Advanced Responsive Hydrogels for Diabetic Wound Healing: Design Principles, Controlled Drug Delivery, Therapeutic Strategies, and Application Prospects
Jiyuan Du, Caihong Xian, and Xiaodan Liang contributed equally to this study.
ABSTRACT
Diabetic wounds characterized by impaired healing and amputation risks, pose clinical challenge worldwide. Hydrogel dressings have emerged as a promising therapeutic strategy due to their ability to absorb exudate, prevent infections, and control therapeutic agents delivery, with over a dozen products clinically approved or in trials. However, these hydrogels rely on passive drug release mechanisms, which do not dynamically respond to the pathological microenvironment of diabetic wounds, such as high glucose, elevated ROS, acidic pH, and increased enzyme activity, resulting in mismatched release kinetics and suboptimal therapeutic outcomes. To address these challenges, researchers have developed smart responsive hydrogels that utilize the wound's endogenous pathological cues as triggers for on-demand, spatiotemporal drug delivery. This approach enables personalized therapy by precisely modulating drug release in response to real-time wound changes, offering a transformative solution for enhancing healing efficacy. Herein, we review the pathological features of diabetic wounds, and then explores the design principles and therapeutic strategies of smart responsive hydrogels. Importantly, the review evaluates the challenges associated with these technologies and outlines future engineering directions to optimize their clinical adoption. This review aims to contribute to the rational design and practical clinical application of smart hydrogels for chronic wound care.
1 Introduction
Diabetes mellitus (DM) is a prevalent chronic metabolic disorder characterized by elevated blood glucose levels due to either intrinsic or acquired deficiencies in insulin production [1-4]. It affects over 10% of the global population, with projections estimating a dramatic increase to 700 million by 2045. DM patients are often accompanied by serious complications such as cardiovascular disease, neuropathy, and diabetic foot ulcers (DFUs) [3-8]. Among them, DFUs are particularly concerning, with prevalence rates alarmingly varying from 1.0% to 30.0% worldwide. Approximately 19%–34% of diabetic patients suffer from impaired wound healing, making DFUs a common and devastating consequence. It is reported that a limb amputation occurs every 30 s due to DFUs, underscoring the alarming fact that 85% of amputations in diabetic patients with poor wound healing are preceded by an ulcer [9]. Current treatments such as debridement, off-loading, antibiotics, and negative pressure wound therapy are widely employed in clinical practice. However, these primarily aim to slow DFU progression and alleviate pain, without fully restoring the complex and prolonged healing process, often leaving wounds vulnerable to recurrence [10]. Diabetic wound healing continues to pose a significant challenge for global healthcare systems.
Wound dressings such as electrospun nanofiber, foam, and hydrogels have emerged as significant advancements in the treatment of DFUs due to their wound-healing capabilities [11-15]. Electrospun nanofibers, characterized by their high surface-area-to-volume ratio and architecture that mimics the extracellular matrix (ECM), show promise for facilitating cellular attachment and growth [16, 17]. However, their clinical application is limited by insufficient mechanical strength and challenges in achieving precise drug delivery. Foam dressings, while absorbent and structurally robust, may promote granulation tissue ingrowth, leading to painful adhesion [18]. Hydrogels, with their three-dimensional (3D) network structures that replicate the ECM environment, exhibit excellent hydrophilicity, good drug-loading capacity, and sustained drug release, making them ideal for diabetic wound care [19-24]. Furthermore, the porous nature of hydrogels facilitates gas exchange and nutrient delivery, which is critical for effective wound repair [25]. Currently, over a dozen hydrogel-based products are either clinically approved or undergoing trials for diabetic wounds management (summarized in Table 1) (source: National Library of Medicine). They aim to achieve outcomes such as reduced wound size, increased epithelialization rates, complete wound closure, and pain relief [26]. For example, Healoderm, Aquaform, and Intrasite Gel are in clinical trials, while Fitostimoline and Woulgan have completed Phase IV studies demonstrating improved healing processes. Furthermore, bioactive formulations such as RMD-G1 (erythropoietin-loaded hydrogel) and GAT@F-nanoenzyme-hydrogel complexes are under evaluation for their potential to enhance granulation and accelerate tissue regeneration. Stem cell-derived platforms, including ALLO-ASC-DFU and TWB-103, represent a new frontier of biofunctional hydrogel therapy with regenerative potential. Nonetheless, most clinically tested hydrogels rely on passive drug release mechanisms, which do not dynamically respond to the pathological microenvironment of diabetic wounds. This limitation often results in mismatched release kinetics and suboptimal therapeutic outcomes.
| Hydrogel dressing | Clinical phases | Outcome measure | Status | Clinical trials numbers | Date |
|---|---|---|---|---|---|
| Fitostimoline | 4 | Proportion of patients' complete responders | Completed | NCT05661474 | 2021.02-2022.12 |
| Woulgan | 4 | Wound Size | Completed | NCT02631512 | 2015.10-2019.04 |
| AmeriGel | 4 |
|
Terminated (recruitment problems) | NCT01350102 | 2012.02-2014.03 |
| Lavior Diabetic Wound Gel | 2/3 |
|
Completed | NCT05607979 | 2022.12- 2024.05 |
| TTAX01 (AMBULATE DFU II) | 3 | Time for wound healing | Completed | NCT04450693 | 2020.11-2025.03 |
| TTAX01 (AMBULATE DFU) | 3 | Time for wound healing | Completed | NCT04176120 | 2020.07-2024.06 |
| ALLO-ASC-DFU | 3 | Proportion of patients who complete wound closure | Completed | NCT03370874 | 2018.06- 2020.02 |
| ALLO-ASC-SHEET | 2 | Proportion of patients who complete wound closure | Recruiting | NCT04497805 | 2020.09- Present |
| GAT@F nanoenzyme hydrogel complex | 2 |
|
Active, not recruiting | NCT06492811 | 2024.04-Present |
| P1G10 | 2 | 100% epithelization | Completed | NCT03700580 | 2012.08 −2016.10 |
| A hydrogel containing erythropoietin (RMD-G1) | 1/2 |
|
Completed | NCT02361931 | 2016.03-2018.06 |
| IZN-6D4 Gel | 2 | Percent reduction in wound area at week 4 | Completed | NCT01427569 | 2012.03-2015.08 |
| TWB-103 (mixture of TWB-102 cell and TWB-103 hydrogel) | 1/2 |
|
Completed | NCT03624023 | 2019.12-2021.07 |
| Treprostinil iontophoresis | 1/2 | Comparison of wound closure | Terminated (recruitment difficulties) | NCT03654989 | 2020.01- 2021.12 |
| Hydrogel with 3% sodium pentaborate pentahydrate | 1 | Wound healing | Unknown status | NCT02241811 | 2014.09- Present |
- Source: https://www.clinicaltrials.gov/.
In contrast to the well-defined phases of normal wound healing (inflammation, proliferation, remodeling), diabetic wound healing is hindered by persistent hyperglycemia, elevated ROS levels, acidic pH, and overexpressed matrix metalloproteinases (MMPs). These factors lead to excessive inflammation, impaired angiogenesis, and delayed re-epithelialization [27-31]. To overcome the limitations of clinical diabetic wound dressings, smart responsive hydrogels are being engineered to respond to specific diabetic wound environments. By achieving precise and controlled drug release on demand, they aim to ameliorate the harsh microenvironment at the diabetic wound site. To achieve personalized therapy, single- and dual-responsive hydrogels targeting key biomarkers such as glucose, ROS, pH, and MMPs have been developed [32-39]. These hydrogels precisely modulate drug release in response to real-time changes within the wound microenvironment. This enables stage-specific interventions, modulating the inflammatory response during the inflammation phase, improving vascular formation in the proliferation phase, and promoting tissue remodeling in the remodeling phase, thereby enhancing overall healing efficacy. Consequently, these smart responsive hydrogels hold significant promise as wound dressings to offer advanced treatment for diabetic wounds.
In this review, we systematically introduce the pathological microenvironment of diabetic wounds. Targeting the aberrant signals within this environment, we elaborate on the design principles of responsive hydrogels. Furthermore, we elucidate the controlled drug release in these intelligent hydrogels for diabetic wound repair, highlighting their therapeutic strategies to ameliorate hyperglycemia, modulate inflammation, inhibit bacterial colonization, exert antioxidant effects, and promote angiogenesis. Finally, we discuss the advantages and challenges of smart responsive hydrogels in diabetic wound therapy and provide insights into their future clinical translation for enhanced diabetic wound healing (Figure 1).
2 The Pathophysiology of DFU
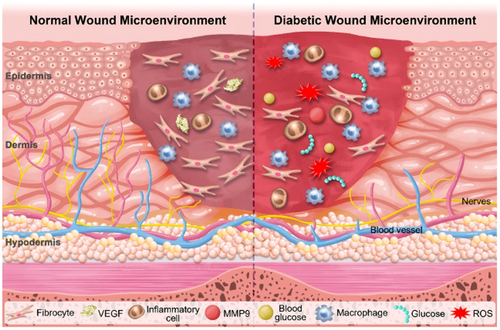
The body initiates multiple complex mechanisms to protect itself through repair and reconstruction immediately following an injury [40]. The normal wound healing processes are categorized into four physiological stages: hemostasis, inflammation, proliferation, and remodeling [41, 42]. However, the wound healing process of DFU is disrupted in multiple mechanisms at different stages due to the increase of advanced glycosylation end products (AGEs) in the bloodstream [43, 44]. Hyperglycemia and AGE levels fosters an inflammatory condition. Unlike the normal wound healing progresses that macrophages transition from an inflammatory M1 phenotype to an anti-inflammatory M2 phenotype, facilitating cell proliferation and wound closure [45-47], they remain in the pro-inflammatory M1 state due to pathogen activation, continuously releasing inflammatory mediators like interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-alpha (TNF-α) in individuals with diabetes [48-50].
Additionally, AGEs directly trigger immune cells to overproduce ROS, thereby increasing oxidative stress, disturbing the cellular redox equilibrium and worsening metabolic complications within the wound site [50-52]. The high level of ROS and the large number of pro-inflammatory cytokines at wound site also prompt the synthesis of MMPs, which degrade the ECM and hinders the formation of granulation tissue, disrupting the wound healing [53-55]. Moreover, MMPs induce a hypoxic and nutrient-deficient microenvironment in wound site by limiting tissue oxygenation and impeding angiogenesis, substantially slowing the healing and regeneration processes [56]. Meanwhile, diabetic wound displays cellular phenotypic abnormalities in both the epidermis and dermis, characterized by reduced growth factor receptor density and inhibited cell proliferation. These abnormalities compromise the capacity of functional cells to swiftly react to signals that promote wound healing [57].
Furthermore, diabetic wound has a higher vulnerability to infections caused by pathogenic bacteria. The rapid multiplication of bacteria and/or the anaerobic glycolysis resulting from the hypoxic microenvironment conducive to bacterial survival can cause an acid wound microenvironment [58, 59]. This acid microenvironment further supports bacterial proliferation, complicating the wound healing process by exacerbating inflammation [60, 61]. Overall, the diabetic wound microenvironment, characterized by high blood glucose and ROS levels, hypoxia, abnormal protease activity, and acidic pH, promotes persistent inflammation, ultimately arresting the wound healing (Figure 2).
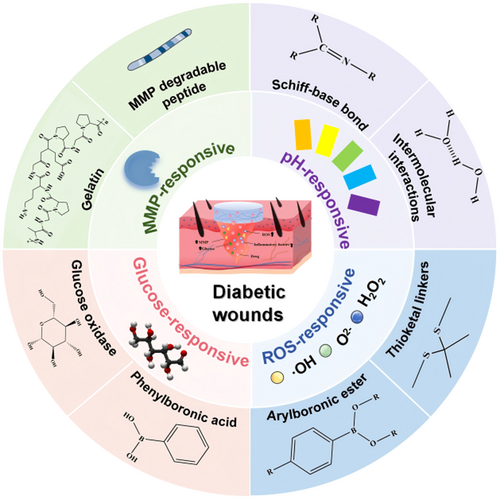
3 Design Principles of Responsive Hydrogels
Stimuli-responsive hydrogels represent a class of intelligent biomaterials capable of undergoing programmed physicochemical transformations in response to specific endogenous or exogenous cues. Beyond their inherent hydrophilicity and 3D network structure, these hydrogels are now being molecularly engineered to achieve spatiotemporally controlled drug release. Leveraging polymer chemistry, diverse stimulus-sensitive moieties can be incorporated into the hydrogel backbone or crosslinking domains. This design enables selective recognition of pathological changes characteristic of diabetic wounds (e.g., pH fluctuations, elevated ROS, hyperglycemia, MMP overexpression). Upon recognition, these hydrogels can alter their network structure and swelling behavior, thereby facilitating the on-demand release of therapeutic agents. This section outlines the fundamental design principles associated with each stimulus modality, with emphasis on the underlying responsive mechanisms of pH-, ROS-, glucose-, and MMP-responsive hydrogels.
3.1 Design Strategies of pH-Responsive Hydrogels
pH-responsive hydrogels are engineered through the incorporation of pH-sensitive linkages that undergo targeted structural transformations or cleavage in specific pH environments, enabling spatiotemporally controlled drug release. Primary design strategies involve integrating acid-labile covalent bonds (e.g., imine bonds and boronate esters) or ionizable moieties (e.g., citraconic anhydride and dimethylmaleic anhydride) into the polymer network [62, 63]. Among them, Schiff base linkages, including imines, hydrazones, and oximes, are widely employed due to their sensitivity to mildly acidic conditions. These dynamic covalent bonds form via condensation reactions between primary amines and carbonyl compounds (aldehydes/ketones), generating hydrolytically labile imine bonds (C = N) [64]. Under acidic conditions, these linkages undergo rapid hydrolysis, leading to network disintegration and therapeutic release, yet remain stable at physiological pH to preserve hydrogel's structural integrity. In addition to covalent systems, non-covalently cross-linked hydrogels can also achieve pH responsiveness. For example, pH-labile hydrogen bonds, characterized by weak, reversible interactions, undergo dissociation upon protonation of polymeric chains in acidic microenvironments, thereby facilitating on-demand drug release [65].
3.2 Design Strategies of ROS-Responsive Hydrogels
ROS-responsive hydrogels are designed by incorporating redox-sensitive motifs that undergo structural or chemical transformation upon ROS exposure. These systems often integrate ROS-labile linkages through covalent, coordination, or supramolecular interactions [66]. The selection of ROS-sensitive functional groups and their integration into polymeric frameworks plays a pivotal role in tuning both responsiveness and stability. A widely employed class involves phenylboronic acid esters (PBAEs), which are oxidized by ROS to yield hydrophilic phenols and boric acid. This transformation not only weakens the hydrophobic interactions within the hydrogel matrix, promoting swelling and drug diffusion, but also confers intrinsic ROS-scavenging capability [67]. An alternative design strategy involves utilizing sulfur-based functionalities, including thioacetals, thioketals, and thioethers [68]. Among these, thioketal linkages, synthesized via the condensation of ketones and dithiols, maintain stability under physiological conditions but undergo selective C-S bond cleavage when exposed to hydrogen peroxide or hydroxyl radicals. This irreversible cleavage mechanism enables precise spatiotemporal hydrogel degradation [69].
Moreover, redox-active natural amino acids can be incorporated into hydrogel matrices to impart ROS sensitivity. Disulfide bonds (-S-S-), formed through oxidative coupling of cysteine residues, constitute redox-switchable units that facilitate network disassembly in ROS-rich microenvironments [70, 71]. These bonds undergo stepwise oxidation to generate a series of sulfur-containing intermediates, ultimately facilitating the controlled disassembly of hydrogel [72]. In addition to cleavage-based strategies, ROS-responsive systems can also be constructed using bioinspired oxidative conversion pathways. For example, l-Arginine, a precursor of nitric oxide (NO), undergoes nonenzymatic oxidation by hydrogen peroxide to generate NO in situ, providing a biomimetic route for spatiotemporally controlled NO release [73].
3.3 Design Strategies for Glucose-Responsive Hydrogels
Glucose, the primary metabolic substrate for most tissues, is tightly regulated under physiological conditions. However, pathological hyperglycemia elevates glucose concentrations to levels markedly exceeding the norm, disrupting metabolic homeostasis [74, 75]. Capitalizing on this dysregulation, glucose-responsive hydrogels have been developed by incorporating glucose-sensitive components, including natural proteins (glucose oxidase (GOx) and concanavalin A (ConA)), and synthetic moieties like phenylboronic acid (PBA). GOx is a flavin-containing glycoprotein that catalyzes the oxidation of glucose into gluconic acid and hydrogen peroxide [76]. This enzymatic reaction is highly dependent on glucose concentration, thereby inducing alterations in key physicochemical hydrogel properties, including swelling kinetics, degradation profiles, and mechanical integrity. ConA, a plant-derived lectin with affinity for α-d-glucose and α-d-mannose, enables reversible noncovalent crosslinking with glycosylated polymers, facilitating glucose-responsive network assembly and disassembly [77]. Conversely, PBA serves as a synthetic glucose-recognition unit, capable of forming reversible covalent bonds with cis-diols through dynamic boronate esterification. PBA exists in equilibrium between a neutral trigonal configuration and an anionic tetrahedral boronate form, with the latter demonstrating enhanced glucose-binding affinity. Under hyperglycemic conditions, cyclic boronate ester formation modulates hydrogel properties, altering crosslinking density, swelling behavior, and inducing sol-gel transitions [78, 79]. This modular design allows for precise glucose-triggered drug release without reliance on biological components.
3.4 Design Strategies for MMPs-Responsive Hydrogels
Enzyme-responsive hydrogels leverage the high substrate selectivity and catalytic efficiency of specific enzymes to achieve controlled matrix remodeling or drug release. Among these systems, MMPs-responsive platforms have garnered attention due to their well-characterized substrate specificity and tunable enzymatic activity. MMPs serve as biologically programmable triggers capable of initiating hydrogel degradation or payload liberation with spatiotemporal precision [80]. MMP-responsive hydrogel design typically follows two complementary strategies. Molecular engineering incorporates enzyme-cleavable peptide sequences into polymer networks via physical adsorption or covalent conjugation. These peptides undergo site-specific cleavage upon MMP exposure, enabling programmable hydrogel degradation and controlled therapeutic release [81]. Another alternative and synergistic design leverages gelatin that inherently retains MMP-sensitive domains. When formulated as hydrogel matrices or micro/nanoparticulate carriers, gelatin undergoes enzymatic degradation upon MMP recognition. This process modifies network porosity and triggers matrix disassembly, ultimately facilitating targeted drug delivery [82-84].
4 Advanced Therapeutic Strategies of Single-Responsive Hydrogels for Diabetic Wound Healing
The diabetic wound microenvironment exhibits dynamic changes both spatially and temporally, including localized inflammation, oxidative stress, and fluctuating glucose and protease levels. The heterogeneous progression of pathological features across wound healing stages challenges conventional hydrogels, which deliver drugs in a passive and nonadaptive manner. To address these limitations, stimulus-responsive hydrogels are designed by integrating stimuli-responsive moieties into the polymer network. This integration facilitates spatiotemporally controlled drug delivery that ameliorates the hyperglycemic microenvironment, modulates inflammatory responses, inhibits bacterial colonization, exerts antioxidant effects, and promotes angiogenesis. The following sections elaborate on how different classes of single-responsive hydrogels exploit the pathological stimuli within the diabetic wound microenvironment to enable spatiotemporally controlled drug delivery, aligned with the dynamic healing process, for effective treatment of diabetic wounds.
4.1 Ameliorate the Hyperglycemia Microenvironment
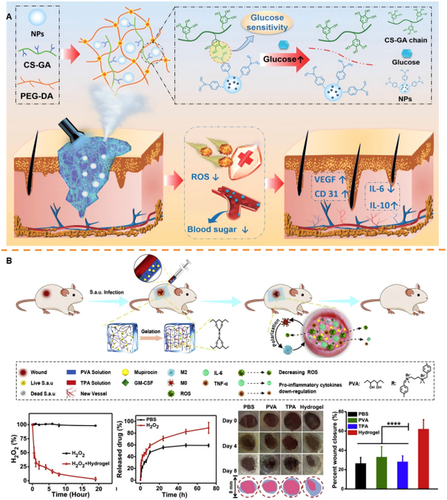
The hyperglycemic microenvironment impedes normal tissue repair by promoting advanced glycation end-product (AGE) formation, impairing immune responses, inducing oxidative stress, and suppressing angiogenesis [85]. Consequently, modulating local glucose levels at diabetic wounds using glucose-responsive hydrogels is critical. Most current glucose-responsive hydrogels employ GOx as the sensing mechanism. GOx catalyzes glucose oxidation, generating gluconic acid and H₂O₂, thereby simultaneously depleting local glucose and creating an acidic microenvironment that triggers drug release [86-93]. Beyond glucose depletion, controlled insulin release is essential. Insulin not only regulates glycemia, mitigating hyperglycemia-induced inflammation and oxidative stress, but also directly promotes collagen synthesis and angiogenesis, key processes for wound closure and regeneration. To achieve this, diverse hydrogel designs have been developed. For example, our group developed a hybrid hydrogel (PPIC) based on a PEG-DA matrix integrated with gallic acid (GA)-grafted chitosan (CS). Insulin nanoparticles (NPs) were encapsulated within PBA-modified polyethylenimine (PEI) network. Glucose binding to PBA triggered controlled insulin release, while the polyphenol backbone provided ROS-scavenging activity. This dual-function system improved glucose regulation, enhanced collagen deposition, and accelerated diabetic wound closure in vivo (Figure 3A) [94]. Additionally, to address limitations in response speed and spatial precision, microneedle (MN)-based glucose-responsive systems have emerged. Xue et al. fabricated an insulin-loaded hydrogel MN (Gel-AFPBA-ins MN) using gelatin methacryloyl (GelMA), the glucose-responsive monomer 4-(2-acrylamidoethylcarbamoyl)−3-fluorophenylboronic acid (AFPBA), and glucose-conjugated insulin (G-insulin). Under hyperglycemia, glucose binding to anionic boronates increased osmotic pressure, inducing matrix swelling and facilitating insulin release. In vivo studies confirmed enhanced collagen deposition and improved healing in diabetic wounds using this MN patch [96].
4.2 Modulate Inflammatory Response
The complex interplay of immune cells during inflammation necessitates precise therapeutic modulation. Responsive hydrogels offer targeted strategies to regulate immune/inflammatory responses through controlled drug release triggered by specific wound microenvironment cues [97-99]. For instance, pH-responsive hydrogels leverage acidic microenvironments to trigger controlled drug release that modulates macrophage polarization. Xuan et al. developed a chitosan hydrogel modified with 4-formylphenylboronic acid (FPBA) to load formyl-methyl-leucine-phenylalanine (fMLP) and SiO₂-FasL. Acidification (pH ~5.5) cleaved Schiff base bonds, releasing SiO₂-FasL to induce neutrophil apoptosis and promote M2 macrophage polarization, accelerating healing [100]. Similarly, Cai et al. synthesized an HA-collagen hydrogel crosslinked via hydrazone bonds, rapidly gelling under neutral/alkaline conditions. In acidic DFU environments, bond cleavage triggered metformin release, enhancing fibroblast proliferation and macrophage modulation [101]. For ROS-responsive strategies, Liu et al. engineered a composite hydrogel (TPA-PVA) using a ROS-cleavable TPA crosslinker. Elevated ROS levels degraded the linker, enabling spatiotemporal release of mupirocin and GM-CSF. This controlled delivery modulated the immune microenvironment and promoted angiogenesis (Figure 3B) [95]. Guo et al. utilized a 3D-printed hydrogel incorporating a ROS-degradable polyurethane (PFKU) membrane loaded with doxycycline hydrochloride (DOXH). The thioketal bonds in PFKU undergo cleavage under high ROS conditions, facilitating sustained DOXH release that stimulated endothelial migration and M2 macrophage polarization, significantly improving wound repair in diabetic mice [102]. Extending beyond ROS-responsive systems, Zhang et al. engineered an MMP-9-responsive hydrogel by crosslinking oxidized dextran with an MMP-9-cleavable peptide (PG-6) to encapsulate M2 macrophage-derived exosomes. During initial healing phases, this hydrogel primarily functions to maintain a moist wound environment. As inflammation progressed and MMP-9 levels increased, accelerated degradation triggered exosome release, reprogramming macrophages and enhancing regeneration [103]. This MMP-9-responsive design provides a dynamic “integrated diagnosis and treatment” strategy by synchronizing therapy with the inflammatory state.
4.3 Inhibit Bacterial Colonization
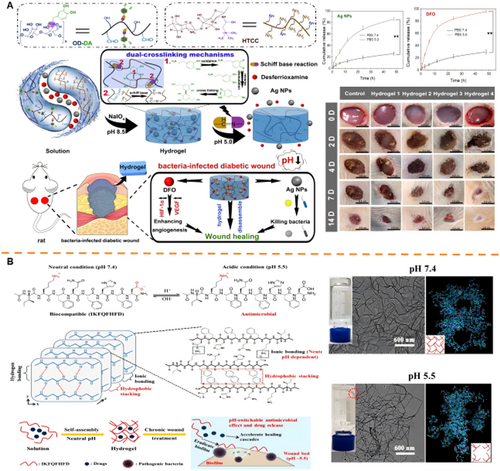
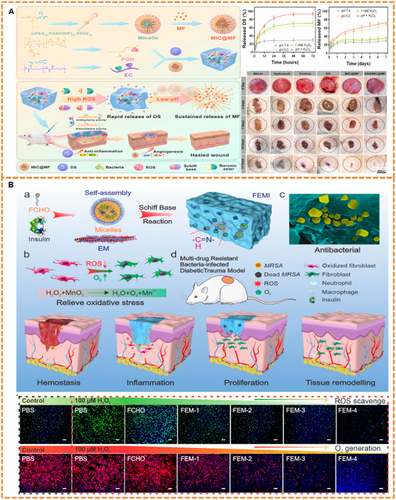
Responsive hydrogels enable targeted antibacterial action through controlled drug release triggered by pathological microenvironment cues. Key approaches include pH-, ROS-, and glucose-responsive antibacterial systems. For pH-responsive antibiotic-dependent platforms, Shao et al. engineered an OCMC-PEI/Tob hydrogel where acidic conditions (pH=5.0) cleave Schiff bases to enable spatiotemporal tobramycin release. This system demonstrated broad-spectrum antibacterial activity through synergistic Tob/PEI action, accelerating healing in P. aeruginosa-infected diabetic wounds [104]. To address antibiotic resistance concerns, hydrogels with intrinsic antimicrobial activity like chitosan derivatives have been engineered [105-111]. Wang et al. developed a dual-crosslinked HTCC/OD-DA hydrogel incorporating silver nanoparticles (Ag NPs), where acid-triggered Schiff base cleavage released Ag NPs at over threefold physiological concentrations, thereby enhancing both antibacterial and angiogenic functions (Figure 4A) [112]. Similarly, Li et al.'s pH-responsive IKFQFHFD peptide hydrogel self-assembles at neutral pH but dissociates in acidity, releasing inherent biofilm-disrupting antimicrobials that promote diabetic wound repair (Figure 4B) [113]. Building on the concept of stimuli-responsive hydrogels, Yang et al. designed a ROS-responsive hydrogel system, TSPBA/PVA/l-Arg. In this system, elevated levels of hydrogen peroxide (H₂O₂) oxidize l-arginine (l-Arg) to generate nitric oxide (NO). Sustained NO release reacts with ROS to produce peroxynitrite, conferring prolonged antibacterial activity while modulating collagen deposition [114]. Complementing these approaches, glucose-responsive cascade systems have been engineered to exert antibacterial effects through the controlled release of metal ions. For instance, Yang et al. developed the IDA-Zn²⁺/GOx/DFO hydrogel (DG@Gel), which exploits glucose-derived acidification (pH≈4.3) to release Zn²⁺ ions, thereby achieving concurrent antibacterial and angiogenic effects [115]. Furthermore, Fenton reaction platforms have been employed. A representative example is Liu et al.'s CS@SBE-β-CD@Fe²⁺-GOx hydrogel, which utilizes glucose-generated H₂O₂ to drive Fe²⁺-mediated •OH production via Fenton reactions. These radicals exerted potent antibacterial effects in hyperglycemic environments while promoting epithelialization [116]. Similar •OH-generation strategies employ CuO/Cu-MOF nanoparticles integrated with GOx-loaded hydrogels [117, 118].
4.4 Exert Antioxidant Effects
Given the spatially localized and temporally fluctuating nature of oxidative stress in diabetic wounds, ROS-responsive hydrogels offer a promising strategy for achieving spatiotemporal drug release through site-specific, redox-triggered mechanisms [119-125]. Among synthetic ROS-responsive units, PBAEs and their derivatives are prominent for their selective cleavage in ROS-enriched microenvironments [126-128]. For instance, Niu et al. developed a novel ROS-responsive hydrogel by sequentially incorporating DL-Dithiothreitol (DTT), poly(ethylene glycol) diacrylate (PEGDA), and phenylboronic acid-modified ε-Poly-l-lysine (EPL-PBA). This hydrogel effectively scavenged H₂O₂, enhanced EPL release, modulated the wound microenvironment, and accelerated healing [129]. Beyond synthetic systems, naturally derived amino acids, particularly cysteine, enable the design of ROS-responsive biomaterials. The strategic linkage of amino acids via amide bonds facilitates the synthesis of functional peptides with diverse sequences [130, 131]. Crucially, cysteine possesses a thiol group (-SH) that undergoes ROS-triggered oxidation to form disulfide bonds [132]. Li et al. demonstrated this principle using genetically encoded hydrogels containing RGD and cysteine-proline-proline-cysteine (CPPC) peptides. H₂O₂ exposure induced disulfide cross-linking between CPPC terminal cysteines, while subsequent ROS elevation cleaved these bonds, triggering a gel-to-sol transition, ROS scavenging, and accelerated epithelial regeneration [133]. Natural polyphenolic antioxidants, such as catechin and epigallocatechin gallate (EGCG), have also been integrated into responsive hydrogels [134-141]. Sun et al. synthesized a glucose-responsive hydrogel (GMPE) by modifying gelatin methacryloyl (GelMA) with 4-carboxyphenylboronic acid (CPBA) and encapsulating EGCG. At diabetic wound sites, elevated glucose triggered cleavage of the boronic ester bonds between CPBA and EGCG's ortho-dihydroxy groups, enabling rapid EGCG release and promoting healing [142]. Similarly, our group developed a glucose-responsive hydrogel (HMPC) by modifying hyaluronic acid methacrylate (HAMA) with PBA to load catechin. High glucose concentrations at the wound site cleaved the PBA-catechin borate ester bonds, facilitating spatiotemporal catechin release. The HMPC hydrogel protected cells from ROS in vitro and significantly accelerated diabetic wound healing over 3 weeks by reducing inflammation and promoting angiogenesis [143]. Furthermore, enzyme-responsive systems, such as MMP-responsive hydrogels, enable precise drug release in protease-rich wound environments [144-147]. Li et al. fabricated a hydrogel by grafting EGCG and GelMA onto oxidized hyaluronic acid for delivering resveratrol nanoparticles. This hydrogel exhibited rapid degradation (residual mass ~18.06% within 48 h) in MMP-rich conditions, primarily due to the MMP-sensitive GelMA component. In vitro and in vivo studies confirmed the precise release of polyphenol nanoparticles, effectively mitigating oxidative stress in diabetic wounds [148].
4.5 Promote Angiogenesis
Chronic inflammation and hyperglycemia in diabetic wounds drive sustained overexpression of MMPs, leading to excessive ECM degradation and proteolysis of essential proangiogenic growth factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β) [149-151]. To counteract this pathological microenvironment, MMP-responsive hydrogels have been engineered using cleavable peptide substrates, metal-chelating agents, or natural ECM analogs [152-154]. One representative strategy utilizes MMP-cleavable peptides covalently integrated into hydrogel networks. Chen et al. developed an MMP-degradable polyethylene glycol hydrogel (MMP-PEG) via Michael addition between thiol groups on an MMP-sensitive peptide and maleimide groups on 4-arm-PEG-MAL. This hydrogel encapsulated adipose-derived stem cell exosomes for diabetic wound treatment. It demonstrated high responsiveness, releasing > 90% of exosomes in MMP-2 solution over 20 days versus < 10% in PBS. In vivo, the MMP-triggered release significantly accelerated wound repair by enhancing neovascularization and collagen deposition [155]. Alternatively, MMP-sensitive hydrogels can leverage natural ECM component degradation. Hu et al. fabricated a hydrogel through Schiff base formation between oxidized hyaluronic acid (HA-CHO) and hydrazide-modified gelatin (Gel-ADH), loading the TRPA1 agonist cinnamaldehyde (CA). This hydrogel underwent complete degradation under MMP-rich conditions in vitro within 5 days. The rapid, MMP-responsive degradation enabled sustained CA release, which inhibited high glucose-induced endothelial cell ferroptosis, alleviated vascular dysfunction, and promoted angiogenesis [156].
5 Dual-Responsive Hydrogels for Diabetic Wound Healing
The inherently dynamic and heterogeneous nature of diabetic wounds poses significant challenges for single-function diabetic wound dressings, which often lack the adaptability to synchronize drug delivery with the evolving microenvironment. While early research centered on single-responsive systems, the complexity and dynamic nature of the wound healing process have underscored the need for more precise and adaptable approaches. Single-responsive systems often fail to address the multifaceted challenges of chronic wounds. In response, dual-responsive strategies have gained prominence, promoting precise intervention at different stages of healing. This multi-triggered mechanism enables a more refined spatial distribution of therapeutics to diseased tissue, while also ensuring that release occurs only when and where pathological conditions are present. In the following section, we discuss representative dual-responsive hydrogel platforms and how their integrated responsiveness aligns with the pathological cues of diabetic wound healing to achieve coordinated and timely drug delivery.
5.1 pH/ROS-Responsive Hydrogels for Diabetic Wound Healing
Diabetic wounds exhibit a pathological microenvironment characterized by spatiotemporal fluctuations in acidity and elevated ROS levels, which exacerbate bacterial infection, metabolic dysregulation, and chronic inflammation. To address this dynamic milieu, dual pH/ROS-responsive hydrogels have been engineered for spatiotemporally controlled drug release [157, 158]. These systems exploit two synergistic mechanisms: (i) the formation of reversible boronic ester bonds between phenylboronic acid (PBA) and diols at physiological pH (pKa 7.8–8.6), hydrolyzing under acidic conditions [159]; and (ii) the dissociation of Schiff base linkages (imine bonds) between aldehydes and amines in acidic microenvironments [160]. Both bonds exhibit ROS sensitivity, enabling dual-triggered hydrogel disassembly. Wang et al. engineered an interpenetrating network from caffeic acid-grafted ε-polylysine (CE) and PBA-grafted oxidized dextran (POD). Acidic/ROS-rich wound conditions triggered cleavage of boronic ester and Schiff base bonds, releasing diclofenac sodium and mangiferin to accelerate healing (Figure 5A) [161]. Similarly, Li et al. synthesized HA-CHO-BA hydrogel via Schiff base conjugation of hyaluronic acid (HA-CHO) and 3-aminophenylboronic acid (BA) within a PVA matrix. The pH/ROS-responsive hydrogel, loaded with silver-doped polydolamine nanoparticles and recombinant human collagen III, mitigated inflammation and enhanced angiogenesis through triggered disintegration [163]. Advanced designs encapsulate therapeutics within stimuli-sensitive carriers embedded in hydrogel matrices, enabling phase-specific release [164-166]. Kong et al. incorporated ROS-sensitive paeoniflorin micelles into a pH-responsive gelatin-dextran hydrogel. Acidic-triggered ZnO nanoparticle release controlled initial infection, while subsequent ROS-mediated micelle degradation released paeoniflorin to promote angiogenesis [167]. To augment ROS scavenging, nanozymes mimicking superoxide dismutase (SOD)/catalase (CAT) activities have been incorporated [168-172]. These degrade O₂•⁻ to H₂O₂ and further to H₂O/O₂, alleviating oxidative stress [173-177]. Zhang et al. developed an in situ-gelling system using ε-polylysine (EPL)-coated MnO₂ nanozymes and aldehyde-modified F127 micelles encapsulating insulin. ROS-triggered MnO₂ catalysis and Schiff base cleavage promoted gel degradation, enabling spatiotemporal insulin release while scavenging ROS, supplying O₂, and modulating glucose (Figure 5B) [162]. Clinical translation of ROS/pH dual-responsive hydrogels faces challenges. Balancing pH and ROS responsiveness within a single hydrogel system remains complex due to significant microenvironmental variations across diabetic wounds. Moreover, biodegradability and biocompatibility require rigorous optimization, particularly when incorporating nanoparticles or drug conjugates.
5.2 pH/Glucose-Responsive Hydrogels for Diabetic Wound Healing
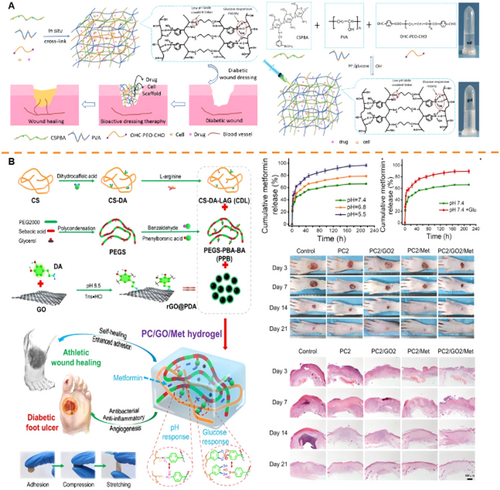
Diabetic wounds exhibit an acidic and hyperglycemic microenvironment, rendering pH/glucose dual-responsive hydrogels a strategic platform for spatiotemporally controlled drug delivery. PBA serves as a pivotal moiety in these systems, responding to pH fluctuations and glucose via reversible binding with 1,2-diol structures [178-181]. This enables glucose-mediated spatial targeting and pH-triggered temporal release synchronized with inflammatory dynamics. Synthetic polymer matrices functionalized with PBA demonstrate targeted drug release. Ni et al. synthesized a hydrogel (PP) via PBA-diol crosslinking between poly(acrylamide-co-dimethylaminopropylacrylamide-co-methacrylamidophenylboronic acid) (PB) and PVA. Embedded antibiotic-loaded mesoporous silica nanoparticles (MSN@PDA-TH) exhibited accelerated release under acidic/high-glucose conditions through PBA-glucose competition and PBA-PVA bond dissociation, effectively targeting infected diabetic wounds [182]. In addition, natural polymer systems leverage PBA functionality. Cao et al. developed an injectable HQB hydrogel from hyaluronic acid-PBA (HA-PBA), quaternized chitosan (QCS), and Mg²⁺-containing bioactive glasses (MgBGs). pH/glucose-triggered boronate ester cleavage enabled controlled MgBG release, accelerating wound repair [183]. Furthermore, hybrid matrices incorporating Schiff bases enhance responsiveness [184]. Zhao et al. engineered a hydrogel using phenylboronic acid-modified chitosan (CSPBA), PVA, and benzaldehyde-capped PEG (OHC-PEG-CHO), encapsulating insulin and fibroblasts. Synergistic disruption of Schiff base bonds and PBA-glucose interactions under acidic pH (6.5) and high glucose (3 g/L) significantly enhanced insulin release versus physiological conditions, promoting neovascularization and collagen deposition (Figure 6A) [185]. Extending this strategy to type II diabetes (90%–95% of cases), Guo et al. loaded metformin and polydopamine-coated graphene oxide (rGO@PDA) into a Schiff base-linked hydrogel (PC). Acidic-triggered hydrolytic cleavage (30.4% increased release at pH 5.5) combined with glucose-induced matrix collapse enabled spatiotemporal delivery, suppressing oxidative stress and accelerating angiogenesis in vivo (Figure 6B) [186]. Despite their promise, key limitations persist. Dynamic pH/glucose fluctuations may cause inconsistent drug release, while PBA integration compromises mechanical integrity in hyperglycemic environments [187-189].
5.3 Other Dual-Responsive Hydrogels for Diabetic Wound Healing
Although pH-responsive hydrogels dominate current dual-responsive systems for diabetic wounds, the complexity of wound pathophysiology necessitates strategies integrating broader biological triggers [190-197]. Platforms responsive to cues beyond pH remain under-explored [198, 199]. Addressing this, Fan et al. developed a glucose/MMP-9 dual-responsive hydrogel composed of cross-linked polyvinyl alcohol (PVA) and phenylboronic acid-modified chitosan (CS-BA). This hydrogel was engineered to co-load insulin and gelatin microspheres encapsulating celecoxib (GMs@Cel). In this system, the PBA groups conferred glucose sensitivity, enabling reversible swelling and controlled insulin release via competitive glucose binding. Simultaneously, gelatin microspheres undergo enzyme-triggered degradation upon encountering elevated MMP-9 levels, significantly accelerating celecoxib release. Concurrent glucose and MMP-9 stimulation, hydrogel disintegration was accelerated, enabling synchronized release of insulin and celecoxib. Notably, release kinetic analysis revealed that insulin release was predominantly diffusion-driven, while celecoxib release followed a non-Fickian model influenced by both microsphere degradation and molecular diffusion. This design strategically aligns dual-stimuli responsiveness with spatiotemporally distinct cues in the diabetic wound environment. Glucose acts as a systemic metabolic marker. MMP-9 serves as a localized indicator of inflammatory tissue remodeling. By coupling these cues, the hydrogel achieves on-demand, sequential drug release of anti-inflammatory and metabolic-regulating agents, effectively coordinating the early and late phases of diabetic wound healing. This dual-responsive platform demonstrated accelerated wound closure, enhanced collagen deposition, and improved modulation of the local immune microenvironment in vivo [200]. Therefore, integrating multiple pathological cues beyond pH represents a promising strategy for adaptable and precise diabetic wound therapies.
6 Conclusions and Prospects
The pathological microenvironment of diabetic wounds, characterized by hyperglycemia, elevated ROS, acidic pH, and aberrant enzyme activity, induces chronic inflammation and healing impairment, significantly increasing risks of infection and amputation. This complexity poses a major global clinical challenge. Hydrogel dressings offer promise through exudate management, infection barrier function, and therapeutic agent delivery, with over a dozen products in clinical use or trials. However, conventional hydrogels rely on passive drug release mechanisms that fail to dynamically adapt to pathological cues, resulting in mismatched release kinetics, and suboptimal therapeutic outcomes during prolonged healing. To overcome these limitations, smart responsive hydrogels have emerged that utilize endogenous pathological signals (pH/glucose/ROS/enzymes) as triggers to achieve on-demand spatiotemporal drug delivery, real-time modulation of release profiles, and active improvement of the wound microenvironment. Beyond enhanced drug control, these intelligent dressings may reduce long-term treatment costs through decreased dressing frequency and recurrence rates. Nevertheless, several critical challenges must be addressed for the clinical translation of these hydrogels.
First, stimulus-responsive precision requires refinement to address the spatiotemporal heterogeneity of diabetic wound microenvironments. Single- and dual-trigger systems (e.g., glucose/pH-responsive) frequently fail to synchronize drug release with dynamic biochemical fluctuations in DFUs. Real-time wound assessment is necessitated by fluctuating biomarker concentrations to enable personalized hydrogel selection, while standardized protocols for matching hydrogel responsiveness to individualized wound progression stages remain lacking.
Second, persistent biocompatibility and safety concerns demand resolution. Chronic exposure risks, particularly immune activation from degradation byproducts (e.g., phenylboronates) or residual crosslinkers, require rigorous toxicological assessment and longitudinal biodistribution studies. Concurrently, manufacturing complexity impedes scalability, where multistep syntheses, batch-dependent crosslinking efficiency, and sterilization-induced instability of biological components (e.g., growth factors) compromise the production of responsive hydrogels.
Third, inadequate preclinical validation constrains clinical predictability. Most studies rely on rodent models that neglect critical DFU features, notably polymicrobial biofilms and human-relevant chronicity, while standardized large-animal models emulating human wound chronification remain deficient. Ambiguous regulatory pathways for combination products (device + drug/biologic) further complicate translation.
Future research should focus on optimizing hydrogel design by integrating multiple stimuli-responsive elements to better address the complex and dynamic microenvironment of diabetic wounds. Emerging advances in 3D bioprinting, bio-orthogonal chemistry, and machine learning guided formulation offer new opportunities for developing next-generation disease-adaptive platforms. Integration with tissue-engineered constructs, cell-based therapies, or gene delivery systems may further enable synergistic wound healing strategies. As these challenges are progressively addressed, stimuli-responsive hydrogels hold transformative potential in diabetic wound management, shifting from passive symptom control to precision-guided, microenvironment-responsive therapy. Realizing this vision will require continued interdisciplinary collaboration across materials science, bioengineering, and clinical medicine to drive translation and achieve optimal wound healing.
Author Contributions
Jiyuan Du: writing – original draft. Caihong Xian: conceptualization, writing – review and editing. Xiaodan Liang: validation, data curation. Shirou Fan: data curation. Liying Wang: supervision. Jun Wu: supervision, funding acquisition, writing – review and editing. All authors have read and approved the final manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 52173150 and U22A20315), the Open Research Funds from the Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People's Hospital (No. 202301-211) and Guangzhou Science and Technology Program City-University Joint Funding Project (Nos. 2023A03J0001 and 2024A03J0604). We gratefully acknowledge Dr. Miaojun Huang from Base of Red Bird MPhil, College of Future Technology, The Hong Kong University of Science and Technology (Guangzhou) for helpful conversations and suggestions.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




