A review on polymers in ocular drug delivery systems
Abstract
Amid the escalating prevalence of eye diseases and the intricate nature of the eye as a crucial target organ for drug delivery, researchers face significant challenges in developing delivery systems tailored specifically for ocular complications. Addressing the gaps in the current conventional ocular drug delivery system (ODDS) is crucial and this can be achieved by incorporating polymers while designing newer ODDS. This review aims to offer a concise overview of the diverse polymers utilized in the development of ODDS, designed to address various eye conditions and disorders, enhance treatment outcomes, and ensure patient adherence. Introducing the anatomy of the eye and different ocular routes of administration, alongside the barriers encountered, this review presents polymer-based ODDS, renowned for their unique properties facilitating the engineering of specialized devices for enhanced drug delivery. Further discussions delve into the applications of polymers in ophthalmology. Emphasis is placed on emerging polymer-based technologies available in the market for treating ocular diseases, underscoring their potential for revolutionizing ocular healthcare. The review also addresses challenges in translating these advancements into clinical practice, while highlighting the versatility of polymers in treating diverse eye diseases and disorders through customizable properties and sustained drug delivery.
1 INTRODUCTION
In 2020, the World Health Organization documented a substantial number of global cases of eye diseases, encompassing conditions such as age-related macular degeneration (AMD), diabetic retinopathy (DR), glaucoma, infectious keratitis, and cataracts (Figure 1).1-7 The United States alone had a substantial number of people affected by various eye conditions. Left untreated, these diseases can lead to complete vision loss. Ocular drug delivery faces challenges due to the intricate anatomy of the eye and its isolation from the rest of the body. The eye's structure comprises the anterior and posterior segments.8 Cataracts and glaucoma primarily affect the anterior segment, where the lens is responsible for vision. Aging can lead to cataracts due to oxidative damage and protein aggregation. Glaucoma, on the other hand, involves imbalances in fluid drainage pathways and increased intraocular pressure (IOP), potentially causing vision loss. Posterior segment disorders include retinal diseases such as AMD, DR, and diabetic macular edema.9 These pose treatment challenges due to limited accessibility and disease complexity. AMD involves inflammation, protein aggregation, and abnormal blood vessel growth.2, 10 DR results from elevated glucose levels, leading to blood vessel damage. Approved treatments for these conditions include steroids, antibiotics, and biological pharmaceuticals. Experimental therapeutics explore antioxidants, complement factor inhibitors, stem cell therapies, and innovative delivery methods. Local drug delivery is often necessary due to the blood–retinal barrier (BRB), but challenges in long-term efficacy and patient compliance persist.
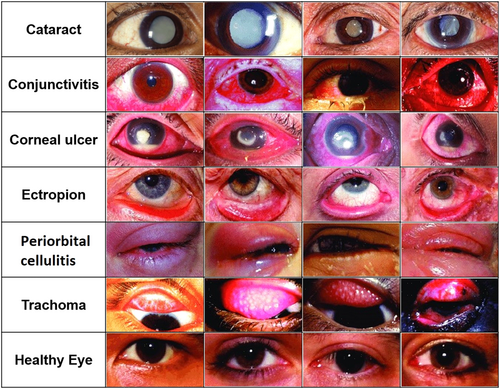
Current advancements in ocular drug delivery research are increasingly directed towards utilizing polymeric biomaterials to overcome challenges and enhance treatment efficacy, with the goal of reducing application frequency. This visual review delineates various ocular drug delivery systems (ODDS), targeted diseases/disorders, and polymeric biomaterials—large macromolecules used in biomedical applications. These polymers, constructed from either synthetic monomers or natural elements like amino acids or sugars, provide adaptable chemical and physical characteristics, enhancing drug delivery and therapeutic dosing. In clinical settings, polymers are utilized to enhance drug solubility, regulate release rates, and improve drug retention in the eye. Synthetic polymers and biopolymers represent the two primary categories of polymers for drug delivery, each with distinct differences, advantages, and specific applications.
2 EYE AND THE OCULAR BARRIERS
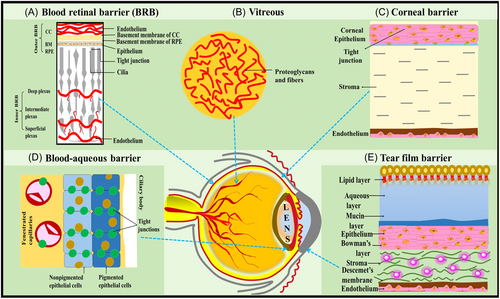
The eye, being a complex and anatomically specialized sensory organ, exhibits unique pharmacodynamic and pharmacokinetic properties, divided into two major segments: the anterior and posterior parts. The anterior segment includes structures such as the conjunctiva, cornea, iris, ciliary body, aqueous humor, and lens, comprising approximately one-third of the eye. In contrast, the posterior segment comprises the retina, optic nerve, choroid, sclera, vitreous humor, and retinal pigment, encompassing the remaining two-thirds of the eye.11 These distinct characteristics render drug delivery to the targeted ocular site an inherently fascinating yet challenging endeavor.12 Considerable challenges confront ODDS, primarily attributed to physiological and anatomical barriers. These barriers encompass factors such as nasolacrimal drainage, blinking, and the cornea, sclera, and blood–ocular barriers. The barriers in the eye are categorized into the blood–aqueous barrier in the anterior segment and the BRB in the posterior segment.13 Additionally, dynamic barriers like conjunctival blood flow, lymphatic clearance, and tear drainage contribute further complexity to the landscape of drug delivery in ocular applications (refer to Figure 2). These barriers function as natural protective mechanisms, simultaneously limiting drug entry into the eye. Novel ODDS have emerged as a promising solution to overcome these challenges, offering advantages over traditional ophthalmic dosage forms.8, 14, 15
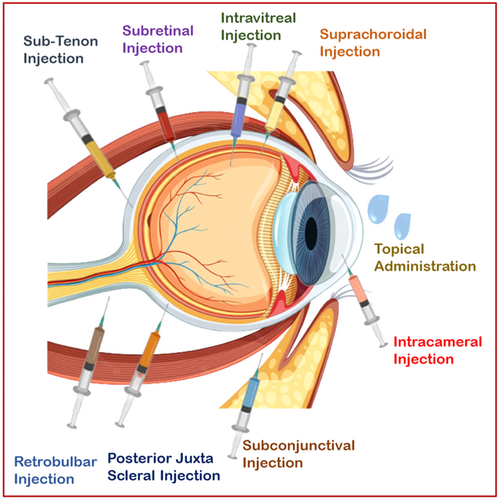
3 ROUTES OF ADMINISTRATION
Ocular drug administration involves three main routes: topical, local ocular (comprising periocular, intravitreal, and intra-cameral methods), and systemic approaches (see Figure 3). The choice of administration route depends on the specific location within the eye that requires treatment. The local ocular route encompasses periocular techniques, such as subconjunctival, sub-Tenon, retrobulbar, and peribulbar injections, as well as intravitreal and intracameral procedures.16-20
3.1 Eyedrops
The widespread utilization of eyedrops in administering diverse medications for ocular disorders is attributed to their user-friendly application, cost-effectiveness, and generally favorable patient adherence.21, 22 Nonetheless, the shortcomings of eyedrops as a drug delivery system (DDS) have prompted substantial research endeavors aimed at enhancing their efficacy or devising more efficient alternatives in recent years.23 Although eyedrops demonstrate commendable delivery efficiency for topical eye diseases, their effectiveness notably diminishes when employed to transport pharmacologic agents to specific tissues within the eye.24-26
3.2 Subconjunctival injections and implants
Injecting pharmacologic agents presents an attractive alternative route for delivering drugs to ocular tissue. Subconjunctival injection, in particular, facilitates the release of drugs adjacent to the sclera, bypassing corneal barriers to entry.27 The enhanced permeability of the scleral layer facilitates easy penetration of drugs, potentially allowing markedly more efficient delivery to the interior of the eye, especially the posterior segment.28, 29
3.3 Ointments
Ointments designed for ocular drug delivery have undergone extensive development. Their viscous nature provides prolonged eye contact, enhancing bioavailability compared to liquid formulations. However, this viscosity may cause temporary blurred vision and challenges in accurate dosing.30 To address this, a common choice for ointment base is white petrolatum, known for its appropriate melting point, resulting in reduced viscosity postadministration.31
3.4 Ocular inserts
Ocular inserts are solid ophthalmic devices with a diameter of around 8 mm. They contain a drug dispersed in a polymer reservoir or matrix system, aiming to prolong precorneal contact time, improve bioavailability, and maintain therapeutic drug concentrations in the targeted ocular tissues.32
3.5 Intraocular implants
Intraocular implants are surgically placed into the eye to gradually release drugs over an extended duration. Initially, these implants were nonbiodegradable, necessitating surgical removal and posing associated risks. However, the exploration of biodegradable polymers in ocular implant development has revolutionized the application by eliminating the need for surgical removal.33
3.6 Intravitreal injections
Administering injections directly into the vitreous humor is a common approach for delivering drugs to the posterior segment of the eye. Nevertheless, in addition to being an invasive and uncomfortable procedure for the patient, these injections come with several risks, including the potential for endophthalmitis or retinal detachment, among other complications.34
4 POLYMERS IN ODDS
A diverse range of polymers meets the criteria for ophthalmic administration and can be categorized based on their origin into natural, semisynthetic (chemically modified natural polymers), and synthetic polymers.35
4.1 Natural polymers
Natural polymers exhibit inertness, biodegradability, biocompatibility, and a lack of immunogenicity, making them promising carriers for DDSs.36 Classification of natural polymers with their physical and physicochemical characteristics in ocular drug delivery. In view to this albumin is a polyanionic natural protein that can be isolated from various sources, including egg white (ovalbumin, bovine (bovine serum albumin), and human plasma (human serum albumin).35 Albumin possesses biodegradable properties and contains functional groups suitable for binding various ligands and complex drugs.37, 38 Another one is Alginate, an anionic polysaccharide, is naturally derived from soil bacteria and brown algae, including species such as Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera.39
4.2 Semisynthetic biopolymers
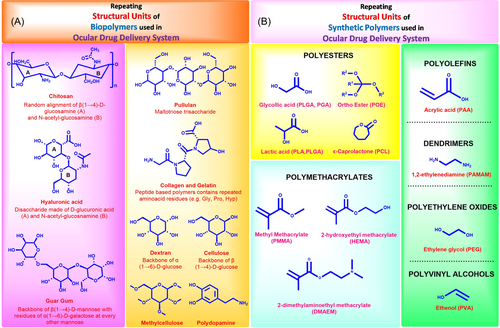
The spotlight on semisynthetic biopolymers is intensifying within polymeric applications, leveraging advancements in production technology and a deeper understanding of their material properties. Originating from natural sources, including animal, plant, fungi, and bacterial-based monomers, these derivatized polymers typically exhibit remarkable biocompatibility, swift degradation in aqueous environments, and a broad range of viscoelastic properties. These characteristics make them well-suited for crafting biomaterials designed for ocular drug delivery.40 Frequently utilized biological polymers in ocular biomaterials and DDSs include chitosan, ethyl cellulose (EC), hyaluronic acid (HA), carboxymethyl cellulose (CMC), collagen, dextran, guar gum, pullulan, gelatin, and polydopamine (see Table 1). Figure 4A illustrates the monomers and recurring units responsible for these biological polymers.
| S. no. | Polymer | Subtype | Charge | Formulation (references) |
|---|---|---|---|---|
| 1. | Sodium hyaluronate | Polysaccharide | Negative | Solutions,41, 42 in situ gels,43 bioadhesive NPs44 |
| 2. | Gellan gum | Polysaccharide, microbial | Negative | In situ gel,45 scleral implants46 |
| 3. | Gelatin | Proteins, animal | Amphoteric | Ocular inserts47 |
| 4. | Chitosan | Polysaccharide, animal | Positive | Liposomes,48, 49 niosomes,50, 51 emulsion,52 solid lipid NPs53, 54 |
| 5. | Carrageenan | Polysaccharide, microbial | Negative | Lambda-microspheres,55 films56 |
| 6. | Collagen | Proteins, Animal | Amphoteric | Inserts,57 collagen discs and shields58 |
| 7. | Guar gum | Polysaccharide, plant | Non-ionic | Artificial tear solution59 |
| 8. | Locust bean | Polysaccharide, plant | Non-ionic | Viscous solutions60 |
| 9. | Xanthan gum | Polysaccharide, microbial | Negative | In situ gels,61 solution enhancing viscosity62 |
| 10. | Sodium alginate | Polysaccharide | Negative | Ocular minitablets,63 in situ gels64 |
| 11. | Tamarind gum | Polysaccharide, plant | Nonionic | Solutions,65 in situ gels66 |
| 12. | Bletilla striata | Polysaccharide, plant | Nonionic | Solutions67 |
| 13. | Gum cordia | Polysaccharide, plant | Negative | NPs68 |
| 14. | Waxy maize starch | Polysaccharide, plant | – | Ocular minitablets69 |
| 15. | Arabinogalactan | Polysaccharide, plant | Negative | Solutions70 |
- Abbreviation: NP, nanoparticle.
4.2.1 Chitosan
Chitosan, a positively charged polysaccharide, consists of copolymers of glucosamine and N-acetylglucosamine. These units of N-acetyl-2-amino-2-deoxy-d-gluco-pyranose are connected by β-d-glycosidic linkages.71 It is sourced from fungal cell walls and is industrially obtained by subjecting chitin from the shells of crustaceans such as crabs, lobsters, and shrimps to alkaline deacetylation, imparting unique properties to chitosan, eliminating cellulose-like characteristics. Notably, chitosan exhibits a positive charge, enabling the formation of polyelectrolyte complexes and nitrogen derivatives.72, 73
4.2.2 Starch (drum-dried waxy maize starch, pregelatinized starch)
The cultivation of waxy maize, a result of genetically modified corn variants, is characterized by a highly branched amylopectin unit in its starch composition. Unlike potato starch, waxy maize starch possesses a less stringy and less cohesive texture. It demonstrates a notably high degree of polymerization and a low rate of retrogradation, imparting enhanced transparency and swelling characteristics.74
4.2.3 Gelatin
Gelatin, derived from the partial hydrolysis of collagen, is characterized by a distinctive sequence of amino acids with a high content of glycine, proline, and hydroxyproline. Available in translucent sheets, granules, or powder, it presents as a light-amber to faintly yellow-colored, vitreous, and brittle solid. Gelatin is practically odorless and tasteless. While it exhibits mucoadhesive properties, its mechanical strength is relatively low. Consequently, modified forms of gelatin are predominantly employed.75
4.2.4 Sodium hyaluronate
HA is prevalent in the connective tissue, umbilical cord, vitreous humor, and synovial fluid throughout the human body. Additionally, it can be derived from the rooster comb, and microorganisms from the Streptococcus species can produce sodium hyaluronate through fermentation. From a chemical standpoint, HA is characterized as a high-molecular-weight polyanionic mucopolysaccharide, consisting of alternating units of N-acetylglucosamine and d-glucuronic acid.76, 77 Utilized for stabilizing and hydrating cells and tissues in the body, this polymer plays a crucial role. In intraocular surgery, a 1% solution of sodium hyaluronate functions as a viscoelastic substance for both the anterior and posterior segments. Its application is directed towards maintaining the eyeball's shape and protecting the cornea during surgery, capitalizing on its gel-forming attributes and optical properties reminiscent of the vitreous.78
4.2.5 Sodium alginate
This white to buff-colored hydrophilic colloidal polysaccharide is an odorless and tasteless sodium salt of alginic acid, a substance naturally occurring in the cell wall of brown seaweeds such as Laminaria, Macrocystis, and Ascophyllum (Class Phaeophyceae). From a chemical perspective, it is a polyuronic acid composed of d-mannuronic acid and l-guluronic acid.38
4.2.6 Gellan gum
Gellan gum, is an extracellular polysaccharide generated by specific gram-negative strains, including Pseudomonas, Sphingomonas paucimobilis, and Auromonas elodea.79 Gellan gum, previously known as PS-60 and S-60, and Gelrite, which is commercially available in a purified form, undergo a transition from sol to gel when exposed to cations (divalent and monovalent) present in the lacrimal fluid, making them ideal for in situ gelling systems. This characteristic makes them well-suited as vehicles for in situ gelling systems.80
4.2.7 Collagen
Collagen, a natural polymer, is an insoluble fibrous protein. With a diameter of approximately 1.5 nm and a length of 300 nm, collagen adopts a triple-stranded helical structure comprising three coiled subunits. The fundamental repeating units in the collagen structure include amino acids such as glycine, proline, and hydroxyproline.81 Collagen exhibits high biodegradability and biocompatibility, resulting in absorption within a span of 84 days.82
4.2.8 EC
Derived from cellulose through an alkaline treatment followed by ethylation with chloroethane, EC is a semisynthetic polymer. This process involves converting some of the hydroxyl groups in glucose units into ethyl ether groups. EC is widely employed to sustain the release of various drugs and is considered safe for use in US Food and Drug Administration (US FDA) approved pharmaceutical products. It finds extensive use in sustaining the release of various drugs.38
4.2.9 CMC sodium
CMC sodium is a natural polymer known for its thermoresponsive properties. Presently, it is employed in formulations designed to address dry eyes.83 Polymers derived from cellulose are frequently utilized in ophthalmic preparations, leveraging their viscosity-modifying capabilities. Methylcellulose, in particular, has demonstrated ocular wound-healing properties and the capacity to serve as a tear substitute.84
4.3 Synthetic polymers
Synthetic polymers are a valuable choice for ocular drug delivery due to their versatility, offering various mechanical, chemical, and degradation properties. Several synthetic polymers, such as poly(ethylene glycol) (PEG), poly(glycolic acid) (PGA), poly(vinyl alcohol) (PVA), poly(lactic-co-glycolic acid) (PLGA), poly[2-(dimethylamino)ethyl methacrylate], poly(caprolactone) (PCL), poly(amidoamine), and poly(acrylic acid) (PAA), have gained approval from the US FDA for ocular applications.24 These polymers are actively used in clinical practice. However, there are numerous other polymers available for experimental use or approved for different applications beyond ocular drug delivery. Figure 4B illustrates the monomeric subunits commonly employed in the synthesis of these synthetic polymers.
4.3.1 Polyethylene oxides
PEG is a transparent and colorless hydrophilic polymer composed of ethylene oxide monomers. It is a non-ionic, synthetic polymer characterized by its hydrophilic properties. PEG is recognized for its capacity to improve the biocompatibility, solubility, and bioavailability of integrated therapeutics.85 PEG is obtained in diverse forms, such as liquid and solid, with a range of molecular weights, linear configuration or multi-armed, and tetrafunctional or bio-inert activity. It has gained general recognition as safe from the FDA and is approved for various applications, including its use in ophthalmic applications.86
4.3.2 Polyvinyl alcohols
The polymer structure of PVA enables tunable permeability, making it suitable for controlled release applications. PVA stands out as a water-soluble and biodegradable polymer commonly used for solubilizing hydrophobic drugs. Notably, it provides chemical resistivity and is known for its ease of processing. The synthesis methods applied to PVA can result in state of hydration and molecular weight variability.87
4.3.3 Poly(ε-caprolactone) (PCL)
PCL is an aliphatic polyester with a synthetic origin, exhibiting a semicrystalline nature. It is derived through the ring-opening polymerization of epsilon-caprolactone. Notably, the polymer possesses a carboxyl terminal group that imparts a negative charge to its structure.88 In terms of safety, PCL is deemed a nontoxic and biocompatible polymer, and it is extensively employed in pharmaceutical products that have received approval from the US FDA.38
4.3.4 Poly(lactic acid) (PLA)
PLA is a hydrophobic and aliphatic polyester that is synthetically derived through the ring-opening polymerization of lactide. Depending on the precursor used in the synthesis, PLA exists in different forms, such as d-PLA and l-PLA, resulting in PDLA and PLLA, respectively. Both PDLA and PLLA exhibit a semicrystalline nature.89, 90
4.3.5 PLGA
PLGA is a synthetic copolymer formed by the combination of PLA and PGA. PLA can exhibit both semicrystalline or amorphous characteristics, while PGA is highly crystalline due to the absence of a methyl group in the side chain, thereby limiting its ability to solubilize in various solvents.91 Hence, PGA is not employed in its pure form and is used by combining PLA. The exceptional biodegradability, biocompatibility, and sustained release characteristics of PLGA have made it one of the most thoroughly explored polymers for sustained ocular drug delivery.92 Concerning safety considerations, PLGA has obtained approval from the US FDA for various DDSs. It is biodegradable, and the degradation products are nontoxic. Nonetheless, it is crucial to acknowledge that the degradation process may result in the formation of acidic byproducts, including lactic and glycolic acid. This can increase local acidity, posing the potential for surrounding tissue irritation.93
4.3.6 Poly(orthoester) (POE)
Hydrophobic polymers with hydrolytically labile orthoester bonds, known as POEs, are classified into four generations, extensively reviewed in various sources. Notably, investigations in the context of ocular applications have primarily focused on POE type III and IV, with the latter being explored for ODDS.94
4.3.7 Polycarbophil (PCP)
PCP is indeed a high-molecular-weight acrylic acid polymer that is crosslinked with polyalkenyl ethers/divinyl glycol. It contains numerous carboxyl (COOH) groups along the chain. As a pharmaceutical excipient, PCP is generally considered safe and does not produce any side effects.95 Carbomer is indeed often used as a thickening or gelling agent in various pharmaceutical formulations, including topical products. On the other hand, PCP is recognized for its mucoadhesive properties, making it suitable for ODDS. Its use can enhance bioavailability by promoting longer residence time on the corneal surface. The small irritation associated with PCP further contributes to its suitability for ophthalmic applications.96
4.3.8 Polysulfone
Polysulfone is a hydrophobic polymer that is impermeable to water but allows the passage of both lipophilic and hydrophilic compounds. One notable advantage of this polymer is the presence of deep macrovoids in the outer membrane, which enhances the surface area for drug diffusion and release. While polysulfone-based implants, such as polysulfone capillary fibres implants, can be sterilized, it's important to note that they need to be removed once emptied.97
4.3.9 Poly(acrylate)s and poly(methacrylate)s
A variety of synthetic polymers, including those derived from acrylates and methacrylates, have been thoroughly investigated for ocular drug delivery. Notably, copolymers derived from esters of acrylic and methacrylic acid, commonly known by the brand name Eudragit®, are extensively studied in this context and are primarily commercialized by Evonik®.98 Within the Eudragit® family, four major families are available. Despite the extensive range of Eudragit® prototypes, the RS and RL subtypes have been the primary focus in the exploration of ocular nano-drug delivery. This preference is likely due to the superior control they offer over drug release kinetics.99 Moreover, these polymers possess a pH-independent positive charge, enabling effective interaction with negatively charged mucosa, conjunctiva, and cornea. This interaction plays a crucial role in prolonging the residence time of the encapsulated drug, enhancing the overall efficiency of drug delivery.100
4.3.10 Poly(2-hydroxyethyl methacrylate) (pHEMA)
pHEMA, or poly(2-hydroxyethyl methacrylate), is a biocompatible hydrophilic polymer known for its nonbiodegradable nature and optical transparency. It forms a hydrogel by swelling and absorbing water or biological fluids, facilitated by its hydrophilic pendant groups. In its dry state, pHEMA is hard and brittle, but upon swelling, it becomes flexible and soft, allowing for easy cutting. The transparent nature of pHEMA-based hydrogels enables the diffusion of liquids and oxygen, making them highly permeable to small molecules.101
4.3.11 Polyolefins
PAA, commercially recognized as Carbopol®, is a synthetic polymer composed of acrylic acid monomers. It displays high water solubility and possesses properties that contribute to viscosity enhancement. While it is biodegradable, the byproducts of acrylic acid during degradation can potentially cause inflammation.102
4.4 Applications of polymers in ODDS
Currently, polymers are being employed clinically to improve drug retention in the eye, manage therapeutic release rates, and increase a drug's solubility in the target microenvironment.103, 104 The FDA approved synthetic polymers (refer Figure 4B) are widely being adapted in building novel ocular technologies such as ocular implants, hydrogels, films, nanoparticles (NPs), contact lenses topical eyedrops because of their hydrophilicity, biocompatibility, slower degradation rate, easily functionalized, and cost-effective advantages (Table 2).106, 110, 112, 113 However, they are synthesized from aggressive solvents they might perform flaws like, slow degradation rate, non-biodegradability, degradation to form acidic byproducts etc.107 To address these challenges, the utilization of biologically derived polymers in ODDS proves beneficial. Examples include polysaccharide biopolymers, protein biopolymers, and polymers like polydopamine (employed in intraocular lenses, as outlined in Table 3). These polymers find widespread use in formulating hydrogels, films, eyedrops, ocular inserts, NPs, and so on, offering advantages such as enhanced mucoadhesion, biocompatibility, non-toxicity, cationic characteristics (e.g., chitosan), favorable rheological properties, biodegradability, and the ability to provide sustained drug release in topical formulations.115, 116, 120 Numerous polymer structures have been employed to enhance and control ocular drug delivery on both macro and nanoscales. Both synthetic and biopolymers have the potential to be shaped into various forms, including nanospheres, nano-capsules, liposomes, hydrogels, dendrimers, NPs, nanomicelles, and microneedles. Furthermore, nanoscale polymers can be integrated into composite materials, for example integration of NPs in contact lenses122 as demonstrated in Figure 5. Although substantial efforts are currently underway to advance the field of polymer-based ODDS, a pivotal hurdle remains in the successful transition of these systems into clinical practice. Over the past few decades, several products have achieved market approval. Eyedrops, being the most established drug delivery platform, naturally feature a considerable array of polymer-based products. A multitude of formulations within this category have received approval for the treatment of various conditions, including but not limited to glaucoma, bacterial conjunctivitis, and uveitis.27, 28, 123
| Polymer name | Ocular indication (GRAS) | Experimental FDA-approved dosage form | Advantages | Disadvantages |
|---|---|---|---|---|
| PEG105 | Yes | (Yes) Parenteral, topicals, rectal and nasal | Water soluble, biocompatible | Fast degradation compared to other synthetic polymers |
| PLA106, 107 | Yes | (Yes) Absorbable sutures, medical devices, food packaging | Synthesized from natural sources, easily processed | Slow degradation rate |
| PGA106 | Yes | (Yes) Absorbable sutures and medical devices | Fast degradation rate | Weak mechanical properties, brittle |
| POE11 | No | (Yes) Therapeutic drug delivery | Degrades via surface erosion | Not heavily investigated for drug delivery uses |
| PVA108 | Yes | (Yes) Coating agent, food additive, and packaging | Slow degradation rate | Synthesized with aggressive solvents |
| PCL109 | No | (Yes) Medical implants, drug delivery devices | Easily modified, inexpensive | Not FDA-approved for ocular applications |
| PLGA110 | Yes | (Yes) Medical implants, drug delivery, and medical devices | Controlled degradation rate, water-soluble, most common polymer used in ocular drug delivery | Forms acidic degradation by-products |
| PAA111 | No | (Yes) Topical drug delivery | Highly water-soluble, mucoadhesive | Biodegradation into acidic byproducts |
- Abbreviations: FDA, Food and Drug Administration; GRAS, generally recognized as safe; PAA, poly(acrylic acid); PCL, poly(caprolactone); PEG, poly(ethylene glycol); PGA, poly(glycolic acid); PLA, Poly(lactic acid); PLGA, poly(lactic-co-glycolic acid); POE, Poly(orthoester); PVA, poly(vinyl alcohol).
| Biopolymer name | Ocular indication | Experimental FDA-approved dosage form | Advantages | Disadvantages |
|---|---|---|---|---|
| Gelatin114 | Yes | (Yes) Medical devices, food excipient | Easily obtained, biocompatible, less immunogenic, | Still immunogenic, difficult to safely crosslink |
| Chitosan73, 115 | No | (Yes) food additive, wound dressing | Mucoadhesive, positively charged at physiologic pH | Insoluble in neutral or alkaline solutions, brittle in hydrogel form, strong electrostatic behavior |
| Guar Gum116 | Yes | (Yes) Food excipient | Mucoadhesive, antioxidant, low antigenicity, biodegradable, | Brittle, excessive swelling, low recoverability |
| Pullulan117 | Yes | (Yes) Food additives, tablet coatings | Easily derived, stable, good biodegradable, nontoxic | Very slow diffusion |
| HA118 | No | (Yes) Cosmetic fillers, injectable for osteoarthritis, topicals | Biocompatible, mucoadhesive, good rheology, naturally occurring | Challenging to functionalize |
| Carboxy-methylcellulose119 | Yes | (Yes) Disintegrants, dental devices | Biodegradable, biocompatible, capable of sustained release, pH-sensitive | It is challenging to develop proper viscous solutions |
| Cellulose120 | Yes | (Yes) Food additives and topical formulations | Biocompatible, nontoxic, high molecular loading potential, nano-construct fabrication feasible | Low solubility |
| Collagen121 | Yes | (Yes) Food additives, cosmetic parenteral, wound dressings | Biodegradable, biocompatible, bioactive, significant existing use in medicine | immunogenicity risks, variable quality, concerns about animal sources |
- Abbreviations: FDA, Food and Drug Administration; HA, hyaluronic acid.
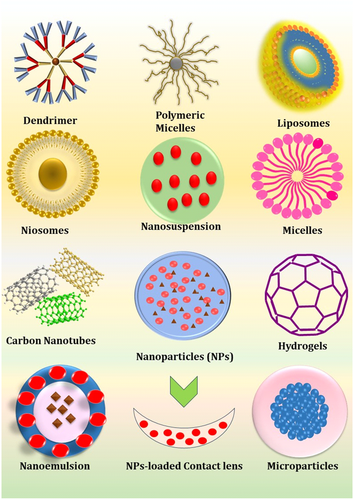
Polymers find diverse applications in various formulation models and strategies for popular DDSs. An exemplary instance can be observed in the research conducted by Vildanova et al., who innovatively engineered a biodegradable hydrogel by employing modified chitosan and HA. Their primary objective was to enhance the controlled release of drugs, particularly mitomycin C and 5-fluorouracil. Through their investigations, formulations featuring higher molecular weight of chitosan demonstrated notable promise and efficacy in extending the duration of drug release.124 In line Chun et al. adopted a gelatin-based hydrogel for the administration of small interfering RNA (siRNA), targeting siSPARC (siRNA for secreted protein, acidic and rich in cysteine), with the aim of mitigating subconjunctival scarring post-trabeculectomy. Their hydrogel delivery system for siSPARC demonstrated significant effectiveness in reducing subconjunctival scarring following glaucoma filtration surgery. Furthermore, it exhibited non-cytotoxic properties compared to a conventional anti-scarring agent frequently utilized in such procedures.125, 126 Taking this into consideration, Zafar et al.127 devised a chitosan-coated carteolol niosome (CH-CT-NIM) as a DDS utilizing the thin-film hydration method. The refined formulation, coated with chitosan, not only improves biodegradability but also enhances bioadhesion, facilitating the delivery of carteolol (CT). Drug release investigations demonstrated a more sustained release profile for CH-CT-NIM compared to both CT niosome and CT solution formulations.127 Subsequently, Song et al.128 employed a coaxial electrospray technique to encapsulate PLGA NPs with phosphatidylserine (PS), thereby enhancing entrapment efficiency and corneal permeation for the topical delivery of brinzolamide (Brz). Through release studies conducted in simulated tear fluid, the sustained release profile of Brz from Brz-PS-PLGA was observed, alongside nontoxic effects on cells as revealed by cytotoxicity assessments. PLGA facilitated the corneal penetration of Brz and demonstrated efficacy in reducing IOP.128 Following this, Lorenzo-Soler et al.129 utilized cyclodextrin (CAP) NPs for the delivery of angiotensin receptor blockers, specifically candesartan and irbesartan. This approach aimed to mitigate the conventional side effects associated with oral administration while achieving comparable reductions in IOP to those observed with timolol eyedrops. Pharmacokinetic investigations have demonstrated that both formulations effectively deliver therapeutic doses of the drug to intraocular tissues by enhancing drug permeation through the aqueous tear film and the eye wall, consequently augmenting bioavailability.129
Thereafter, Nepp et al.130 conducted a study on the efficacy of chitosan-NAC (N-acetyl cysteine) eyedrops (marketed as Lacrimera™) for patients diagnosed with dry eye disease, revealing sustained improvements over a 1-month treatment duration. Evaluation via corneal staining indicated that Lacrimera™ is both safe and effective in the management of dry eye disease. The attributed wound healing effect of chitosan-NAC is linked to its thiol group, which facilitates polymer-mucin interaction, leading to enhanced tear adherence to the corneal epithelium and subsequent protection of the ocular surface.130 In a parallel study, Chae et al.131 developed a HA hydrogel microneedle injection loaded with an in situ-forming gel to expand the suprachoroidal space. The injection of commercial HA hydrogel into the suprachoroidal space resulted in a reduction in IOP for ~1 month, while the optimized HA hydrogel formulation extended this reduction to about 4 months. Given that HA is naturally present in the eye as a component of various structures and is a biocompatible substance used in formulations injected into the eye and other body parts, it serves as a suitable candidate for such applications.131 Adjacent to this research, Jain et al.132 documented a formulation featuring a polymeric membrane comprising sodium CMC (NaCMC) and PVA, designed to confer mucoadhesive and biodegradable properties to the formulations. NaCMC, recognized as a viscosity-inducing polymer, yields a translucent solution and membrane characterized by low mechanical strength, thus restricting its usage primarily to ocular biomaterial applications. The objective of this endeavor is to leverage the mechanical attributes of PVA along with the adhesion characteristics of NaCMC to achieve sustained drug delivery over an extended period.132 In light of these findings, Abrego et al. developed NPs loaded with pranoprofen and dispersed them into a hydrogel matrix. The formulation utilized PLGA as the polymer for NP formation, while carbomer 934 (polyacrylic acid) served as the base for the hydrogel. With the aim of enhancing the biopharmaceutical profile of the selected nonsteroidal anti-inflammatory drug, optimized nanosuspensions were incorporated into carbomer hydrogels or hydrogels containing 1% azone. This strategy aimed to prolong the ocular contact time of pranoprofen, enhancing its retention in the organ and consequently improving its anti-inflammatory and analgesic efficacy.133
Following this, Christiansen et al. developed various scaffolds for the delivery of retinal progenitor cells (RPCs), including PCL short nanowires (SNWs), PCL electrospun, and PCL smooth scaffolds. These scaffolds were compared in terms of their fabrication methods and subsequent effectiveness in facilitating RPC delivery. The utilization of different fabrication techniques for PCL membranes undeniably resulted in varying degrees of flexibility, consequently impacting the ease of implantation into the eye. Notably, PCL-SNW demonstrated the highest ease of insertion into the subretinal space, attributed to its high permeability and slow degradation kinetics, which minimized the formation of an acidic microenvironment compared to polymers with higher molecular weights.134 Subsequently, Xu et al.135 devised chitosan oligosaccharide valyl valine-stearic acid (CSO-VV-SA) nanomicelles and hydrogen-castor oil 40/octyl alcohol 40 (HCO-40/OC-40) hybrid nanomicelles for topical ocular drug delivery. Both formulations exhibited negligible cytotoxicity and demonstrated similar effects on human corneal epithelial cells and human conjunctival epithelial cells. In vivo studies on precorneal retention revealed that dexamethasone from both nanomicelles remained detectable for more than 3 h in rabbits.135 Building upon this research, Jiao et al.136 employed a pioneering approach by utilizing a novel polyacrylamide semi-interpenetrating network hydrogel composed of quaternary ammonium chitosan and tannic acid to develop an innovative antibacterial and antioxidant contact lens. The resulting hydrogels demonstrated elevated water content, enhanced light transmittance, and improved resistance to swelling. Additionally, the hydrogel exhibited antioxidant properties attributed to the presence of tannic acid, which effectively alleviates oxidative stress and provides protection against reactive oxygen species-induced toxicity.136, 137
Later on, Dukovski et al.138 engineered a functional cationic ophthalmic nanoemulsions (NE) containing 0.05% (w/w) chitosan and loaded with nonsteroidal anti-inflammatory drugs. Chitosan served as the cationic surfactant, while lecithin acted as the anionic surfactant, effectively addressing solubility challenges and prolonging drug residence on the ocular surface, thereby stabilizing the tear film. Results indicated that the NE formulation with 0.05% (w/w) chitosan emerged as the primary formulation, displaying mucoadhesive properties and excellent biocompatibility.138 Following these studies, Ibrahim et al. illustrated that chitosan NPs extended the duration of brimonidine's effect until 23.2 h postadministration, in contrast to the drug solution, which ceased its effect after 7 h. The NPs were synthesized using the emulsification solvent diffusion method, resulting in uniform drug content across formulations. Due to chitosan loading, all formulations exhibited a sustained lowering effect on IOP compared to brimonidine tartrate (BT) eyedrops.139 Continuing this line of research, Ghelardi et al. showcased the effectiveness of tamarind seed polysaccharide as a delivery system for ocular administration of both hydrophilic and hydrophobic antibiotics. This polysaccharide demonstrated a notable increase in intra-aqueous penetration across various conditions. Consequently, it was proposed that the polysaccharide facilitates prolonged precorneal residence of antibiotics, leading to enhanced drug accumulation in the cornea. This suggests its potential as a promising vehicle for topical treatment of bacterial keratitis.140, 141
Subsequently, Lin and Sung142 developed a Carbopol-Pluronic solution for ophthalmic drug delivery, which can function as an in situ gelling system to enhance bioavailability. Through experimentation, the optimal concentrations for Carbopol solution and Pluronic solution to form the in situ gel were determined to be 0.3% (w/w) and 14% (w/w), respectively. The resulting solution exhibited a notable increase in gel strength, particularly under physiological conditions. In vivo studies further confirmed that the Carbopol/Pluronic solution retained the drug more effectively compared to solutions containing Carbopol or Pluronic alone.142 Additionally, Gurny and colleagues.143 formulated bioadhesive ophthalmic gentamycin inserts employing a combination of hydroxypropyl methylcellulose (HPMC), EC, and PVA. To extend the release of gentamycin sulfate (GS), inserts incorporating GS/CAP inclusion complex, solid dispersion, GS/EC/CAP coprecipitate, and HPMC were investigated, revealing superior efficacy in terms of duration of action. However, inserts containing GS/EC/CAP and HPMC exhibited higher levels of irritation.143 Likewise, Hornhof et al.144 fabricated ocular inserts utilizing PAA 450-cysteine conjugates and 450 kDa thiolated PAA through direct compression. Experimental findings indicated that inserts comprised of thiolated PAA exhibited insolubility yet possessed favorable cohesive properties, thereby facilitating controlled drug release from the system. These inserts demonstrated sustained fluorescein concentration on the eye surface for over 8 h, with a rapid decline upon the application of aqueous eyedrops.144
Another study reported by Cavalli et al.145 assessed solid lipid NPs as carriers for tobramycin, comparing them with commercial eyedrops. Their findings indicated significantly higher bioavailability and no evidence of ocular irritation compared to the commercial formulation. The enhanced bioavailability could potentially be attributed to the longer preocular retention and extremely small size of the solid lipid NPs. This approach holds promise as a potential replacement for tobramycin-loaded subconjunctival injections in cases of pseudomonal keratitis.145, 146 In a separate investigation, Liu et al.147 devised an in situ nanogel system for curcumin delivery. Their findings revealed that the nanogel incorporating a lipid carrier exhibited an extended mean residence time within the eye. The formulated nanogel demonstrated zero-order drug release kinetics, resulting in a noteworthy enhancement in maximum drug concentration (Cmax) and mean residence time, indicative of a controlled-release formulation.147 Continuing this line of research, Pignatello et al.148 developed a nanosuspension containing ibuprofen and Eudragit RS100 for intraocular delivery, achieving higher concentrations compared to ibuprofen solution in rabbit models. The nanosuspensions were prepared using the quasi-emulsion solvent diffusion technique, resulting in NPs with a mean size of approximately 100 nm, rendering them suitable for ophthalmic applications. In vitro studies suggested a controlled release profile of the NPs, further highlighting their potential for sustained drug delivery in ocular therapies.148 In a similar vein, Lancina et al.149 explored a novel topically administered nanofiber loaded with BT and polyamidoamine dendrimers. The dendrimer-based nanofiber (DNF) exhibited no toxicity or ocular irritation in rat models. Comparisons between DNF and BT solution at a single dose revealed equivalent IOP outcomes. However, long-term applications demonstrated improved efficacy of the DNF over a 3-week test period, suggesting its potential for sustained therapeutic benefits in ocular conditions.149 The majority of these polymer-based eyedrop formulations leverage the unique properties of polymers to enhance the retention time of the drops and optimize drug release efficiency. In addition to these established applications, ongoing investigations are exploring the potential of polymer nanocarriers and thermosetting gels to further extend the duration of drug release and enhance drug penetration.27, 123
5 NEWER ODDS TECHNOLOGIES
Investigating the potential of employing eyedrops for delivering drugs to the posterior segment presents substantial potential, offering the prospect of revolutionary strides in ocular drug delivery. Conversely, advancements in the intravitreal domain have been relatively gradual, securing regulatory approval for only a few intravitreal polymer systems, primarily tailored for specific diseases.28, 123, 150, 151 Various intravitreal implants, such as Retisert®, Vitrasert®, Ozurdex®, Iluvien®, Dextenza®, Yutiq®, and DEXYCU®, integrate a variety of polymers in their composition. Table 4 expands upon these breakthroughs and integrating insights from polymers employed in various ODDS offers a promising and viable approach to drive advancements in polymer-based systems targeting the posterior segment of the eye.
| Product | Polymer(s) | Drug | Route of administration | Therapeutic indication |
|---|---|---|---|---|
| TRIESENCE® | Carboxymethyl-cellulose | Triamcinolone Acetonide | IV Inj. | Uveitis, temporal arteritis, |
| Optive Fusion | HA/CMC | HA/CMC | ED | Dry Eye Syndrome |
| Ozurdex® | PLGA | Dexamethasone | IV Imp | Diabetic macular edema, uveitis |
| Dextenza | PEG-Fluorescein | Dexamethasone | IV Imp | Ocular inflammation |
| Yutiq® | Polyimide | Fluocinolone Acetonide | IV Imp | Posterior uveitis |
| SUSVIMOTM | Polysulfone/Silicone | Ranibizumab | RIO Imp | Wet AMD |
| ACUVUE® and others | PHEMA, PMMA | None (contact lenses) | AEP | Vision correction |
| Optive | CMC | CMC | ED | Dry Eye Syndrome |
| Macugen® | PEG | Pegaptanib sodium | IV Imp | Late-stage AMD |
| Tears Naturale® | HPMC, Dextran70 | HPMC | ED | Dry Eye Syndrome |
| Xen Gel | Gelatin | None (glaucoma drainage) | Subconjunctival implant | Glaucoma |
| Azasite® | PCP | Azithromycin | ED | Bacterial conjunctivitis |
- Abbreviations: AEP, anterior eye placement; CMC, carboxymethyl cellulose; DDS, drug delivery system; ED, eyedrop; HPMC, hydroxypropyl methylcellulose; IV Imp, intravitreal implant; IV Inj, intravitreal injection; PCP, polycarbophil; PEG, poly(ethylene glycol); PLGA, poly(lactic-co-glycolic acid); RIO Imp, refillable intraocular implant.
6 SUMMARY AND FUTURE PERSPECTIVES
The first intravitreal implants were approved in 1996, while topical applications began in the 1970s. However, many polymer applications in ODDS are still in research, with tremendous untapped potential to improve treatment capability, quality, and simplicity. Polymer-based ODDS preclinical and clinical trials will expect to expand during the next decade. Eyedrop systems have successfully developed and approved polymers to lengthen drop residence duration on the cornea. However, corneal penetration issues warrant more study. Researchers are translating nano-formulations and gelling agents to clinical applications to improve eyedrop administration. Subconjunctival and suprachoroidal injections and implants can develop swiftly once suitable polymer formulations are found. Although natural polymers exhibit superiority in topical drug delivery for both conventional and novel ophthalmic formulations, their utility is hampered by challenges in efficient scale-up technologies and sterility issues related to ophthalmic dosage forms. Efforts at an industrial scale are required for the extraction, purification, and authentication of natural polymers to overcome challenges during regulatory approval. Polymer-based drug delivery has been a focal point of extensive research, but there are still advancements needed to fully harness the beneficial properties of these materials. The potential of polymeric DDSs and nanotechnology holds promise for the delivery of larger molecules, including proteins and peptides, to the eye. However, persistent challenges in corneal penetration require further exploration. Ongoing research aims to translate technologies such as nanomicelles and gelling agents into clinical applications, with the goal of enhancing the efficacy of ODDS. Although there have been advancements, there are still opportunities for further improvement in this field. Similarly, the efficacy of topically administered chemotherapeutic drugs for treating ocular melanoma, including uveal, corneal, and conjunctival melanoma, is limited by the challenge of inadequate drug penetration into deeper tissues, formulating delivery systems loaded with chemotherapeutic drugs and marked with specific moieties provides a potential strategy for targeting melanoma cells and achieving sustained release. In conclusion, the future appears promising for the utilization of polymers in ODDS. Supported by established clinical technologies and numerous ongoing clinical trials assessing advanced delivery systems for enhanced efficiency, ongoing research in polymer science is poised to explore new applications in ocular delivery.
AUTHOR CONTRIBUTIONS
Amol C. Bisen conceptualized, wrote, edited, and revised the manuscript content. Arpon Biswas, Ayush Dubey, Sachin N. Sanap, Sristi Agrawal, Karan S. Yadav, Vaishali Singh, Priyanka Rawat, and Sudhansu Sagar wrote separate topics, edited, revised, and proof-read the manuscript. Madhav N. Mugale and Rabi S. Bhatta edited the manuscript, guided, and administered the work. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors Amol C. Bisen, Arpon Biswas and Sristi Agrawal are thankful to the Indian Council of Medical Research, New Delhi, India, for providing Senior Research Fellowship grant 3/1/3(3)/OPH/20-NCD-II. The CSIR-CDRI, institutional communication number allotted to this paper is 10760.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict and interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this paper.




