Non-Porous Hydrogel Scaffolds for Locoregional Chemotherapy: A One-Pot Synthesis Approach
Abstract
Although surgery is common in the treatment of solid tumors in cancer, the risk of recurrence after operation is high in malignant tumors. This study focuses on fabricating doxorubicin-loaded, non-porous POEGMEMA (Poly(oligo(ethylene glycol) methyl ether methacrylate)) and PLGA (Poly(D,L-lactide-co-glycolide))/POEGMEMA scaffolds for locoregional chemotherapy. Polymer synthesis, hydrogel formation, and drug-loading processes are performed using a one-pot approach. The scaffolds were characterized by mechanical, swelling, degradation, and release tests. 10% PLGA content, causes PLGA/POEGMEMA scaffolds to give a 2.5 times lower swelling ratio and sixfold higher compression stress than POEGMEMA scaffolds. Also, PLGA addition causes an increase in the biodegradation half-life of POEGMEMA-based hydrogel scaffolds. While PLGA/POEGMEMA scaffolds exhibit a degradation-dependent release profile, POEGMEMA scaffolds give a burst release due to their high water uptake ratio. The burst release behavior of POEGMEMA scaffolds causes a high antiproliferative effect (viable cells: 5–10%) against HT-29 and MCF-7 cancer cells in the short term. In contrast, the controlled and sustained release profile of PLGA/POEGMEMA scaffolds shows an antiproliferative effect (viable cells: 50–60%) dependent on the release ratio. This hydrogel scaffold platform allows tuning physical and functional properties to deal with diverse physiological conditions at the region after tumor surgery for locoregional chemotherapy.
1 Introduction
The most commonly used method for the treatment of solid tumors in cancer is surgical removal of the tumor mass.[1] However, even if successful results are obtained with surgical treatment in benign tumors, recurrence after treatment is quite common in malignant tumors. To prevent recurrence, adjuvant treatments such as chemotherapy are continued after surgery to destroy any remaining cancer cells.[2] However, the inability to target drugs to tumor tissue in chemotherapy limits the effectiveness of the treatment. Drawbacks such as drug toxicity, first-pass elimination, low bioavailability of chemotherapy agents at the tumor region, and multidrug resistance cause systemic chemotherapy to fail.[3] Therefore, effective adjuvant treatment strategies that can eliminate the limitations of conventional chemotherapy are required to prevent tumor recurrence after cancer surgery.
In recent years, biomaterials have offered alternative solutions for preventing recurrence after surgical operations. Wang et al. developed a personalized local chemotherapy method for the treatment of osteosarcoma. Biodegradable 3D-printed PLLA (poly-l-lactic acid) implants were used in the study. According to in vivo experiments, 3D-printed PLLA implants maintained high drug concentrations at the tumor site for ≈12 weeks and provided a significantly higher concentration than systemic drug administration. In the implant group, 30% lower systemic side effects were observed compared to conventional chemotherapy, and tumor growth was inhibited 70% more effectively.[4] Bastiancich et al. developed anticancer drug-loaded hydrogel systems for local chemotherapy in the treatment of glioblastoma. Temozolomide (TMZ)-loaded hydrogels maintained high drug concentration in glioblastoma resected areas for ≈4 weeks and inhibited tumor growth by 85%. In the hydrogel group, survival time increased by 50% compared to the control group.[5] Wang et al. developed an in situ photo-crosslinked hydrogel loaded with doxorubicin (DOX) and the TLR4 antagonist resatorvid (TAK-242) to prevent recurrence after breast cancer surgery. Their study showed that this DOX/TAK-242 loaded gel suppressed recurrence by modulating the inflammatory tumor microenvironment caused by surgical injury. The DOX/TAK-242 loaded gel inhibited tumor growth by 70% and significantly reduced the tumor recurrence rate by increasing the concentrations of DOX and TAK-242 at the tumor site.[6] Bressler et al. developed a DOX-loaded PCL (poly(caprolactone))-based electrospun polymer network to prevent recurrence after surgical resection of soft tissue sarcoma. Animal experiments showed that these DOX-loaded meshes increased survival fourfold and almost completely prevented local tumor recurrence compared to systemic doxorubicin treatment. Additionally, 23% less cardiotoxicity was observed compared to systemic DOX administration, demonstrating a major advantage of switching to biomaterial-based local therapy.[7]
In the design of drug carrier biomaterials, the form of the biomaterial,[8] the network formation mechanism[9] and pore structures[10] are the main factors that determine the performance of the designed biomaterial. These factors directly affect the drug release rate, the time to reach the target tissue, and the treatment efficacy. For example, the hydrogel form of the biomaterial can increase the diffusion rate of the drug by expanding the distance between the polymer chains thanks to its high water retention capacity.[11, 12] Since the diffusion-controlled release is more dominant in non-porous hydrogels, it can provide a slower and controlled release profile.[13, 14] This feature enables the release of the drug in a more controlled dose and time in the applied region. In addition, it is reported that non-porous structures increase the stability of the drug by protecting it from environmental factors and therefore can be preferred in controlled drug release systems.[15, 16] The use of semi-IPN (Interpenetrating Polymer Network) methodologies in the design of non-porous hydrogels also offers various advantages.[17] Semi-IPNs are structures formed by two different polymer networks that are not chemically bonded to each other but are physically intertwined. These structures can better control the swelling behavior and drug release rate with the presence of a second polymer network. Therefore, the semi-IPN strategy can be advantageous in the design of non-porous hydrogels for a more stable and controlled drug release.
Considering the diversity of cancer tissues and drugs, different biomaterial designs are needed for the development of controlled drug delivery systems. In this study, we developed DOX-loaded (Poly(oligo(ethylene glycol) methyl ether methacrylate)) (POEGMEMA) and (Poly(D,L-lactide-co-glycolide)) (PLGA)/POEGMEMA scaffolds in a non-porous hydrogel structure. POEGMEMA scaffolds were produced by polymerization of monomer alone, while PLGA/POEGMEMA scaffolds were produced by polymerization of monomer in the presence of PLGA. Gelation was achieved thanks to the mid-chain radicals (MCR) formed as a result of free radical polymerization that causes cross-linking in concentrated conditions without the use of any crosslinker.
PLGA is a biocompatible and biodegradable polymer approved by the Food and Drug Administration (FDA) and plays an important role in the design of IPNs in the field of controlled drug delivery systems.[18-21] PLGA is degraded, metabolized, and converted into non-toxic by-products in the body.[22] At the same time, thanks to its biodegradable feature, the drug release profile can be controlled in the long term by adjusting the PLGA ratios.[23] Finally, thanks to the chemical bonds it contains, it offers an advantage in the design of drug delivery systems that can directly target the acidic environment of the tumor microenvironment by degrading it in an acidic environment.[24, 25]
Another advantageous structure for the design of IPNs is PEG (polyethylene glycol), another polymer approved by the FDA, and PEG-based polymers.[26] POEGMEMA is a brush-type polymer with short PEG side branches synthesized by polymerization of OEGMEMA monomer. The brushed structure increases the strength of physical cross-linking with steric effects, thus enhancing the mechanical strength and structural stability of the hydrogel. In semi-IPN, the brushed POEGMEMA structure can contribute to the formation of a robust network by steric entanglements. The ester bonds connecting the side branches to the backbone are broken in the acidic tumor microenvironment, causing the side branches to break and the polymer to disintegrate.[27] The low molecular weight end products formed as a result of this disintegration are excreted from the body through the kidneys.
We performed the polymer synthesis, hydrogel formation, and drug loading processes in a single step with the one-pot synthesis methodology to create drug-loaded non-porous hydrogel scaffolds. One-pot synthesis provides time-saving, less material loss, and high product yield by performing all processes in a single step. Additionally, the absence of intermediate processes helps create intelligently designed hydrogels while maintaining structural integrity.[28, 29] The methodology used is advantageous in terms of efficiency and functionality, especially in controlled release systems. Moreover, This POEGMEMA-based hydrogel scaffold with the addition of PLGA possesses a platform with tunable mechanical, swelling, degradation, and release properties for locoregional chemotherapy.
The primary aim of this study is to develop and characterize doxorubicin-loaded, non-porous hydrogel scaffolds using a one-pot synthesis approach, specifically designed for locoregional chemotherapy applications. By eliminating the need for cross-linkers and enhancing the controlled release of chemotherapy agents, we intend to provide more effective and localized cancer treatment solutions.
2 Results and Discussion
2.1 Synthesis of the Hydrogel Scaffolds
POEGMEMA and PLGA/POEGMEMA scaffolds were produced by free radical polymerization of OEGMEMA monomer found in scaffold precursors. In the production of POEGMEMA scaffolds, only monomer and initiator were used in the scaffold precursor, while in the production of PLGA/POEGMEMA scaffolds, PLGA polymer was also included in the precursor solution. Drug-loaded versions of both hydrogel types were prepared by adding DOX to the scaffold precursor solutions. The production steps of DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds are schematized in Figure 1.
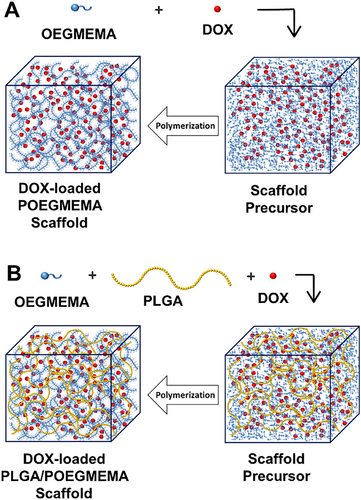
During free radical polymerization, mid-chain radicals (MCR) are formed in the polymer chain as a result of side reactions such as hydrogen abstraction. This formation can occur generally in two ways: First, by the intramolecular transfer mechanism, the radical at the end of the polymer chain can abstract a hydrogen atom from a different position on its chain. Second, by the intermolecular transfer mechanism, the radical abstracts a hydrogen atom from another polymer chain and transfers the radical function to that chain. MCRs lead to branching and cross-linking.[30-32]
High monomer concentration increases the cross-linking rate by allowing free radicals to react more rapidly and intensively with the polymer chains.[33] This process promotes the formation of tight cross-link structures and provides physical integration of the polymer network, leading to gelation. Similarly, the synthesis of PLGA/POEGMEMA hydrogels was carried out in an ethyl acetate-ethanol mixture containing a monomer (90%) – polymer (10%) mixture and an initiator.
Passos et al. synthesized pHEMA hydrogels by free radical polymerization using only HEMA (2-hydroxyethyl methacrylate) monomers without using solvents or crosslinkers. The monomer ratio being 100% increased the viscosity of the system during polymerization and limited the diffusion rate, which led to the activation of a diffusion-controlled mechanism. This process increased the crosslinking rate and triggered the self-acceleration effect called the “gel effect”.[34] In another study, Passos et al. synthesized PHEMA-PLLA (poly (L-lactic acid)) based semi-IPN hydrogels by free radical polymerization. It was reported that crosslinking in PHEMA-PLLA hydrogels occurred by the H abstraction mechanism.[35] We synthesized naturally occurring semi-IPN structured PLGA/POEGMEMA hydrogels without using any crosslinkers by using the crosslinking effect of the H abstraction mechanism. The cross-links formed by OEGMEMA monomer as a result of MCRs trapped the PLGA polymer chains in the medium and enabled the formation of a semi-IPN system.
Doxorubicin-loaded hydrogels were synthesized using the same method by adding doxorubicin (0.5% by weight) to the precursor solution. The hydrogels were obtained with a 98% yield. The PLGA/POEGMEMA and DOX-loaded PLGA/POEGMEMA hydrogel precursors before the reaction and the hydrogels obtained after the reaction are shown in Figure 2.
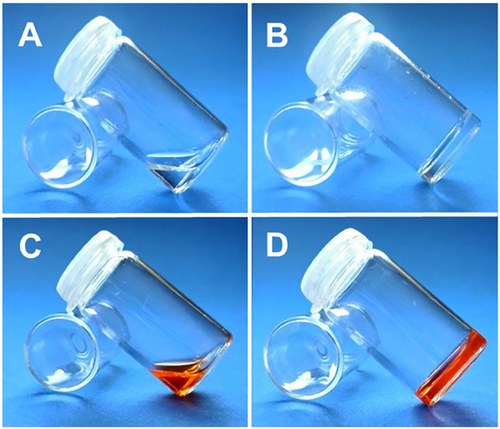
In Figure 2, images A and C show precursor solutions before gelation, while images B and D show the formed hydrogel structures. The presented images confirm that the methodology used provides gel formation. The synthesis of drug-loaded hydrogels was optimized using the “one-pot synthesis” methodology, in which polymer synthesis, hydrogel synthesis, and drug loading processes are performed in a single step. This method provided both time-saving and material usage efficiency by combining the processes. In addition, the elimination of intermediate steps increased the structural integrity of the obtained hydrogel, allowing for the creation of a more stable and effective system. The methodology used offers significant advantages in terms of both high product yield and functionality in systems for controlled drug release. In the literature, the “one-pot synthesis” methodology has been widely used in hydrogel synthesis and biomedical applications to simplify processes and save time. For example, Li et al. synthesized hydrogels with self-adhesive properties using a similar “one-pot synthesis” method. These hydrogels were prepared by combining polyacrylamide (PAM), natural chitosan (CS), and tannic acid/ferric ion (TA@Fe3⁺) chelates. N,N-methylene bisacrylamide (MBAA), used as a crosslinker, provided chemical crosslinking with TA@Fe3⁺ during polymerization, which provided strong mechanical strength and structural integrity to the hydrogels. At the same time, TA@Fe3⁺ contributed to physical crosslinking and formed a hybrid network structure, both chemical and physical. All polymerization, hydrogel formation, and chemical agent loading processes were carried out in a single reaction, saving time and material. In addition, the synthesis without intermediate steps increased the homogeneity and mechanical strength of the structure. These hydrogels designed for photothermal therapy showed high efficiency for both controlled drug release and targeted therapy applications.[36] Kim and colleagues synthesized cross-linked hydrogels with glycol chitosan and polyphenol-structured EGCG (epigallocatechin gallate) molecules by enzyme-mediated “one-pot synthesis” method. EGCG used as crosslinker was oxidized by tyrosinase enzyme and formed covalent bonds with primary amine groups on glycol chitosan. EGCG was used in this study both in cross-linking the hydrogel structure and partially remained free in the structure and showed biological functions such as antioxidant and anti-inflammatory. This method combined the polymerization, hydrogel formation, and active agent loading processes in a single pot, saving time and material usage. Since the process does not require chemical modification, structure homogeneity and biocompatibility increased.[37]
In addition, it is important to realize cross-linked structures with a one-pot synthesis methodology without using crosslinkers to avoid the toxic effects of chemical crosslinkers.[38] In this context, POEGMEMA and PLGA/POEGMEMA hydrogels developed with the one-pot approach in this study can make significant contributions to biomedical applications by ensuring homogeneous and functional hydrogel structures while avoiding the toxic effects of chemical crosslinkers. Also, this one-pot methodology ensures homogeneous distribution of the drug throughout the hydrogel matrix and maximizes loading efficiency.
2.2 SEM Analysis of the Hydrogels
SEM analysis was performed after the hydrogels were completely hydrated, freeze-dried, and subjected to the necessary gold coating process. Figure 3 shows SEM images of POEGMEMA and PLGA/POEGMEMA hydrogels.
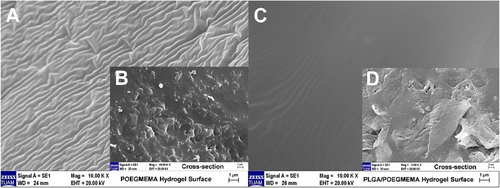
When the SEM images were examined, homogeneous morphology was observed in both hydrogel types. The images reveal that the hydrogels have a non-porous character. This non-porous hydrogel morphology is similar to the non-porous hydrogels reported by Franke et al.[39] and Aziz et al.[40] SEM analyses include not only the surface morphology but also the cross-sections of the hydrogel. As shown in Figure 3B,D, cross-sectional examinations reveal that the homogeneous non-porous structure is preserved in the internal sections of both hydrogels. These results confirm that the hydrogels are non-porous throughout their entire volume, not just on the surface. This non-porous structure was obtained due to the hydrogel formation process which was carried by the polymerization of OEGMEMA monomer in an anhydrous and concentrated medium. Considering that the hydrogels were completely hydrated and then frozen and lyophilized, the fact that they did not show a porous or hollow structure even after these processes confirms their network homogenization and stability.
2.3 Spectral Characterization of the Hydrogel Scaffolds
POEGMEMA and PLGA/POEGMEMA scaffolds were analyzed using FTIR spectrometry. Figure 4 shows the FTIR spectra of OEGMEMA monomer, PLGA, POEGMEMA scaffold, and PLGA/POEGMEMA scaffold.
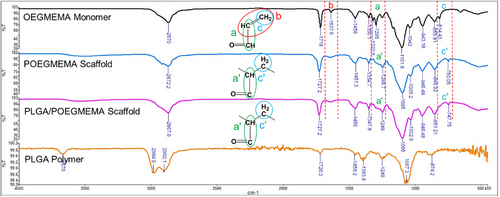
The characteristic bands in the spectrum provide information about the chemical changes in the structure with the polymerization process. The characteristic band (b) originating from the carbon-carbon double bond (C═C) observed at 1637.5 cm⁻¹ in OEGMEMA monomer disappeared in the POEGMEMA scaffold and PLGA/POEGMEMA scaffold obtained as a result of polymerization. This situation proves that the C═C double bonds of the monomers are broken during polymerization and are involved in the polymerization process. This verification is critical because incomplete polymerization can lead to suboptimal mechanical properties and inconsistent drug release rates, which play a significant role in the success of localized chemotherapy applications. Cherifi et al. also proved the polymerization by the disappearance of the characteristic bands of the C═C double bonds observed in vinyl acetate monomer (1646.07 cm⁻¹) in poly(vinyl acetate) polymer.[41] Similarly, Larrañaga et al. also proved the polymerization by observing that the characteristic bands belonging to the C═C double bonds observed in acrylic acid monomers (1637 cm⁻¹) disappeared in poly(acrylic acid) polymer.[42] At the same time, the band (a) belonging to the carbon-carbon single bonds (C─C) adjacent to the C═C double bond at 1295 cm⁻¹ in OEGMEMA monomer shifted to 1246.7 cm⁻¹ and 1249 cm⁻¹ (a’) in POEGMEMA scaffold and PLGA/POEGMEMA scaffold, respectively, due to the disappearance of the C═C double bond as a result of polymerization. Finally, the band at 814.43 cm−1 (c) originating from the CH2 group forming the C═C double bond in OEGMEMA monomer shifted to 750.05 and 747.75 cm−1 (c’) in POEGMEMA scaffold and PLGA/POEGMEMA scaffold, respectively. This shift shows the change in bond energies around the CH2 group during polymerization.[42]
2.4 Mechanical Properties of the Scaffolds
Compressive stress-strain behaviors of POEGMEMA and PLGA/POEGMEMA scaffolds are shown graphically in Figure 5. The POEGMEMA scaffold showed a maximum compressive stress of 32.1 kPa at 78% strain. In contrast, the PLGA/POEGMEMA scaffold showed maximum compressive stress of 184.3 kPa at the same strain level. This indicates that the PLGA/POEGMEMA scaffold offers higher resistance to deformation (≈6 times) compared to the POEGMEMA scaffold. This remarkable difference is due to the contribution of PLGA to the mechanical strength of the POEGMEMA scaffold.
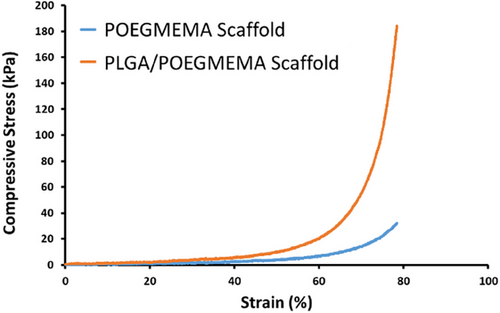
Yusop et al.,[43] Johansson et al.,[44] and Miao et al.[45] also demonstrated that the mechanical strength of the scaffolds increased with the addition of PLGA to the scaffold structures. The improved mechanical properties with the addition of PLGA indicate that it increases the intermolecular interactions and the overall network stability of the composite material. While the mechanical strength in hydrogels is generally increased by using crosslinkers,[46, 47] in the study, the mechanical strength is increased by incorporating PLGA polymer into the structure in addition to the crosslinks formed by the H-abstraction mechanism during the polymerization of OEGMEMA monomer.
Hydrogels can be used in tissue engineering as well as in locoregional chemotherapy applications. While hydrogels with higher mechanical strength are generally preferred in tissue engineering, those developed for locoregional chemotherapy are designed to degrade for completely release drugs. The robustness (compressive strength) of the hydrogel scaffolds was determined in the range of ≈30–180 kPa. These hydrogels are designed specifically for soft tissue tumors. In vivo studies with hydrogels having similar mechanical properties have shown successful results in various tissues. Yang et al.[48] have developed hydrogels with a compressive strength of 53.5 kPa for locoregional chemotherapy applications in melanoma. These hydrogels have been tested in vivo on the back skin of C57BL/6 mice. Wang et al.[49] have developed hydrogels with a compressive strength of 33 kPa for breast cancer treatment applications, conducting in vivo experiments in the mammary tissues of mice, and observed that these hydrogels reduce tumor recurrence. Both studies confirm the potential of hydrogels with similar compressive strength in locoregional chemotherapy applications.
The compressive strength of POEGMEMA and PLGA/POEGMEMA scaffolds were measured at 32.1 and 184.3 kPa respectively. The compressive strength of 184.3 kPa for the PLGA/POEGMEMA scaffold is compatible with the mechanical properties of various human tissues such as the liver, kidney, uterus, cervix, meniscus, and human articular cartilage. For example, the compressive strength in the liver ranges from 66 to 386 kPa in males and 89–308 kPa in females,[50] indicating that the PLGA/POEGMEMA scaffold has an appropriate strength level for liver applications. Additionally, the measured values of 180.32 kPa under axial loads and 95.64 kPa under transversal loads for kidney tissue[51] show the potential use of the PLGA/POEGMEMA scaffold in renal applications. The compressive strength of the cervix during pregnancy ranges from 80 to 150 kPa and the uterus from 20 to 1400 kPa,[52] the meniscus shows strength from 10 kPa to 200 kPa,[53] and human articular cartilage ranges from 0.1 to 1 MPa (100–1000 kPa)[54] also indicate the suitability of the PLGA/POEGMEMA scaffold for these tissues. Furthermore, the compressive strength of 32.1 kPa for the POEGMEMA scaffold aligns well with the strength of the uterus ranging from 20 to 1400 kPa,[52] meniscus from 10 to 200 kPa,[53] human skin from 1 to 100 kPa,[55, 56] and human tongue from 12 to 123 kPa.[57] These findings demonstrate the potential for the POEGMEMA scaffold in uterine, meniscus, skin, and lingual tissues. The mechanical properties of both POEGMEMA and PLGA/POEGMEMA scaffolds match with a wide range of tissues. That highlights their potential use in various tissues by tuning mechanical properties by altering the PLGA/POEGMEMA ratio.
2.5 Swelling Properties of the Scaffolds
The swelling test was performed for POEGMEMA and PLGA/POEGMEMA scaffolds until the scaffolds reached saturation (3 consecutive same measurements). The tests were performed in 3 different pH environments: pH: 4.0 (lysosomal pH), pH: 5.5 (tumor microenvironment pH), and pH: 7.4 (healthy tissue physiological environment pH). The swelling profiles of the scaffolds are shown in Figure 6.
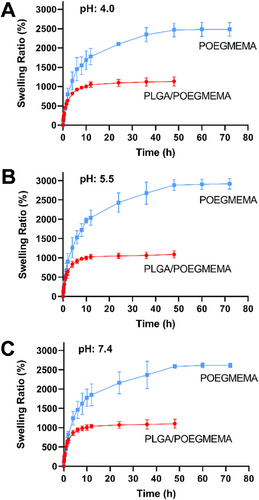
As a result of the hydrophilic character of the POEGMEMA polymer, POEGMEMA, and PLGA/POEGMEMA hydrogel scaffolds showed high swelling behavior. Due to its non-porous structure, the swelling process occurred by the diffusion of water and the water retention process continued until maximum saturation was reached.[39] POEGMEMA hydrogels continued to swell for 48 h due to their high hydrophilic character in all tested pH conditions, while the swelling behavior of PLGA/POEGMEMA hydrogels ended after 12 h due to the low hydrophilic character of the structure with the addition of PLGA polymer. Despite their high hydrophilic content, the relatively long swelling saturation times are due to their non-porous character. Since the polymer chains of these non-porous hydrogel scaffolds are densely packed, they provide strictly limited water transport via diffusion.[58] Similarly, studies on non-porous hydrogels indicate that the water uptake mechanism occurs independently of porosity and due to the hydrophilic content and through diffusion.[59, 60]
In all 3 pH conditions tested, POEGMEMA scaffolds showed a swelling capacity of ≈2500%, while this value was ≈1000% in PLGA/POEGMEMA scaffolds. The different pH conditions tested did not have a significant effect on the degree of swelling. Hydrophilic structure content is the most important parameter playing a role in swelling kinetics. While hydrophilic structure increases the degree of swelling of hydrogels, hydrophobic content significantly reduces the degree of swelling.[33, 61] The results show that POEGMEMA scaffolds have ≈2.5 times higher swelling ratio than PLGA/POEGMEMA scaffolds. This difference was due to the effect of hydrophobic PLGA content in PLGA/POEGMEMA scaffolds. At the same time, PLGA increased the morphological stability of the structure and gave the structure a shape memory feature (Figure 7B). In contrast, POEGMEMA tissue scaffolds showed swelling behavior without shape memory. (Figure 7A). The shape memory property observed in PLGA/POEGMEMA hydrogels is thought to be related to the higher mechanical properties of these scaffolds compared to POEGMEMA scaffolds.
This characteristic demonstrates the scaffold's ability to return to its original form after deformation, a critical attribute for applications requiring resilience in dynamic physiological environments. The mechanical robustness provided by the integration of PLGA not only supports this shape memory function but also impacts the swelling behavior of the hydrogels. As mechanical integrity increases, the strength of the network decreases the swelling capacity, which in turn controls the rate of fluid absorption. This relationship between mechanical strength and swelling behavior, as detailed by Kolahdoozan et al.,[62] is crucial for maintaining the functional stability and effectiveness of the scaffold in delivering therapeutic agents, particularly in environments where precise control over drug release and scaffold morphology is necessary.
2.6 Degradation Study
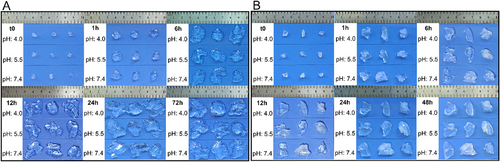
Degradation tests of POEGMEMA and PLGA/POEGMEMA scaffolds were carried out at 37 °C in 3 different pH environments: pH: 4.0, pH 5.5, and pH: 7.4. Figure 8 shows the degradation profiles of POEGMEMA and PLGA/POEGMEMA scaffolds. Figure 9 shows dried scaffold images during the degradation study.
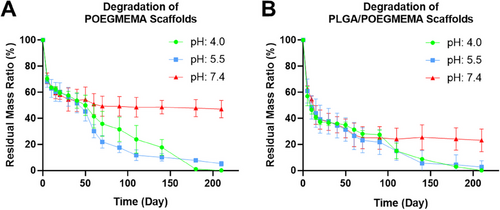

Hydrogel scaffolds appear completely degraded in acidic environments in ≈6–7 months. This period seems to be longer in neutral conditions. Such long-term degradation periods are preferred especially for locoregional treatments.[63] When evaluating degradation rates in addition to complete degradation, the degradation half-life (Dt1/2), which means the time for 50% degradation of the total mass, is an important parameter for evaluating the drug release performance.[64] At pH: 4.0, the Dt1/2 value for the POEGMEMA scaffold was 47.7 days, for the PLGA/POEGMEMA scaffold the value was 7.9 days. The Dt1/2 value was determined as 41.2 days for the POEGMEMA scaffold, 13.0 days for the PLGA/POEGMEMA scaffold at pH: 5.5, and 52.3 days for the POEGMEMA scaffold and 11.0 days for the PLGA/POEGMEMA scaffold at pH: 7.4 (Table 1).
| Degradation half-life (Dt1/2) values (Day) | |||
|---|---|---|---|
| SCAFFOLD | pH: 4.0 | pH: 5.5 | pH: 7.4 |
| PLGA/POEGMEMA | 7.9 ± 2.6 | 13.0 ± 10.0 | 11.0 ± 0.9 |
| POEGMEMA | 47.7 ± 12.8 | 41.2 ± 13.5 | 52.3 ± 19.4 |
- Results were shown as mean ± standard deviation (SD), n = 3 for each sample.
The scaffolds showed relatively similar degradation profiles in two different acidic conditions, pH: 4.0 and pH: 5.5. The degradation rate of both scaffolds in acidic conditions was higher than the degradation rate in pH: 7.4. This may be associated with the breaking of ester bonds in the structure of POEGMEMA and PLGA polymers in acidic conditions.[65, 66] However, in pH: 7.4 conditions, PLGA/POEGMEMA scaffolds exhibited a significantly faster degradation profile than POEGMEMA scaffolds. This may be explained by the fact that ester bonds in the structure of PLGA are more susceptible to hydrolysis and that lactic acid and glycolic acid formed during the degradation of PLGA catalyze degradation by increasing the local acidity.[67] However, at pH: 7.4, the degradation rate remained constant for both scaffold types from day 70 to day 210, and ≈50% degradation was observed in POEGMEMA scaffolds and ≈75% degradation in PLGA/POEGMEMA scaffolds (Figures 8 and 9).
Although POEGMEMA scaffolds are more resistant under neutral pH conditions, it is known that the rate of biological degradation rate will be higher in vivo due to the effects of enzymes and other physiological processes.[68]
On the other hand, the LA (lactic acid) and GA (glycolic acid) ratios in the PLGA structure are among the factors affecting the degradation rate of PLGA.[69] The degradation period of PLGA with a 50:50/LA:GA ratio used in the study is ≈1–2 months.[70] However, in this study, 10% PLGA was used in PLGA/POEGMEMA scaffolds. Due to the low PLGA ratio and the more stable nature of the POEGMEMA polymer in these conditions, a longer degradation time was obtained compared to structures containing 100% PLGA. However, PLGA/POEGMEMA containing PLGA showed a faster degradation than the one without the POEGMEMA scaffold.
As a result, the different degradation rates of POEGMEMA and PLGA/POEGMEMA scaffolds constitute an alternative for situations where faster or slower degradation is desired. For the potential of the post-surgical resected area to be neutral or acidic,[71] the structure with appropriate drug release and degradation rate can be preferred. Of course, determining these qualities under in vivo conditions will allow for more accurate guidance in choosing suitable biomaterial.
2.7 The Drug Release Study
The pH-dependent release profiles of DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds were tested at three different pH environments (pH: 4.0, pH: 5.5, and pH: 7.4) using an orbital shaker. Drug release was monitored in triplicate for DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds at 37 °C. Figure 10 shows the DOX release graph of DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds at different pH conditions. At the end of the first 12 h, the DOX-loaded POEGMEMA scaffold released 56% of the drug at pH: 7.4, 70% at pH: 5.5, and 82% at pH: 4.0 (Figure 10A). In contrast, the DOX-loaded PLGA/POEGMEMA scaffold released 40% of the drug at pH: 7.4, 47% at pH: 5.5, and 49% at pH: 4.0 (Figure 10B).
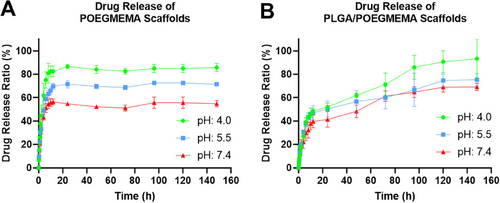
Although the PLGA/POEGMEMA scaffold is less in proportion, both scaffolds appear to have burst release in the first 12 h. The higher DOX release in the first 12 h of POEGMEMA scaffolds compared to PLGA/POEGMEMA scaffolds can be explained by the fact that POEGMEMA scaffolds exhibit ≈2 times more swelling ratio compared to PLGA/POEGMEMA scaffolds. Another related situation is that DOX, a hydrophilic drug, exhibits better diffusion in the POEGMEMA scaffold with high hydrophilic content. The solubility of DOX in water significantly impacts its interactions with different materials in drug delivery systems such as hydrogels. In hydrophilic environments, DOX dissolves easily, which often leads to faster release rates in hydrophilic structures like PEGMEMA. As shown by Cardoso et al., increasing the ratio of PEG in PLGA-co-PEG nanoparticles significantly increases the release rates of doxorubicin. This effect is attributed to the increased hydrophilicity provided by the PEG content, which facilitates water uptake and supports faster and more extensive drug diffusion. Incorporating a hydrophobic structure like PLGA into an environment containing DOX significantly alters the interaction dynamics. Cardoso et al. showed that in formulations with low PEG content, an increased proportion of PLGA extends the release duration.[72] Within a hydrogel matrix, the molecular chains of PLGA form a network-like barrier that physically confines DOX molecules. The network density and hydrophobic nature of PLGA limit the available areas and pathways for DOX to move. When the hydrogel swells in an aqueous environment, it alters the structural integrity of these hydrophobic barriers, creating more pathways and thus potentially increasing the diffusion rate of DOX. Additionally, over time, the degradation of PLGA through the hydrolysis of its ester bonds leads to the gradual breakdown of the polymer chains. As this network structure loosens, new pathways are formed, facilitating DOX diffusion.
When we look at the effect of pH conditions on DOX release, it is seen that the release rate increases as the pH value decreases, i.e., as acidity increases, in both scaffolds. This can be explained by the pH-sensitive degradation profiles of POEGMEMA and PLGA/POEGMEMA scaffolds due to their breakable ester bonds in acidic environments. The pH-sensitive release profiles of the scaffolds offer the potential to increase therapeutic efficacy by enabling the drug to target the tumor microenvironment.[73]
In our study, due to cross-linking formed during the polymerization of the OEGMEMA monomer, non-porous POEGMEMA-based tissue scaffolds exhibit remarkable properties. Despite their high water retention capacities and pronounced hydrophilic nature, these scaffolds substantially retain the hydrophilic DOX molecule after 12 h, especially under neutral pH conditions. This retention is primarily due to the scaffolds' non-porous structure, which restricts the diffusion rate of the drug throughout the matrix. The burst release observed in the first 12 h is largely attributed to the rapid diffusion of DOX molecules near the hydrogel surface, a common characteristic in highly hydrophilic polymer networks.
To gain a deeper understanding, it is critical to consider the molecular interactions between the polymer chains and the drug molecules. The tight cross-linking within the non-porous structure forms a dense network that physically restricts the movement of DOX molecules, thereby slowing the release rates after the initial burst. This phenomenon is consistent with the findings of Siboro et al.,[33] who demonstrated that the drug release rate could be decelerated in non-porous hydrogel structures due to their tightly cross-linked networks. Such behavior is advantageous for therapeutic applications where controlled release is necessary to maintain effective drug levels over an extended period without compromising the initial dose needed to effectively combat tumor cells.
The addition of the hydrophobic PLGA polymer in the structure has led to a more controlled DOX release rate due to the increased hydrophobicity of the environment in PLGA/OEGMEMA scaffolds. After 12 h, the drug release, which almost stopped in POEGMEMA scaffolds, continued slowly and in a controlled manner in PLGA/POEGMEMA scaffolds with the addition of PLGA as a factor that increased degradation.
Figure 11 includes photographs showing DOX release from POEGMEMA and PLGA/POEGMEMA scaffolds. The release can be observed by the decrease in red color which belongs to the DOX molecule that the hydrogel scaffolds retain despite swelling during the release period and release over time.
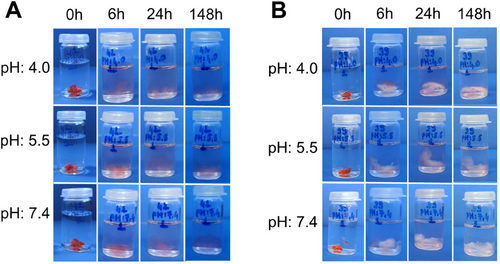
In many local drug applications, controlled release is preferred instead of burst release. Therefore, the PLGA/POEGMEMA scaffold seems advantageous for local drug applications due to its better-controlled release profile than the POEGMEMA scaffold. However, considering the resistance developed by cancer cells against drugs at low drug concentrations,[3] it is thought that systems that initially release a portion of the loaded drug with burst release, providing the loading dose and then providing the maintenance dose with the following controlled release in the region will possess advantages in locoregional chemotherapeutics. Therefore, it is thought that both POEGMEMA and PLGA/POEGMEMA scaffolds can be used as alternatives to each other in different physiological conditions.
2.8 Proliferation Properties of the Scaffolds
The proliferation properties of DOX-loaded and plain scaffolds on HT-29 and MTT cells were determined by MTT assay by comparison with the free DOX and the control group. Applied scaffold and DOX formulations on HT-29 and MCF-7 cells are given in Table 2.
| Groups | DOX Concentration (µM) | Scaffold Mass in a well (mg) |
|---|---|---|
| DOX Loaded PLGA/POEGMEMA | 10 | 1,2 |
| 15 | 1,8 | |
| 20 | 2,4 | |
| DOX Loaded POEGMEMA | 10 | 1,2 |
| 15 | 1,8 | |
| 20 | 2,4 | |
| Free DOX | 10 | 0 |
| 15 | 0 | |
| 20 | 0 | |
| POEGMEMA | 0 | 1,2 |
| 0 | 1,8 | |
| 0 | 2,4 | |
| PLGA/POEGMEMA | 0 | 1,2 |
| 0 | 1,8 | |
| 0 | 2,4 | |
| Control (RPMI) | 0 | 0 |
- n = 4 for each sample.
The proliferative effect of scaffolds on HT-29 and MCF-7 cells was investigated at 3 different DOX concentrations for drug-loaded scaffold groups and free DOX: 10, 15, and 20 µm for 72 h. The DOX concentration range used for the study was determined as where free DOX efficiently stops proliferation. Plain scaffolds were prepared to be equivalent to the masses of DOX-loaded scaffolds. Proliferation test results for HT-29 and MCF-7 cells are shown in Figure 12A,B, respectively.
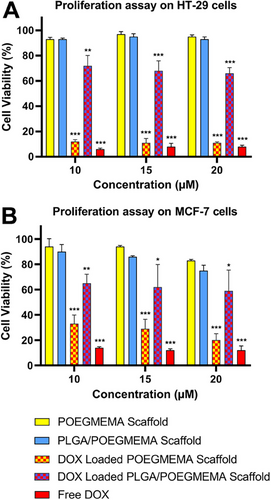
It is observed that free DOX effectively reduces cell viability to ≈5% in the HT-29 line and ≈10% in the MCF-7 line compared to the control groups in the tested concentration range. The tested cell lines showed high proliferation in the presence of plain POEGMEMA and PLGA/POEGMEMA scaffolds. Plain scaffolds showed no statistical differences compared to the control groups for both cell lines tested. Despite the high scaffold content tested, both scaffolds exhibited a 90% and above proliferation in the HT-29 cell line. In the MCF-7 cell line, proliferation values were 83% and above for POEGMEMA scaffolds and 77% and above for PLGA/POEGMEMA scaffolds. The fact that cells can maintain cell viability at a level that meets the biocompatibility criteria (>70% cell viability)[74] determined by ISO 10993–5 standards despite high scaffold exposure is due to the biocompatible polymers used in the design.
Studies examining the cytotoxic effects of drug-loaded scaffolds also emphasize that the release profiles of hydrogels can significantly affect cytotoxicity and therefore treatment efficacy.[75, 76] The proliferative effects of DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds are similarly associated with their release profiles. When we remember that DOX-loaded POEGMEMA scaffolds released ≈80% of DOX in acidic conditions with burst release, it can be understood why they showed an antiproliferative effect close to free DOX (≈10% cell viability) in the HT-29 cell line (Figure 12A). DOX-loaded PLGA/POEGMEMA scaffolds reduced cell viability in the HT-29 cell line to 72%, 68%, and 66% in a dose-dependent manner (10, 15, and 20 µm, respectively). On the other hand, PLGA/POEGMEMA scaffolds showed less antiproliferative effect than POEGMEMA scaffolds in the same cell line. It is related to their burst release being as low as half of the POEGMEMA scaffolds (≈40%).
The antiproliferative effects of DOX-loaded POEGMEMA and PLGA/POEGMEMA scaffolds on the MCF-7 cell line were similar to the ones on the HT-29 cell line (Figure 12B). In the MCF-7 cell line, cell viability decreased to ∼20% with increasing concentration of POEGMEMA scaffolds, while it remained at ≈60% levels in PLGA/POEGMEMA scaffolds. Similarly, the results were related to the DOX rates released by the scaffolds to the medium by burst release.
It has been determined that the DOX-loaded POEGMEMA scaffold provides a rapid therapeutic effect with burst release in vitro. Considering that it can still retain 50% of the loaded drug after burst release under neutral conditions, DOX-loaded POEGMEMA scaffolds may provide an effective strategy for the rapid elimination of residual tumor cells that have the potential to grow rapidly after surgery and to prevent possible drug resistance by providing high local drug concentration in the short term. In addition, the results show that DOX-loaded PLGA/POEGMEMA scaffolds have an antiproliferative effect in proportion to the drug they release due to their controlled drug-release properties. In cases of potential for recurrence in the long term after surgical dissection, such structures with a sustained release profile have the potential to prevent recurrence.
3 Conclusion
Although surgical treatment in cancer is the most widely used primary and most common treatment for solid tumors, recurrence rates after surgery are quite high, especially in malignant tumors. This situation reveals the need for effective adjuvant treatment strategies that complement surgery. In this study, we aimed to develop a platform that can provide tunable mechanical, degradation, and drug release properties by fabricating nonporous hydrogel scaffolds with potential applications, especially for locoregional chemotherapy. In this context, POEGMEMA-based tissue scaffolds were developed with 98% efficiency without crosslinkers. Compared to POEGMEMA-based scaffolds with high water retention capacity (≈%2500) and relatively low mechanical properties (32.1 kPa), in PLGA/POEGMEMA scaffolds prepared by adding only 10% PLGA to the hydrogel precursor, water retention capacity was reduced by 2.5 times (≈%1000) while mechanical properties were increased by 6 times (184.3 kPa). In addition to these physical properties, significant differences occurred in functional properties such as degradation and drug release with the addition of PLGA to the structure. Biodegradation half-life values of POEGMEMA scaffolds varied between 49–62 days under different pH conditions, while values varied between 7 and 12 days for PLGA/POEGMEMA scaffolds. In addition, although it is accepted that it will be faster under in vivo conditions, the fact that POEGMEMA scaffolds maintained their structural integrity by 50% and PLGA/POEGMEMA scaffolds by 30% for 6–7 months under neutral in vitro conditions demonstrates the potential of this platform for locoregional chemotherapy where long-term drug release is required. The drug-loaded versions of these scaffolds, whose biocompatible structures were shown by the proliferation tests, showed different antiproliferative effects against the tested cancer cells since they showed different drug release profiles. While POEGMEMA scaffolds with a high burst DOX release rate increased cell viability to 10%, PLGA/POEGMEMA scaffolds with a more prominent prolonged release character decreased cell viability to 60% in the short term. These different release profiles provide sustainable release in slightly acidic or neutral pH conditions of completely resected tumor regions while offering the potential for faster drug release in the acidic microenvironment of the remaining tumor tissue when complete resection is not possible. The results present a dataset to adjust the mass of the hydrogel, the ratio of hydrophilic to hydrophobic polymers, and the amount of drug loaded according to the size of the application area and specific pH conditions. This nonporous hydrogel scaffold platform with its tunable physical and functional properties, has the potential for many local drug applications, especially locoregional chemotherapy.
4 Experimental Section
Materials
Poly(D,L-lactide-co-glycolide) (lactide:glycolide 50:50, Mw: 24000-38000), Oligo(ethylene glycol) methyl ether methacrylate, Mn: 500 (OEGMEMA), 2,2′-Azobis(2-methylpropionitrile) (AIBN), ethanol, Dimethyl sulfoxide (DMSO), Acetic acid, 3–4,5-dimethyl-thiazolyl-2,5-diphenyltetrazolium bromide (MTT), Phosphate buffered saline (PBS), Sodium acetate trihydrate, Fetal Bovine Serum (FBS) and Penicillin-Streptomycin were purchased from Sigma–Aldrich. Acetic acid was purchased from Honeywell. RPMI 1640, and Trypsin-EDTA purchased from Biowest. High-purity nitrogen gas was purchased from Linde company. Doxorubicin HCl (DOX) was obtained from Kocak Pharma.
Synthesis of POEGMEMA and DOX Loaded POEGMEMA Scaffolds
A monomer-only synthesis method was used for the synthesis of POEGMEMA scaffolds. OEGMEMA (1000 mg) and AIBN (0.5 mg) were dissolved in ethyl acetate/ethanol mixture (1.66 mL:0.5 mL). The mixture was then placed in vials, sealed, and purged with high-purity nitrogen gas for 3 min. The reaction was carried out at 70 °C for 24 h. At the end of the reaction, the prepared tissue scaffolds were purified by washing three times in 3 mL ethyl acetate solution using a sonicator. Finally, the resulting tissue scaffolds were dried in the oven at 37 °C for 24 h. Synthesis of DOX Loaded POEGMEMA scaffolds was carried out similarly. In difference, a DOX solution (5 mg/0.5 mL) was prepared using ethanol, while OEGMEMA and AIBN were dissolved in ethyl acetate (1.66 mL). Finally, both solutions were thoroughly mixed to ensure the homogeneous distribution of DOX within the hydrogel.
Synthesis of PLGA/POEGMEMA and DOX Loaded PLGA/POEGMEMA Scaffolds
A hybrid synthesis method involving polymer-monomer mixtures carried out synthesis of PLGA/POEGMEMA scaffolds. PLGA (100 mg), OEGMEMA (900 mg), and AIBN (0.4 mg) were dissolved in ethyl acetate/ethanol mixture (1.66 mL:0.5 mL). The mixture was then placed in vials, sealed, and purged with high-purity nitrogen gas for 3 min. The reaction was carried out in an oven at 70 °C for 24 h. The resulting scaffolds were purified by washing in 3 mL ethyl acetate using a sonicator. The purification process was performed in 3 repetitions. Finally, the scaffolds were dried at 37 °C for 24 h. Synthesis of DOX Loaded PLGA/POEGMEMA scaffolds was carried out similarly. Differently, a DOX solution (5 mg/0.5 mL) was prepared using ethanol. Monomer-polymer mixtures were dissolved separately in ethyl acetate (1.66 mL). Finally, both solutions were thoroughly mixed to ensure the homogeneous distribution of DOX within the hydrogel.
SEM Imaging
The morphological properties of POEGMEMA and PLGA/POEGMEMA hydrogels were examined using scanning electron microscopy (SEM) (LEO Co., Cambridge, England, Model: 1430VP). Before SEM analysis, the samples were swollen with water for 48 h. All samples were freeze-dried for 24 h using a lyophilizer before SEM observation. Hydrogel samples were gold coated under 100 mA current for 20 s using a gold coating device (Bal-Tec SCD005 Sputter Coater). SEM images were obtained by applying 20 kV voltage.
Characterization of OEGMEMA and PLGA/OEGMEMA Scaffolds
Characterization of OEGMEMA monomer, PLGA polymer, POEGMEMA scaffolds, and PLGA/POEGMEMA scaffolds was performed using ATR-FTIR (Perkin Elmer/Spectrum Two). The spectrum was recorded between wavelengths 4000–450 cm⁻¹.
Mechanical Test
The mechanical characteristics of POEGMEMA and PLGA/POEGMEMA scaffolds were tested using a universal testing machine (Autograph AGS-J Table-top Precision Universal Testing Machine, Shimadzu) at 25 °C. For compression tests, cylindrical scaffolds with dimensions of 4 mm (height) × 10 mm (diameter) were utilized. The compressive stress (𝜎) was calculated using the formula 𝜎 = F/A, where F represents the applied force and A denotes the cross-sectional area. Similarly, the compressive strain (𝜖) was determined using the formula 𝜖 = (l/l₀) × 100, where l is the measured length and l₀ is the initial length of the sample.
Swelling Test
W0 is the dry weight of the scaffolds at the beginning, and Ww is the wet weight at the specified time. All experiments were performed in 3 replicates.
Degradation Study
W0 is the dry weight of the scaffolds at the beginning, Wd is its dry weight at the specified time. All experiments were performed in 3 replicates.
Drug Release
The release kinetics of drug-loaded scaffolds were investigated in 3 different pH solutions: pH: 4.0 (acetate buffer), pH: 5.5 (acetate buffer), and pH: 7.4 (PBS). First, the initial doxorubicin concentrations of the scaffolds were calculated. Then, the scaffolds were immersed in the related solution (5 mL), sealed, and incubated in a shaker incubator at 37 °C. At certain time intervals, the absorbance values of the solutions were recorded at ≈480 nm with a UV–vis spectrophotometer (Shimadzu UV-1280).[78] Released doxorubicin concentrations were calculated with the calibration curve equation of doxorubicin obtained with the UV–vis spectrophotometer. All experiments were performed in 3 replicates.
Cell Culture
HT-29 (ATCC HTB-38) and MCF-7 (ATCC HTB-22) cell lines were cultured with RPMI 1640 containing 10% FBS and 100 U/mL penicillin-streptomycin. Cells were incubated at 37° C in the presence of 5% CO2.
Proliferation Assay
To evaluate the cytotoxicity of the scaffolds, cytotoxicity tests were performed using MTT according to the literature procedure.[79] 24 well plates were used and 10.000 cells (MCF-7, HT-29) were planted in each well. Cytotoxicity assay was investigated in 5 groups (free DOX, DOX loaded PLGA/POEGMEMA, DOX loaded POEGMEMA, plain POEGMEMA, and plain PLGA/POEGMEMA scaffolds) compared to the control group. Scaffolds were sterilized under UV light for 20 min before adding to the wells. Mediums including 10, 15, and 20 µm DOX (1 mL) were applied to HT-29 and MCF-7 cells, respectively, for each group. Cells were treated for 72 h. At the end of 72 h, scaffolds were removed, mediums were aspirated, and 200 µL of MTT solution (0.05 mg/1mL media) was added to each well and incubated for 3 h. At the end of the incubation, the MTT solutions were aspirated and 200 µL DMSO was added to each well and incubated for 15 min. Finally, the absorbance values of each well were read at 620 nm with a plate reader (Thermo Fisher Scientific). The results were converted into graphics using GraphPad. All experiments were performed in 4 replicates.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8 software. Results are the mean ± standard deviation (SD) of at least three replicates. Statistical analyses between multiple groups were evaluated using one-way ANOVA and the significance of differences between groups was tested using the Tukey HSD test. Statistically significant results were expressed as *p < 0.033, **p < 0.002, ***p < 0.001.
Acknowledgements
This study was supported by the Afyon Kocatepe University Scientific Research Projects Coordination Unit (Project No: 22.FEN.BİL.12).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
The authors both helped in preparing the manuscript. They both read and approved the final manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.