Light Based 3D Printable Photochromic WO3 Polymer Gel Nanocomposites for Heat Management and Decorative Applications
Abstract
Materials with functionalities such as photochromicity are needed to extend the palette of materials for additive manufacturing. Herein, gel nanocomposites (gel NCs) with photochromic properties are designed for light-induced 3D printing. The photochromic resin is prepared by using a photocurable acrylic formulation, containing tungsten oxide nanoparticles (WO3 NPs) in the presence of aqueous and alcohol plasticizers. After photopolymerization, gel NCs transition reversibly from a highly transparent colorless state to a dark blue-colored state when exposed to UV light. The addition of ethylene glycol to water as a co-plasticizer and a lithium salt improves the gels coloration kinetics while providing anti-freezing and anti-drying capabilities. The gels demonstrate high luminous transmission (Tlum) of 89.24% and optical modulations (ΔTsol) of up to 75.96%, with a coloration speed of less than 1 min. Energy-saving simulations show that the WO3 gel NCs can be used for heat management applications. The preparation of costume-made 3D designs using the photocurable resin with a digital light processing (DLP) 3D printer, demonstrates that the resin can be used for the additive manufacturing of photochromic gels.
1 Introduction
Chromogenic materials are capable of changing their optical properties reversibly when exposed to different external stimuli such as heat, electricity, or light.[1, 2] Photochromic materials experience a change in coloration after being exposed to light irradiation of certain wavelengths, with the coloration bleaching after removing the light. This property has been found to be useful in smart windows to modulate incoming light transmittance and reduce energy demand for indoor temperature regulation.[3] Photochromic materials have also been employed for information storage,[4] anti-counterfeiting,[5] textiles[6] and smart displays.[7] Organic photochromic molecules such as azobenzenes[8] and spiropyrans,[9] show remarkable color diversity, flexibility, and fast response times, although with the significant drawbacks of high degradability and costly synthetic processes, which limit their potential applications. Conversely, inorganic photochromic compounds like transition metal oxides (TMOs) show higher thermal and chemical stability, low toxicity, and better cost efficiency.[10]Some of the most notable photochromic TMOs are WO3,[11] molybdenum oxide (MoO3),[12] titanium oxide (TiO2)[13] and Niobium oxide (Nb2O5).[14] Photochromic TMOs are usually prepared by aggregating nanoparticles into thin films, however, their poor mechanical properties produce cracked films, which scatter incoming light and greatly diminish the overall performance of the material.[15] Blending photochromic TMOs within polymers or hydrogels has been proposed as an alternative route to improve the mechanical properties.
Hydrogels are a class of soft materials formed by 3D networks made by polymers that absorb a large quantity of water. Hydrogels with photochromic properties can be achieved by either functionalizing the polymeric chains forming the 3D network or by dispersing photochromic compounds into the hydrogel matrix.[16] The flexibility and softness of photochromic hydrogels have made them attractive prospects for many different applications, including flexible sensors,[17] biomedical devices[18, 19] and smart windows.[20] Nevertheless, hydrogels freeze under cold temperatures and dry when exposed to air, which limits their potential applications. Both the mechanical properties and the stability of these photochromic gels have been improved by using organic solvents or mixtures of water and organic solvents. For example, Yang et al.[21] prepared tough and transparent photochromic organohydrogels via cross-linking of glycerol and poly(vinyl alcohol), adding WO3 NPs into the mixture. There have been at least two examples of TMO nanoparticle based photochromic gels prepared by photopolymerization. Eglītis et al.[15] developed photochromic organogels based on TiO2 quantum dots by photopolymerization of polyethylene glycol diacrylate (PEGDA) in an ethanol/N,N-dimethylformamide solution, while Luu et al.[22] prepared TiO2 based photochromic microgels by photopolymerizing 2-hydroxyethyl methacrylate (HEMA) and TiO2 nanoparticles functionalized with polymerizable ligands. Photopolymerization as a method for hydrogel synthesis offers several advantages over traditional methods like thermal curing, such as absence of volatile organic compounds, high polymerization speeds, room temperature synthesis, and overall high efficiency and precision.[23] This makes the polymerization process more precise, allowing for applications such as 3D printing.[24]
Products made by additive manufacturing (AM) are increasingly present in our day-to-day lives due to many recent innovations in this field, including the elaboration of 3D printable smart materials.[25, 26] Photochromic materials have been successfully fabricated by AM.[27] However, when it comes to photochromic TMOs few methods have been studied, most notably laser sintering[28] and inkjet printing.[29] Light-based 3D printing or vat photopolymerization is an AM process in which a photopolymer resin is cured layer-by-layer via light irradiation. This photopolymer resin (consisting of monomers, photoinitiators and other additives) determines the specific properties of the final structure, such as flexibility, transparency and toughness, among others. The preparation of photochromic gels based on TMOs by UV curing opens up the possibility of using advanced manufacturing techniques like low-cost light-based 3D printing, allowing for the development of 3D hydrogels with complex configurations and structures with high resolutions.[30]
There are many light-based 3D printing techniques, most of them demonstrating high printing resolutions and fast printing speeds. Some of these techniques have been used for the 3D printing of photochromic materials, including stereolithographic (SLA) 3D printing,[31] solution mask liquid lithography (SMaLL),[32] two-photon photolithography (2PP),[33] and DLP 3D printing.[34, 35]In these cases, organic compounds have been used to give the gels photochromic properties. To the best of our knowledge, there are no reported studies of inorganic TMO-based photochromic hydrogels prepared by vat photopolymerization. However, TMO nanoparticles could be added to photocurable resins as additives to prepare photochromic hydrogels, although the possible inhibition effect that TMOs with strong photocatalytic properties (for example ZnO and TiO2) could have on the photopolymerization process is something that needs to be considered.[36]
Herein, we propose a method to prepare photochromic gel NCs including WO3 NPs via photopolymerization. The WO3 NPs were prepared and used with acrylic monomers to elaborate a photocurable resin. The low viscosity and fast curation times of the photocurable resin made it a promising candidate for light-based 3D printing, hence we prepared 3D structures printed through DLP. The addition of WO3 allows for the functionalization of the photocurable resin, allowing for the elaboration of complex 3D structures that can change their color when exposed to sunlight. In order to prepare the gel NCs, we used a water and ethylene glycol (H2O:EG) binary solution, which showed good mechanical properties, photochromic activity as well as anti-freezing and anti-drying capabilities. The influence of factors such as the composition and the addition of lithium salt to the gels have in the coloring/bleaching kinetics was also studied. The WO3 gel NCs were highly transparent in their bleached state, but show a very high absorbance in their colored state, which makes interesting materials for smart window applications, and therefore, their temperature control ability was investigated. Finally, DLP 3D printing of the photochromic resins for decorative purposes was demonstrated.
2 Results and Discussion
2.1 Preparation of PHEA-WO3 gel NCs
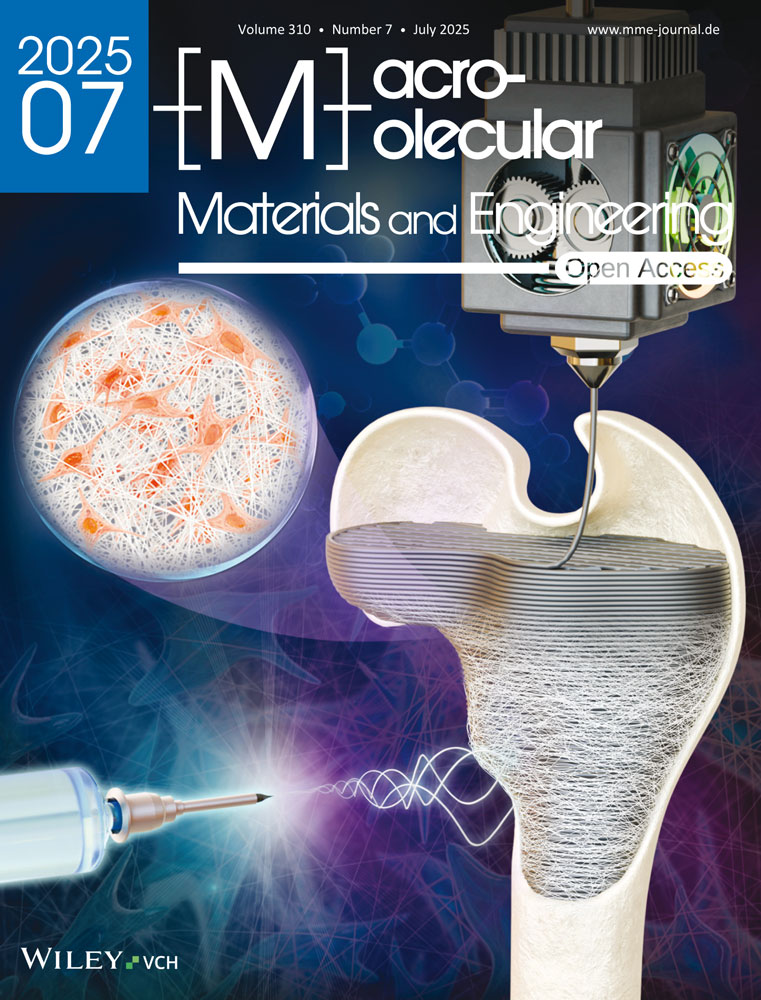
Hence, the hydroxyl groups in ethylene glycol accelerate proton propagation thanks to the increased hydrogen bond donor activity, which enhances the photochromic capabilities of the WO3 NPs.[38]
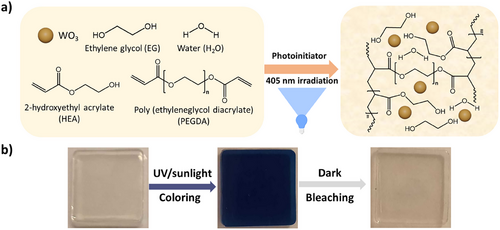
As mentioned before, the effect of water and different binary water: alcohols on the resulting gels are reported in Figure S1 (Supporting information). In the following experiments, ethylene glycol was chosen as a co-solvent alongside water as it showed better optical contrasts over other H2O: alcohol binary solutions (1:1 volumetric ratios in all cases). The dispersion of the WO3 NPs in the PHEA-WO3 organohydrogels was observed by TEM, as shown in Figure 1a. The size of the nanoparticles was calculated by using an image-processing program called image J, which gave a mean particle size of 29.7 ± 9.7 nm for the hydrated WO3 NPs (Figure S2, Supporting information). Fourier transformed infrared (FTIR) spectra were measured for the photocurable ink (containing HEA monomer, water:EG, PEGDA and fine turner FT1) before and after UV curing. In the photocurable inks spectra found in Figure 1b, two C═C peaks at 1637 cm−1 and 935 cm−1 corresponding to the HEA monomer and PEGDA disappear after photopolymerization (PHEA gels), demonstrating the formation of the crosslinked polymer matrix. Also in Figure 1b, the spectra for the PHEA-WO3 gel NC shows no significant difference compared to the PHEA gel.
Interestingly, ethylene glycol is well known as an anti-freeze agent when mixed with water. Many polyols form strong hydrogen bonds with water molecules, resulting in a water:polyol binary solution capable of withstanding temperatures well below water's freezing point, while also demonstrating anti-drying capabilities due to lower vapor pressures.[39, 40]Any effect on gel resilience was tested by comparing a reference PHEA-WO3 hydrogel NC and a PHEA-WO3 gel NC with H2O:EG (1:1 volumetric ratio) under different conditions (photographs shown in Figure S3, Supporting information). The anti-freezing properties of the gels were tested by placing them in a freezer for 12 h at −20 °C. As expected, the hydrogels lost transparency due to ice crystal formation, while PHEA-WO3 gel NCs with ethylene glycol and water in equal volumes maintained their transparency, making it possible to see through them clearly (reported in Figure S3 photograph c, Supporting information). If the gels are exposed to UV light right after freezing, the hydrogel remains mostly colorless while the ethylene glycol containing gel becomes blue (shows a lighter blue than usual, possibly due to lower ion mobility, as reported in Figure S3, photograph D, Supporting information). On the other hand, after a week during which the gels were exposed to air, the hydrogel showed a brownish-orange color, slightly shrunk in size (becoming harder and less gel like), and dried significantly, while the binary H2O:EG gel NC maintained its colorlessness and transparency, showed the same size and was still soft and flexible. When exposed to UV light both gels became blue, although the hydrogels reversibility lessened significantly (Figure S3 photographs E and F, Supporting information). Overall, the addition of ethylene glycol seems beneficial not only for improving the optical properties but also to bring anti-freezing characteristics and stability over time to the photochromic gel NCs.
Next, rheological tests were carried out to observe whether the presence of WO3 NPs in the gels affects their mechanical properties. In the amplitude sweep experiment presented in Figure 1c, all gels regardless of WO3 concentration showed a linear viscoelasticity up to almost 10% oscillation strain. The gels with 0 and 25 mg mL−1 WO3 concentration showed the widest linear viscoelastic region, which remained almost unchanged until going beyond 100% oscillation strain. It appears that this concentration strengthens the polymeric chain, while lower concentrations make the gel softer, and higher concentrations, such as 35 mg mL−1 WO3, could be impeding crosslinking activity. In the frequency sweep experiment shown in Figure S4 (Supporting information), the elastic modulus of the gels remained stable and higher than the loss modulus regardless of the applied frequency, indicating high stability of the internal structure. There is also no significant change in G’, for both 0 mg mL−1 and 25 mg mL−1 PHEA-WO3 gels, which was higher than G’’ when heating and cooling between a range of 0 and 80 °C, as it can be observed in Figure 1d. This could indicate a strong thermal stability and homogeneity in the 3D network for the tested temperature range as the mechanical properties did not change. In all cases, the storage modulus is remarkably higher than the loss modulus, indicating a mostly elastic behavior for the reference and acrylic-WO3 gel NCs. Additional mechanical tests were carried out to study the effect of doping the PHEA gels with WO3 NPs, which are shown in Figure 1e,f. Succinctly, the tensile strength appears to be very similar between the doped (0.29 MPa) and non-doped samples (0.25 MPa), while the compression strength at almost 300% strain increases notably from 0.6 MPA to 2.0 MPA when doping the gel with WO3 NPs.
2.2 Investigation of the Photochromic Properties of the Acrylic WO3 gel NCs
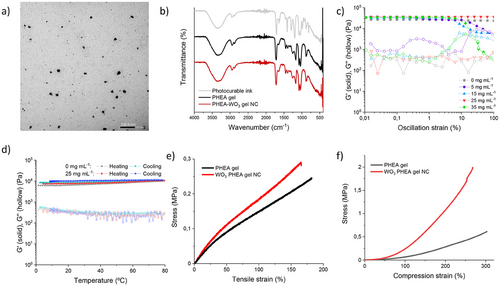
The previous experiments indicate that WO3 NPs were successfully merged into the acrylic gels, consequently granting them photochromic properties when exposed to UV irradiation. The UV spectra for the gels are shown in Figure 2a, where little difference can be observed between the gels with and without WO3 just after gel formation. Applying UV light on the former triggers its coloration in seconds into a deep dark blue, changing its spectra by increasing absorbance beyond 400 nm. A step-by-step spectra change over increased UV light can be seen in Figure 2b. Although this color change is reversible, the bleaching process is much slower and it takes a few hours. Preparing gels with different concentrations of WO3 produced gels with different intensities of the same shade of blue, with more concentrated gels achieving a darker blue color at a faster rate. The WO3 gel NCs proved to be reversible over multiple cycles of coloring and bleaching, with a loss of an initially high transparency from a transmittance of 90% to ≈75%, which can be observed in Figure 2c. As mentioned earlier, lithium perchlorate (LiClO4) was added to improve the coloration of the PHEA-WO3 gel NCs. Therefore, the effect of lithium ions was studied by comparing gels with and without the addition of LiClO4. Figure 2d shows how under the same 370 nm lamp light irradiation for 120s, gels with Li+ underwent color change at a faster rate and achieved a darker blue color. This change can be observed in the photographs shown in Figure 2g). The most likely reason being the increase in cations, which would improve the WO3 photochromic mechanism. As shown in Figure 2e, bleaching takes place gradually and is completed after a few hours exposed to air. The gels with Li+, despite reaching a darker blue color, have a faster bleaching speed than those without it (Figure 2h), showing a more gradual decline in its absorbance. The kinetic parameters of the PHEA-WO3 gel NCs with and without LiClO4 are shown in Table S1 (Supporting information). Essentially, the addition of lithium salt improves the gels reversibility over time, showing greater transparency in their bleached state and a darker blue color in their photochromic state when compared to gels without any additional Li+ ions. Interestingly, salts such as LiClO4 have also been used in other publications of electrochromic gels and as a mean to improve hydrogel anti-freezing and anti-drying properties.[39] Increasing the temperature fastens the bleaching speed of the PHEA-WO3 gel NCs, reducing the absorbance by an additional 54% after 2 h at 60 °C (Figure 2f).
2.3 Energy Saving Performance of a PHEA-WO3 Gel NC Window
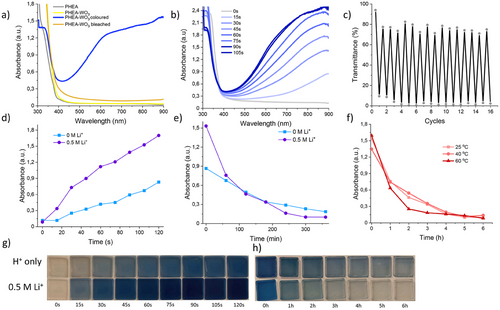
The energy-saving potential of the photochromic gels was tested by studying its transmittance in the Visible and near infrared (NIR) regions. To test their transmittance, the photocurable resins were placed between glass slides that were separated by spacers with a thickness of 0.21 mm. PHEA-WO3 gel NCs with different WO3 concentrations (all with the same concentrations of solvent, PEGDA and LiClO4) were tested to study its effect at blocking heat transmittance. The transmittances reported in Figure 3a demonstrate almost full transparency in the visible spectrum, progressively dropping in the NIR spectrum. The peaks can be attributed to the polymer and the solvents, for they do not appear in the clean glass slide spectra. While the sample containing only acrylic resin shows a few minor peaks (most notably ≈2100 and 2275 nm), the spectra of the blank sample (containing the solvent (H2O:EG)) show more prominent peaks, with significant decreases in transmittance at 1445 nm and 1930 nm. Figure 3b displays the gradual decrease in transmittance due to the increased amount of WO3. This decrease is more significant in the NIR region with very low transmittances for highly concentrated gels. The samples with the highest WO3 concentrations (35 mg mL−1) show almost no light transmittance at all between 750 and 1500 nm wavelengths, meaning that there is almost complete opacity in this spectral region. As the concentration of WO3 increases, there is also a gradual decrease in light transmittances of the gels in the visible spectrum although at a smaller rate compared to the NIR region.
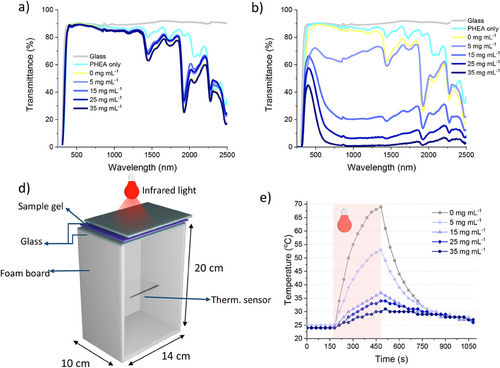
The optical properties of the WO3 gels were determined to evaluate their potential in energy saving. The results for luminous transmittance (Tlum), NIR transmittance (TNIR), and the solar modulation efficiency (ΔTsol) are displayed in Table S2 (Supporting information). In their bleached state, all samples showed very high Tlum, being ≈88% for all gels regardless of the doped WO3 concentration. Conversely, in the colored state Tlum decreases significantly for every WO3 concentration increment, while the TNIR showed an even steeper decrease. The regulation of incoming solar radiation (light and heat) is described by the ΔTsol parameter. This quantity increased remarkably for every increment in WO3 doping, reaching very high values of 52.52%, 66.59%, and 75.96% when using the samples with 15, 25, and 35 mg mL−1 of WO3 respectively. Therefore, the samples with high WO3 concentrations can block most NIR light in their colored state while having very high transmittances in their bleached states, showing promising properties for energy-saving applications.
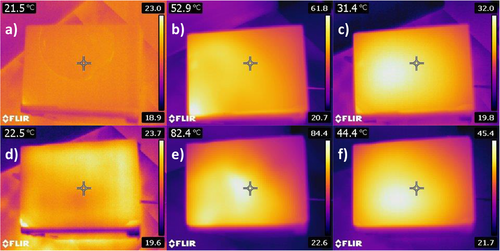
The potential energy-saving efficiency of WO3 gel containing double-glazed windows was studied by performing a real life simulation in the laboratory. To carry this experiment out, we used the customized setup reported in Figure 3c. An IR lamp was used as a heat source, irradiating a closed box through a smart window. The latter was prepared by sealing the WO3 gel between two glass sheets. The temperature inside the box was monitored with a thermocouple inside the box. The performance of the WO3 gel containing double-glazed windows is reported in Figure 3d. When using the blank gel window, which contains no WO3, the temperature inside the box increases rapidly to almost 70 °C in 3 min. The increase in temperature inside the box is reduced significantly as the WO3 particle content increases. This is due to the absorption of light, especially in the NIR region, which blocks thermal radiation through the WO3 gels. This is demonstrated in the thermal imaging of the double glazed windows shown in Figure 4. The double glazed window with 25 mg mL−1 WO3 PHEA gel NC has a temperature increase to 82.4 °C, while the temperature inside the box was ≈35 °C. In comparison, as shown in Figure S5 (Supporting information), the double glazed window with the non-doped gel has a surface temperature of 52.9 °C, while the temperature inside the box was 71 °C. After switching off the lamp, the temperature decreases at different rates, showing a correlation between higher WO3 concentration and a slower cooling time. Storing heat for a longer time when cooling down would suggest a higher thermal storage capacity, which is a useful feature for environments with high diurnal air temperature variations. The results demonstrate that the windows with photochromic PHEA-WO3 gels possess significant thermal insulation and thermal storage capacity, which are potential characteristics for smart windows with high-energy efficiency.
2.4 Light-Based DLP 3D Printing of PHEA-WO3 Photochromic Gels
Many TMOs like TiO2 and ZnO show strong photocatalytic effects,[36]so we evaluated first whether WO3 NPs could have any effect on the photopolymerization process. Photorheological studies were carried out by preparing solutions with different amounts of WO3 NPs and measuring their elastic and loss modulus at different irradiation times. A minimum of 10 s irradiation time was required to achieve enough crosslinking to obtain a gel, while 30 s were required to achieve the maximum cross-linking degree (G´≈ 104 Pa). As there is no remarkable correlation between WO3 concentration and gelation kinetics (Figure 5a; Figure S6, Supporting information), WO3 NPs do not have any significant effect on favoring or preventing the UV curing of the resin. Moreover, the relatively fast curing speeds together with the photocurable resins high transparency, show that the employed formulations possess some promising features for light-based 3D printing.
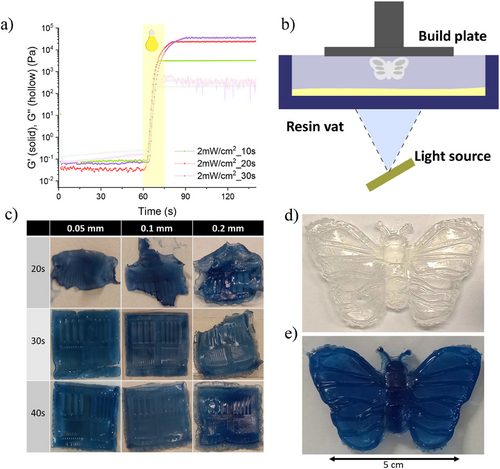
Next, the photocurable resins potential for light-based 3D printing was tested by using a DLP 3D printer. The photopolymerizable precursor prepared for this study showed favorable properties for VAT photopolymerization, including low viscosity, transparency, and fast photocuring.[41] In DLP 3D printing, as shown in Figure 5b, light irradiation is projected onto the vat containing the photocurable resin, where the digital light projector will cure entire layers at once in a layer-by-layer basis toward the Z-axis. Full gel formation will take place before photochromic color change is triggered, obtaining 3D printed figures in their bleached state. Applying UV light after printing not only changes the figures color, but it also ensures full UV curing. The optimal printing parameters were determined by preparing the same structure at different light exposure times and layer heights (Figure 5c). Exposure times of 20 s proved to be insufficient, as the structure did not fully form. Printing at layer heights of 0.2 mm was also troublesome as there was some leftover gel forming on the structure sides. The best resolution to printing time ratio was obtained with an exposure time of 30 s and a layer height of 0.1 mm, showing high resolution and no discernible defects in the fastest printing speed.
These parameters were used for the 3D printing of acrylic WO3 gels using the DLP 3D printer. Pre-set designs were sketched using the photon workshop program. The printed structures proved to be able to change color reversibly after being exposed to sunlight or UV lamps, as shown in Figure 5d,e. Moreover, the anti-freezing and anti-drying properties of the printed 3D structures ensure a longer durability when exposed to air and different temperatures, maintaining their photochromic properties over time.
We have compared different photochromic materials prepared by 3D printing and light based 3D printing techniques in Table 1. To the best of our knowledge, this is the first study where TMO nanoparticle based photochromic materials have been prepared by vat photopolymerization. Moreover, our material is the only one capable of going from a transparent and colorless state into a dark blue one, although there have been other reports of color changes going from a colorless state to orange[34] and purple.[35] Most of these works do not get into detail about the reversibility of their materials. The bleaching capability by Wales et al.[31] decreases significantly with each cycle, while another study by Ulrich et al.[33] shows good reversibility for 5 cycles. In this work, we have reported fairly consistent reversibility for 16 cycles, in which the WO3 based gels go from a transparency ≈80% to almost full opacity in its colored state at a wavelength of 700 nm.
| Photochromic Material | Photochromic material type | Matrix | 3D printing method | Color change | Reversibility | Source |
|---|---|---|---|---|---|---|
| WO3 NPs | Transition metal oxide | None | Laser sintering | Colorless to black, white, and pink | Thermal reversibility | [28] |
| Poly (ionic liquid)-polyoxometalate | Polyoxometalate | Poly (ionic liquid) | SLA | yellow to blue | Thermal reversibility | [31] |
| diarylethene | Organic molecule | Epoxides and acrylates | SMall | green to colorless | Thermal reversibility | [32] |
| donor-acceptor Stenhouse adduct | Organic molecule | thiol-ene photo-clickable resin | 2PP | violet to colorless | Thermal reversibility | [33] |
| Triphenylethylene | Organic molecule | PEGDA | DLP | colorless to orange | Light reversibility | [34] |
| Photochromic pigments | Unknown | Commercial resin | DLP | colorless to purple | Thermal reversibility | [35] |
| WO3 NPs | Transition metal oxide | PHEA gel | DLP | colorless to dark blue | Thermal reversibility | [this work] |
3 Conclusion
In summary, this article shows the preparation of photochromic gels including WO3 NPs by means of photopolymerization. The gels with WO3 NPs displayed high transparency, a dark blue color state, good mechanical properties together with anti-freezing and anti-drying properties. The addition of ethylene glycol to water as a co-plastisizer and a lithium salt improves the gels coloration kinetics while providing anti-freezing and anti-drying capabilities. The photochromic gels showed very favorable optical properties, with Tlum of 88% and ΔTsol of 75.96% when using 35 mg mL−1 of doped WO3. They also proved to be efficient at retaining heat, making them suitable materials for smart window applications.
The WO3 NPs did not show any negative effect on the photocurable resin, allowing it to photopolymerize at high speeds. Finally, the photocurable resin was used for DLP 3D printing, resulting in a detailed structure that undergoes a reversible coloration when exposed to sunlight.
4 Experimental Section
Materials
Tungsten powder (fine powder, 99%), HEA (96%), PEGDA (Mw 700) were purchased from Sigma-Aldrich. Lithium perchlorate trihydrate (98%) was obtained from Panreac, a fine tuner FT1 photoinitiator was supplied by 3Dresyns, hydrogen peroxide (35%) and ethylene glycol were purchased from Fischer scientific. All reagents were used without further purification.
Methods
Methods—Tungsten Oxide Hydrate Nanoparticle Preparation
WO3 NPs were prepared by adapting an already reported procedure.[42] In short, 3.3 g of tungsten fine powder (99%) was added into a cooled down (between 5–10 °C) 46.7 g solution of hydrogen peroxide (35%) and stirred overnight. The resulting solution was filtered and heated to 80 °C to catalytically decompose any leftover hydrogen peroxide until a yellow solution was formed. This solution was evaporated at room temperature resulting in a pale yellow powder.
Methods—Photopolymerizable Solution Preparation
The photopolymerizable solution was prepared by a simple and scalable method to accommodate the required ink amount. Succinctly, taking the preparation of 40 mL ink with 25 mg/mLWO3 and 0.5 M of LiClO4 as an example, 500 mg of hydrated WO3 powder was dispersed in a 20 mL H2O:EG (1:1 volumetric ratio) solution, by vigorously stirring it until it became clear. 1.6 g of LiClO4·2H2O were also added also added alongside the WO3 powder in this first step. Then, 20 mL of HEA (1:1 volumetric ratio regarding the solvent) were added to the solution and mixed by stirring. Finally, 200 µg of PEGDA and 20 µg fine tuner FT1 (1 and 0.1 wt.% regarding the monomer, respectively) were added while stirring. The solution was then wrapped in aluminum foil and stored in a freezer until used.
Methods—Coated Double-Glazed Window Preparation
The photocurable resin was placed on a glass sheet between double faced tapes acting as spacers (0.21 mm thick) and another glass sheet was placed on top, sealing the solution inside. This resin was then photopolymerized by UV light irradiation from a 390 nm lamp (kessil PR160-390 nm). This light was irradiated from a distance of 15 cm from the target with an intensity of 7.7 mW cm−3, for a duration of 120 s. The newly formed gel adhered to both glass slides, helping to maintain the glass sheets in a fixed position.
Methods—3D structures by DLP 3D Printing
A DLP 3D printer of the model Any Cubic Photon Mono X 6K was used for 3D printing tests. The photocurable resin was poured into the 3D printer vat and then exposed to UV light irradiation (405 nm wavelength and 2 mW cm−2 of power) forming previously set printing patterns. These patterns were designed by using the software program photon workshop. The printing was optimized by testing different UV light exposure times, layer sizes, and lifting speeds. The irradiation time was not optimized for the burn-in-layer. An irradiation time of 60 s for four initial layers printed in for the burn in layer was used on all tests and figures shown in this work. When the printing is finished, the printed figure was irradiated with UV light until obtaining a dark blue color to ensure full photopolymerization.
Characterization Methods
Imaging by transmission electron microscopy (TEM) were obtained using a JEOL JEM 1400 Plus with a tungsten filament. The images were obtained at 100 kV using a sCMOS digital camera integrated into the devices software. Fourier transformed infrared (FTIR) characterization was performed using a brucker alpha II spectrophotometer equipped with a platinum ATR module that has a diamond window. Rheological experiments were performed with an AR-G2 rheometer (TA Instruments) by using a 20 mm parallel-plate geometry. Amplitude sweeps were carried out from 0.01 to 1000% strain, at a constant frequency of 1 Hz, and frequency sweeps were carried out from 0.01 to 10 Hz at 1% strain and 25 °C. Temperature sweeps were performed at 1 Hz and 1% strain, in the range of 2 to 80 °C, at heating and cooling rates of 5 °C min−1. Tensile and compression strength tests were carried out using a TA.HDPlus Texture Analyzer (Texture Technologies, Hamilton, MA, USA). These tests were performed at room temperature at a stretching rate of 100 mm per minute. The samples for the tensile test were prepared in a mold of 7 cm in length and 1 cm in width. Samples for the compression tests had a 1 cm diameter and 1 mm height.
Heat efficiency tests were performed using a customized setup. To simulate a room, we built a box with foam boards with the dimensions of 10 × 14 × 20 cm3 on all sides except on the top, which is where the double glazed window would be placed. An Ex pro flir thermal camera was used for thermal imaging of the double glazed windows surface.
Photorheological measurements were carried out with an AR-G2 rheometer (TA Instruments) using a UV-light lamp (wavelength = 365 nm, power = 2 mW cm−2), oscillation stress of 100 Pa, and 0.1 Hz frequency. A kinetic study was performed to determine the gel point through a continuous registration of the elastic modulus (G′) and loss modulus (G′′) before and after UV irradiation. To that aim, the samples were placed on a glass parallel plate of 20 mm diameter and stabilized for 60 s to be subsequently irradiated for 10, 20, and 30 s, and continuing to register G′ and G′′ until a plateau was reached.
Acknowledgements
This work was supported by a Grant for the Basque Government through grant TECMAT ZL-2021/0027. A. U. is grateful to Spanish MCIN/AEI for the predoctoral fellowship (PRE2021-097649).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.