Mesenchymal Stem Cell-Engrafted Bacterial Cellulose and Graphene Oxide Scaffolds Enhance Peripheral Nerve Repair in a Rat Model
Funding: The study was funded by the Scientific Research Committee of Marmara University under protocol number TTU-2022-10513.
ABSTRACT
Peripheral nerve injuries result in significant functional impairment, and limited regenerative capacity within the central nervous system further complicates recovery. This study investigates the effects of graphene oxide-decorated bacterial cellulose (BC/GO) scaffolds, with or without mesenchymal stem cells (MSCs), on axonal regeneration following sciatic nerve injury in rats. Twenty-seven male rats were assigned to autograft, BC/GO, and BC/GO+MSCs. The sciatic functional index (SFI), electromyography (EMG), and histopathological analysis were evaluated at 8 weeks. Although SFI scores showed no significant differences, compound muscle action potential (CMAP) values at 4 weeks were significantly higher in both the BC/GO and BC/GO+MSCs groups compared to autografts. Macroscopic examination revealed extensive tissue adhesions in the BC/GO and BC/GO+MSCs groups. Histological analysis indicated regeneration across all groups. The autograft group showed no inflammation, whereas the BC/GO group demonstrated the highest levels of inflammation and degeneration. The BC/GO+MSCs group exhibited reduced inflammation, likely due to the immunomodulatory effects of MSCs. While BC/GO scaffolds promoted early regeneration, the inflammatory response compromised the long-term outcomes. These findings suggest BC/GO scaffolds can facilitate initial nerve repair but require further refinement to sustain long-term functional recovery.
1 Introduction
Peripheral nervous system (PNS) injuries impose significant functional, psychological, and socioeconomic burdens, not only on affected individuals but also on caregivers and society. The greatest limitation in treating injuries and diseases of the central nervous system (CNS) is its low regenerative capacity. As a result, most of these damages are irreversible. However, the PNS, in contrast, possesses a partial ability to regenerate [1, 2].
Surgical interventions for peripheral nerve injuries aim to relieve compression, remove debris resulting from trauma, or reconnect nerve endings through primary repair (resuturing), autografting, or the use of synthetic or biologically derived graft materials. The ultimate goal is to optimize the regenerative potential of the PNS. Current research efforts focus on developing these surgical techniques, identifying optimal graft materials, and exploring stem cell-based technologies to further enhance nerve regeneration [3].
Treatment strategies vary according to the size of the nerve defect. Primary suturing and autografting are effective for smaller defects. Recent studies have shown promising results using stem cell therapies in combination with entubulation techniques, especially in the presence of large defects. Among the various stem cell types, including pluripotent, neural, and mesenchymal stem cells, mesenchymal stem cells (MSCs) are of particular interest due to their multipotency and self-renewal capabilities [4]. Adipose tissue stands out as an ideal source of adult MSCs because of its availability and high stem cell content [5]. Moreover, adipose-derived mesenchymal stem cells (AdMSCs) have been successfully induced to differentiate into functional neuronal cells using specialized culture media enriched with growth factors and cytokines [6].
A variety of scaffolds from natural, synthetic, and hybrid biomaterials have been developed to replicate the native cellular microenvironment to maintain stem cell viability after transplantation, promote proliferation, and facilitate efficient and targeted integration. Graphene and its derivatives are frequently used in tissue engineering due to their unique chemical, electrical, and mechanical properties, which can direct stem cells to differentiate into specific lineages [7, 8]. Graphene oxide (GO), in particular, offers a high surface area and abundant functional groups (e.g., hydroxyl and carboxyl) that enhance cell adhesion and conductivity [7, 9]. However, van der Waals forces and π–π stacking interactions between GO nanosheets can lead to aggregation within the polymer matrix, reducing effective surface area between the GO and the surrounding matrix and limiting the material's potential to enhance scaffold properties [8, 10]. GO can be easily dispersed in bacterial cellulose hydrogel, a promising natural polymer, thereby enhancing the properties of the hydrogel. Bacterial cellulose (BC), which has a 3D network structure and is produced extracellularly by Gluconacetobacter xylinus, possesses characteristics such as high porosity, water-holding capacity, biodegradability, and a unique fibrous structure. This fibrous structure also supports cell adhesion and proliferation. Since BC scaffolds resemble the extracellular matrix (ECM), they are well-suited for applications such as promoting tissue regeneration [11-15].
To our knowledge, the therapeutic strategy presented in this study has not been previously reported. This novel composition has been used to promote functional nerve regeneration. In this study, we aimed to evaluate the effect of a biomaterial scaffold composed of a bacterial cellulose and graphene oxide mixture (BC/GO) on axonal healing after experimentally induced sciatic nerve injury in Wistar rats, both alone and in combination with mesenchymal stem cells (MSCs) derived from rat adipose tissue.
2 Results
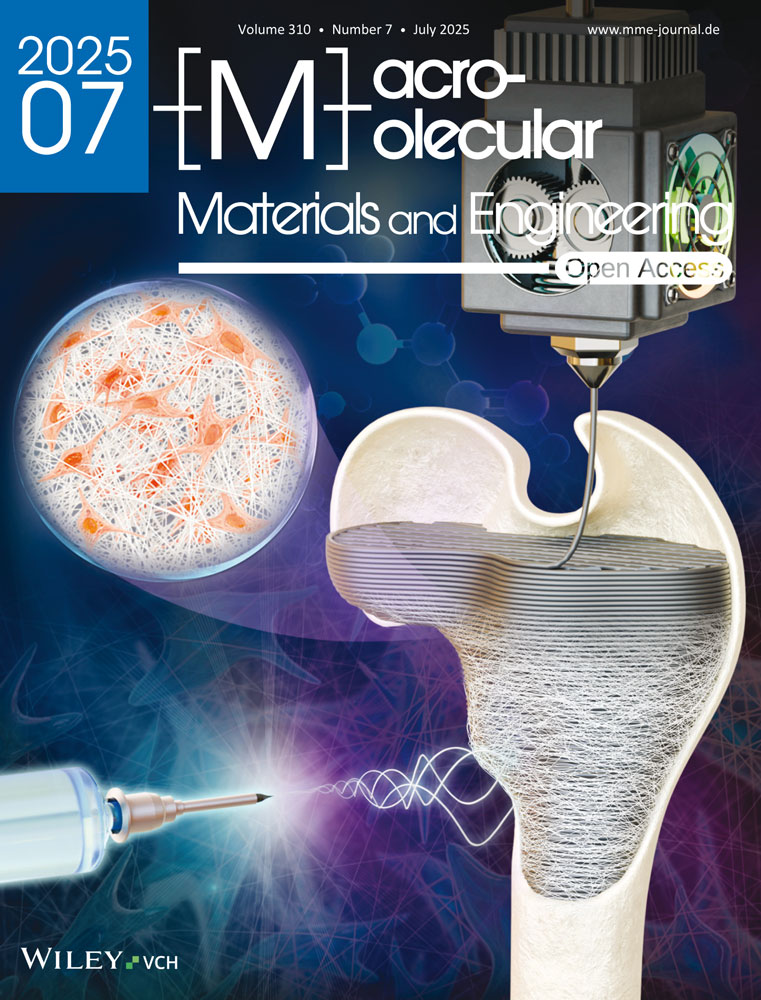
BC and BC/GO scaffolds were imaged using scanning electron microscopy (SEM). The BC scaffold exhibited a fibrillar structure (Figure 1A), while the BC/GO scaffold showed graphene oxide dispersed in granular form along the BC fibrils (Figure 1B).
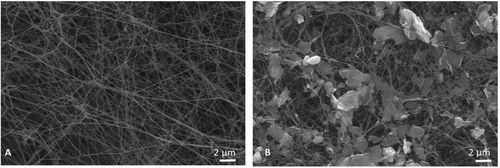
The FTIR spectra of pure BC, GO, and BC/GO are shown in Figure 2A. For pure BC, the peak at 3300 cm−1 corresponds to hydrogen-bonded O─H stretching, while the peaks at 2800−2900, 1620, and 1151 cm−1 are attributed to C─H stretching, amide I (C═O), and C─O─C stretching, respectively [16, 17]. The FTIR spectrum of GO showed characteristic peaks at 3410, 1641, 1721, and 1386 cm−1, assigned to OH, C═C, C═O, and C─O stretching, respectively [18]. The BC/GO composite scaffold exhibited all characteristic bands of both BC and GO.
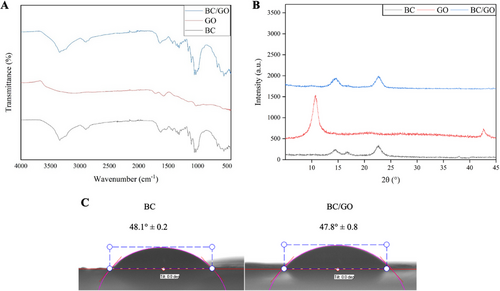
Figure 2B shows the XRD peaks of BC, GO, and BC/GO nanocomposites. In the XRD pattern of GO obtained via the improved Hummers’ method, a sharp characteristic peak can be observed at 2θ = 10,7° (001 plane) and another at 2θ = 42,6° (100) as reported by Gul and Alrobei [19]. BC exhibited characteristic peaks at 2θ = 14.5°, 16.7°, and 22.7° corresponding to the (110), (110), and (200) crystal planes. These peak positions remained unchanged in the BC/GO nanocomposite, indicating that BC's crystalline structure remained intact upon GO incorporation [20, 21]. Surface hydrophilicity of the BC/GO nanocomposites was assessed by measuring water contact angles (WCA) (Figure 2C). WCA was measured by depositing water droplets on the surfaces of dried BC and BC/GO samples. Both BC and BC/GO exhibited strong hydrophilic behavior, as indicated by low contact angle values (<60°). The incorporation of GO further enhanced the hydrophilicity of the BC scaffold, reducing the WCA from 48.1° ± 0.2 to 47.8° ± 0.8.
Adipose-derived stem cells were isolated from rat inguinal visceral adipose tissue and verified to meet International Society for Stem Cell Research (ISSCR) criteria via direct observation of self-renewal ability and flow cytometry analysis of cell surface markers [22].
Scaffolds used in tissue engineering and their degradation products should not be toxic to the cells. BC/GO scaffold and 2D controls were seeded with cells and cultured for 1 and 4 days (Figure 3, Upper Graph). The MTT results showed that after 1 day of incubation, cell viability on BC/GO scaffolds was more than 60% compared to 2D, while after 4 days of cell incubation, viability increased and was approximately 84% compared to 2D. However, the BC/GO scaffold did not have a negative effect on viability. Based on MTT analysis, the scaffolds were shown to be biocompatible. The morphology of AdMSCs was visualized using confocal laser scanning microscopy. The nuclei and cytoskeletons of cells on the BC/GO scaffold were stained with DAPI and F-actin, respectively. As shown in the confocal images (Figure 3, Lower row), after 4 days of incubation, rounded nuclei and developed cytoskeletons were clearly observed in cells on the BC/GO scaffold. Extending the incubation to 4 days promoted cellular attachment, enhancing cytoskeletal organization and cell–cell interaction.
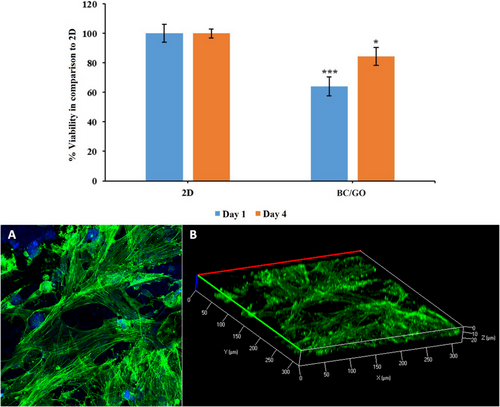
Figure 3B shows Z-stack images of top-to-bottom optical slices through the nanofibers, with a slice thickness of 2.5 µm, along with 3D reconstructions of cells within the nanofiber scaffold. Optical slicing in the Z-direction enabled visualization of cell penetration into the scaffolds after 4 days of incubation. It was observed that cells infiltrated the nanofibers to a depth of 20 µm from the upper surface. These results demonstrate successful cell growth, proliferation, and infiltration within the BC/GO scaffold, indicating its suitability for tissue engineering applications.
The morphology of AdMSCs was also confirmed using scanning electron microscopy (SEM). On day 4 after seeding AdMSCs onto the BC/GO scaffold, SEM images clearly showed that the dispersed cells had attached to the scaffold surface. The BC/GO scaffold provided a larger 3D surface area for cell growth. The results clearly demonstrated that the cells were spreading and proliferating on the surface (Figure 4A,B). These images indicated strong cell–material adhesion. The BC/GO scaffold exhibited favorable biocompatibility and supported cell interaction.
When the SFI results of the Wistar rats in the autograft group were compared between the 4th and 8th weeks for the left foot, no statistically significant difference was found [−49.7((−77.3)- (−25.6)) vs −54.2 (−62.9)–(−45.9); p = 0.4961]. Similarly, in the BC/GO graft group, there was no significant difference in SFI between the 4th and 8th weeks [65.9((−67.6)–(−54.3)) vs −62.5((−65.7) –(−53.8)); p = 0.1289]. For the BC/GO + MSCs group, SFI comparison between the two time points also did not reveal a statistically significant difference [−60.3 (−71.8)–(−44.4) vs −56.1(−72.9)–(−43.4); p = 0.4258].
The degree of recovery in EMG studies was assessed by analyzing the difference between the left and right limbs, where a decrease in this difference was interpreted as functional improvement. In the autograft group, the difference between the left and right feet significantly decreased in the 8th week compared to the 4th week [4th week left EMG CMAP: 397 (50–490) and right EMG CMAP: 786 (255–898); p = 0.0012 vs 8th week month left EMG CMAP: 300 (80–860) and right EMG CMAP: 630 (415–950); p = 0.0244]. However, when CMAP ratios were compared between the 4th and 8th weeks, the difference was not statistically significant (4th week CMAP rate: 0.47; 0.06–0.70) vs 8th week CMAP rate: 0.54(0.15–0.92; p = 0.1641) (Tables 1, 2 and Figure 5).
| N | 4th Week CMAP Rate | c p Value | ||
|---|---|---|---|---|
| Autograft (A) | BC/GO (B) | BC/GO+MSc (C) | ||
| 1 | 0.276 | 0.958 | 0.481 | |
| 2 | 0.546 | 0.895 | 0.255 | |
| 3 | 0.562 | 0.866 | 0.970 | |
| 4 | 0.471 | 0.905 | 0.707 | |
| 5 | 0.349 | 0.681 | 0.527 | |
| 6 | 0.472 | 0.671 | 0.978 | |
| 7 | 0.700 | 0.680 | 0.865 | |
| 8 | 0.058 | 0.747 | 0.810 | |
| 9 | 0.442 | 0.630 | 0.774 | |
| Mean | 0.43 | 0.78 | 0.71 | |
| SD | 0.19 | 0.12 | 0.24 | |
| Median | 0.47 | 0.75 | 0.77 | 0.0046 |
| Min | 0.06 | 0.63 | 0.26 | |
| Max | 0.70 | 0.96 | 0.98 | |
- Comparisons, P: A-B: <0.01 A-C: <0.05 B-C: >0.05
- cKruskal-Wallis Test (Nonparametric ANOVA) with post test (Dunn's Multiple Comparisons Test)
| N | 8th Week CMAP Rate | c p Value | ||
|---|---|---|---|---|
| Autograft (A) | BC/GO (B) | BC/GO+MSCs (C) | ||
| 1 | 0.482 | 0.271 | 0.708 | |
| 2 | 0.154 | 0.260 | 0.618 | |
| 3 | 0.905 | 0.971 | 0.147 | |
| 4 | 0.921 | 0.558 | 0.408 | |
| 5 | 0.569 | 0.256 | 0.242 | |
| 6 | 0.476 | 0.167 | 0.825 | |
| 7 | 0.642 | 0.522 | 0.791 | |
| 8 | 0.310 | 0.859 | 0.727 | |
| 9 | 0.542 | 0.265 | 0.772 | |
| Mean | 0.56 | 0.46 | 0.58 | |
| SD | 0.25 | 0.29 | 0.25 | |
| Median | 0.54 | 0.27 | 0.71 | 0.6469 |
| Min | 0.15 | 0.17 | 0.15 | |
| Max | 0.92 | 0.97 | 0.82 | |
- cKruskal-Wallis Test (Nonparametric ANOVA) with post test (Dunn's Multiple Comparisons Test)
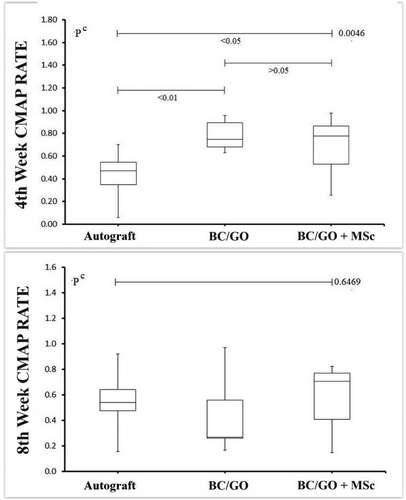
In the BC/GO graft group, comparison of EMG CMAP values between the left and right feet at the 4th and 8th weeks revealed a borderline significant difference at the 8th week (p = 0.0547) [4th week: left EMG CMAP: 511(332–690) and right EMG CMAP: 720(420–830); p = 0.0400 vs 8th week left EMG CMAP: 185(101–389) and right EMG CMAP: 573(245–711); p = 0.0003]. In terms of CMAP ratios, the difference was statistically significant between the two time points (4th week CMAP rate: 0.75(0.63–0.96) vs 8th week. CMAP rate: 0.27(0.17–0.97); p = 0.0195) (Tables 1 & 2).
For the BC/GO + MSCs group, EMG CMAP values showed a statistically significant improvement in the left foot between the 4th and 8th weeks [4th week left EMG CMAP: 450(140–743) and right EMG CMAP: 660(520–760); p = 0.0305 vs. 8th week left EMG CMAP: 525(106–755) and right EMG CMAP: 742(508–978); p = 0.0040]. However, when CMAP ratios were compared between the two time points, no significant difference was found (4th week CMAP rate: 0.77 (0.26–0.98) vs. 8th week CMAP rate: 0,71 (0.15–0.82); p = 0.308) (Tables 1 & 2).
When comparing the 4th-week CMAP ratios among the three groups, a statistically significant difference was found (p < 0.0046). The BC/GO + MSCs group showed significantly higher CMAP ratios than the autograft group (p < 0.01 and p < 0.05, respectively) (Table 1).
At the 8th week, although the CMAP ratio of the BC/GO + MSCs group remained higher, no statistically significant difference was observed between the three groups (p < 0.6469). A Pearson correlation analysis evaluating the relationship between SFI and CMAP data across all experimental and control groups showed no statistically significant correlation (r = 0.054, p = 0.6981) (Table 2).
Hematoxylin-Eosin-stained sections of tissues from the autograft, BC/GO, and BC/GO + MSCs groups were evaluated under a light microscope. In the tissue sections from the autograft group, regularly regenerated epineurium, perineurium, and nerve fascicles were observed. Examination of the Schwann cells within the fascicles revealed a normal histological structure, with well-organized nuclei and axons in cross-section. No inflammatory cell infiltration was detected in the autograft tissue sections.
Significant necrosis and intense inflammatory reaction were observed in the transverse sections of the BC/GO graft group, particularly in the inner regions. Neutrophils, lymphocytes, macrophages, and multinucleated giant cells were noted within the inflamed zones. Schwann cells were scattered in the outer parts of these sections, and the axons showed irregular orientation. An increase in fibroblast presence and connective tissue layers was also noted. While some sections exhibited significant fascicular regeneration, degenerative changes were still evident in some axons (Figure 6).
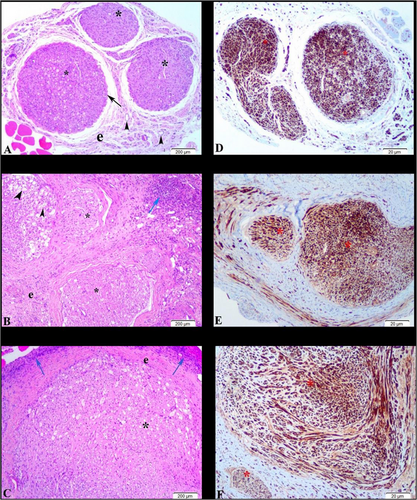
Tissue sections from the BC/GO + MSCs group similarly showed necrotic areas and intense inflammation in the inner regions. Increases in fibroblast numbers and connective tissue layers were also observed in the stem cell-treated grafts. However, axonal organization within the regenerating fascicles was more regular compared to the BC/GO group alone (Figure 6).
Peripheral nerve regeneration was further evaluated by immunohistochemical analysis using S100 immunoexpression across all three groups. The most prominent regeneration was detected in the fascicles of the autograft sections. Significant S100 immunoexpression was observed in a few regenerating fascicles of the BC/GO and BC/GO + MSCs tissue sections. While immunoexpression was sparse in the inner, necrotic regions of both groups, it was more pronounced in the Schwann cell populations located in the outer parts of the tissues.
3 Discussion
The most limiting factor in the treatment of injuries and diseases related to the CNS is its low self-renewal capacity, making such damage largely irreversible. In contrast, the PNS has a partial regenerative ability. Injuries to the PNS, along with the associated functional losses, not only affect the health of the individual but also have broader psychological and socioeconomic impacts on society. The slow pace of peripheral nerve regeneration eliminates the possibility of spontaneous healing in relatively large injuries. As Sullivan et al. have noted, the gold standard for treating peripheral nerve damage is primary repair for small defects and autologous nerve grafting for larger ones; however, this technique can result in functional loss at the donor site [23].
Stem cell transplantation offers a promising alternative, with MSCs standing out among available stem cell sources [3]. The ability of these cells to self-renew, their multipotency, and their broad paracrine effects make them strong candidates for neural regenerative therapies. MSCs can differentiate into neural and/or Schwann-like cells at the injury site and secrete neurotrophic and neuroprotective cytokines that can provide a microenvironment necessary for regeneration [6]. Compared to other MSC sources, AdMSCs are frequently used due to their ease of isolation using minimally invasive techniques such as liposuction, which causes less morbidity. Moreover, adipose tissue yields approximately 500 times more stem cells than the same volume of bone marrow [6, 24].
However, the proinflammatory microenvironment and morphological changes that develop after peripheral nerve injury limit the success of stem cell transplantation by impairing cell adhesion. Collagen hyperplasia and scar tissue formation are additional challenges that negatively affect regenerative outcomes. Therefore, current research on peripheral nerve repair aims to address these limitations and enhance the effectiveness of stem cell therapies [25, 26].
In cases where primary repair is not feasible, the entubulation technique is often preferred. Biological and synthetic tubular grafts provide a potential solution to some of the issues associated with stem cell application. Although tendons or vascular structures were historically used as graft materials, they have largely been replaced by synthetic alternatives, including silicone, plastic, polyglycolic acid, polyethylene, and polyvinyl, increasing research on these materials [27, 28].
Apart from providing guidance for axonal growth and contact support for Schwann cells, these materials can be supplemented with fillers such as stem cells or various regenerative cytokines. Although this strategy aims to enhance regenerative efficiency, foreign body responses, alloimmunization, or inflammation may limit the success of these biosynthetic materials [26, 29]. Despite these potential complications, entubulation strategies allow for nerve repair over longer distances compared to primary repair.
Sakar et al. stated that the entubulation technique is designed to create a physical bridge between the damaged nerve endings and to restore communication between the regenerative activities occurring on both sides of the lesion [30]. Tubular grafts guide regenerating axons toward their target and prevent adhesion to surrounding tissues, thereby reducing neuroma and scar formation [31].
Despite these benefits, the choice of an appropriate material is critical to the success of tubular grafts. An ideal scaffold should be capable of supporting stem cell delivery while eliciting minimal immune response. Furthermore, it must be biocompatible, biodegradable, and exhibit low toxicity [32]. The scaffold should also mimic the extracellular matrix to support regenerating tissue and possess mechanical and physical properties necessary for stem cell adhesion and proliferation [2].
Biosynthetic materials combine the advantages of both natural and synthetic components. One such material is bacterial cellulose, also referred to as biocellulose. Bacterial cellulose is an ideal candidate for grafting due to its biocompatibility, biodegradability, permeability, and biomechanical properties essential for effective scaffolding. It also facilitates cell migration and encapsulation. Among the few studies on this subject, Kowalska et al. showed that bacterial cellulose tubules reduce neuroma and scar formation. However, they suggested that bacterial cellulose alone is not superior to allograft in improving motor functions [31].
It has also been shown that bacterial cellulose can be effective in the regenerative treatment of other tissues with neural structures, such as the enteric nervous system, in intestinal repair [33, 34]. FTIR analysis confirmed the successful grafting of GO onto bacterial cellulose, while SEM imaging demonstrated that the structure of GO remained more stable within bacterial cellulose nanofibers, effectively preventing GO agglomeration. Scaffolds composed of BC and graphene foam hybrids have been found suitable for neural stem cell proliferation in in vitro spinal cord injury models. However, there is no prior study investigating damage to the peripheral nervous system using a BC/GO composite, nor its combination with AdMSCs.
Evaluation of peripheral nerve function using the SFI revealed that the autograft group showed no statistically significant difference between the 4th and 8th week values for the left foot. Similarly, no significant differences were observed between the 4th and 8th weeks for SFI scores in the BC/GO and BC/GO +MSCs groups. The consistently high negative SFI values across all three groups suggest that axonal regeneration is insufficient for effective conduction to the motor unit. The autophagic behavior observed in some Wistar rats, such as gnawing of the operated foot, may help explain the lack of significant improvement in SFI scores.
Considering the EMG data, although the improvement observed in the 8th week was not statistically significant in the autograft group, a time-dependent upward trend in CMAP values was evident in autografted Wistar rats.
In the graft group, when the degree of improvement in the left foot was compared with the right foot (control), the EMG CMAP values revealed a greater difference in the 8th week compared to the 4th week. This increase was possibly due to the presence of alloantigens in bacterial cellulose, which may have immunogenic potential. Since bacterial cellulose is commonly produced by bacteria such as Acetobacter, Sarcina ventriculi, and Agrobacterium, it may carry alloantigenic components. These alloantigens can trigger the production of alloantibodies, initiating an immune response that peaks between 5 and 16 weeks, similar to the response seen with blood group antigens [24]. After the development of alloantibodies, the immune response increases, leading to the destruction of antigenic structures and increased inflammation. Following exposure to incompatible blood or tissue, alloantigens typically become undetectable in the blood within about two months, while alloantibodies begin to appear [35].
When comparing CMAP rates at the 4th and 8th weeks in the graft group, a statistically significant impairment was observed at two months, indicating a time-dependent decline in nerve function.
″When the degree of improvement in the left foot of the BC/GO +MSCs group Wistar rats was compared with the right foot (control), and the EMG CMAP results measured at the 4th and 8th weeks were examined, there was a tendency for the difference between the left and right foot to increase in the 8th week compared to the 4th week. This difference, indicating deterioration, was statistically significant. However, although this increase in the 8th week was less pronounced than in the graft group, it was not statistically significant. This finding was possibly due to the presence of alloantigens with immunogenic potential in the bacterial cellulose material. Nevertheless, it is suggested that the additional administration of stem cells may have limited the inflammation due to their immunomodulatory properties.
Importantly, no time-dependent deterioration was detected in CMAP rates for rats treated with BC/GO +MSCs. This suggests that stem cells may have acted as immunomodulators, limiting the immune response and suppressing inflammation.
Histopathological evaluation was performed to assess axonal regeneration. During incision, the macroscopic adhesion of the sciatic nerve to the surrounding muscle tissue and its ease of dissection were also observed. In the autograft group, the nerve could be easily separated and showed no significant adhesion to surrounding tissues. In contrast, in the other two groups, the nerve was covered with a membranous layer, though this was less pronounced in the BC/GO +MSCs group. En bloc dissection was challenging due to the nascent adhesions. Consistent with the EMG findings, these observations suggest that an immune response developed in the graft group, while the administration of AdMSCs may have attenuated this response through their immunomodulatory effects.
Significant necrosis and intense inflammatory reactions were noted in both groups, particularly in the inner regions of the tissue cross-sections. Neutrophils, lymphocytes, macrophages, and multinucleated giant cells were identified within the inflammatory zones. Although some areas in the graft group showed significant fascicle regeneration, degenerative changes were noted in certain axons within these regions. An increase in fibroblasts and connective tissue layers was also observed in the BC/GO +MSCs group; however, axon organization within regenerating fascicles was more regularly oriented. These histo-morphological findings confirm our hypothesis of an allo-immunization-like response triggered by the graft.
Based on these findings, we demonstrated that the BC/GO graft elicited an immune response, and the administration of AdMSCs partially mitigated the associated degenerative effects. Notably, no existing study in the literature has reported that bacterial cellulose possesses alloantigenic properties, underscoring the need for more comprehensive investigations using advanced molecular techniques to explore this possibility.
In addition, although the 8th week findings indicated an inflammatory response, the 4th-week EMG results suggest that BC/GO grafts may still hold therapeutic potential in the early stages of treatment. However, considering the possible immune response that may develop beyond the 8th week, it will be helpful to clarify the effectiveness of appropriate treatment protocols accordingly through extended and long-term studies. It is necessary to elucidate the immune response mechanisms associated with bacterial cellulose derived from Gluconacetobacter xylinus and confirm these findings using advanced models.
4 Conclusions
The BC/GO nanocomposite scaffold was successfully confirmed through FTIR spectroscopy and characterized via XRD analysis. The FTIR spectra verified the integration and stabilization of GO particles within the composite matrix. SEM analysis revealed that GO was well dispersed within the bacterial cellulose microfibrils and penetrated the scaffold pores, potentially influencing the scaffold's physicochemical properties. Incorporation of GO also enhanced the hydrophilicity of the BC scaffolds. Our findings demonstrate that although BC/GO scaffolds may trigger an immune and inflammatory response in the absence of immunosuppressive strategies, they still demonstrate potential in supporting early axonal regeneration. The mechanisms underlying this immune reaction, particularly with bacterial cellulose produced by Gluconacetobacter xylinus, require further investigation. Importantly, combining the scaffold with AdMSCs exhibited partial neuroprotective effects, likely attributable to the immunomodulatory properties of AdMSCs, as evidenced by EMG and histopathological analyses. Despite inflammatory and degenerative responses in the graft groups, early EMG improvements suggest that BC/GO-based scaffolds may play a beneficial role in the initial phases of peripheral nerve repair. Further studies incorporating optimized immunosuppressive protocols and long-term functional evaluations are essential for improving this approach for enhanced nerve regeneration.
5 Materials and Method
5.1 Fabrication of the BC/GO Scaffolds
Bacterial cellulose (BC) membranes were produced using Gluconacetobacter xylinus cultured in Hestrin and Schramm (HS) medium, composed of 2.0% (w/v) D-glucose, 0.5% (w/v) peptone, 0.5% (w/v) yeast extract, 0.27% (w/v) disodium hydrogen phosphate, and 0.115% (w/v) citric acid. The pH was adjusted to 5.0–6.0 using acetic acid [36]. After incubation in a static incubator at 28°C for 7 days, membranes were purified in 0.1 M NaOH for 24 h and rinsed thoroughly with deionized (DI) water. Membranes were sterilized in an autoclave at 121°C for 20 min, followed by freezing at −50°C for 24 h.
Graphene oxide (GO) was synthesized from graphite powder using a modified Hummers method. In brief, graphite flakes (3.3 g) and NaNO₃ (2.5 g) were mixed with 100 mL of concentrated H₂SO₄ in an ice bath and stirred for 30 min. KMnO₄ (15 g) was added gradually, and the mixture was stirred for another 30 min, then heated to 35 °C and stirred for an additional 2 h. Distilled water (150 mL) was slowly added, followed by 15 mL of 30% H₂O₂ to terminate the reaction. The resulting suspension was stirred for 1 h, filtered, washed with a 1:10 HCl:H₂O solution, and dried in a vacuum oven. Finally, 15 mg of the obtained graphite oxide powder was dispersed in aqueous solvent by ultrasonication, yielding a stable brown dispersion of GO flakes [37].
For scaffold preparation, GO was dispersed in distilled water and sonicated for at least 30 min to ensure uniform distribution. A total of 10 mL of GO solution (1 mg/mL) was added to the BC membranes and incubated on a linear shaker at room temperature for 24 h to allow impregnation. The resulting composite was referred to as the BC/GO scaffold.
5.2 Characterization of BC/GO Scaffolds
The cellular morphology on BC/GO scaffolds was evaluated using SEM. On day 4 of cell culture, samples were fixed in 2.5% glutaraldehyde (Sigma-Aldrich) and subsequently dehydrated with serial ethanol dilutions. The samples were then sputter-coated with gold and imaged using SEM. The structural features of pure BC, GO, and BC/GO composites with varying GO concentrations were characterized using Fourier transform infrared (FTIR) spectroscopy (Model 4600, Jasco, Japan). FTIR spectra were collected over a wavenumber range of 400–4000 cm−1. The crystalline structures of the nanocomposites were analyzed by X-ray diffraction (XRD, Shimadzu-6100, Japan) with Cu Kα radiation (λ = 1.54060 A°). Diffraction patterns were recorded over a scan range of 5°–45° at a scanning rate of 2°min−1. The wettability of the nanocomposites was assessed via water contact angle (WCA) measurements using a CAM 200 optical contact angle meter (KSV Instruments, Finland) at room temperature. Droplets of 10 µL were deposited onto the surface of BC and BC/BN nanocomposites, and images were captured immediately and continuously for 1 s.
5.3 Cell Culture Study
AdMSCs were isolated from adipose tissues obtained from the inguinal and umbilical regions of adult Wistar rats, following a previously established protocol [9]. Briefly, freshly harvested adipose tissue was minced with a sterile scalpel and enzymatically digested in collagenase type I solution for 1 h at 37°C with gentle agitation in a water bath. The digested tissue was centrifuged (Beckman Coulter, USA), and the upper lipid layer was discarded. The cell suspension was filtered through 100 µm and 40 or 70 µm filters, washed with 15 mL of phosphate-buffered saline (PBS), and centrifuged again. After discarding the supernatant, the cell pellet was resuspended in 5 mL of DMEM/F12 medium supplemented with 15% fetal bovine serum (FBS), 2 µM L-glutamine (Gibco, USA), 10 ng/mL epidermal growth factor (EGF; Peprotech, USA), and 1% penicillin/streptomycin. Cells were seeded into culture flasks and incubated at 37°C in 5% CO2 for 48 h. Nonadherent cells were removed by replacing the culture medium. Cells were maintained until they reached 80–90% confluency, at which point they were passaged. Third-passage AdMSCs were used for all subsequent grafting procedures.
AdMSCs were characterized via flow cytometry using fluorescence-activated cell sorting (FACS; BD FACSCalibur, BD Biosciences, USA). Cells were positively stained for mesenchymal markers CD90 and CD29, and negatively stained for hematopoietic markers CD45, CD34, and CD14, confirming their mesenchymal origin (Figure S1).
5.3.1 Viability
BC/GO scaffolds were cut into circular discs (0.04 mm in thickness and 8 mm in diameter) and placed into 96-well plates. The scaffolds were sterilized under ultraviolet (UV) light for 12 h. AdMSCs were seeded onto the scaffolds at a density of 1×104 cells/well and incubated at 37°C in a 5% CO2 incubator. A blank well (tissue culture polystyrene, 2D) served as the control. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. After 1 and 4 days of incubation, the scaffolds were gently washed with PBS and incubated with 0.5 mL of 0.5 mg/mL MTT (Glentham Life Sciences) in PBS for 4 h at 37°C in 5% CO2. Following incubation, the supernatant was carefully removed, and 100 µL of dimethylsulfoxide (DMSO; MERCK) was added. The mixture was transferred to a new 96-well plate, and absorbance was measured at 560 nm using a microplate reader (Enspire, Perkin Elmer) (Figure 5, Upper).
5.3.2 Cell Attachment
After 4 days of incubation, the BC/GO scaffolds were fixed with 4% formaldehyde for 20 min and subsequently washed with PBS. The fixed samples were permeabilized using 0.1% Triton X-100 in PBS for 20 min, followed by additional PBS washes. The samples were then stained with DAPI (1 µg/mL) (Thermo Fisher, USA) for 10 min at room temperature, and excess stain was removed by washing with PBS. Cell fluorescence was determined using a fluorescence microscope (Leica Microsystems) for A) 20X magnification and B) 3-dimensional cell distribution.
5.4 Experimental Design
5.4.1 Ethical Consideration
All animal procedures were conducted by the Guidelines of the Animal Experimentation Committee of Marmara University (08.03.2022, “23.2022mar”). Animals were maintained under controlled environmental conditions, including a 12-h light/dark cycle, constant humidity (60%), and temperature (22°C), with free access to food and water. All necessary measures were taken to minimize pain and distress.
5.4.2 Experimental Stage
Adult Wistar rats (250–300 g) were obtained from the Laboratory Animal Centre of Marmara University. A total of 27 male adult Wistar rats were randomly divided into three experimental groups (n = 9 per group): 1) autograft group, 2) bacterial cellulose and graphene oxide mixture (BC/GO) group, 3) BC/GO scaffolds seeded with AdMSCs via cell culture techniques (BC/GO +MSCs). Each rat was housed in a separate cage and provided food and water without restriction. After an acclimation period, surgical procedures were performed. Each scaffold used in the study measured 10 mm in length with a 2-mm internal diameter. Functional, electrophysiological, histological, and morphological assessments were conducted 8 weeks postsurgery.
5.4.3 The Surgical Procedure
General anesthesia was administered intraperitoneally using ketamine (100 mg/kg) and xylazine (10 mg/kg). Following anesthesia induction, rats were positioned prone, and a linear skin incision was made from the left hip to the popliteal region to expose the left sciatic nerve. The mid-portion of the left sciatic nerve was sharply transected, creating a 10 mm nerve gap. In the autograft group, the excised 10 mm nerve segment was reversed (rotated 180°) and re-implanted as an autograft. In the BC/GO and the BC/GO +MSCs groups, nerve scaffolds measuring 12 mm in length were used. The proximal and distal nerve stumps were inserted 1 mm into each end of the scaffold, effectively bridging the 10 mm nerve gap. For the BC/GO +MSCs group, each scaffold was seeded with 6×105 AdMSCs and incubated at 37°C in a 5% CO2 incubator for four days. Immediately prior to implantation, the cell-seeded scaffolds were washed with phosphate-buffered saline (PBS), and the surgical procedure was performed. All nerve reconstructions were secured with 10/0 polyamide microsutures (Ethicon Inc.). After irrigating the surgical field with sterile saline, the muscle and skin layers were closed using 3/0 polyamide sutures (Ethicon Inc). Animals were given free access to food and water upon recovery.
A walking test was performed on all subjects at the end of the 4th and 8th weeks, and the sciatic functional index (SFI) was calculated. Nerve function was assessed using the SFI test based on footprint analysis following the Bain-Mackinnon-Hunter method. According to the formula, an SFI of -100 corresponds to a complete sciatic nerve transection, while 0 indicates a fully functional sciatic nerve. In other words, as the SFI value becomes less negative, functional improvement due to regeneration increases. Electrophysiological evaluation was conducted using EMG. Two parameters were utilized: the direct EMG CMAP values, ranging between 300 and 900, and the comparative CMAP rate (%CMAP), calculated as %CMAP = 100 × CMAP (left) / CMAP (right). Histopathological evaluations were performed after sacrificing with a high-dose anesthetic at the end of the 8th week.
5.4.4 Histomorphological Evaluation
Tissues fixed in 10% buffered formaldehyde for 24 h were processed through alcohol, xylene, and paraffin series for tissue preparation. Sections of 3 µm thickness were cut from paraffin-embedded tissues using a microtome (Leica RM2125) and placed on slides, followed by deparaffinization at 60°C for 1 h. Subsequently, Hematoxylin-Eosin staining was performed. The slides were first placed in xylene for complete deparaffinization, then rehydrated through a descending alcohol series. Nuclear staining was carried out with Gill III hematoxylin, followed by washing under running water. Cytoplasmic staining was completed by immersing the sections in eosin. After a final wash with water, the sections were dehydrated through 70%, 80%, and 96% ethanol series and coverslipped. Histomorphological changes were evaluated under a light microscope (Olympus CX41) using 4× and 40× objectives.
5.4.5 Immunohistochemistry Staining
To demonstrate S100 expression, the HRP-polymer immunohistochemistry staining method was used. Sections of 3 µm thickness were taken from paraffin-embedded tissues and incubated overnight at 37°C. The sections were first deparaffinized using three changes of xylene, then hydrated with two changes of 96% ethanol. Endogenous enzyme activity was blocked with 3% hydrogen peroxide (in methanol) for 20 min. After washing with distilled water, antigen retrieval was performed using EDTA (pH 8.0) in a microwave oven. Once cooled to room temperature, the slides were washed in phosphate-buffered saline (PBS, pH 7.4). To prevent nonspecific staining, a protein blocking solution was applied to the sections. Following this, a ready-to-use mouse anti-S100 primary antibody (330M-18, Cell Marque, USA) was applied and incubated at room temperature for 1 hour. The sections were washed again with PBS, and HRP-polymer was applied. The chromogenic reaction was developed using 3,3’-diaminobenzidine (DAB). After nuclear counterstaining with Mayer's Hematoxylin, the sections were dehydrated with 96% ethanol and coverslipped with mounting medium.
5.5 Statistical Analysis
IBM SPSS version 25 software was used for statistical analysis. Unpaired t-test (ANOVA) was used to compare the parametric data of two independent groups, while the Mann-Whitney Test was used to compare non-parametric data. The Wilcoxon matched-pairs signed-ranks test was used to compare the non-parametric data of two dependent groups. Kruskal–Wallis Test (Nonparametric ANOVA with post-test, Dunn's Multiple Comparisons Test) was performed to compare the non-parametric data of three independent groups. Pearson correlation analysis was used for correlational analysis of parametric data.
In addition, to determine the study's power, posthoc power analysis was performed using the PS power and a sample size program at the end of the study. With a type I error probability of 0.05, a standard deviation of 0.19, and a mean difference of 0.35, the study's power was calculated as 0.954.
Acknowledgements
The study was funded by the Scientific Research Committee of Marmara University under protocol number TTU-2022-10513.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.