The Potential of Ruthenium(II) Tris-Bidentate Complexes as Multifunctional Photo-Initiators
Abstract
An optically active antibacterial ruthenium(II) phenanthroline complex (tris(3,4,7,8-tetramethyl-1,10-phenanthroline) ruthenium(II) dichloride), is investigated for its potential as a multifunctional photo-initiator. Antibacterial and cytotoxic studies indicated that the complex is biologically active, and viable below <0.5 mm. The complex is shown to photo-polymerize gelatin using visible light in a similar manner to tris(2,2′-bipyridyl)ruthenium(II) dichloride and is demonstrated to retain antibacterial activity post-fabrication of a gelatin hydrogel. Incorporation of the photo-initiator into the interstitial fluid of a hybrid gelatin granular scaffold generated a multifunctional scaffold that retained cell viability and exhibited injectable properties.
1 Introduction
The use of photo-initiated radical-based crosslinking of hydrogels allows for the formation of constructs with unique architectures, primarily through the spatio-temporal control of light combined with the chemical properties of the macromer backbone.[1-3] In the past decade, multiple synthetic and natural photo-crosslinkable hydrogels have been employed for a range of tissue engineering and regenerative medicine applications, mostly due to the tailorable physio-mechanical properties and highly hydrated environment that benefits 3D cellular growth.[4-6] Examples of applications include delivery of cells and bioactive molecules, fabrication of in vitro tissue models, and development of constructs that facilitate wound healing.[7-9]
In most cases, the photo-initiators used in these crosslinking reactions fall into two overarching categories: Type I (Lithium phenyl-2,4,6-trimethylbenzoylphosphinate, Irgacure 2959, etc.) and Type II (tris(2,2′-bipyridyl)ruthenium(II) dichloride hexahydrate (RuBipy), Rose Bengal, etc.).[10, 11] Type I photo-initiators are consumed to fuel the required photo-chemical crosslinking. On the other hand, Type II photo-initiators generate the required radicals through a complimentary process, where a co-initiator is consumed to fuel the radical production. Depending on the photo-initiator of choice, this can proceed through a catalytic reaction, where the photo-initiator is not consumed or drastically changed in the hydrogel crosslinking process. These persistent photo-initiators then exist in the resulting hydrogel construct and will be removed via passive diffusion to the surrounding environment. In in vivo applications, one requirement of such a photo-initiator is to be inherently inert so as not to negatively impact the surrounding tissues (irritation, inflammation, etc.). However, the persistent nature of these chemicals also allows for their exploitation for secondary purposes.[12, 13] By engineering catalytic photo-initiators to possess additional biologically relevant properties, multi-functionality can be imparted to the photo-initiators beyond their primary hydrogel crosslinking purpose, allowing for further depth of customization of the resultant hydrogel constructs.[14]
Ruthenium(II) complexes with N-N chelators (e.g., 2,2′-bipyridyl or 1,10-phenanthroline) have been extensively researched due to the photophysical, chemical, and biological properties they possess.[15-17] The chemistry of the ruthenium(II) core allows for a modular design, where during synthesis the composition of the ligands can be replaced, generating a host of complexes of either homoleptic or heteroleptic nature. These complexes have found use as photosensitizers, catalysts, antibacterial agents, and anticancer agents.[18-22] RuBipy is a Type II photo-initiator that is frequently used for the photo-crosslinking of hydrogels designed for cell and therapeutic delivery, as well as bioinks/bioresins for 3D bioprinting.[23-25] The ability of RuBipy to absorb light in the visible spectrum has led to the complex being used to crosslink cell-laden systems, as the longer wavelength of light has reduced photo-toxicity upon light exposure.[26, 27] Commonly used with persulfate to generate sulfate radical ions, the complex can act as a strong oxidant and oxidize aromatic residues on proteins to mediate crosslinking.[28, 29]
Building off the RuBipy core, it can be rationalized that closely related complexes would display a similar ability to mediate radical photo-crosslinking.[30, 31] Tris(1,10-phenanthroline) ruthenium(II) dichloride and related phenanthroline ruthenium complexes have been shown to mediate photocatalysis.[32-35] Lalevee et al. described the use of tris(1,10-phenanthroline)ruthenium(II) dichloride for dual photo-crosslinking using radical and cationic generated species.[36] Thangavel et al. utilized a modified tris(1,10-phenanthroline) ruthenium complex appended to gold nanoparticles to mediate the photo-crosslinking of collagen via the formation of di-tyrosine crosslinks.[37] However, they observed that the non-conjugated complex was not as effective due to the presence of the free thiol group. Removing this functional group from the complex may allow it to mediate photo-polymerization. Structurally related tris(1,10-phenanthroline) ruthenium(II) complexes were previously investigated for their antibacterial properties by Dwyer et al.[38, 39] With the rise of antibacterial resistance, ruthenium complexes are gaining interest for their potential use as antibacterial agents.[22, 40, 41] Tris(3,4,7,8-tetramethyl-1,10-phenanthroline) ruthenium(II) dichloride (RuMePhen) was investigated by Keene et al. against Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus.[42] They observed that the more lipophilic nature of the complex, relative to the non-methylated version (tris(1,10-phenanthroline) ruthenium(II) dichloride), allowed it to acquire antibacterial efficacy. As such, there exists an overlap for this complex in its ability to photo-crosslink tyrosine residues while providing antibacterial activity to the hydrogel construct after photo-crosslinking.
This study investigates the complex RuMePhen for its ability to facilitate photo-crosslinking of a hydrogel construct and to determine whether the antibacterial expression is affected. The antibacterial and biofilm inhibitory activity of RuMePhen was validated against S. aureus. The dose-dependent cytotoxicity of the complex was evaluated with human dermal fibroblasts (HDFs). Furthermore, photo-crosslinking kinetics and efficiency were explored using gelatin as the macromer, and the antibacterial activity post-photopolymerization was confirmed in the hydrogel constructs. Lastly, the multi-functional advantages of RuMePhen to facilitate hydrogel photo-crosslinking and exhibit anti-bacterial properties were demonstrated as injectable granular hydrogels for wound healing applications. This proof-of-concept study highlights the usage of RuMePhen in delivering cell-laden microgels to an exposed wound-site with the additional advantage of infection control during the wound healing process.
2 Results and Discussion
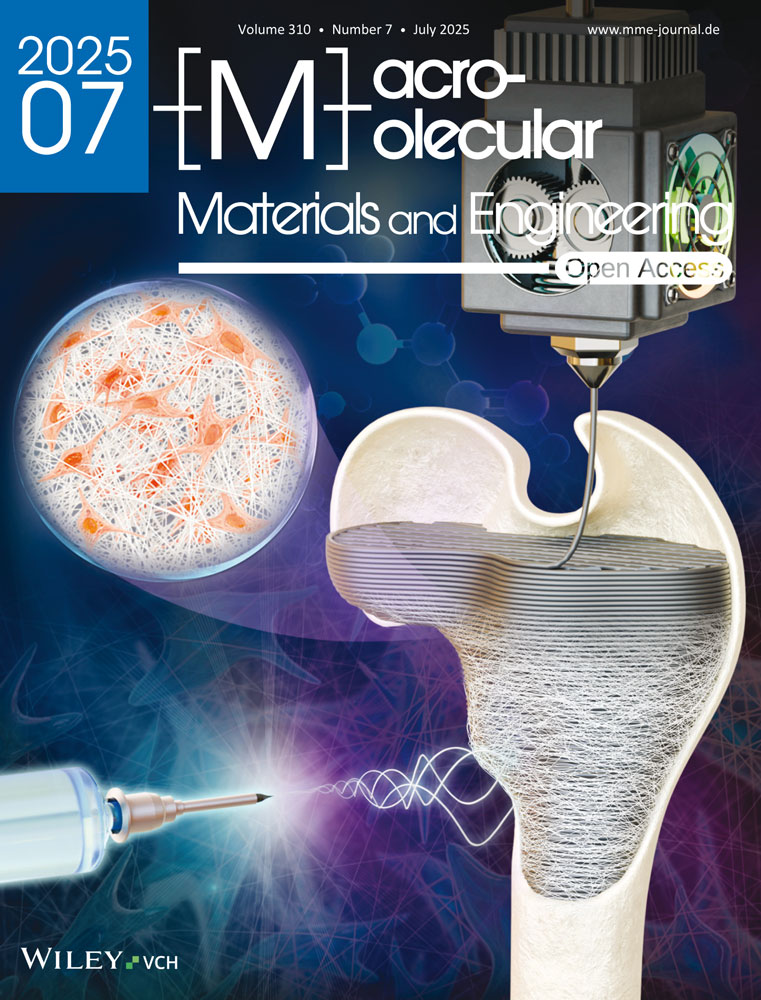
2.1 Synthesis and Characterization of RuBipy and RuMePhen
The ruthenium(II) complexes were prepared in a facile manner using methods adapted from the literature, which exploited microwave-assisted synthesis.[43, 44] Briefly, the precursor complex cis-dichlorotertakis(dimethyl sulfoxide)ruthenium(II) ([Ru(DMSO)4(Cl)2] was synthesized from the irradiation of ruthenium(II) trichloride hydrate (RuCl3·H2O) in the presence of DMSO.[43] This complex was then combined with the appropriate ligand (2,2′-bipyridine or 3,4,7,8-tetramethyl-1,10-phenanthroline) and irradiated (200 W) to generate the complexes RuBipy (Figure 1A,B) and RuMePhen (Figure 1A,C) in good yields (94 and 95% respectively) with characterization data (Supporting Information: 1H NMR, ESIMS, and elemental analysis) in agreement with the literature.[44-46] The UV–vis spectra of these complexes were measured in phosphate-buffered saline (PBS) to examine their spectroscopic properties in a physiologically relevant solvent (Figure 1D). Due to solubility issues, RuMePhen was first dissolved in milli-Q (MQ) water and then diluted with PBS before measurement. RuMePhen exhibited a metal-to-ligand charge transfer (1MLCT) absorption band, though with lower extinction coefficients compared to RuBipy across the spectra (ε at 440 nm: 13300 (RuBipy); and 4050 (RuMePhen) L mol−1 cm−1). As such, RuMePhen will absorb fewer photons than RuBipy, leading to fewer complexes acquiring the required energy to promote electrons via the MLCT to the lowest unoccupied molecular orbital (LUMO), an orbital with 1MLCT character, before intersystem crossing occurs to generate the long-lived 3MLCT state. This electron in the 3MLCT excited state is transferred to the sodium persulfate in the crosslinking process and implies that RuMePhen will be less efficient at generating the required radicals needed for the photopolymerization. The RuMePhen spectra in PBS differed from the spectra in MQ water reported in the literature in that the absorbance did not extend as far into the visible region (Figure 1D).[47] In the literature, the complex absorbed weakly across the 500–650 nm range in MQ water, while in PBS the absorption was observed to tail of at 560 nm. This hypsochromic shift may be due to the differences in polarity between PBS and MQ water.[48, 49] The complex absorbed light in the green region of the visible light spectrum (495–570 nm) but had a lower absorption coefficient compared to RuBipy across this portion of the spectrum (Figure 1E, 1330 (RuMePhen) versus 2830 (RuBipy) L mol−1 cm−1). While this indicated that RuMePhen has the potential to mediate photo-crosslinking via the use of green light (and be less efficient than RuBipy), it would be unable to do so with red light. This would have been beneficial as there is a clinical need to shift the photo-irradiation used in hydrogel formations to longer wavelengths, to reduce the photo-toxicity from using high energy ultraviolet or blue light, as well as low energy visible light (green–infrared) providing deeper tissue penetration for potential in situ photo-polymerization post-injection.[50, 51]
2.2 Antibacterial Activity of Synthesized Ruthenium Complexes
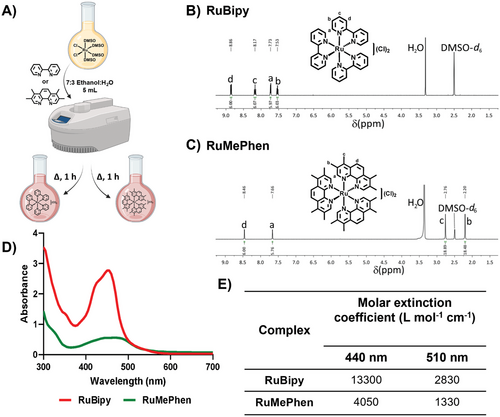
The antibacterial activity of the complexes against S. aureus (ATC25925) by zone of inhibition disk diffusion assays (Figure 2A) was explored. As expected, RuBipy exhibited no antibacterial activity up to 1 mm, whereas RuMePhen exhibited antibacterial activity from as low as 0.1 mm (Figure 2A,C). This observation is in agreement with previous work reported by Keene and co-workers, where RuMePhen exhibited antibacterial activity against a different strain of S. aureus with a minimum inhibitory concentration (MIC) of 6 × 10−4 mm (0.5 µg mL−1).[42] It is known that by increasing the lipophilicity of 1,10-phenanthroline and 2,2′-bipyridine ruthenium(II) complexes, there is a corresponding increase in bacterial activity for Gram-positive bacteria.[38, 42] Keene and co-workers related this to the complexes ability to traverse the cellular membrane, and showed that the non-methylated tris(1,10-phenanthroline)ruthenium(II) chloride complex was inactive against their strain of S. aureus. As RuBipy is less lipophilic relative to RuMePhen (which is significantly methylated), this potentially explains RuBipy’s lack of bacterial activity against S. aureus for this work, as it has difficulty crossing the bacterial cell membrane. While the MIC for RuMePhen reported in the literature is more potent than the concentration observed via the zone of inhibition data reported here, the hydrophobicity of the ligands coordinated to the complex hinders its diffusion through the aqueous Mueller-Hinton agar (MHA). As the concentration decreases, the corresponding diffused concentration will also decrease, leading to a reduction observed in the zones of inhibition. The antimicrobial effects of the complexes were further investigated on biofilms formed by S. aureus (ATCC25925) via confocal microscopy (Figure 2B,D). Bacteria were incubated with varied concentrations of the complexes (0.1, 0.5, or 1 mm), and the biofilm formed by the bacteria was stained with SYTO9 and propidium iodide before visualization. RuBipy had no observable effect on the bacteria's ability to form biofilms even at high concentrations (1 mm). In contrast, RuMePhen exhibited effective prevention of biofilm formation at a concentration as low as 0.1 mm (Figure 2B,D). A 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay (which indicates metabolic activity of live bacterial cells via absorbance of XTT) showed a reduction in the absorbance for RuMePhen, with no statistical difference between the positive control and RuMePhen at 0.1 mm (Figure 2D). The difference in antibacterial activity for RuMePhen and RuBipy highlights RuMePhen’s potential use as an antibacterial photo-initiator. The low concentration required for the antibacterial and biofilm prevention properties (0.1 mm) is within the range required by RuBipy to photo-crosslink hydrogels.[52-54] The ability to express this effect via diffusion into an aqueous gel and generate an antibacterial zone is promising, as RuMePhen may impart a similar diffusion-based inhibition to a hydrogel it is incorporated into, allowing for localized infection control at the periphery/interface of the hydrogel.
2.3 Cytotoxicity Evaluation of the Synthesized Ruthenium Complexes
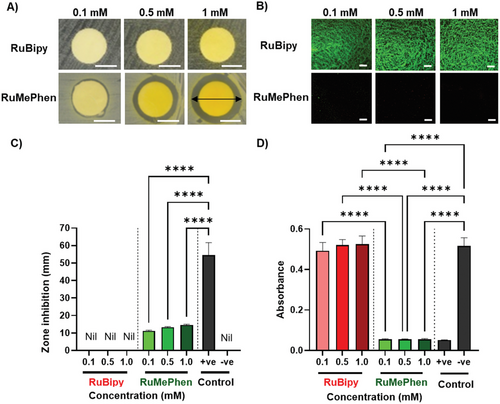
Limited cytotoxicity to human cells is essential for the effective use of ruthenium-based complexes for antibacterial applications. The cytotoxicity of RuBipy and RuMePhen was therefore assessed by seeding HDFs onto TCP in the presence of the photo-initiators in the culture media for 48 h (Figure 3). In the absence of any photo-initiator (Figure 3A, control), fibroblasts adopted a spindle-like morphology (DAPI, F-actin) and were actively proliferating, as evidenced by the expression of the Ki-67 proliferation marker. The supplementation of culture media with ≤0.5 mm RuBipy did not affect cell number or cell morphology, indicating that RuBipy was not toxic at these concentrations, but RuBipy at 1 mm resulted in a significant decrease in cell number compared to 0.1 and 0.5 mm groups (p < 0.0001 and p < 0.001 respectively) and the positive control (p < 0.001). Whilst the reduction in cell number at the higher concentration was accompanied by a decrease in total area coverage (Figure 3B), increasing the RuBipy concentration from 0.1 to 0.5 mm to 1 mm did not affect the area covered by individual cells (Figure 3D). It was therefore postulated that the elevated RuBipy concentration impeded HDF proliferative rate during the 48 h incubation, but did not affect the cell spreading as evidenced by the similar cell morphology between all groups and the proliferative capacity evidenced by Ki-67 positive staining. RuMePhen demonstrated a similar concentration-dependent effect to RuBipy, albeit the supplementation of the culture media with RuMePhen had a more pronounced effect on the HDFs. Concentrations of RuMePhen higher than 0.5 mm yielded no cell attachment on the TCP surface after 48 h (results not shown). At 0.5 mm RuMePhen, fibroblast behavior was significantly affected, as evidenced by a change in cell morphology, a reduction in cell number, and a consequential reduction in the total coverage area. Similar trends were, however, observed for the 0.1 mm RuMePhen as described for the 1 mm RuBipy; where a reduction in cell number was observed, but the area coverage of individual cells was not affected.
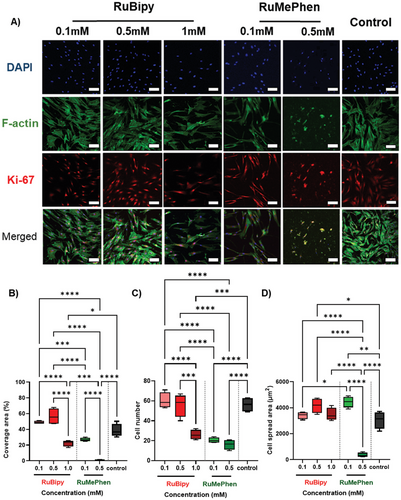
RuBipy has been extensively used as a photo-initiator for the fabrication of cell-laden hydrogels for in vitro and in vivo culture.[26, 52, 53, 55] Within these studies, concentrations as high as 1 mm RuBipy yielded > 80% cell viability for a range of cell types including breast cancer cells, chondrocytes, and mesenchymal stromal cells (Table S1, Supporting Information).[26, 56-59] These studies meet the accepted standard for cytocompatibility (<30% drop in cell viability over a 24 incubation in accordance with ISO10993), thus establishing the cytocompatibility of ≤1 mm RuBipy.[27] These experiments were conducted where cell-laden hydrogel precursor solutions were photo-crosslinked directly into cell-laden hydrogels. Small molecules such as photo-initiators are expected to leach out into their surroundings within the first 24 h after hydrogel fabrication, thus limiting the exposure time of the initiators to cells. The disparity between these observations and the slight cytotoxic response observed within our study may therefore be explained by the relatively long time (48 h) that the HDFs were exposed to the photo-initiators. These results align well with our hypothesis that although RuBipy slightly impeded the proliferative rate of the HDFs in our work, it does not contribute to a cytotoxic effect. A similar observation may be made for the 0.1 mm RuMePhen group, whereas concentrations above 0.5 mm RuMePhen exhibited high levels of cytotoxicity, indicating that 0.5 mm RuMePhen may be the upper threshold for the viable use of RuMePhen as a photo-initiator. Combined with the discussed antibacterial and anti-biofilm properties of RuMePhen at this concentration, these results indicate a potential use of RuMePhen as a multifunctional photo-initiator.
2.4 Evaluation of the Synthesized Ruthenium Complexes as Photo-Initiators to Fabricate Hydrogels
The use of RuBipy and RuMePhen as photo-initiators for hydrogel fabrication was investigated next. The hydrogel fabrication process involves the preparation of a hydrogel precursor solution containing a macromer, photo-initiator, and a secondary sacrificial molecule. Sol-to-gel transition of this precursor solution to form a hydrogel is dependent on the light-activated photon absorbance by the ruthenium photo-initiator, resulting in the generation of excited radical species that initiate crosslinking between macromers to form a polymer network. Hence, the ability for RuBipy and RuMePhen to form hydrogels was assessed initially by investigating the ability of these initiators to facilitate sol-to-gel transition after light irradiation. It was demonstrated that both RuBipy and RuMePhen could be used to initiate sol-to-gel transition of a hydrogel precursor solution composed of gelatin and this photo-initiator upon exposure to light (Figure 4A,B). This observation aligned well with reports that have established that RuBipy facilitates the formation of di-tyrosine crosslinks between gelatin chains upon light irradiation.[53] Whilst RuMePhen demonstrated antibacterial and anti-biofilm properties, its use as a photo-initiator for hydrogel fabrication has not been explored. The ability to utilize RuMePhen as a photo-initiator for gelatin hydrogel fabrication was therefore explored and compared to gelatin hydrogels fabricated with RuBipy as a baseline.
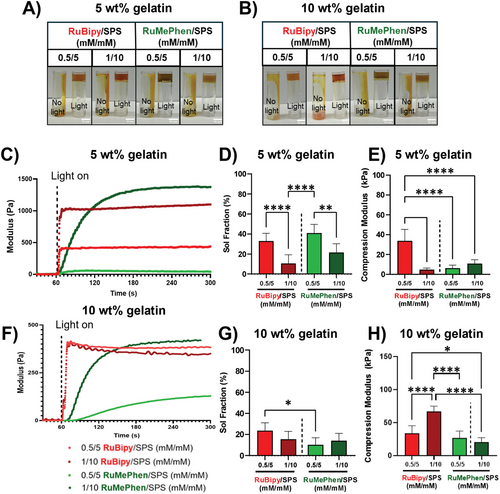
Both photo-initiators were able to form hydrogels at the tested concentrations (photo-initiator/SPS: 0.5/5 or 1/10 mm) from 5 and 10 wt.% gelatin (Figure 4A,B). To further investigate the difference between the photo-initiators, photo-rheology was used to determine the dosage required for each condition to reach the maximum amount of crosslinking per condition (Figure 4C,F). RuBipy reached crosslinking saturation for both gelatin concentrations within a few seconds, with little difference in the rate of reactions observed with all concentrations tested. In contrast, RuMePhen resulted in a slower, concentration-dependent crosslinking rate, which is evident in the gentle gradient compared to RuBipy. A single exposure time (3 min) was then chosen to ensure each condition reached crosslinking saturation and the physical properties of the resultant hydrogels were investigated (Figure 4D,E,G,H). For 5 wt.% gelatin crosslinked using either RuBipy or RuMePhen, there was a significant decrease in sol fraction (Figure 4D) with increasing the photo-initiator concentration (p < 0.0001 for RuBipy and p < 0.01 for RuMePhen). However, a significant decrease in compressive modulus was only observed with RuBipy with increasing photo-initiator concentration (Figure 4E). It should be noted that for both RuMePhen concentrations, the compressive modulus of the hydrogels was significantly lower than that of the RuBipy gels. When either photo-initiator was used to crosslink 10 wt.% gelatin, there were no differences observed in sol fraction (Figure 4G), but there was an increase in compressive modulus with increasing photo-initiator concentration (Figure 4H and p < 0.0001 for RuBipy and p < 0.01 for RuMePhen). Confirmation of di-tyrosine crosslinking was acquired by exploiting the natural fluorescence of the di-tyrosine crosslinks (Figure S1, Supporting Information). RuBipy was observed to generate more di-tyrosine crosslinks compared to RuMePhen at either 0.5/5 or 1/10 mm (Ru/SPS) and showed an increasing trend in the number of di-tyrosine crosslinks formed with increasing concentration. The di-tyrosine crosslinking for RuMePhen at 5 wt.% was practically identical between the concentrations of 0.5/5 and 1/10 mm (Ru/SPS). Intriguingly, at 10 wt.%, RuMePhen exhibited the opposite to RuBipy, where the lower concentration 0.5/5 mm gave an increased number of di-tyrosine residues as compared to 1/10 mm.
RuBipy has widely been used as a photo-initiator to fabricate hydrogels, whereas RuMePhen has not been directly established to facilitate hydrogel formation.[27, 52, 53] While there is evidence that a very structurally related ruthenium(II) 3,4,7,8-tetramethyl-1,10-phenanthroline complex can crosslink collagen when appended to gold nanoparticles, the ability and properties of RuMePhen-formed hydrogels remain unclear.[37] Here, it was demonstrated that RuMePhen is indeed able to crosslink gelatin hydrogels, in a similar manner to conventionally used RuBipy. However, there are differences in the cross-linking rate and resulting physical properties of the hydrogel formed. Slower crosslinking saturation observed with photo-rheology agree with the data showing the molar extinction coefficient was lower for RuMePhen compared to RuBipy at both 440 and 510 nm (Figure 1E). Sol fraction behavior with RuBipy as a photo-initiator was also consistent with previous reports using GelMA/collagen blends.[26] In line with this work, a similar trend was observed for RuMePhen at low macromer concentration, where higher concentration of the photo-initiator system led to a drop in the sol-fraction. This will be due to a higher number of crosslinks being formed between the gelatin backbones. However, the compressive modulus for RuMePhen-formed hydrogels typically differed to their RuBipy counterparts, with equivalent concentrations giving a lower compressive modulus. Taken in conjunction with the slower photo-kinetics observed for RuMePhen compared to RuBipy, this indicated RuMePhen is less efficient at forming crosslinks compared to RuBipy, forming gels that are structurally softer. Taken all together, the photo-kinetics and physical characterization results showed that RuMePhen can be used as a photo-initiator to form hydrogels.
2.5 Antibacterial Properties of RuMePhen Photo-Crosslinked Hydrogels
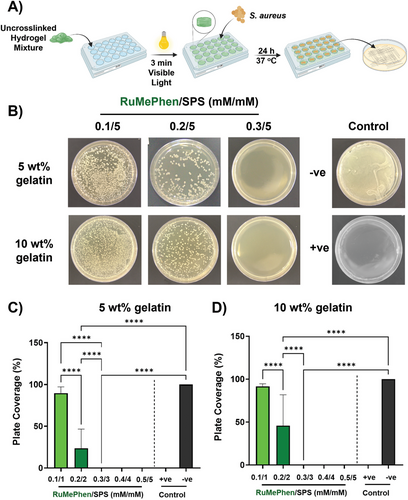
With the knowledge that RuMePhen can photo-crosslink gelatin into stable hydrogels, the preservation of the antibacterial efficacy of the photo-initiator was validated post-crosslinking in a hydrogel system. Due to the lack of antibacterial activity expressed by RuBipy against S. aureus, it was omitted from any further antibacterial studies in this work. Gelatin (5 or 10 wt.%) was combined with RuMePhen at varied concentrations (0.1, 0.2, 0.3, 0.4, 0.5 mm) while the SPS concentration was kept constant at 5 mm and the mixture photo-crosslinked with white light (400 klux, 3 min). Post-photo-crosslinking, S. aureus (108 CFU mL−1) was introduced onto the hydrogels and the plates incubated for 24 h. Post-incubation, a portion of the mixture was transferred onto TSA plates and incubated to look for viable growth of bacterial cells (Figure 5A,B). The percent coverage of the bacterial growth on the plate was quantified (Figure 5C,D). At lower RuMePhen concentrations (0.1 and 0.2 mm), the bacterial growth was reduced in the presence of the hydrogel system but was still viable when plated onto TSA agar. This result indicated that these concentrations bordered between bacteriostatic and bactericidal. This was the case for both 5 and 10 wt.% gelatin, while 5 wt.% gelatin exhibited increased activity (Figure 5C,D, 10 wt.%: 92 ± 3% (0.1 mm), 46 ± 36% (0.2 mm); 5 wt.%: 90 ± 8% (0.1 mm), 24 ± 23% (0.2 mm)). This difference might be due to the gelatin content acting as a food source for the bacterial growth through digestion via bacteria-secreted gelatinase.[60] However, at higher RuMePhen concentrations (≥0.3 mm), there was a complete reduction of bacterial growth. This indicated that 0.3 mm is the minimum bactericidal concentration (MBC) for this photo-initiator system, and that the antibacterial efficacy was preserved post-photo-crosslinking of the hydrogel system. The increase in MBC post-photo-crosslinking (0.3 mm) relative to MIC reported in the literature by Keene and co-workers (6 × 10−4 mm) has the potential to be due to inefficient catalytic turnover of RuMePhen, leading to the loss of antibacterial complexes post-photo-crosslinking. The upper bound for the dose-dependent cytotoxicity was 0.5 mm. This gave a range of 0.3–0.5 mm (308–512 µg mL−1) where RuMePhen could be employed to efficiently photo-crosslink a hydrogel construct, where it will exhibit both antibacterial efficacy and retain cell viability. This allows a multifunctional hydrogel construct to be derived from a multifunctional photo-initiator. Current approaches to antibacterial hydrogels either center around the incorporation of an antibacterial agent (silver nanoparticles (AgNP), antibacterial drug, etc.) that does not participate in the photopolymerization process or through the use of an antibacterial polymer backbone (either chemically or naturally derived).[61-65] Depending on the polymer backbone used or the incorporated antibacterial agent, the antibacterial efficacy of these systems is able to be utilized at low micromolar concentrations.[66, 67] This system in comparison is modest in terms of its antibacterial activity. However, the construction of this multifunctional hydrogel that expresses antibacterial activity differs from the current approaches in the literature in that it utilizes an already present component of the photo-polymerization process. This reduces the inherent complexity of the system (as there are fewer additives) and broadens the scope of polymer backbones that can be utilized in the formation of the hydrogel, as there is less potential for components to be incompatible. To the best of the authors knowledge, this approach has only been employed before by Banin et al.[14] The authors photo-crosslinked PEGDA hydrogels that utilized zinc oxide nanorods (UV light, 365 nm) and observed that leaching of the Zn2+ ions from the hydrogels induced excellent reductions in CFU mL−1 for various strains of bacteria. Due to the utilization of zinc oxide nanorods, they were limited to the use of UV light for their photo-polymerization, which has limited depth of penetration for in situ polymerization compared to the white light employed in this study. Modulation of this photo-absorptivity is inherently linked to the composition and morphology of the zinc oxide nanorods, with acquisition of visible light absorption being trickier for these nanorods than modulation of the ruthenium core of RuBipy or RuMePhen by ligand exchange.[68-70] This potentially limits the chemical flexibility of their system compared to the one reported herein. In terms of scalability and cost-to-efficiency, ignoring the cost of manual labor, the zinc oxide nanorods eclipses both RuBipy and RuMePhen due to the simplicity of their synthesis and low-cost materials (Table S2, Supporting Information). However, zinc oxide toxicity is well known, and is linked to the concentration of Zn2+ ions that can leach out of the system and accumulate in the intracellular matrix.[71, 72] The zinc nanorods were shown to bleed Zn2+ post-photo-polymerization of their hydrogel system, but no cytocompatibility studies were performed to quantify their potential cytotoxicity.
2.6 Fabrication and Characterization of Functional Hybrid Granular Hydrogels
Finally, to demonstrate an application of RuMePhen as a photo-initiator toward biomedical applications, hybrid gelatin granular scaffolds were fabricated by annealing microgels (fabricated using RuBipy) together utilizing a gelatin filler photo-crosslinked via RuMePhen (Figure 6A). The rheological properties of the annealed injectable granular hydrogels were investigated to assess their practicality for clinical applications. The microgel-hydrogel precursor solution was photo-crosslinked using a white LED light source (400 klux, 3 min) before rheological analysis. The annealed microgel scaffold demonstrated viscoelastic properties that allow them to be injected, exhibiting shear-thinning behavior with decreased viscosity while increasing shear rate (Figure 6B). Strain sweeps of annealed granular hydrogel showed that despite being mixed with microgels, the constructs behaved as elastic hydrogels, with low strain, but yielding at higher strains. The hybrid gelatin granular scaffold was strain-yielding with a storage modulus (G’) of 60 Pa and a yield strain of 80% (Figure 6C). This indicated this composition forms a soft annealed microgel formation, that possessed a high yield strain due the soft microgels ability to deform significantly before shear-thinning occurs. Finally, the self-healing properties of granular scaffolds were characterized using a cyclic time sweep, where the G’ recovery was measured before and after shearing at high strain (500% strain, 1 Hz, 2 min) shown in Figure 6D. Here, the results showed that annealed granular constructs possessed a quick and reversible solid-liquid transition. The annealed granular hydrogels were able to be injected through a clinically relevant syringe nozzle (20 G) whilst maintaining their filament shape (Figure 6E). Observations under fluorescence microscopy show that FITC-BSA-labelled microgels were closely packed together and held together by the annealing solution, shown by the fewer non-fluorescent gaps between the fluorescent microgels (Figure 6E; Figure S7, Supporting Information). To further demonstrate the capacity of the annealed granular hydrogel as an injectable material, a biopsy-punched hole was created in porcine skin and the solution was injected into the cavity. Results show that the annealed granular hydrogel was able to fully fill the cavity and retain good contact with the surrounding tissue (Figure 6F). Together, the results show that gelatin photo-crosslinked with RuMePhen can be used as an annealing solution for gelatin microgels to form a hybrid granular construct that can be successfully injected into a cavity.
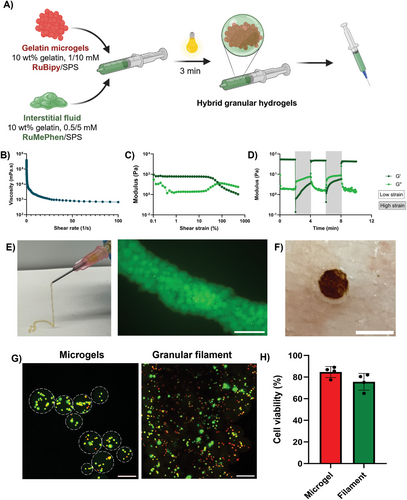
The effect of the microgel fabrication and annealing process on healthy cells was examined to validate the application of the hybrid granular system for injectable cell delivery. HDFs were encapsulated in gelatin microgels (10 wt.%) using RuBipy/SPS (1 mm/10 mm), then combined with RuMePhen/SPS (0.5 mm/5 mm) dissolved in gelatin (10 wt.%) as the annealing solution. Cell viability was assessed 24 h after fabricating the construct to determine if the fabrication process or leachable products were cytotoxic to the encapsulated cells using live/dead staining. The viability of fibroblasts encapsulated within microgels remained relatively high (Figure 6G, ≈85%,) and annealing the microgels with gelatin containing RuMePhen only slightly reduced the viability, but not significantly compared to cells encapsulated in microgels that were not annealed with RuMePhen (Figure 6H).
Injectable microgels provide a powerful platform for minimally invasive cell delivery compared to conventional injectable hydrogels, improving the viability of delivered cells.[73] Annealing microgels using an interstitial fluid provides the opportunity to impart multifunctionality in a single construct.[74, 75] In the absence of cell encapsulation, hybrid granular constructs compared to bulk 10 wt.% gelatin for both RuBipy and RuMePhen photo-initiators possessed much lower storage moduli. This indicates that the extrudability is relatively independent of the composition of the microgel when at low microgel packing densities similar to previous reports.[76, 77] Microgel formulations have been observed to possess lower G’ than their corresponding bulk counterparts due to the difference in interparticle crosslinks relative to the bulk hydrogels and lower crosslinking efficiency dependent on the fabrication method.[78] In addition, the compressive modulus of interstitial fluid directly affects the formulation's compressive modulus.[75] This experiment utilized a microgel formulation (with a stiff bulk hydrogel modulus) with a softer interstitial fluid, and the resulting soft microgel formulation agrees with the observations in the literature.[75] When HDFs were encapsulated in gelatin microgels using RuBipy as the photo-initiator while utilizing RuMePhen with its antibacterial properties as the annealing solution, the cell viability after microgel encapsulation remained high (Figure 6G,H). This is consistent with previous cell-laden microgel studies, with human MSCs in PEGDA or chitosan microgels, and 3T3 fibroblasts in NorHA microgels.[76, 79, 80] This is attributed to the flowability and elasticity of the microgels and granular scaffolds that can protect encapsulated cells from shear stresses, as demonstrated in previous studies.[81] Furthermore, using RuMePhen as the photo-initiator in the annealing solution and the fabrication process did not significantly alter the viability of the fibroblasts. This suggests that the diffusion of RuMePhen into the cell-laden microgels and the stresses of the secondary light exposure did not significantly influence the viability of the cells. Therefore, in conjunction with previous antibacterial hydrogel results, it demonstrates the potential for RuMePhen to be used as a multifunctional photo-initiator, where it can mediate gelatin photo-crosslinking and provide antibacterial activity to the hydrogel while retaining cell viability.
The incorporation of RuMePhen into a hydrogel construct can have a significant benefit, as by preventing bacterial infiltration, the hydrogel can provide a stable, moist environment for tissue regeneration.[82] AgNP, a hot topic in recent years with the rise of antibacterial resistance, is known to cause cytotoxic effects to cells through reactive oxygen species generation, DNA interference, and oxidative stress.[83, 84] As such there exists work aimed at improving their cytocompatibility through selective control of released silver ions, modulation of the nanoparticle size, and changes to their surface chemistry.[85-87] As there is a current need for antibacterials, a similar train of thought can be applied to the proof of concept described herein. Future work centered around modification of the lead complex RuMePhen (through modification of the ligands around the ruthenium core) or modulation of the fabricated hydrogel properties has the potential to the broaden the cytocompatible range for this formulation. More importantly, the proof of concept described herein opens the door to the potential exploitation of other photo-responsive complexes for their uses as multifunctional initiators, with there being a wide variety of antibacterial, antifungal, and anticancer agents whose core revolves around an optically active metal ion.[88-93]
3 Conclusion
The ruthenium complex, RuMePhen was shown to impart antibacterial activity through diffusion into an aqueous gel, and while cytotoxic, it exhibited dose-dependent toxicity to both mammalian and bacterial cells. The complex was shown to mediate photo-crosslinking of gelatin with comparative sol fractions to RuBipy photo-crosslinked gels, but with a lower compressive modulus for direct concentration comparisons. These RuMePhen photo-crosslinked gels were shown to retain their antibacterial activity against S. aureus post-photo-crosslinking, highlighting the complex's potential as a multifunctional photo-initiator. By incorporating the antibacterial RuMePhen photo-crosslinked hydrogel as an interstitial fluid between gelatin microgels, a novel injectable hybrid granular hydrogel was prepared. This injectable material was shown to retain cell viability post-extrusion into a filament structure, confirming its proof-of-concept. The multifunctional properties exhibited by the ruthenium complex RuMePhen open the door for its incorporation into other hydrogel constructs, with the potential to impart antibacterial activity while retaining a simple and effective hydrogel crosslinking. Due to the modular nature of the photo-initiator (a ruthenium(II) core with three bidentate binding ligands), modification of the complex can impart new functionality to the system. This could be through either ligand substitution during synthesis (as heteroleptic ruthenium complexes are well known, with facile synthetic pathways) or through direct modification of the 3,4,7,8-tetramethyl-1,10-phenanthroline ligand post coordination. However, as this and other works have demonstrated, careful consideration needs to be taken while adapting RuMePhen, as these modifications will not only impact its biological activity, but also its photo-crosslinking efficiency. This study also paves a pathway to exploiting other transition metal complexes that exhibit interesting properties as potential new photo-initiators in the future, for example complexes that display anticancer, antiviral, or antifungal activity may possess the ability to act as multifunctional photoinitiators and in turn impart those features into existing hydrogel formulas and constructs. By incorporation into hydrogel systems that already display those features, such as antimicrobial polymers or polymer-drug conjugates, they can strengthen the inherent effect of these systems, and potentially allow for multiple modes of action in a single construct.
4 Experimental Section
Antibacterial Assays—Bacterial Culture and Suspension Preparation
Staphylococcus aureus ATCC 25925 (S. aureus, the American Type Culture Collection, Manassas, USA) were maintained on tryptic soy agar at 4 °C and grown in tryptic soy broth at 37 °C for 24 h with shaking at 150 rpm.
Antibacterial Assays—Antimicrobial and Susceptibility Test
Due to its low solubility, RuMePhen stock solutions were dissolved in MQ water before dilution into PBS for the experiments. Antimicrobial susceptibility of RuBipy and RuMePhen against S. aureus (ATCC 25925) were tested using disc diffusion assays as described by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).[94] Briefly, overnight cultures of the S. aureus (ATCC 25925) were suspended in 150 mm of PBS to the density of a McFarland 0.5 standard (1–2 × 108 CFU mL−1). The suspension was spread onto a MHA plate. Sterile paper discs loaded with RuBipy or RuMePhen at varied concentrations (0.1, 0.5, or 1 mm) were applied to the inoculated agar plates. Pen-Strep (Penicillin: 100 unit mL−1 and Streptomycin: 100 mg mL−1) were used as the positive control. MQ water was used as the negative control. The plates were incubated at 35 °C for 20 h prior to measuring zones of inhibition, which was recorded as the smallest diameter that could be measured across the inhibited zone.
Antibacterial Assays—Biofilm Formation Assays
Biofilm formation assays were performed in 96-well plates and assessed using a XTT reduction assay.[95] Briefly, bacterial cells (1 × 105 CFU mL−1) were suspended in fresh TSB supplemented with 1% glucose with RuBipy or RuMePhen at varying concentrations (0.1, 0.5, or 1 mm). Pen-Strep and MQ water was used as the positive and negative control respectively. 100 µL of the mixture was incubated statically in a well of a 96-well plate at 37 °C for 48 h. After incubation, the liquid media was removed from the well, and the biofilm formed on the bottom of the well was gently washed three times with PBS to remove loosely attached cells. 50 µL of XTT assay mixture consisting of XTT salt (1 mg mL−1), freshly prepared menadione solution (0.4 mm), and PBS at a ratio of 20:1:79 were added to the well and incubated in the absence of light at 37 °C for 3 h. After incubation, absorbance at 492 nm was measured using a plate reader (Spectra Max 340 tuneable microplate reader, Molecular Devices Ltd, CA). All assays were performed in octuplicate using independently grown cultures. Biofilms formed in a 96-well plate were then stained with SYTO 9 and propidium iodide (LIVE/DEAD BacLight Bacterial Viability Kit) and imaged using a C2 CLSM confocal scanning microscope (Nikon, Japan).[96] Experiments were performed in octuplicate.
Cell Interaction Studies—Cell Culture
HDFs were cultured in αMEM with 10% FBS and 1% v/v Pen-Strep and incubated in conditions of 37 °C and 5% CO2. Media was refreshed every 3–4 days and cells were sub-cultured every 7 days or when cells reached ≈90% confluency.
Cell Interaction Studies—Cell Adhesion Assay
To prepare seeding solution, the media was discarded, the flask was rinsed in PBS and HDFs were trypsinized from the flask using 0.25% trypsin/EDTA for 5 mins at 37 °C, 5% CO2, and seeded at a density of 5×104 cells mL−1 in a 96 well plate. RuBipy or RuMePhen (0.1, 0.5, 1, or 2 mm) was incubated along with the seeded HDFs for 48 h in 37 °C, 5% CO2. Wells were subsequently washed with PBS and fixed with formalin for 15 min at room temperature.
Cells were then washed in PBS and permeabilized with 0.25% Triton X-100 for 30 min at room temperature. After washing with PBS, wells were then blocked with 1% BSA solution for 1 h at 37 °C. Cells were then probed with an anti-rabbit Ki-67 antibody at a 1:1000 dilution in blocking solution overnight at 4 °C. On the following day, wells were washed with PBS and then incubated with an AF594-tagged goat anti-rabbit IgG (H&L) secondary antibody (1:1000 dilution in blocking solution) for 1 h at room temperature. After washing with PBS, DAPI and FITC-Phalloidin (50 µg mL−1) was used to stain the cell nuclear and actin respectively. After washing with PBS, samples were kept in PBS at 4 °C prior to imaging. Cells were then imaged using an A1R Advanced Confocal microscope (Nikon, Japan) using a 20x objective. All samples were imaged using the same imaging settings. To quantify confocal images, cell surface coverage was determined by quantifying the number of F-actin+ pixels expressed as a percentage of the total number of pixels per field of view using ImageJ. Cell number was determined using the DAPI channel and was counted using ImageJ using the “Analyze particle” function. Cell spreading was calculated by counting the number of F-actin+ pixels and dividing that by the number of cells determined by the DAPI channel per field of view.
Hydrogel Fabrication and Characterization—Cast Hydrogels Fabrication
Gelatin dissolved in PBS at either 5 or 10 wt.% solutions were mixed with varied in situ concentrations of RuBipy/SPS (0.5/5 or 1/10 mm) or RuMePhen/SPS (0.5/5 or 1/10 mm). 50 µL of each mixture was cast into silicon molds with a depth of 2 mm and diameter of 5 mm. Photo-crosslinking of hydrogel precursor solution was done by light exposure from a white LED light source (Jobmate, New Zealand) at 400 klux for 3 min. Hydrogel mixtures for Figure 4A,B, were generated by mixing gelatin dissolved in PBS at either 5 or 10 wt.% with varied in situ concentrations of RuBipy/SPS (0.5/5 or 1/10 mm) or RuMePhen/SPS (0.5/5 or 1/10 mm) in duplicate. For each of the hydrogel precursor mixtures, one duplicate was stored in the absence of light for 3 min at 37 °C, and the other photo-crosslinked via white LED light source (Jobmate, New Zealand) at 400 klux for 3 min at 37 °C. After exposure, the hydrogel mixtures were inverted simultaneously and the images were captured (Canon).
Hydrogel Fabrication and Characterization—Photo-Rheological Analysis
Photo-kinetic measurements were conducted by loading 200 µL of gelatin (5% or 10%) mixed with either RuBipy/SPS (0.5/5 or 1/10 mm) or RuMePhen/SPS (0.5/5 or 1/10 mm) in PBS onto an Anton-Paar MCR 702e MultiDrive (Anton-Paar, Germany) equipped with an optical Peltier plate, with an upper plate geometry of 25 mm. Light exposure was mediated by an Omnicure S1500 (Excelitas Technologies Corp, USA), equipped with a ROSCO IR/UV filter to remove UV light from the exposure. The experiments set to run for were run for 5 min, with light exposure commencing 1 min after initiation of the experiment. Samples were measured at 37 °C, with a frequency of 0.1 Hz, amplitude of 0.1%, and a 0.2 mm gap between the plates. Light intensity was set to either 1 klux or 160 klux and the storage (G′) and loss (G″) moduli were recorded and G′ was plotted as a function of time.
Hydrogel Fabrication and Characterization—Physical Characterization of Photo-Crosslinked Hydrogels
Unconfined compression (n = 3) was performed on freshly cast hydrogel disks (prepared as described in section Cast Hydrogels Fabrication) using an Anton-Paar MCR 702e MultiDrive (Anton-Paar, Germany) equipped with a lower linear drive. Samples were loaded between a 25 mm upper and 25 mm lower plate and preloaded to 0.05 N. The samples were compressed at a rate of 0.2 mm s−1, with measurements recorded every 0.1 s until fracture of the hydrogel. The sample area was determined using ImageJ on captured photos (Canon) prior to compression. Load-extension curves were converted to stress-strain curves with the corresponding compressive modulus determined from the linear region (5–15%) of the corresponding smoothed stress-strain curve.
Antibacterial Hydrogel Assay
The antimicrobial hydrogel testing was evaluated using S. aureus (ATCC 25925) prepared as described in section Cast Hydrogels Fabrication. Hydrogel solutions were prepared for gelatin at 5 or 10 wt.% dissolved in PBS with RuMePhen at in situ concentrations of 0.1, 0.2,0.3, 0.4, and 0.5 mm and the SPS concentration was kept constant at 5 mm. 200 µL of each solution was pipetted into a 48-well flat bottom tissue culture plate and photo-crosslinked with a white LED source (Jobmate, New Zealand) at a light intensity of 400 klux to generate a hydrogel bed. 50 µL of a bacterial suspension of S. aureus (108 CFU mL−1) in TSB was pipetted on top of the hydrogel and the plates incubated at 37 °C for 24 h. 5 or 10 wt.% gelatin hydrogels photo-crosslinked with RuBipy/SPS (0.5/5 mm) with and without 1% Pen-Strep was used as the positive and negative control respectively. PBS alone was used as the growth control. The following day, 200 µL of TSB was pipetted into each well and the plates incubated for a further 2 h at 37 °C. 200 µL of the bacterial suspension was pipetted out of and dispersed onto a TSA bacterial plate and spread to generate a bacterial lawn. The bacterial plates were then incubated for 24 h at 37 °C. The following day, the resulting bacterial growth was photographed by a high-resolution camera (Canon). Bacterial growth on each plate was measured as a percentage of total plate coverage using ImageJ.
Hybrid Granular Hydrogel Fabrication—Microgel Fabrication
Gelatin microgels were created using 10 wt.% gelatin solution in PBS, mixed with RuBipy/SPS (1/10 mm), and prepared using a droplet-based microfluidic system. The polymer dispersed phase was introduced with a flow rate of 8 µL min−1 into a continuous oil phase (3% v/v Span 80 in mineral oil) using a flow-focusing microfluidic device (SmartMCs, Australia) at a flow rate of 64 µL min−1, which were controlled by two independent syringe pumps (Nexus 6000 and New Era NE-4002X). The water-in-oil microgels were run through a coil with a travel time of 5 min where the microgels were photo-crosslinked (400 klux) using a white LED light source (Jobmate, New Zealand). The microgels were then thoroughly washed with PBS (with 1% wt./v Pluronic F127), followed by PBS, with the supernatant decanted after centrifugation (3000 rpm, 5 min) between each wash.
Hybrid Granular Hydrogel Fabrication—Hybrid Granular Hydrogel Formation and Characterization
Microgels were collected using a vacuum filter (Steriflip filter, 41µm) and then mixed with annealing fluid of 10 wt.% gelatin solution containing RuMePhen/SPS (0.5/5 mm) at a 10:1 volume ratio (e.g., 100 µL microgels: 10 µL annealing fluid). The microgel-hydrogel precursor mixtures were transferred to either a 5 mL syringe or a mold (5 mm diameter, 2 mm depth) and exposed to a white LED light source (Jobmate, New Zealand) for 3 min (400 klux) for interparticle photo-crosslinking. To create hybrid granular filaments, a 20 G nozzle was used to extrude the photo-crosslinked hybrid from a 5 mL syringe. For fluorescent imaging experiments, microgels were prepared as described in section Microgel Fabrication. with the inclusion of (100 µg mL−1 FTIC-BSA) in the polymer dispersed phase. The hybrid granular hydrogels were then prepared with the labeled-microgels as described in section Cell Interaction Studies. Images were captured on a Carl Zeiss fluorescent microscope. Rheological properties of the annealed microgels were characterized on an Anton-Paar MCR 702e MultiDrive (Anton-Paar, Germany) equipped with an optical Peltier plate, with an upper plate geometry of 25 mm. Samples were measured at 37 °C and a plate gap of 1.5 mm. Strain sweeps were performed from 0.05 to 500% at 1 Hz. Frequency sweeps were performed from 0.1 to 100 Hz at 1% strain. Shear thinning properties were investigated by measuring the viscosity as the shear rate was increased from 0.01 to 100 s−1. A cyclic time sweep was performed on the system to investigate shear recovery, with the sample exposed to alternating low (0.5%) and high shear strains (500%) every 2 min at 1 Hz. Samples were performed in octuplicate.
Cell-Laden Microgel and Granular Hydrogel Assays
For cell-encapsulated microgels, HDFs were prepared as described in section Cell Adhesion Assay. Following trypsinization of cells and preparation of the cell pellet, the supernatant was removed and resuspended in gelatin solution containing RuBipy/SPS at a density of 5 × 106 cells mL−1. Cell-encapsulated microgels were then prepared using a droplet-based microfluidic system as described in section Microgel Fabrication. For cell-laden microgels within the hybrid granular hydrogel, cell-encapsulated microgels were vacuum filtered, collected, and processed as described in section Hybrid Granular Hydrogel Formation and Characterization to create the hybrid constructs. The cell-laden microgels and granular scaffolds were incubated (37 °C, 5% CO2) for 24 h before cell viability was assessed. The microgels and granular scaffolds were stained with Calcein AM (1 µg mL−1) and propidium iodide (1 µL mL−1) for 15 min. The stained constructs were then washed with PBS and imaged on an A1R Advanced Confocal microscope (Nikon, Japan) using a 10x objective.
Statistical Analysis
All experimental data was collected in triplicate (N = 3), and repeated in triplicate (n = 3), unless otherwise specified. Data are expressed as mean ± standard deviation where applicable. Results between experimental groups were analyzed using GraphPad Prism 10.4.0, through either one-way ANOVA or two-way ANOVA followed by Tukey post-hoc test. Statistical differences are displayed as * (p ≤ 0.05), **(p ≤ 0.01), ***(p ≤ 0.001), or ****(p ≤ 0.0001).
Acknowledgements
Q.V.C.V.H. and V.K.D. contributed equally to this work. K.S.L. acknowledges funding from the Australian Research Council (FT230100249) and New South Wales Health (H22/98586). J.R.-K. would like to acknowledge funding support from the Australian Research Council (FT210100668) and the UNSW Scientia Program. The authors acknowledge the facilities as well as the scientific and technical assistance of the Microscopy Australia (micro.org.au) node at the University of Sydney: Sydney Microscopy and Microanalysis. The authors acknowledge the facilities and the scientific and technical assistance of Sydney Analytical, a core research facility at The University of Sydney. This research was partially supported by the Australian Government through the Australian Research Council's LIEF Projects funding scheme (project LE100100118).
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.