vector derived from ΦC31 phage, one low-copy number derived from SCP2*, and one high-copy number derived from pIJ101—have been combined with the SEVA origins of replication RK2, pBBR1, and pUC to create a collection of SEVA shuttle vectors that would be functional in numerous bacterial species, including E. coli, Pseudomonas putida KT2440, and S. albus J1074. In this way, all cloning processes can be performed in laboratory strains of E. coli and the assembled plasmid transferred directly into multiple cell factories, such as P. putida or Streptomyces for industrial production of added-value compounds such as antibiotics and recombinant proteins, simplifying downstream purification steps and reducing working time.
At the same time, the antibiotic resistance marker aac(3)IV gene was added to the SEVA collection. This cassette confers resistance to apramycin, an aminoglycoside with a broad spectrum of action against Gram-negatives and Streptomyces. Moreover, chloramphenicol, kanamycin, and streptomycin resistance markers were combined into these new SEVA shuttle frames and its functionality was tested on S. albus J1074.
An intrinsic property of all SEVA plasmids is that the backbone includes the oriT DNA region of the broad host range and self-transmissible RK2/RP4 plasmid to facilitate the transfer of these vectors between strains/species by conjugation through the use of a mating helper strain that provides the transfer/mobilization functions in trans (Simon, Priefer, & Pühler, 1983). This feature will facilitate the transfer of plasmids from E. coli cells to Streptomyces germinated spores by mating (Mazodier, Petter, & Thompson, 1989), avoiding protoplasts transformation, which can be tedious in some actinomycete strains (Rocha, Ruiz-Villafán, Manzo, Rodríguez-Sanoja, & Sánchez, 2018).
Furthermore, the expression of a fluorescent reporter placed under the control of two constitutive promoters (PEM7 and PermE*) was tested, and the production level of the plant flavonoid apigenin in S. albus J1074 was evaluated using a set of these new shuttle vectors with different copy numbers.
These new plasmids are distributed by SEVA-DB and freely available.
2 MATERIALS AND METHODS
2.1 Growth media and conditions
The bacterial strains used in this work are described in Table 1. E. coli strains were cultured in LB medium (Kieser et al., 2000) or tryptic soy broth (TSB, Merk) at 37°C and 250 rpm, while P. putida KT2440 was grown in LB and incubated with agitation at 30°C. The solid media was prepared by adding 1.5% (w/v) agar to the liquid media. S. albus J1074 was cultured in TSB liquid medium at 30°C and 250 rpm (Kieser et al., 2000). To grow S. albus J1074, the solid media used was Bennet agar (Kieser et al., 2000) to induce sporulation, R5A (Fernández et al., 1998) for flavonoid production, or the selective mannitol soy agar plates (MS) (Kieser et al., 2000). The antibiotics used in this work were ampicillin (100 μg/ml), chloramphenicol (30 μg/ml for conjugation experiments and 50 μg/ml for plasmid selection), kanamycin (50 μg/ml), gentamycin (10 μg/ml), streptomycin 50 μg/ml for E. coli, 100 μg/ml for P. putida, and 200 μg/ml for S. albus, apramycin (100 μg/ml for S. albus J1074 and 40 μg/ml for E. coli and P. putida KT2440), nalidixic acid (60 μg/ml).
| Strain | Description | Genotype | Characteristics | Reference | |
|---|---|---|---|---|---|
| Escherichia coli | |||||
| CC118 | Cloning host for plasmids construction | (ara-leu) araD ΔlacX174 galE galK phoA20 thi−1 rpsE rpoB argE (Am) recA1. | SpR, RifR, Thi–, Leu– | Herrero, De Lorenzo, and Timmis (1990) | |
| HB101 | Mating helper strain carrying plasmid pRK600 | F– λ– hsdS20 (rB–mB–) recA13 leuB6(Am) araC14 Δ(gpt-proA)62 lacY1 galK2(Oc) xyl−5 mtl−1 thiE1 rpsL20 glnX44B(AS)B | SmR, Thi–, Leu–, Pro– | Boyer and Roulland-dussoix (1969) | |
| Pseudomonas putida | |||||
| KT2440 | Wild-type strain | mt−2 derivative cured of TOL plasmid | Bagdasarian et al. (1981) | ||
| EM42 | KT2440 genome reduce derivative | Martínez-García et al. (2014) | |||
| Streptomyces albus | |||||
| J1074 | Wild-type strain | Ilv−1, sal−2 | Chater and Wilde (1980) | ||
2.2 DNA manipulation techniques
Plasmid DNA miniprep extractions were carried out either using the NaOH denaturalization protocol (Sambrook & Russell, 2001) or using the QIAprep plasmid purification kit (Qiagen). All restriction enzymes were purchased from Thermo Scientific or New England Biolabs and used according to manufacturer instructions. The purification of DNA fragments from agarose gels was carried out using the GeneJET Gel Extraction Kit (Thermo scientific). DNA synthesis was outsourced to GeneCust Europe (Luxembourg).
Ligations were carried out using T4 DNA ligase (Fermentas) and were performed overnight at room temperature. The vector's DNA fragment ratio was 1:3 in all cases. 5 µl of the ligation product was transformed in calcium-competent E. coli cells following the protocol as described in Sambrook and Russell (2001). After transformation, bacteria were plated on TSA (tryptic soy agar), which is a medium that permits faster growth, with the appropriate antibiotic and incubated for 24 hr at 37°C.
2.3 PCR amplifications
In the cases of the integrative plasmids (those containing the integrase-attP locus), their presence in the Streptomyces chromosome as an episome was always checked by PCR. For this, 500 µl mycelium from the corresponding 24-hr-old TSB culture was centrifuged. Then, 2 µl of the cellular pellet was used as the DNA template for colony PCR, using the designed primers CGG-integrase-up and CGG-integrase-rp that anneal inside the integrase gene (Table 2). In case of a successful insertion into the S. albus genome, the expected size of the DNA amplicon is 1 kb, whereas no amplification is expected for noninsertion events. PCR amplifications were carried out with Amplus polymerase (EURx), according to manufacturer instructions.
| Name | Sequence 5’ → 3’a | Utilization in this work | Source |
|---|---|---|---|
| CGG-integrase-up | TGCAACGCGAGCTGGGCGG | Used with CGG-integrase-rp to verify the proper insertion in the genome of S. albus of the SEVA integrative plasmids | This work |
| CGG-integrase-rp | GCCTCTGCTGCCTCCAGCTC | Used with CGG-integrase-up to verify the proper insertion in the genome of S. albus of the SEVA integrative plasmids | This work |
| CGG-PermE-up | ATTAATTAACGAGTGTCCGTTCGAGTG | Used with CGG-PermE-rp to amplify the PermE* promoter | This work |
| CGG-PermE-rp | AAAGAGCTCGAGATCTTCGTCCTCCTAC | Used with CGG-PermE-up to amplify the PermE* promoter | This work |
| 2-pUC-R | GAGTAGGACAAATCCGCCGCCCTAGACAGCTGGGCGCGCCCCGTAGAAAAGATCAAAGGATCTTCTTG | Used with 2-pUC-F to obtain the fragment containing the pUC ori for Gibson assembly | This work |
| 2-pUC-F | TGCCTAATGAGTGAGCTAACTCAC | Used with 2-pUC-R to obtain the fragment containing the pUC ori for Gibson assembly | This work |
| 2-pIJ-R | GCGCAACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCCGCGGGGCGCCGGCACTTCGTG | Used with 2-pIJ-F to obtain the fragment containing the pIJ101 ori for Gibson assembly | This work |
| 2-pIJ-F | AACGGGAATCCTGCTCTGCGAGGCTGGCCGTAGGCCGGCCGGAGCGAGTTAGTGCGAAGTGGGCCC | Used with 2-pIJ-R to obtain the fragment containing the pIJ101 ori for Gibson assembly | This work |
| 2-pIJ-2-F | GGCGTCGTCCTCGGTCATGCCG | To sequence the pSEVA88c1 plasmid generated by Gibson assembly | This work |
| 2-pIJ−3-F | TGATCCACTCGACCACGGCGGC | To sequence the pSEVA88c1 plasmid generated by Gibson assembly | This work |
| 2-pIJ-4-F | GAAGTCGGCCCAGTCGCAACCG | To sequence the pSEVA88c1 plasmid generated by Gibson assembly | This work |
| 2-pIJ-5-R | CAAGACGGCGACCGGCGGGA | To sequence the pSEVA88c1 plasmid generated by Gibson assembly | This work |
| PS2 | GCGGCAACCGAGCGTTC | SEVA backbone | Silva-Rocha et al. (2013) |
| PS5 | CCCTGCTTCGGGGTCATT | SEVA backbone | Silva-Rocha et al. (2013) |
| PS6 | GGACAAATCCGCCGCCCT | SEVA backbone | Silva-Rocha et al. (2013) |
- a Restriction site sequences in the oligonucleotides are depicted in bold. Italics indicate the 40-bp overlapping DNA sequences required for isothermal assembly.
The PermE* promoter region was amplified with the high fidelity Herculase II Fusion DNA polymerase (Agilent), according to the manufacturer instructions.
2.4 S. albus protoplast formation and transformation
This procedure was performed using the PEG-mediated protocol (Kieser et al., 2000). Briefly, the mycelium from the actinomycete was grown in baffled Erlenmeyer flasks containing YEME (17% sucrose w/v) medium and incubated at 30°C with 250 rpm. Then, 25 ml of the exponentially cultured mycelium was centrifuged, resuspended in P-buffer (Kieser et al., 2000), treated with 1 mg/ml lysozyme for 1 hr (to eliminate the cell wall), and gently resuspended in P-buffer. After that, 20 µl of the corresponding plasmid DNA (~100 ng/µl) was added to a 200 µl aliquot of the protoplasts in the presence of 500 µl PEG 6000 (25% w/v in P-buffer), mixed, and plated out on four R5A Petri dishes. After a 24-hr incubation at 30°C, plates were overlaid with 1.5 ml of sterile distilled water, each containing the corresponding antibiotic for selection (Kieser et al., 2000).
2.5 Triparental mating
The mini-Tn5 delivery method (Martínez-García, Aparicio, De Lorenzo, & Nikel, 2017) and the plasmid DNA conjugation protocol for Streptomyces (Kieser et al., 2000) were the starting points for developing a new protocol to carry out the mating between E. coli and S. albus bacterial species. First, two preinocula—the donor strain E. coli CC118 carrying pSEVA82c1 to be introduced into S. albus and the E. coli HB101 strain harboring the mating helper plasmid pRK600—were prepared in LB medium, 100 μg/ml apramycin, and 30 μg/ml Cm, respectively. After 18-hr incubation at 37°C with agitation at 250 rpm, the optical density of both bacterial cultures was measured and adjusted to an OD600 of 1 with PBS 1x buffer, in a final volume of 1 ml. Elsewhere, ~108 spores were prepared by incubating a lawn of S. albus cells on Bennet medium agar plates for 7 days at 30°C. These were subsequently collected and filtered through sterile cotton syringes, centrifuged at 8,000 g for 2 min, and resuspended in 500 μl of LB medium. Then, these spores were submitted to a heat-shock treatment at 56°C for 10 min in order to activate the spores’ germination.
After this, the two E. coli preinocula and the heat-treated spores were centrifuged at 7,200 g for 2 min and the three cellular pellets were resuspended in 1 ml of 10 mM MgSO4. The two E. coli cultures were mixed in a 1:1 ratio with 108 heat-treated spores (150 μl each one) using a 1.5-ml tube, and this suspension was centrifuged at 7,200 g for 2 min. Finally, this pellet was resuspended in 25 μl of 10 mM MgSO4 and spotted onto three MS medium agar plates without antibiotics in order to obtain sufficient isolated colonies, and then incubated at 30°C overnight. After this incubation period, each plate was overlaid with 1.5 ml 60 μg/ml nalidixic acid, so as to eliminate the E. coli donor and mating helper strains (S. albus is resistant to this antibiotic, as are all Gram-positives), and 100 μg/ml apramycin. It was then incubated at 30°C until colonies appeared (about one week).
As negative controls, 10 μl of each of the two E. coli strains cultures (donor and mating helper) were plated out on MS medium agar plates with 60 μg/ml nalidixic acid. Also, a 10 μl of the suspension of S. albus spores was plated out on MS medium agar plates with 100 μg/ml apramycin. No growth was observed in any of these three cases after incubation at 30°C for 168 hr.
2.6 Confocal fluorescence microscopy
Images were collected with Leica TCS-SP8X confocal microscopies (Servicios Científico-Técnicos, Universidad de Oviedo) using a 63×/1.40 oil objective. GFP was excited using a white light laser (496 nm line), and fluorescence emission was detected at 505–550 nm. Leica Application Suite X (LASX) was used for data acquisition (Leica Microsystems Heidelberg GmbH (Germany)). The mycelia obtained from a 24-hr-old TSB culture of wild-type S. albus and cells carrying the plasmids pSEVA82c1 (negative control plasmid), pSEVA82c13-GFP, and pSEVA82c-PermE*-msfGFP were washed with 1× PBS before they were observed under the confocal microscope.
2.7 Lab scale production, extraction, and analysis of apigenin
107 spores/ml from the different S. albus J1074 recombinant clones harboring pSEVA28a-PermE*-API or pSEVA88c-PermE*-API plasmids (Table 3) were incubated for 184 hr in 30 ml of liquid medium R5A with agitation at 250 rpm. Apigenin extraction was carried out using three volumes of ethyl acetate and extracting separately the supernatant and the disrupted mycelium pellet (after acetone treatment). These extraction mixtures were incubated for 1 hr in an orbital shaker at room temperature. After this incubation, the organic phases were filtered, mixed together and concentrated by rotary evaporation, and kept at −20°C for later use. All cultivation experiments were carried out in triplicates.
| Plasmid | Resistancea | ORIb | Cargoc | Reference | |
|---|---|---|---|---|---|
| Gram- | Strept | ||||
| pSEVA181 | Ap | pUC | MCS | Silva-Rocha et al. (2013) and Martínez-García et al. (2015) | |
| pSEVA221 | Km | RK2 | MCS | Silva-Rocha et al. (2013) | |
| pSEVA331 | Cm | pBBR1 | MCS | Silva-Rocha et al. (2013) | |
| pSEVA451 | Sm/Sp | RSF1010 | MCS | Silva-Rocha et al. (2013) | |
| pSEVA22b1 | Km | RK2 | SCP2* | MCS | This work |
| pSEVA18b1 | Ap | pUC | SCP2* | MCS | This work |
| pSEVA33b1 | Cm | pBBR1 | SCP2* | MCS | This work |
| pSEVA22a1 | Km | RK2 | int- attP | MCS | This work |
| pSEVA18a1 | Ap | pUC | int- attP | MCS | This work |
| pSEVA33a1 | Cm | pBBR1 | int- attP | MCS | This work |
| pSEVA82c1 | Am | RK2 | pIJ101 | MCS | This work |
| pSEVA88c1 | Am | pUC | pIJ101 | MCS | This work |
| pSEVA32b1 | Cm | RK2 | SCP2* | MCS | This work |
| pSEVA28b1 | Km | pUC | SCP2* | MCS | This work |
| pSEVA38b1 | Cm | pUC | SCP2* | MCS | This work |
| pSEVA48b1 | Sm/Sp | pUC | SCP2* | MCS | This work |
| pSEVA23b1 | Km | pBBR1 | SCP2* | MCS | This work |
| pSEVA32a1 | Cm | RK2 | int- attP | MCS | This work |
| pSEVA28a1 | Km | pUC | int- attP | MCS | This work |
| pSEVA38a1 | Cm | pUC | int- attP | MCS | This work |
| pSEVA48a1 | Sm/Sp | pUC | int- attP | MCS | This work |
| pSEVA23a1 | Km | pBBR1 | int- attP | MCS | This work |
| pSEVA22c1 | Km | RK2 | pIJ101 | MCS | This work |
| pSEVA32c1 | Cm | RK2 | pIJ101 | MCS | This work |
| pSEVA28c1 | Km | pUC | pIJ101 | MCS | This work |
| pSEVA38c1 | Cm | pUC | pIJ101 | MCS | This work |
| pSEVA48c1 | Sm/Sp | pUC | pIJ101 | MCS | This work |
| pSEVA237-PEM7 | Km | pBBR1 | PEM7 → GFP | Silva-Rocha et al. (2013) and Martínez-García et al. (2015) | |
| pSEVA238-msfGFP | Km | pBBR1 | xylS-Pm → msfGFP | Calles B. et al in preparation | |
| pSEVA82c13-GFP | Am | RK2 | pIJ101 | PEM7 → GFP | This work |
| pSEVA82c-PermE*-msfGFP | Am | RK2 | pIJ101 | PermE*→ msfGFP | This work |
| pSEVA82c8-msfGFP | Am | RK2 | pIJ101 | xylS-Pm → msfGFP | This work |
| pAPI | Ap, Thio | pUC | pJVI | PermE*→ MCS | 31 |
| pSEVA28a-PermE*-API | Km | pUC | int- attP | PermE*→ APIGC | This work |
| pSEVA88c-PermE*-API | Am | pUC | pIJ101 | PermE*→ APIGC | This work |
- a Antibiotic markers: Km, kanamycin; Ap, ampicillin; Cm, chloramphenicol; Am, apramycin; Sm, streptomycin; Sp, spectinomycin; Thio: thiostrepton.
- b Gram-: the origin of replication that works in Gram-negative bacteria (broad host range: RK2 and pBBR1; and the narrow-host range: pUC). Strept: the origin of replication for S. albus.
- c MCS: multiple cloning site; GFP: green fluorescent protein; msfGFP: monomeric superfolder GFP; APIGC: apigenin biosynthetic gene cluster.
Dry extracts were dissolved in 200 µl of methanol:DMSO (1:1), filtrated (0.4-µm cellulose filters), and analyzed by liquid chromatography–electrospray ionization mass spectrometry (HPLC-ESI-MS/MS, Agilent technologies 1,290 Infinity, Triple Quadrupole). This chromatography was carried out using a Zorbax Eclipse Plus C18 column (50 mm × 2.1 mm, 1.8 µm) in the positive ion mode. The analytes were eluted at a flow rate of 0.3 ml/min using a gradient of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B) at 0%–10% (v/v) of B for 1 min, which was increased to 35% (v/v) for 3 min and maintained at 35% (v/v) for 1 min; then increased to 80% (v/v) for 3 min and maintained at 80% (v/v) for 2 min; and finally decreased to 10% (v/v) for 1 min.
Apigenin quantification was carried out in multiple reaction monitoring (MRM) mode in MS/MS. To accomplish this, the following ion sets were selected to detect the transitions of the parent ions to the product ions specific to the analytes: naringenin 272 > 119 Da and 272 > 151 Da; apigenin 270 > 117 Da and 270 > 150 Da. Pure flavonoid standards were purchased from Sigma-Aldrich (apigenin and naringenin) (Marín et al., 2017).
3 RESULTS AND DISCUSSION
3.1 General architecture and construction of new SEVA shuttle vectors
The aim of this work was to build a number of shuttle vectors that would allow replication in both Gram-negative bacteria and in the Streptomyces genus, as well as in other actinomycetes. To do that, the SEVA standard was adopted to implement a number of new shuttle vectors with different properties, such as different copy number (ORI) and selection markers (AbR).
The basic structure of the new shuttle plasmids is depicted in Figure 1. Following the SEVA architecture, all plasmids were divided into three main modules: antibiotic selection marker, replication origin, and cargo region.
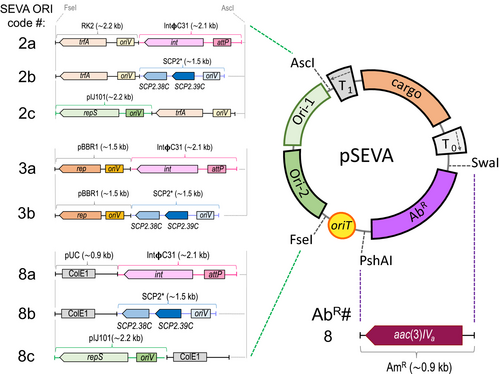
In this work, the selection marker that confers resistance to apramycin was added to the SEVA repertoire. This new antibiotic resistance cassette is encoded by the aac3(IV) gene, has been “SEVArized”, and assigned with SEVA code #8. Also, several shuttle replicons were constructed such that the ORI module, flanked by the enzymes AscI and FseI, is a combination of two different replication units: one that allows maintenance in Gram-negative bacteria and the other in S. albus J1074 and other streptomycetes. To that end, either the broad host range ORIs RK2 (ORI #2 in the SEVA collection) and pBBR1 (#3), or the narrow-host range pUC (#8) were combined with the following three different replicons of Streptomyces: the low-copy SCP2* (Freeman, Bibb, & Hopwood, 1977), the integrative derived from ΦC31 (Chater, Bruton, Springer, & Suarez, 1981), and the high-copy pIJ101 (Kendall & Cohen, 1988).
First, a synthetic ORI derived from SCP2* plasmid of S. coelicolor A3(2) (NC_003904; DNA region from 28,549 to 30,077 bp) was designed with the removal of restriction site sequences. In a similar way, the integrative region containing the integrase gene (int) plus the attP site from the ΦC31 bacteriophage of Streptomyces sp. (NC_001978; DNA region from 38,151 to 40,262 bp) was designed. In both cases, the restriction sites AscI and MluI (compatible with AscI) were introduced, respectively, at the 5´ and 3´ ends of the designed fragments, and the DNA cassettes outsourced for de novo synthesis. Then, these DNA fragments and the plasmids pSEVA221, pSEVA181, and pSEVA331 were AscI-restricted and ligated to render the new shuttle vectors: pSEVA22a1, pSEVA18a1, and pSEVA33a1 (int-attP versions; Table 3) and pSEVA22b1, pSEVA18b1. and pSEVA33b1 (SCP2* versions; Table 3). Next, the high-copy ORI derived from the pIJ101 (M21778.1) plasmid from Streptomyces lividans was used to design the ~2.2 kb DNA region that contains the replicon. This DNA fragment was curated from the restriction sites stipulated in the SEVA rules (Silva-Rocha et al., 2013) and assembled in silico to a pSEVA221 backbone where the Km resistance cassette was substituted by the aac3(IV) gene of plasmid pU0909 (Prado et al., 1999). The complete plasmid was chemically synthesized and termed pSEVA82c1 (Table 3 and Figure 1). Finally, the plasmid variant pSEVA88c1 was assembled with the Gibson Assembly Cloning Kit (New England BioLabs).
Following that, the plasmid pSEVA82c1 was digested with AscI and FseI and the fragment without the ORI module (~1.6 kb) was purified from an agarose gel. Subsequently, the 8c ORI module was generated by two combining two different PCR fragments, one for the pUC ORI (~0.9 kb) and other for the pIJ101 ORI (~2.2 kb). First, the 2-pUC-R and 2-pUC-F primers (Table 2) were used to amplify the pUC (#8) ORI. The 2-pUC-R primer has the corresponding 40-bp complementary sequence to the pSEVA18b1 plasmid backbone (in italics in the oligo sequence in Table 2). Second, the 2-pIJ-R and 2-pIJ-F primers Table 2) were used to amplify the pIJ101 ORI. The 2-pIJ-R primer has an extra 40-bp sequence complementary to the pUC ORI (in italics in the oligo sequence in Table 2), while the 2-pIJ-F has an extra 40-bp sequence complementary to the pSEVA82c1 plasmid backbone (in italics in the oligo sequence in Table 2). The PCR fragments were amplified using Q5 DNA polymerase (New England BioLabs) and purified from agarose gels. Then, the three DNA fragments were quantified, combined, and assembled following the manufacturer instructions. Finally, the assembled regions of the resulting plasmid were verified by DNA sequencing using the following primers: 2-pIJ-2-F, 2-pIJ-3-F, 2-pIJ-4-F, 2-pIJ-5-R, PS2, PS5, and PS6 (Table 2).
As stated in the SEVA nomenclature, the second position in the code stands for the ORI, which in the case of these shuttle vectors is a combination of a number (#2, #3 and #8; Gram-negative replicons) and a lower-case letter (#a, #b, and #c; Streptomyces replicons).
Finally, variants with different antibiotic selection markers were obtained by PshAI and SwaI enzymatic restriction of the shuttle destination plasmid and the corresponding antibiotic resistance marker (ApR, KmR, CmR, AmR, and SmR) from the appropriate SEVA donor plasmid. As a result, a total of 23 SEVA shuttle plasmids were constructed. Table 3 shows the list of all plasmids generated in this work, and their most relevant characteristics are depicted in Figure 1.
3.2 Host range of SEVA shuttle vectors
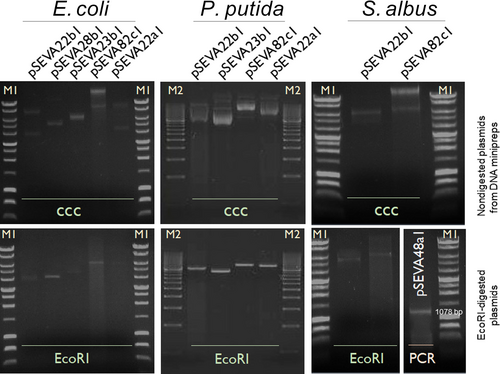
To test the maintenance and stability of these new plasmids, a selection of shuttle vectors (pSEVA22b1, pSEVA28b1, pSEVA23b1, pSEVA82c1, pSEVA22a1, and pSEVA48a1) were transformed into three representative bacterial hosts: E. coli, P. putida KT2440, and S. albus J1074. In the case of Gram-negative bacteria, plasmids were introduced into E. coli by chemical transformation and by electroporation into P. putida KT2440 as described (Martínez-García & Lorenzo, 2012). Then, plasmid DNA was isolated from overnight cultures of E. coli and P. putida KT2440 transformants, and the integrity was verified upon enzymatic restriction and the result visualized in an agarose gel (Figure 2). In the particular case of S. albus J1074, all plasmids were first introduced by protoplast formation (Kieser et al., 2000). As plasmids obtained directly from Streptomyces are not visualized properly in agarose gels, they were isolated from S. albus J1074 transformants and then retransformed into E. coli. Plasmid DNA was extracted from E. coli transformants, and the integrity was verified by enzymatic restriction (Figure 2). As for the integrative plasmid pSEVA48a1, it integrates (due to integrase expression) in a site-specific locus of the genome that codes for a nonessential chromosome condensation protein (XNR3086) (Bilyk & Luzhetskyy, 2014). Thus, integration was tested by PCR amplification of the resultant transformant colonies using a pair of primers that hybridize inside the integrase (int) gene (CGG-integrase-up and CGG-integrase-rp; Table 2). Figure 2 confirms the proper functionality of the different types of SEVA shuttle plasmids in three bacterial species, E. coli, P. putida, and S. albus.
The functionality of the chloramphenicol resistance cassette was also verified in S. albus using plasmid pSEVA33a1 (data not shown). This new strain was cultivated on solid Bennett media containing Cm at a final concentration of 50 μg/ml, and gDNA from the growing colonies was used for PCR amplification of the using CGG-integrase-up and CGG-integrase-rp primers.
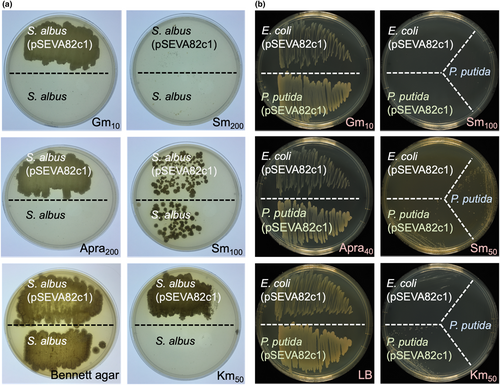
The cross-resistance of the apramycin cassette against other antibiotics was also tested, and the results are shown in Figure 3. In S. albus, plasmid pSEVA82c1 confers cross-resistance to the aminoglycosides gentamycin and kanamycin and not to the streptomycin 200 µg/ml used as a negative control (Figure 3a). The same result was observed in E. coli and P. putida KT2440, where the pSEVA82c1 plasmid showed a cross-resistance against gentamycin and to a lesser level to kanamycin, while cells did not grow on streptomycin 100 µg/ml (Figure 3b).
3.3 Conjugal transfer of shuttle plasmids from E. coli to S. albus
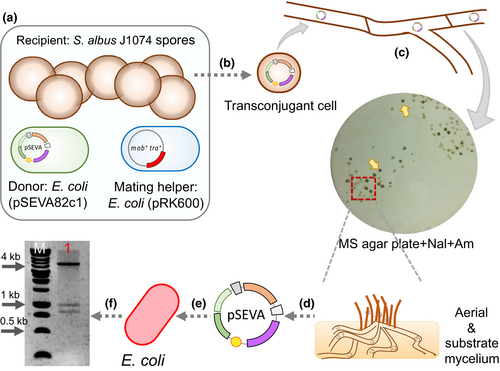
The plasmid transfer by conjugation from E. coli to S. albus J1074 was then analyzed. To do so, a modified triparental mating protocol was performed using E. coli CC118 (pSEVA82c1) donor cells, an E. coli HB101 mating helper (pRK600) strain, and S. albus J1074 as the recipient (Figure 4). Briefly, the two E. coli strains were mixed at a 1:1 ratio with ~ 108 heat-treated spores of S. albus J1074. This mixture was spotted and incubated on MS agar overnight at 30°C. Then, plates were overlaid with 1.5 ml of distilled water containing nalidixic acid to counterselect E. coli donor and mating helper strains, and apramycin for the proper selection of S. albus exoconjugants. When exoconjugants appeared (on average about 1 exoconjugant per 3 × 106 spores), they were transferred to a fresh plate to obtain enough mycelium biomass for plasmid extraction. Next, the obtained plasmid DNA from S. albus mycelium was used to transform E. coli competent cells. From the resulting E. coli recombinant colonies, plasmid DNA was isolated and double-digested with EcoRI and XhoI to confirm the presence and integrity of the shuttle plasmid used for the test. The restriction pattern obtained (~4.3 kb, ~0.9 kb, and ~0.8 kb; Figure 4) confirmed the correct transfer of the pSEVA82c1 plasmid from E. coli to S. albus J1074 by triparental mating. This presents the possibility of transferring recombinant plasmids from common E. coli laboratory host to S. albus quickly and easily, as plasmid DNA extraction from the donor E. coli strain is not necessary, and neither is the tedious protoplasts preparation from S. albus mycelium.
3.4 Functional validation of a set of expression systems in the different hosts
Ideally, it is good to not only have a broad collection of ORI and AbR modules but also to have a number of promiscuous expression devices, constitutive and inducible, that work among several species. This would allow the heterologous expression of potential gene/s of interest in different bacterial hosts, allowing for the quick selection of an optimal chassis. Therefore, the next goal was to investigate whether different expression systems commonly use in Gram-negative bacteria could also function in S. albus J1074. To validate this concept, two fluorescent reporters already available at the SEVA collection were used, GFP (cargo #7) and msfGFP. First, the gene coding for the green fluorescent protein was placed under control of PEM7 (Choi et al., 2008), a constitutive promoter in Gram-negative bacteria, and the msfGFP under control of erythromycin promoter (PermE*) (Bibb, Janssen, & Ward, 1985), a well-known constitutive promoter for streptomycetes. To obtain those constructions, the cargo region from pSEVA237-PEM7 and pSEVA238-msfGFP was excised with PacI and SpeI and ligated into the appropriate sites of pSEVA82c1 to render pSEVA82c13-GFP and pSEVA82c8-msfGFP (Table 3). Finally, plasmid pSEVA82c8-msfGFP was digested with PacI and SacI and the resulting backbone ligated to a PCR amplified PermE* promoter to generate plasmid pSEVA82c-PermE*-msfGFP (Table 3). The PCR amplification of the PermE* promoter was carried out using the pAPI plasmid (Martínez-García et al., 2017) as a DNA template using the primers CGG-PermE-up and CGG-PermE-rp (Table 2). These two primers contain the SacI and PacI recognition sites at their 5´-ends, which facilitate the cloning of the amplified promoter fragment (~200 bp) into the cargo region of the pSEVA82c8-msfGFP plasmid, replacing the XylS/Pm expression system by the PermE* promoter.
Then, the mycelia from wild-type S. albus (Figure 5a) and cells carrying the plasmids pSEVA82c1 (negative control plasmid; Figure 5b), pSEVA82c13-GFP (GFP gene under the control of the constitutive PEM7 promoter; Figure 5c), and pSEVA82c-PermE*-msfGFP (strong constitutive PermE* promoter; Figure 5d) were observed by confocal microscopy to analyze the fluorescence level produced by each plasmid in S. albus. The images show that S. albus wild-type (Figure 5a) and cells containing pSEVA82c1 (Figure 5b) produced no detectable fluorescence. One step closer, clones harboring the plasmid pSEVA82c13-GFP (Figure 5c) that contains the PEM7 constitutive promoter driving the expression of GFP showed some, but very little, fluorescence signal. Interestingly, S. albus cells harboring pSEVA82c-PermE*-msfGFP (Figure 5d) showed the highest fluorescence level of all constructions tested, as a result of the strong activity of this PermE* promoter.
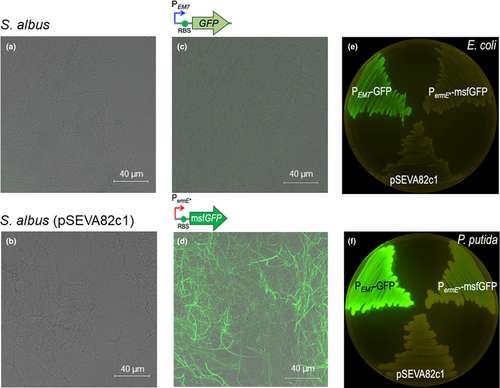
These experiments demonstrate that these vectors are stable in S. albus and are able to direct the heterologous expression of GFP and msfGFP proteins at different levels depending on the expression system used in this bacterium, with higher expression titers in the case of PermE*, a well-known strong constitutive promotor for Streptomyces (Bibb et al., 1985).
This led to exploration of the activity of the PermE* promoter in E. coli and P. putida. For that, plasmids pSEVA82c1 (negative control), pSEVA82c-PEM7-GFP (positive control), and pSEVA82c- PermE*-msfGFP were transformed into E. coli CC118 and P. putida EM42 (Martínez-García, Nikel, & Aparicio, 2014). As shown in Figure 5e,f, the PermE* promotor is able to drive expression of the fluorescent reporter, albeit only at low levels, in P. putida, while in E. coli fluorescence was not observed at detectable levels. PermE* derives from the promoter of the erythromycin resistance gene of Saccharopolyspora erythraea, a high GC content organism (Bibb et al., 1985). This fact might explain the activity of PermE*in S albus (73.3% GC) and P. putida (61.5% GC), but not in the low GC content bacterium E. coli (50.5%). The PermE* promoter could be especially attractive in P. putida when the required expression levels of the GOI are not high.
3.5 Heterologous expression of the apigenin biosynthetic gene cluster
To functionally validate these plasmids, the complete biosynthetic pathway (~ 6 kb; Figure 6a) was cloned to produce the bioactive molecule apigenin in three SEVA shuttle vectors with different copy number values in S. albus (Table 3). Apigenin is an important flavonoid naturally produced by plants, which is interesting for its antitumor activities (Manach, Scalbert, Morand, Rémésy, & Jiménez, 2004; Tong & Pelling, 2013).
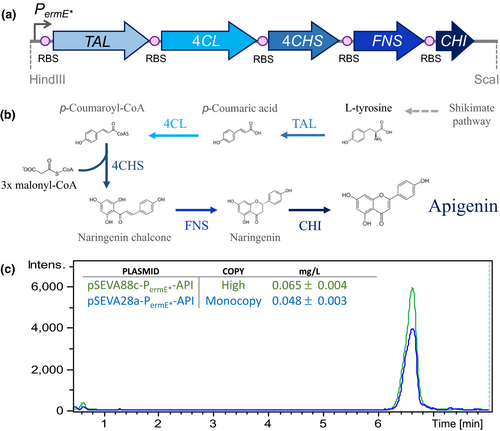
Recently, it has been shown that apigenin can be completely synthesized de novo in S. albus by the heterologous expression of its biosynthetic pathway (Marín et al., 2017). Taking advantage of this, the five genes (TAL, 4Cl, CHS, CHI, and FNS) needed for the production of apigenin from L-tyrosine were selected. Then, the constitutive PermE* promoter was placed to facilitate their expression as a single transcriptional unit, avoiding the need for adding an external inducer to the system (Figure 6b). Briefly, this biosynthetic cluster encodes a tyrosine ammonia lyase from Rhodobacter capsulatus (TAL), a 4-coumaroyl CoA ligase from Streptomyces coelicolor (4Cl), a chalcone synthase (CHS) and a chalcone isomerase (CHI) from Glycine max, and a flavone synthase oxidoreductase from Petroselinum crispum (FNS). With this framework, apigenin can be produced by simply feeding cells with a cheap carbon source, without the need for expensive precursors or inducers which generally increases the final production cost. Recently, all of these elements (GenBank accession numbers LT629810.1, LT629809.1, LT629808.1, LT629807.1, LT629806.1, LT629805.1) were assembled to yield the pAPI plasmid, and its functionality was heterologously validated in S. albus (Marín et al., 2017). Given this previous work, it was decided to test the apigenin production yield with two of the newly designed SEVA shuttle vectors with different copy number values. For that, the constitutive promoter and the complete apigenin biosynthetic pathway were obtained from the pAPI (Marín et al., 2017) plasmid after digestion with HindIII and the blunt end producing enzyme ScaI. The resulting ~ 7,5 kb DNA fragment with all the necessary elements was purified and cloned into two different copy number shuttle vectors developed as part of this work: (a) an integrative (monocopy) vector and (b) a high-copy number vector.
In both cases, the DNA fragment with all the gene elements for apigenin production was cloned into the HindIII/SmaI sites of the pSEVA28a1 and pSEVA88c1 plasmids, respectively, rendering the following plasmids: pSEVA28a-PermE*-API (monocopy) and pSEVA88c-PermE*-API (high-copy). Then, the obtained recombinant plasmids were introduced into S. albus by protoplast transformation. After selecting the correct S. albus clones containing the two different plasmids, these were grown in 30 ml of R5A media for 184 hr to accumulate apigenin. Then, the levels of production of apigenin were determined by HPLC-MS chromatography. The production yield was calculated using a commercial pure standard for apigenin (Sigma-Aldrich). The quantification result showed different production levels depending on the copy number of the plasmid used (Figure 6c). Production rates in the integrative and high-copy number vectors were 0.048 ± 0.003 mg/L and 0.065 ± 0.004 mg/L, respectively (Figure 6c). As expected, the apigenin production values correlated well with the plasmid copy number. Therefore, integrative plasmids, which are more stable, do not have copy number fluctuations among cells, and do not require constant addition of antibiotic to the media for maintenance, may be useful in situations where the genes expressed encode proteins that are toxic when expressed at high levels or in cases when no antibiotic can be added to the growth media. On the other hand, high-copy number plasmids are better suited for overexpression of industrially important metabolites and when the addition of an antibiotic to the media is not a problem. In this case, the apigenin production rates obtained with the new SEVA shuttle vectors (∼0.048 and ∼0.065 mg/L) were similar to the rates reported using the pAPI multicopy plasmid in S. albus (0.089 mg/ml) (Marín et al., 2017). Other works in the literature described the production of apigenin using other gene combinations, plasmids, or different bacterial hosts. When using E. coli as the host, the production rate ranged from 13 to 110 mg/L (Lee, Kim, Kim, & Ahn, 2015; Leonard et al., 2008; Miyahisa et al., 2006). However, to obtain those high yields the media must be supplemented with 543 mg/L of L-Tyr or p-coumaric acid and/or malonate (Lee et al., 2015; Leonard et al., 2008; Miyahisa et al., 2006). In the actinomycete S. venezuelae, apigenin was produced at a concentration of 1.5 mg/ml but with the addition of 136 mg/L of naringenin (a precursor; Figure 6b) to the production medium (Park, Paik, Ahn, Park, & Yoon, 2010).
In summary, this production system has three main advantages: (a) no need for the addition of an expensive precursor that would increase production costs; (b) various plasmid backbones with different copy numbers and selection markers for the user to choose from; and (c) since S. albus is a Gram-positive, no health nor regulatory issues are linked to chemicals produced with those bacteria since they lack the endotoxic lipopolysaccharide, present in the outer membrane of Gram-negative bacteria, such as E. coli (Wright, Ramos, Tobias, Ulevitch, & Mathison, 1990).
4 CONCLUDING REMARKS
The SEVA vector system allows easy exchange of any of the three modules of which these plasmids are composed. In this work, 23 new vectors based on the SEVA architecture have been designed and generated in order to expand the use of this highly convenient and modular vector system to actinomycetes, in general, and Streptomyces species, in particular. These Gram-positive bacteria are of considerable commercial interest, as their metabolic capacities are capable of producing a vast array of antibiotic, antitumor, antifungal, and other bioactive drugs. Expression of some genes (coding for some enzymes or regulatory factors) may be toxic in Streptomyces, and therefore, it is mandatory to keep their expression levels as low as possible. In other cases, it may be helpful to obtain medium or high expression levels, in order to get more of the final metabolite of interest (an industrial enzyme, the metabolite resulting from a given biosynthetic gene cluster, etc.). Furthermore, the coexistence of more than one type of plasmid vector may be necessary if different genes must be controlled at different rates or introduced at different stages in a microbial factory. To allow for all these different requirements, a set of diverse monocopy number (attP integration system), low-copy number (SCP2* replication origin), or high-copy number (pIJ101 replication origin) plasmid vectors has been developed. Each of these three types of vectors contains a collection of diverse antibiotic gene markers widely used in E. coli and Streptomyces species, including resistance gene markers to apramycin, chloramphenicol, kanamycin, and streptomycin. Taking advantage of the SEVA modular structure and the public availability of their sequences, this array of modules provides for a number of hypothetical plasmid combinations for engineering the industrial Streptomyces species, enabling further and easier customization toward new derivatives. The utility of these vectors has been demonstrated by the heterologous expression of a fluorescent reporter and de novo synthesis of the plant flavonoid apigenin in S. albus, a Gram-positive species which possesses advantages with respect to the Gram-negative E. coli host. Specifically, the presence of lipopolysaccharide in the outer membrane makes E. coli toxic for applications in humans and other animals, requiring expensive purification methods. Also, in the case of the present work, no precursors were added to the S. albus host to attain apigenin production, which makes the production more cost-effective. Moreover, this apigenin production is highly efficient, as practically no naringenin intermediary is left in the cytoplasm of the apigenin-producing cells.
ACKNOWLEDGMENTS
The authors wish to thank the research project AGL2017-88095-R (MINECO, Spanish Ministry of Economy and Business), IDI/2018/000120 (Programa de Ayudas a Grupos de Investigación del Principado de Asturias), the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement no. 814650, and the FET Program 766975 for financial support. The authors thank the Scientific-Technical Services (University of Oviedo) for their support with confocal microscopy and HPLC-MS.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Coral García-Gutiérrez: Investigation; Methodology; Validation; Writing-original draft. Tomas Aparicio: Investigation; Methodology; Validation; Writing-original draft. Lucia Torres-Sánchez: Investigation. Esteban Martinez-Garcia: Conceptualization; Funding acquisition; Supervision; Writing-original draft. Victor de Lorenzo: Conceptualization; Funding acquisition; Writing-original draft. Claudio J. Villar: Supervision; Writing-original draft. Felipe Lombó: Conceptualization; Funding acquisition; Supervision; Writing-original draft.
ETHICS STATEMENT
None required.
Open Research
DATA AVAILABILITY STATEMENT
The sequence data are available in GenBank under the accession numbers LT629810.1, LT629809.1, LT629808.1, LT629807.1, LT629806.1, LT629805.1.