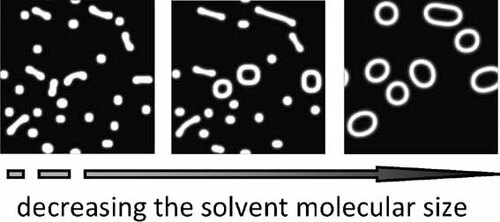
Effect of Solvent Molecular Size on the Self-Assembly of Amphiphilic Diblock Copolymer in Selective Solvent
Wei Li
State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Wei Jiang
State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China Fax: (+86) 431 85262126Search for more papers by this authorWei Li
State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China
Search for more papers by this authorCorresponding Author
Wei Jiang
State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China
Graduate University of the Chinese Academy of Sciences, Beijing, P. R. China Fax: (+86) 431 85262126Search for more papers by this authorAbstract
Real-space self-consistent field theory (SCFT) is employed to study the effect of solvent molecular size on the self-assembly of amphiphilic diblock copolymer in selective solvent. The phase diagrams in wide ranges of interaction parameters and solvent molecular size were obtained in present study. The results indicate that the solvent molecular size is a key factor that determines the self-assembly of amphiphilic diblock copolymer. The self-assembled morphology changes from circle-like micelle to line-like micelle, then to loop-like micelle by decreasing the solvent molecular size in a wide range of solvent selectivity. We analyze and discuss this change in terms of the solvent solubility and the entropy contribution.
References
- 1 J. Chen, G. Xue, Y. Li, Macromolecules 2001, 34, 1297.
- 2 Q. Sun, D. Zhou, X. Wang, G. Xue, Macromolecules 2002, 35, 7089.
- 3 D. Zhou, L. Li, B. Che, Q. Cao, Y. Lu, G. Xue, Macromolecules 2004, 37, 4744.
- 4 D. Zhou, L. Li, Y. Li, J. Zhang, G. Xue, Macromolecules 2003, 36, 4609.
- 5 R. J. Spontak, R. Shankar, M. K. Bowman, A. S. Krishnan, M. W. Hamersky, J. Samseth, M. R. Bockstaller, K. Ø. Rasmussen, Nano Lett. 2006, 6, 2115.
- 6 Y. Q. Li, Q. R. Huang, T. F. Shi, L. J. An, J. Chem. Phys. 2006, 125, 044902.
- 7 J. Hu, R. Wang, G. Xue, J. Phys. Chem. B 2006, 110, 1872.
- 8 J. R. Naughton, M. W. Matsen, Macromolecules 2002, 35, 5688.
- 9 L. Zhang, A. Eisenberg, Science 1995, 268, 1728.
- 10 L. Zhang, K. Yu, A. Eisenberg, Science 1996, 272, 1777.
- 11 L. Zhang, A. Eisenberg, Macromolecules 1996, 29, 8805.
- 12 C. Allen, D. Maysinger, A. Eisenberg, Colloids Surf., B 1999, 16, 3.
- 13 D. Viduna, A. Milchev, K. Binder, Macromol. Theory Simul. 1998, 7, 649.
- 14 C. I. Huang, H. Y. Hsueh, Y. K. Lan, Y. C. Lin, Macromol. Theory Simul. 2007, 16, 77.
- 15 H. B. Du, J. T. Zhu, W. Jiang, J. Phys. Chem. B 2007, 111, 1938.
- 16 S. F. Edwards, Proc. Phys. Soc. 1965, 85, 613.
- 17 E. J. Helfand, Chem. Phys. 1975, 62, 999.
- 18 M. W. Matsen, M. Schick, Phys. Rev. Lett. 1994, 72, 2660.
- 19 M. W. Matsen, F. S. Bates, J. Chem. Phys. 1996, 106, 2436.
- 20 A. C. Shi, J. Noolandi, Macromol. Theory Simul. 1999, 8, 214.
- 21 J. Noolandi, A. C. Shi, P. Linse, Macromolecules 1996, 29, 5907.
- 22 M. Müller, F. Schmid, Adv. Polym. Sci. 2005, 185, 1.
- 23 K. C. Daoulas, M. Müller, J. J. de Pablo, P. F. Nealey, G. D. Smith, Soft Matter 2006, 2, 573.
- 24 F. Drolet, G. H. Fredrickson, Phys. Rev. Lett. 1999, 83, 4317.
- 25 F. Drolet, G. H. Fredrickson, Macromolecules 2001, 34, 5317.
- 26 G. H. Fredrickson, V. Ganesan, F. Drolet, Macromolecules 2002, 35, 16.
- 27 G. H. Fredrickson, “ The Equilibrium Theory of Inhomogeneous Polymers”, Clarendon Press, Oxford, London 2006.
- 28 X. H. He, H. J. Liang, L. Huang, C. Y. Pan, J. Phys. Chem. B 2004, 108, 1731.
- 29 J. T. Zhu, Y. Jiang, H. J. Liang, W. Jiang, J. Phys. Chem. B 2005, 109, 8619.
- 30 Y. Jiang, J. T. Zhu, W. Jiang, H. J. Liang, J. Phys. Chem. B 2005, 109, 21549.
- 31 R. Wang, P. Tang, F. Qiu, Y. L. Yang, J. Phys. Chem. B 2005, 109, 17120.
- 32 J. W. Ma, X. Li, P. Tang, Y. L. Yang, J. Phys. Chem. B 2007, 111, 1552.
- 33 L. S. Zhang, J. P. Lin, S. L. Lin, J. Phys. Chem. B 2007, 111, 9209.
- 34 Y.-Y. Won, A. K. Brannan, H. T. Davis, F. S. Bates, J. Phys. Chem. B 2002, 106, 3354.
- 35 F. F. Tao, J. L. Han, Q. Gu, C. Teng, D. W. Zou, D. S. Zhou, G. Xue, Macromolecules 2008, 41, 9890.