Characterization and Drug Delivery Potential of Biodegradable PCL/PLA Scaffolds Fabricated via Solvent-Cast Direct-Writing
Funding: The authors acknowledge financial support from UCL Mechanical Engineering for funding conference participation and research visits, which have contributed to the development of this research. Work conducted while SHC was at University College London.
ABSTRACT
3D printing of biodegradable scaffolds for drug delivery holds significant promise for patient-specific tissue engineering. Solvent-cast direct-writing (SCDW) is a versatile technique that produces intricate architectures by microextruding polymer solutions that solidify upon solvent evaporation. Unlike conventional 3D printing approaches, which often require high pressures, elevated temperatures, or photocurable resins, SCDW operates under gentle conditions, accommodating a wide variety of biodegradable polymers and thermosensitive agents. This study develops a specialized SCDW protocol to construct complex scaffold geometries using polycaprolactone (PCL) and polylactic acid (PLA) as the polymer matrices, with ibuprofen serving as the model thermosensitive drug. The thermal, physical, and mechanical properties of the PCL/PLA system are characterized, and in vitro dissolution studies assess the impact of polymer composition on drug release kinetics. Results reveal a strong correlation between the polymers’ physical state and release behavior: PCL to PLA ratio of 35:65 achieved the highest cumulative release in a sustained manner, releasing over 40% of the encapsulated drug within three weeks. Ratios richer in PCL triggered an initial burst release, while higher PLA contents decreased the release rate. This study establishes a versatile framework for expanding SCDW-processed biodegradable polymers in advanced drug delivery and tissue engineering applications.
1 Introduction
3D printing of biomaterials has demonstrated significant potential in transforming theoretical applications into practical solutions in tissue engineering and the creation of pharmaceutical dosage forms for targeted delivery and controlled release [1-10]. Today's 3D printing landscape encompasses a broad spectrum of technologies, each defined by its layer-by-layer deposition strategy, achievable resolution, structural complexity, and compatible material palette. One specific method, known as solvent-cast direct-writing (SCDW), operates by extruding pre-prepared ink from a syringe through a nozzle at a controlled flow rate under applied compressional force [11]. As the material is deposited according to the input architecture, the solvent evaporates, leaving behind a polymer-based cast [12-14].
The 3D construction via SCDW relies on solvent evaporation, offering distinct advantages but also certain limitations. It can cause evaporation-induced volumetric shrinkage and leave behind residual solvent, both of which may alter the material's mechanical or biological performance and restrict its ultimate utility. Overcoming these issues demands careful tuning of operational parameters. Despite its limitations, SCDW stands out from other 3D printing techniques by enabling the processing of a vast array of biomaterials. This includes thermolabile biomolecules, such as proteins and growth factors, which are highly susceptible to degradation at elevated temperatures. Additionally, this approach is well-suited for drugs with low melting points, such as ibuprofen, as it effectively preserves the integrity of these compounds, preventing thermal degradation [15, 16].
Extrusion-based 3D printing of thermosensitive drugs primarily relies on water-soluble polymers, which are more suited for oral drug formulations due to their swelling properties and rapid dissolution [17-20]. However, most biodegradable polymers used in tissue engineering scaffolds, such as polycaprolactone (PCL) and polylactic acid (PLA), are water-insoluble and require specific solvents for processing. In this context, SCDW presents a significant advantage by enabling the fabrication of biodegradable polymer scaffolds while concurrently incorporating thermosensitive molecules. This capability sets it apart from high-temperature techniques, such as fused deposition modeling, laser sintering, and photocurable methods that often require toxic photo-initiators [21, 22]. Although previous studies have explored the use of biodegradable polymers with SCDW, there remains a significant gap in research specifically addressing their potential in drug delivery systems [23].
Biodegradability streamlines implantable devices in tissue engineering and drug delivery by eliminating the need for retrieval and enabling programmed degradation to sustain therapeutic drug concentrations [24-26]. PCL and PLA are FDA-approved, biodegradable, biocompatible semi-crystalline thermoplastic polyesters with demonstrated potential as drug carriers [27-31]. PCL's low glass transition temperature (Tg) of approximately −60°C yields a rubbery, highly flexible network (strain > 1000%) that facilitates rapid mass transfer of encapsulated drugs, often resulting in an initial burst release [32, 33]. In contrast, PLA's Tg ranges from 40°C to 60°C, depending on molecular weight and physical form, imparting greater strength and stiffness but lower flexibility and elongation at break [34-36]. While previous studies have investigated PCL- and PLA-based scaffolds for drug delivery, there exists a paucity of studies examining the efficacy of their blends in drug delivery systems, and none have been fabricated using SCDW technology [37-39]. By harnessing the complementary thermal and physical characteristics of each polymer, binary blends offer a versatile platform for tailoring carrier morphology and engineering precise drug-release profiles [40-46].
This study investigates the potential of SCDW for fabricating scaffolds from PCL and PLA, beginning with an assessment of the processing condition requirements for these polymers and the development of a novel fabrication protocol. The study then focuses on characterizing the polymers and their blends following SCDW fabrication and evaluating their suitability as drug delivery systems. Ibuprofen, selected as a model drug, is used to assess the effectiveness of SCDW's mild operating conditions in preserving thermosensitive molecules during processing. Finally, the release profile of the drug from the scaffolds is measured, and the effect of the polymer composition in determining the release profile is evaluated.
2 Results and Discussion
2.1 Novel Solvent-Cast Direct-Writing Protocol
2.1.1 Protocol Development
The printing parameters required for the fabrication of PCL and PLA constructs were determined using a custom-made SCDW 3D printer. To understand the thermal behavior of the raw polymers, their thermal properties were characterized (Figure S1).
PCL fabrication posed challenges due to its susceptibility to warping, which compromised structural stability and hindered the realization of the intended design [47]. A common strategy to mitigate warping during solidification is to extend the crystallization time, thereby reducing internal stress by allowing polymer chains to reorganize more uniformly and minimizing shrinkage differentials [48, 49]. To investigate this, the exothermic crystallization behavior of raw PCL during controlled cooling from the melt was analyzed using differential scanning calorimetry (DSC). At this slow cooling rate of 0.5°C min−1, crystallization began at approximately 43°C (Figure 1A). Based on the endothermic heating curve of raw PCL, the temperature range that could delay crystallization without inducing remelting was identified as 43°C–45°C.
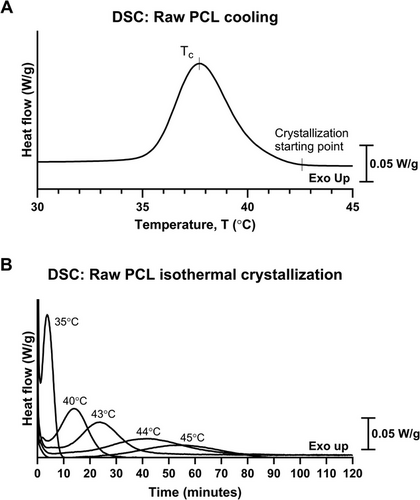
To further examine this, isothermal crystallization of PCL from the melt was analyzed using DSC. Five isothermal temperatures were selected: 35°C, 40°C, 43°C, 44°C, and 45°C. The results indicated that crystallization had already initiated during quench-cooling at 35°C and 40°C. By analyzing exothermic heat flow over time, the crystallization completion time for each isothermal temperature was determined (Figure 1B). The mean crystallization times (n = 2) were 52 min, 72 min, and 85 min at 43°C, 44°C, and 45°C, respectively. These results suggested that crystallization could be delayed by allowing PCL to solidify within the 43–45°C range and maintaining it at this temperature beyond the required time for full crystallization.
PLA, on the other hand, presented additional fabrication challenges owing to its propensity to retain the feed solvent, dichloromethane, within the polymer matrix, necessitating an effective strategy for thorough solvent removal. Dichloromethane is classified as a Class 2 solvent by the FDA and is permissible at concentrations up to 600 ppm in finished products; consequently, residual solvent levels in solvent-cast constructs must be tightly regulated to ensure safety and compliance [50]. In practice, thermogravimetric analysis (TGA) is often employed as a rapid, preliminary screening tool for residual solvent content, whereas gas chromatography offers the precision required for trace-level quantification to assess potential toxicity [51]. Structural integrity was not a concern, as PLA has been demonstrated to be processable via this method in previous studies [11, 12]. A simple yet effective approach was to fabricate above the boiling point of the feed solvent, dichloromethane (39.8°C), to accelerate solvent evaporation during the solidification process [52]. To simultaneously mitigate warpage in PCL, the highest isothermal temperature of 45°C, followed by a 90-min post-isothermal processing step, was identified as the optimal condition for addressing fabrication challenges in both PCL and PLA using the SCDW method.
2.1.2 Design, Assembly, and Implementation
To implement the required processing conditions in the SCDW method, a drying apparatus was designed to heat the printing region and ventilate the space, accelerating solvent evaporation. This led to designing an extruder with an integrated dryer (Figure 2A). The design comprises four main components: the body, gear transmission, compressor, and dryer. The body connects the high-torque stepper motor (400 steps per revolution), secures the gear housing, holds the ink-loaded syringes, and mounts the extruder to the linear guide rail. A ×3 torque multiplier was achieved via the pulley system. Linear translation for syringe compression was facilitated by an M8 threaded drive screw (lead = 1.25 mm). The extruder operated with 15360 e-steps per revolution. The customized extruder assembly was mounted on a disassembled 3D printer, Ender 3 Pro (Creality, Shenzhen, China), with the Marlin firmware configured accordingly (Figure 2B).
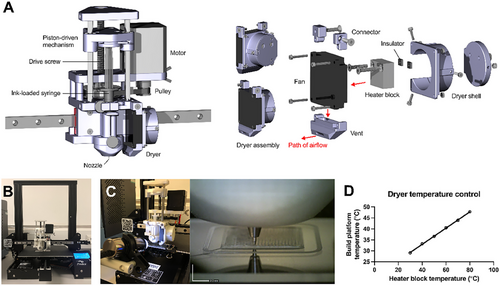
A portable microscope was used to monitor the printing process, ensuring a continuous flow of extrudates without clogging (Figure 2C). Print region temperature was controlled by adjusting the heater block within the dryer, directing heated air toward the extrusion point. The linear relationship between the heater block temperature and the build platform temperature within the given domain enabled precise temperature control (Figure 2D).
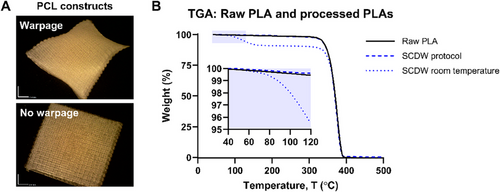
The custom-designed SCDW 3D printer was employed to fabricate PCL and PLA constructs under two conditions: (i) dryer inactive (room temperature) and (ii) testing protocol with the dryer active to achieve 45°C with 90 min of post-processing. To assess the impact of the protocol, a 100% infill, square-based construct (15 × 15 × 0.4 mm) was printed using a 400 µm nozzle and a set layer height of 200 µm. Microscopic imaging of PCL post-printing revealed that the protocol effectively eliminated warpage, which was evident in samples printed with the dryer inactive at room temperature (Figure 3A). TGA was conducted to quantify residual solvent content in PLA matrices before and after applying the protocol (Figure 3B). PLA printed with the dryer inactive retained solvent levels exceeding 9% of its initial mass. In contrast, PLA processed using the optimized SCDW protocol exhibited negligible weight loss, comparable to raw PLA. These findings indicate that processing at 45°C, followed by 90 min of post-drying, effectively removed residual solvents, demonstrating the effectiveness of the determined fabrication protocol.
Having established the SCDW protocol to eliminate structural warpage in PCL and residual solvent in PLA, the next objective was to ensure precise layer heights for additive fabrication. A key challenge was the thinness of the resultant films, as the relatively low solid component of the inks (30% w/w polymer concentration) led to substantial volume loss due to solvent evaporation (70% w/w solvent concentration), causing volumetric shrinkage. Given the importance of structural accuracy, layer height correction was implemented by measuring and compensating for volumetric loss (Figure 4A).
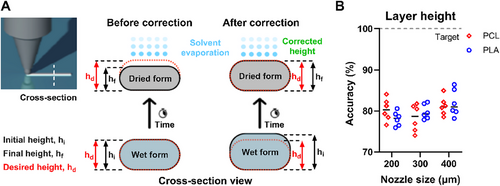
To quantify height loss, 2-layered square-based constructs (15 × 15 × height mm) were fabricated using PCL- and PLA-based inks with 200, 300, and 400 µm nozzles. Layer heights were set at 120, 160, and 200 µm, respectively, ensuring compatibility with the printer's 40 µm z-axis resolution. Thickness was measured to the nearest 0.01 mm and compared to input values to calculate layer height accuracy (Figure 4B). All samples showed ∼80% accuracy, indicating a 20% height reduction. To compensate, manual G-code corrections adjusted subsequent layers. For example, with a 400 µm nozzle and 200 µm layer height, the second layer was printed at 360 µm above the print bed level instead of 400 µm, accounting for the first layer's reduced height (160 µm). The third layer was set at 520 µm, with each layer increasing by 160 µm. Adjustments for other nozzle sizes followed the same principle: 120 µm for 300 µm nozzles and 80 µm for 200 µm nozzles. This correction method enabled precise scaffold fabrication using 200–400 µm nozzles (Figure 5).
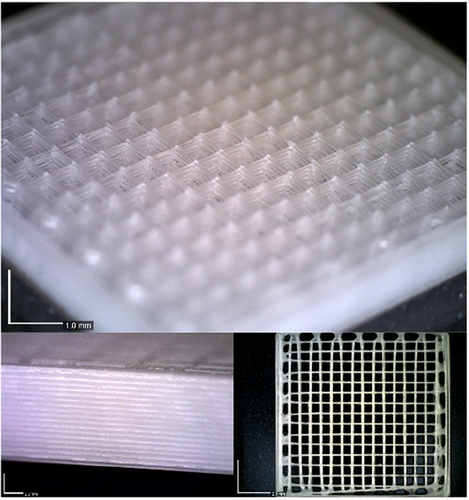
The demonstrated protocol proved effective for fabricating constructs from PCL, PLA, and their blends. This setup successfully addressed two persistent challenges in polymer processing: warpage in PCL and solvent retention in PLA. Volumetric loss of extrudates due to solvent evaporation was subsequently corrected using a layer height correction method. These issues had not previously been resolved within the context of SCDW. By demonstrating that precise thermal and drying control can overcome such limitations, this study establishes a methodological framework that may be extended to other polymer systems. This advancement broadens the applicability of SCDW and enhances the versatility of biodegradable polymers for biomedical applications, particularly in the fabrication of complex drug delivery scaffolds.
2.2 PCL/PLA Binary System
2.2.1 Thermal Analysis
TGA was conducted to assess residual solvent content in the PCL/PLA constructs post-printing via the SCDW protocol. The results indicated minimal solvent retention, with mass loss below 0.4% at 100°C, confirming sufficient drying of the printed scaffolds irrespective of the PCL:PLA ratio (Figure 6A). In the polymer blends, thermal degradation occurred in two distinct stages, as shown by the differential thermogravimetric traces: the first stage, between 300°C and 370°C, corresponded to PLA degradation, while the second, from 360°C to 470°C, reflected PCL decomposition (Figure 6B). These profiles are consistent with previously reported PCL/PLA data prepared via melt compounding [53].
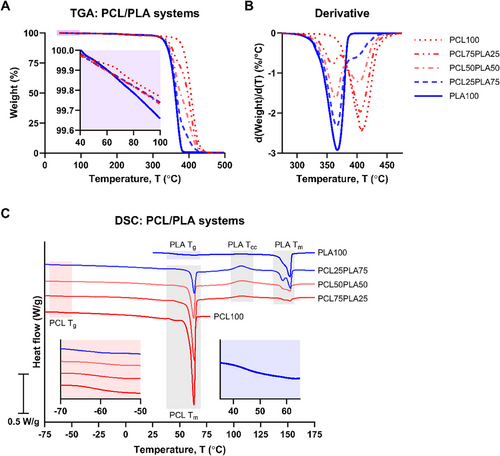
The physical state of PCL and PLA within the matrices was analyzed using DSC, and the resulting traces (Figure 6C) provided thermal property data (Table 1). PCL100 exhibited a glass transition Tg of approximately −61°C, with a melting endotherm ranging from 54°C to 66°C, peaking at 63°C. In the case of PLA100, the Tg was recorded around 49°C, while the endothermic peak appeared between 140°C and 155°C, with a minor shoulder preceding the primary peak at 152°C. As PLA is generally amorphous, it typically undergoes cold crystallization between 100°C and 120°C [54]. The absence of an exothermic peak in PLA100 indicates partial crystallization during the SCDW process.
| Formulation | PCL | PLA | |||
|---|---|---|---|---|---|
| Tg [°C] | Tm [°C] | Tg [°C] | Tcc [°C] | Tm [°C] | |
| PCL100 | −61.3 | 63.3 | — | — | — |
| PCL75PLA25 | −60.0 | 63.3 | — | 107.8 | 152.3 |
| PCL50PLA50 | −59.0 | 62.8 | — | 107.3 | 152.0 |
| PCL25PLA75 | −62.2 | 63.7 | — | 108.0 | 152.3 |
| PLA100 | — | — | 49.4 | — | 152.5 |
A shift in Tg is often indicative of polymer miscibility, as it reflects molecular-level interactions between the components [55]. However, the Tg of PCL in the blends remained relatively unchanged, ranging from −62.2°C to −59.0°C, indicating limited miscibility with PLA. The Tg of PLA in the blends was not clearly defined, likely due to an overlap with PCL's endotherm. Minimal variation in thermal properties, including melting temperature (Tm) and cold crystallization temperature (Tcc), further supports the absence of significant polymer interactions. Cold crystallization of PLA in the blends occurred around 108°C, suggesting a higher proportion of amorphous PLA within the matrix, potentially constrained by the PCL network, which restricted PLA crystallization. These results align with previous studies on PCL/PLA systems [53, 56].
2.2.2 Physical Form
X-ray diffraction (XRD) was used to examine how varying the PCL:PLA ratio influenced the physical structure of the matrices. The PCL100 samples displayed three distinct diffraction peaks at 2θ values of 21.5°, 22.2°, and 23.8°, corresponding to the crystal planes (110), (111), and (200), respectively, along with a broad halo between 15° and 26°, characteristic of semi-crystalline PCL [57]. As PLA content increased to 50% by weight (Figure 7A–C), the halo region broadened, while the position of the PCL peaks remained nearly unchanged (2θ values of 21.5°, 22.2°, and 23.9°), suggesting minimal interaction between PCL and PLA within the matrix. No PLA diffraction peaks were detected even at 50% PLA content, and the expanded halo was attributed to a higher fraction of amorphous PLA. At 75% PLA content, the XRD pattern (Figure 7D) more closely resembled that of pure PLA (Figure 7E), with a minor peak at 23.9° corresponding to residual PCL. For pure PLA, peaks at 16.9°, 19.2°, and 22.5° were associated with crystal planes (200/110), (203), and (015), respectively [58]. The PCL25PLA75 blend exhibited peaks at these same positions.
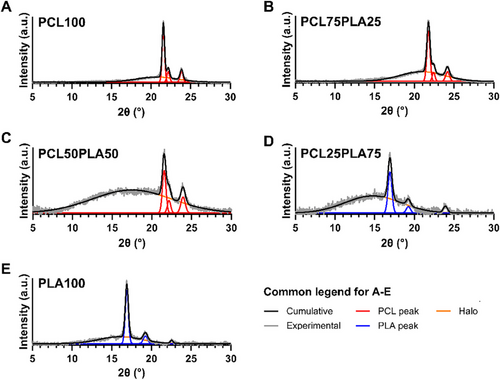
The degree of crystallinity (Xc) of PCL and PLA in the matrix was determined using the XRD data (Table 2). The crystallinity of PCL in the blends decreased with increasing PLA content, and vice versa. A gradual decline in PCL crystallinity was relatively modest in the blend with a high PCL fraction but became significantly more pronounced in compositions where PCL and PLA were present in nearly equal amounts or when PLA was the dominant phase. This behavior is attributed to the restricted molecular mobility imposed by the presence of the other polymer, which inhibits spherulite growth [59]. The crystalline phase in the PCL/PLA systems revealed lower total crystallinity in the blends compared to the homopolymers. The PCL50PLA50 formulation exhibited the lowest crystallinity, suggesting a maximum amorphous phase at this composition. Considering the combined impact of PCL and PLA crystalline phases, the preponderance of the nature of the crystalline material changed from PCL to PLA as the PLA mass proportion increased from 50% to 75%.
| Formulation | Degree of crystallinity | Crystalline phase proportion | ||
|---|---|---|---|---|
| Xc,pcl [%] | Xc,pla [%] | PCL [%] | PLA [%] | |
| PCL100 | 48.3 ± 2.2 | — | 48.3 ± 2.2 | — |
| PCL75PLA25 | 45.7 ± 3.3 | — | 34.3 ± 2.5 | — |
| PCL50PLA50 | 25.4 ± 4.2 | — | 12.7 ± 2.1 | — |
| PCL25PLA75 | 9.2 ± 5.2 | 21.5 ± 3.9 | 2.3 ± 1.9 | 16.1 ± 2.9 |
| PLA100 | — | 38.1 ± 2.5 | — | 38.1 ± 2.5 |
2.2.3 Mechanical Properties
Tensile tests were performed to assess the influence of the PCL:PLA ratio on tensile properties. The tests were conducted at 37°C to ensure consistency with the dissolution study conditions, as this temperature represents the physiological environment of the human body. From the stress-strain profiles, yield stress, ultimate tensile strength (UTS), Young's modulus, and strain at failure were determined (Table 3). The incorporation of PLA into the matrix improved both strength and stiffness. The yield stress of PCL100 was significantly lower than that of the other blends (p < 0.0001), increasing fourfold when PLA content reached 25%. However, this increase became less pronounced at higher PLA concentrations, following a gradual upward trend. Similarly, UTS increased with greater PLA content, with PCL100 differing significantly from blends containing 75% or more PLA (p < 0.05). The UTS increased by 77%, 20%, 18%, and 34% as PLA content rose in 25% increments. Regarding Young's modulus, stiffness, all formulations were statistically different (p < 0.05), except for the two with the highest PLA content, suggesting that at least 50% PCL is required to significantly reduce PLA stiffness.
| Formulation | Yield stress, σy [MPa] | UTS, σmax [MPa] | Young's modulus [MPa] | Strain, ε [mm mm−1] |
|---|---|---|---|---|
| PCL100 | 3.11 ± 0.64 | 7.57 ± 1.49 | 84.67 ± 18.02 | >4a |
| PCL75PLA25 | 12.29 ± 0.58 | 13.38 ± 2.13 | 263.79 ± 27.45 | >4a |
| PCL50PLA50 | 12.86 ± 2.24 | 15.99 ± 1.91 | 425.64 ± 83.10 | 3.10 ± 0.50 |
| PCL25PLA75 | 15.63 ± 0.61 | 18.88 ± 2.55 | 789.81 ± 85.82 | 1.62 ± 0.19 |
| PLA100 | 18.76 ± 1.05 | 25.36 ± 2.65 | 828.49 ± 111.33 | 0.11 ± 0.03 |
- a Exceeded the limit of the range of motion.
The two formulations with the highest PCL content, PCL100 and PCL75PLA25, could not be differentiated based on strain, as they did not fracture within the available range of motion of the DMA machine. The incorporation of PLA into the matrix reduced strain, making the system less ductile. The strain values of the remaining three formulations were significantly different (p < 0.0001). Strain performance decreased by nearly 50% from PCL50PLA50 to PCL25PLA75, followed by an additional 90% reduction as it approached PLA100.
The overall trends in strength, stiffness, and strain with varying PCL:PLA ratios were consistent with previous research [53, 60]. However, the strength and stiffness values observed in this study were comparatively lower. In prior studies, PCL/PLA constructs were cooled from the melt, whereas in this study, the SCDW process led to solidification as the solvent evaporated. Several factors may have contributed to the discrepancies, including material grade, testing methods, and processing conditions. Research indicates that 3D printed materials exhibit lower mechanical properties than injection-molded counterparts of the same composition due to potential voids in the matrix and the anisotropic nature of 3D printed structures [61]. Additionally, the operational temperature used in this process exceeded the boiling point of the feed solvent dichloromethane, causing rapid solvent evaporation, which may have resulted in pore formation within the polymeric matrix [62]. Consequently, the cross-sectional area may have appeared larger than the normalized area, potentially contributing to the observed reduction in mechanical properties.
2.3 PCL/PLA Drug Delivery System
2.3.1 Thermal Analysis
The thermal properties of the raw ibuprofen used in this study were characterized (Figure S2). Prior to assessing PCL/PLA drug delivery systems, homopolymer formulations were prepared to evaluate the effects of ibuprofen on solvent residue and thermal properties. Drug-loaded inks with 2% w/w ibuprofen (excluding solvent mass) were formulated to create PCL100/Ibu2 and PLA100/Ibu2 samples. TGA analysis confirmed the absence of residual solvents, with mass loss below 0.6% at 100°C, indicating effective solvent removal via the SCDW protocol (Figure 8A).
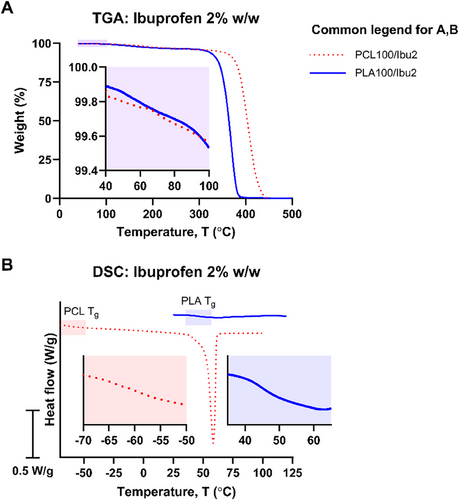
DSC analysis revealed that the melting peak of ibuprofen was not observed in PCL100/Ibu2 and PLA100/Ibu2 (Figure 8B), suggesting that the drug may exist in an amorphous state or its presence may have been undetectable due to the low drug loading. In PCL-based samples, the addition of ibuprofen increased the PCL Tg to −58.4°C and lowered the PCL Tm to 59.1°C, suggesting that the drug disrupted the spherulitic arrangement within the polymer matrix [63]. To prevent instrument damage from the degradation of ibuprofen, DSC measurements were limited to 120°C, which is lower than the melting point of PLA. The Tg of PLA remained largely unaffected by the inclusion of ibuprofen (49.2°C).
2.3.2 In Vitro Dissolution Study
PCL/PLA scaffolds were evaluated as potential drug carriers by incorporating 2% w/w ibuprofen (excluding solvent mass) into inks with varying PCL:PLA ratios from 100:0 to 0:100 in 25% increments. The dissolution study was conducted over 3 weeks (Figure 9A). The PCL100/Ibu2 formulation exhibited burst release, reaching 88.5% within 24 h, whereas PLA100/Ibu2 released 10.5% of the drug over 3 weeks in a sustained manner, consistent with previous studies [35, 64]. The variation in drug release profiles can be attributed to the Tg of the polymer-drug constructs [65]. PLA grades with different Tg values exhibit distinct release kinetics; for instance, a PLA grade with a Tg near the dissolution medium temperature released over 90% of the drug within 2 days, whereas a grade with a Tg more than 15°C higher released only 5.7% over 4 days [35]. At a dissolution temperature of 37°C, PLA remains in a glassy, rigid state, restricting molecular mobility and promoting a slower, sustained drug release. In contrast, the PCL matrix remains in a rubbery state, exhibiting high chain mobility, which facilitates rapid drug diffusion and results in burst release.
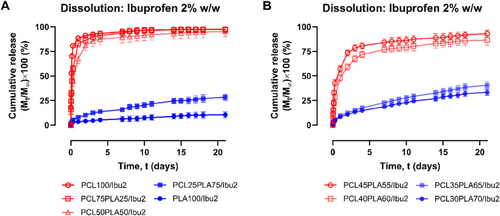
Blended formulations, PCL75PLA25/Ibu2 and PCL50PLA50/Ibu2, showed release patterns similar to PCL100/Ibu2 but slowed with increasing PLA content, releasing over 90% of the drug within eight days. In contrast, PCL25PLA75/Ibu2 exhibited a sustained release profile similar to PLA100/Ibu2 but with nearly three times the drug release (28.4%) by day 21. This highlights the critical role of the PCL:PLA ratio in modulating drug release. As PLA weight content increased from 49.0% (PCL50PLA50/Ibu2) to 73.5% (PCL25PLA75/Ibu2), the corresponding release profile transitioned from burst to sustained release.
The transition in drug release behavior between PCL50PLA50/Ibu2 and PCL25PLA75/Ibu2 raised the question of whether this range could be further refined. To investigate, additional PCL/PLA-based systems were fabricated with PCL:PLA ratios between 45:55 and 30:70 in 5% increments, maintaining 2% drug loading. Dissolution studies were then conducted on these formulations (Figure 9B). Burst release was observed in PCL45PLA55/Ibu2 and PCL40PLA60/Ibu2, while PCL35PLA65/Ibu2 and PCL30PLA70/Ibu2 exhibited sustained release profiles. As PLA content reached approximately 63.7% (PCL35PLA65/Ibu2), sustained release began, with 40.5% of the drug released by day 21, which is about four times the amount released from PLA100/Ibu2. In contrast, formulations with PLA weight of 58.8% (PCL40PLA60/Ibu2) or lower exhibited burst release, with over 75% of the drug released in the first week. The drug-loaded scaffolds exhibiting fast release profiles have potential for use in surgical situations where the therapeutic effect is required over a relatively short period only [66]. Conversely, drug-loaded scaffolds showing sustained release profiles have potential for use as in-dwelling inserts where a constant therapeutic effect can be provided over an extended period [67].
2.3.3 Physical Form
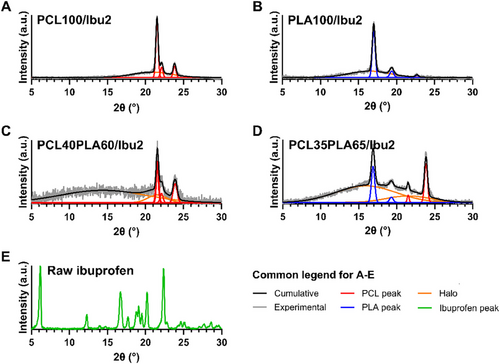
To investigate the differences in release kinetics across the transition range, the physical forms of the drug-loaded formulations were analyzed using XRD. Homopolymer-based formulations and the two systems at the transition boundaries were examined. The addition of 2% w/w ibuprofen did not alter the XRD patterns, as PCL100/Ibu2 (Figure 10A) and PLA100/Ibu2 (Figure 10B) closely resembled their drug-free counterparts (PCL100 and PLA100), with all peak positions matching within 0.1°. The resulting degrees of crystallinity were also comparable (Table 4).
| Formulation | Degree of crystallinity | Crystalline phase proportion | ||
|---|---|---|---|---|
| Xc,pcl [%] | Xc,pla [%] | PCL [%] | PLA [%] | |
| PCL100/Ibu2 | 47.7 ± 2.7 | — | 46.7 ± 2.7 | — |
| PCL40PLA60/Ibu2 | 34.3 ± 8.3. | — | 13.7 ± 3.3 | — |
| PCL35PLA65/Ibu2 | 21.4 ± 9.7 | 13.4 ± 3.5 | 7.5 ± 3.4 | 8.7 ± 2.3 |
| PLA100/Ibu2 | — | 39.9 ± 3.2 | — | 39.1 ± 3.1 |
The XRD pattern of PCL40PLA60/Ibu2 (Figure 10C) closely matched that of PCL100/Ibu2 in peak positions corresponding to PCL (2θ values of 21.6°, 22.1°, and 23.9°, assigned to crystal planes (110), (111), and (200), respectively). PCL35PLA65/Ibu2 (Figure 10D), which exhibited the highest sustained drug release, displayed diffraction peaks for both PCL (21.5° and 23.8°, corresponding to (110) and (200)) and PLA (16.9° and 19.3°, assigned to (200/110) and (203)). The broader halo regions observed in the two systems at the transition boundaries, compared to the homopolymer-based formulations, resulted from a greater amorphous fraction in the blends than in the homopolymer formulations.
In the XRD patterns, no diffraction peaks for ibuprofen were observed (Figure 10E). Despite only a 4.9% difference in PLA content, PCL40PLA60/Ibu2 and PCL35PLA65/Ibu2 exhibited distinct XRD patterns. In PCL40PLA60/Ibu2, the crystalline phase consisted entirely of PCL, whereas in PCL35PLA65/Ibu2, both PCL and PLA contributed to the total. These findings highlight the influence of the PCL:PLA ratio on drug release behavior. Formulations with primarily PCL crystalline phase tend to exhibit burst release, whereas those containing sufficient PLA to form distinct PLA crystalline peaks are associated with sustained release.
A PLA threshold of 63.7% by weight within the drug-loaded matrix was identified, beyond which the drug release profile shifts from an initial burst to a sustained release pattern. This quantitative result offers a practical design parameter for developing polymeric drug delivery systems with predictable and controllable performance. While this threshold may be specific to the SCDW process and the materials and conditions used in this study, the broader implication is that the physical characteristics established during fabrication play a central role in determining release behavior. This finding demonstrates the influence of formulation and processing conditions on functional outcomes in binary polymer systems and may inform the design of a broader range of drug delivery platforms beyond the PCL/PLA blend.
2.3.4 Potential Applications
SCDW extends traditional solvent-casting by enabling 3D deposition of polymer-solvent solutions, thereby producing complex, patient-specific architectures that leverage advances in medical imaging for personalized tissue engineering. By employing volatile organic solvents instead of aqueous media, SCDW accommodates water-insoluble biodegradable polymers, as demonstrated in this study with PCL and PLA, while its mild operating temperature preserves the integrity of thermosensitive drugs such as ibuprofen. The evaluated characteristics of the PCL/PLA-based systems and their drug-delivery potential underscore their relevance for diverse tissue-engineering applications.
The PCL/PLA-based scaffold system developed herein has potential applicability to bone regeneration with integrated analgesic delivery. In this study, the deposited constructs exhibited mechanical properties that could be suitable for non-critical load-bearing defects, such as for calvarial or maxillofacial applications [68, 69]. Nonetheless, the ibuprofen delivery through the PCL/PLA matrix could enable postoperative pain-management targets [70-72]. Furthermore, the SCDW-fabricated architecture could potentially accommodate ceramic fillers, such as hydroxyapatite, distributed homogeneously to augment mechanical stiffness toward cortical-bone-like values while preserving pore interconnectivity essential for osteoblast migration and neovascularization [73, 74].
Another promising avenue involves the fabrication of nerve guidance conduits for peripheral nerve repair with concurrent ibuprofen delivery. Recognition of the Rho/Rho-associated kinase signaling pathway as a critical regulator of axonal outgrowth has prompted strategies to modulate upstream receptors such as peroxisome proliferator-activated receptor gamma (PPAR-γ) [75]. Ibuprofen has been identified as a PPAR-γ agonist that can enhance neurite elongation in preclinical models [76, 77]. Controlled and sustained release of ibuprofen from PCL/PLA matrices could enable local administration for this purpose. However, the intrinsic stiffness of PCL/PLA may exceed the compliance requirements of an ideal biomimetic conduit, potentially impeding regeneration [78, 79]. To address this, one could blend PCL/PLA with softer polymers or plasticizers, incorporate elastomeric or more compliant biodegradable materials, and adjust porosity and filament architecture via 3D deposition. Such refinements may better recapitulate the mechanical microenvironment of peripheral nerve tissue while preserving controlled drug-release kinetics, thereby facilitating axonal extension and improving functional integration.
3 Conclusions
The successful development of an SCDW-based 3D printing strategy enhances the potential for fabricating biodegradable scaffolds with tunable thermal, physical, and mechanical properties, as well as drug delivery capabilities. In this study, material characterization revealed that the PCL:PLA ratio significantly influenced the physical form and mechanical properties. As PLA content increased from 50% to 75%, the dominant crystalline phase shifted from PCL to PLA, enhancing stiffness and strength while reducing ductility. Drug release kinetics were highly dependent on PLA content, exhibiting a burst release below 63.7% PLA and transitioning to sustained release at or beyond this threshold for formulations with 2% w/w drug loading. The controlled-temperature processing and solvent-assisted fabrication demonstrated here extend the applicability of this approach to biomaterials unsuitable for conventional heat- or laser-based 3D printing. Additionally, the successful incorporation of ibuprofen, a thermosensitive drug, highlights the method's adaptability for other thermosensitive materials, reinforcing its relevance in biomedical research. This study underscores the importance of understanding material properties, equipment capabilities, and process parameters to develop a robust manufacturing method for functional multi-component products.
4 Experimental Section
4.1 Materials
PCL pellets (Mn = 80,000 g mol−1, ρ = 1.145 g cm−3), PLA granules (Goodfellow 459-898-81; Mn = 193,300 g mol−1, ρ = 1.24 g cm−3), and dichloromethane (ACS reagent puriss. p.a., boiling point = 39.8°C) were obtained from Sigma–Aldrich (Dorset, UK). Ibuprofen powder (Ibuprofen 25; solubility in phosphate buffer pH 7.2 at 37°C = 5.2 mg mL−1, ρ = 1.18 g cm−3) was sourced from BASF (Rhineland-Palatinate, Germany). Phosphate buffer (pH 7.4) was prepared using PBS tablets (MP Biomedicals 092810305) purchased from Fisher Scientific (Loughborough, UK) and distilled water.
4.2 Ink Preparation
Inks were formulated by dissolving PCL and PLA in dichloromethane. For drug-loaded inks, ibuprofen was first dissolved in the solvent for over 4 h before incorporating the polymers. The polymer concentration was maintained at 30% w/w in the wet formulation. The amount of drug in the ink was adjusted so that the final dried product, excluding the solvent mass, contained 2% w/w concentration. The mixtures were stirred for at least 24 h using a 30 mm magnetic bar in 100 mL reagent bottles to ensure homogeneity.
4.3 3D Printer Customization
A custom-built 3D printer was developed to implement the SCDW technique. This involved designing an extruder that could be integrated into an existing desktop 3D printer frame and controlled via the machine's interface to facilitate extrusion. The extruder was modeled using CATIA V5 (Dassault Systèmes, Vélizy-Villacoublay, France) and fabricated via fused deposition modeling (FDM) using an Ender 3 Pro (Creality, Shenzhen, China) with PLA filament (Verbatim, Eschborn, Germany). Mechanical components, primarily sourced from Accu Limited (Huddersfield, UK), were assembled to construct the extruder, which was then mounted onto a modified FDM printer. The system was operated using the open-source Marlin firmware, with the extruder axis steps per unit (e-steps) calibrated based on the stepper motor's micro-stepping configuration and the gear ratio between the driving and driven gears. To ensure precise layer deposition, the printer's z-axis was manually calibrated by adjusting the build platform's leveling knobs.
4.4 Solvent-Cast Direct-Writing Operation
The printing structures were designed using CATIA V5 (Dassault Systèmes, Vélizy-Villacoublay, France) and exported as STL files. These files were then processed in Cura 4.12 (Ultimaker, Utrecht, Netherlands), which generated G-code instructions based on designated input parameters. A fixed print speed of 5 mm s−1 was applied, with the slicer software automatically determining the required volumetric extrusion and travel speed of the extruder. Ink formulations were dispensed through metal nozzles of 200, 300, or 400 µm diameter (Precision Tip, Adhesive Dispensing, Buckinghamshire, UK) attached to Luer-lock syringes. The input layer height was set to 50% of the chosen nozzle diameter, and any necessary fine-tuning of layer heights was performed manually on the generated G-code. Unless stated otherwise, a 15 × 15 × 0.32 mm construct fabricated with a 400 µm nozzle at 100% infill was used for characterization and dissolution studies.
4.5 Imaging
The Dino-Lite digital microscope (AnMo Electronics Corporation, California, USA) was used for imaging. The device was calibrated before use with the supplied auto-calibration kit using the DinoCapture 2.0 software.
4.6 Thermogravimetric Analysis
TGA was conducted using a Discovery TGA (TA Instruments, Delaware, USA). Samples, weighing 4–5 mg, were placed in pre-tared open aluminum pans (TA Instruments, Delaware, USA). The samples were heated from 40°C to 500°C at a rate of 10°C min−1 under a nitrogen flow of 25 mL min−1. Differential thermogravimetric traces were obtained using the TRIOS software, and peak deconvolution was performed with the Peak Deconvolution feature in OriginPro 2021 (OriginLab Corporation, Massachusetts, USA). Each specimen group was analyzed in duplicate.
4.7 Differential Scanning Calorimetry
DSC was performed using a Q2000 instrument (TA Instruments, Delaware, USA) with Tzero aluminum hermetic pans and lids. Calibration was carried out with indium prior to analysis. Samples (4–5 mg) were sealed and tested under a nitrogen purge (50 mL min−1). For raw PCL cooling, samples were fully melted at 80°C for 15 min to erase thermal history, then cooled at 0.5°C min⁻¹ to obtain the cooling profile. Isothermal crystallization tests involved quench-cooling to predefined temperatures and holding for 2 h. Thermal traces were recorded by heating PCL-containing samples from −80°C and PLA-containing samples up to 180°C, both at 5°C min−1. Samples loaded with ibuprofen were only heated to 120°C to prevent potential instrument damage from material degradation. Data analysis was conducted using TA Universal Analysis 2000 software. Each sample group was analyzed in duplicate, and mean values were reported.
4.8 X-Ray Diffraction
4.9 Dynamic Mechanical Analysis
Tensile tests were conducted using a Q800 DMA instrument (TA Instruments, Delaware, USA) equipped with film tension clamps. The system was calibrated for mass, position, and compliance (< 0.5 µm N−1) before testing. The samples were printed with input dimensions of 25 × 3.6 × 0.16 mm and used as printed. Specimen width was measured to the nearest 0.001 mm using a digital microscope, while thickness was recorded to the nearest 0.01 mm using a digital caliper. Samples were loaded with a 5 mm gauge length (4.95–5.05 mm), with the top and bottom ends secured to the fixed and movable parts, respectively. To minimize pre-yielding, bolts were hand-tightened, then fully secured using a torque screwdriver (QDRIVER3, Snap-on Incorporated, Wisconsin, USA) at 2 in-lb. Tensile tests were performed at 37°C with a displacement rate of 2 mm min−1 until failure or the instrument's range limit (∼25 mm). Data were analyzed in MATLAB R2019b (MathWorks, Massachusetts, USA). The UTS and strain at failure were determined from stress-strain data. Young's modulus was calculated by analyzing the linear region of the stress-strain curve, and yield stress was identified using a 0.2% strain offset method. Each group was tested in triplicate, and results were reported as mean ± SD.
4.10 In Vitro Dissolution Study
4.11 Statistical Analysis
Statistical analysis was performed using Prism V9.5.1 (GraphPad Software, California, USA). Significance was determined using one-way ANOVA, unless stated otherwise, via Tukey's multiple comparisons, with p-values ≤ 0.05 considered statistically significant. Quantitative data are reported as mean ± SD, with significance levels indicated as *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
Acknowledgements
The authors acknowledge financial support from UCL Mechanical Engineering for funding conference participation and research visits, which have contributed to the development of this research. Work conducted while SHC was at University College London.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.