The Effects of Methoxylated Isoindigo on the Optical and Charge Transport Properties of the Corresponding Polymers
Abstract
Although the effects of electron-deficient group substitution on isoindigo on the corresponding conjugated polymers are extensively studied, the modification of isoindigo core with electron-rich groups has not been investigated. It is envisioned that the introduction of the methoxy group on isoindigo will not only tune the highest occupied molecular orbital (HOMO) energy level of the corresponding polymers but also introduce O···S “conformation lock” to increase the coplanarity of the polymers, which should facilitate hole transport. Herein, the syntheses of two methoxylated isoindigos and the investigations on the charge transport behaviors of their copolymers with bisthiophene (2T) and bisthiazole (2Tz) are reported. It is found that the substitution positions have a drastic influence on the UV–vis absorption and electrochemical properties for both monomers and polymers. Theoretical calculations and single crystal structure analysis confirm the existence of O···S “conformation lock”, however, both methoxy substitutions also change the aggregation behaviors of the corresponding polymers to a mixed face-on/edge-on orientation which has an adverse effect for charge transport. Among the four polymers, the polymer of 5,5'-methoxylated isoindigo and 2T exhibit the best hole mobility of 1.9 × 10−1 cm2 V−1 s−1.
1 Introduction
Organic field-effect transistors (OFETs) have garnered considerable attention due to their advantages in fabricating low-cost, large-area, and flexible electronic devices such as flexible circuits, bendable smart cards, sensors, and synthetic skins.[1-5] Donor–acceptor (D–A)-type alternating conjugated copolymers have been widely used as the active layer of OFETs since they are prone to form intramolecularly partially separated charges, which is effective not only in tuning front orbital levels but also in enhancing intermolecular dipole–dipole interactions.[6-12]
Isoindigo (II) has been extensively investigated as the acceptor for the D–A type copolymers in OFETs, and impressive OFET performances have been observed.[13-21] Moreover, the modification of II using electron-deficient groups, such as F, Cl, and cyanide, makes the corresponding materials prone to ambipolar charge transport.[22-24] Polymer based on fluorinated II exhibits significantly lowered LUMO energy level, which boosts the electron mobility up to 0.43 cm2 V−1 s−1, while still maintaining high hole mobility.[22] Chlorinated II shows similar effects and polymer with a nearly balanced hole and electron mobility.[23] The introduction of multiple strong electron-withdrawing groups onto II can further adjust the charge transport preference toward the n-type. For example, Marder and colleagues synthesized tetracyanoisoindigo, which exhibits n-type transport properties with electron mobility around 0.09 cm2 V−1 s−1.[24]
Interestingly, the introduction of the electron-donating group (such as methoxy group) onto II and the study on its influence on the OFET performance of the corresponding polymers, to the best of our knowledge, have not been investigated yet, although theoretical calculation has predicted that 5,5′-methoxy-isoindigo should be an excellent acceptor unit for optoelectronic devices.[25] Nevertheless, as shown in Figure 1, examples of using methoxy/alkoxy groups to modify conjugated molecules are not rare in the literature, and many of them focus on electron-rich substances. Nishihara and colleagues have shown that methoxy substitution has an obvious effect on the picene core's electronic structure, resulting in red-shifted absorption bands, higher HOMO/LUMO energy levels, and narrower energy gaps compared to unsubstituted picene, which varies significantly with the substitution positions and numbers.[26] The crystal structures of methoxy-substituted picenes are also strongly dependent on the methoxy positions, which have a dramatic influence on the hole mobilities. Liang and colleagues synthesized various solar cell donor materials by introducing methoxy onto thiophene-flanked benzodithiophene (BDT). The resultant polymer possesses lower HOMO and LUMO energy levels, greater absorption coefficient, stronger interchain interactions, and better solubility in eco-friendly solvents compared to the unsubstituted BDT polymers, which lead to better OPV performance.[27] More recently, Takimiya and coworkers compared the methoxylation effect with other methylchalcogenation effects on pyrenes.[28] Alkoxy-substitutions on acceptors have also reported. For example, ethoxy-substituted naphthalene diimide (NDI) has been synthesized, however, the influence of ethoxylation on the properties of NDI was not disclosed.[29] It has also been shown that alkoxy-substitution increases the HOMO energy level of the benzothiadiazole (BT) unit while having no effect on the LUMO energy level, and the bandgap of the corresponding polymer is thus narrowed.[30, 31] Hou and colleagues found that the introduction of alkoxy groups significantly reduces the bandgap of a non-fullerene acceptor to 1.34 eV by primarily increasing the HOMO energy level,[32] while the LUMO energy level of the acceptor remains almost unchanged, which facilitates sunlight absorption and results in better photon conversion efficiency (PCE) of organic solar cells.

Moreover, the introduction of a methoxy group in conjugated molecules may have a so-called “conformational lock” effect. Noncovalent conformational lock improves the planarity and rigidity of the conjugated backbone by incorporating O···S, N···S, X···S (where X = halide), and hydrogen bonding interactions.[6, 8, 33-36] Ever since Rosenfield and colleagues first characterized the structural features of noncovalent intra and intermolecular O···S interactions,[37] it has been recognized that these weak through-space forces are involved in a variety of phenomena including self-assembly, charge transport, and molecular recognition. Typical examples are shown in Figure 2. It helps to improve the planar alignment of the material and facilitates the formation of stronger π–π stacks, which can lead to improved device performance.[38, 39].
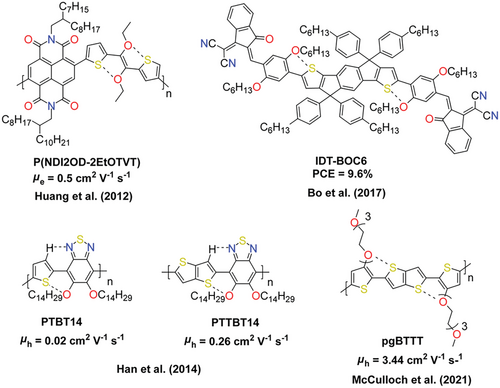
Weak interactions, such as N···S, S···F, and hydrogen bonding, have also been employed in isoindigo-based conjugated polymers, which enhance the planarity and conformational coherence of the polymers and improve the charge carrier mobility.[22, 23, 40-44] However, there was no report on how the methoxylation on II would influence the charge carrier mobility of the corresponding polymers. On one hand, the introduction of methoxy group onto II can effectively increase the HOMO energy level of the material to match up with the Femi level of Au while having less effect on the LUMO energy level, which may form better Ohm contact and facilitating hole injection; On the other hand, it could also add O···S conformational lock to the corresponding polymers, which may improve the coplanarity of the material and hence facilitate the charge transport. Here, we wish to report the synthesis of two methoxy-substituted II isomers, namely 6,6-dibromo-7,7′-dimethoxyisoindigo (7MeOII) and 6,6-dibromo-5,5′-dimethoxy- isoindigo (5MeOII), and their copolymers with bithiophene (2T) or bithiazole (2Tz). It was found that the substitution positions have a drastic influence on the UV–vis absorption for both monomers and polymers, in which 5,5′-methoxy substitution causes a larger bathochromic shift of HOMO→LUMO transition, while 7,7′-methoxy substitution has a more obvious influence on HOMO-2→LUMO transition. Similar to some reported cases,[31, 32] methoxy substitution has little influence on the LUMO energy level but has a more profound influence on the HOMO energy level of isoindigo, among which 5,5′-methoxy substitution exhibits a more obvious HOMO energy level lifting effect. As to the O···S interaction in the methoxylated II, although both the theoretical study and single crystal structure analysis confirm such an effect, the single crystal of thiophene-flanked 7MeOII also confirms the existence of other stable conformations. Moreover, the introduction of the methoxy group on II greatly increases the solubility of the corresponding polymers, which facilitates the processing procedure. Interestingly, in all cases, methoxy substitution changes the aggregation behaviors of the corresponding polymers to a mixed face-on/edge-on orientation, different from the preferable edge-on orientation of polymers based on unsubstituted isoindigo. Among the four polymers, the polymer of 5MeOII with 2T exhibits the best hole mobility of 1.9 × 10−1 cm2 V−1 s−1 with the smallest threshold voltage of −2.7 V, thanks to its HOMO energy level (5.08 V) that matches well with the work function of Au.
2 Results and Discussion
2.1 Monomer Synthesis
Methoxy-substituted II (MeOII) was first reported in 1982,[44] in which its electron spin resonance (ESR) properties were studied. We initially envisioned that ortho-bromination on MeOII should be favored and would lead to the monomer suitable for polymerization, since the methoxy group is an ortho/para directing group and the para-position is too crowded for substitution. Thus, we first chose the commercially available and inexpensive methoxyaniline as the starting material for 7MeOII synthesis.
The corresponding methoxy-isatin 1 was obtained according to the method of Gaussian et al. in 70% yield.[45-47] Compound 1 was alkylated with 1-bromo-octane to give compound 2 in 70% yield, which was in turn converted to t 7,7′-methoxyisoindigo 3 in an almost quantitative (99%) yield by reacting with tris(diethylamino)phosphine, as shown in Route 1, Scheme 1. Unfortunately, the bromination of compound 3 using NBS only resulted in exclusively meta-brominated compound 4 in 97% yield, the structure of which was confirmed by X-ray single crystal structure analysis (inset in Scheme 1). However, compound 4 is not suitable for polymerization, since it will lead to a cross-conjugated polymer. We reasoned that there might be two reasons for meta-bromination: 1). 7,7′-dimethoxyisoindigo is a more complicated aromatic system with three directing groups: methoxy, -NRCOR′, and the central double bond, where NRCOR′ is also electron-donating which may compete with the methoxy group, and the final outcome is the result of overall directing effect; 2) the alkyl chain on the lactam may restrict the free-rotation of the methoxy group due to the steric hindrance between them and force the methyl group to adopt a conformation closer to the ortho-position, as evidenced by the crystal structure, which may block the ortho-position from bromination.
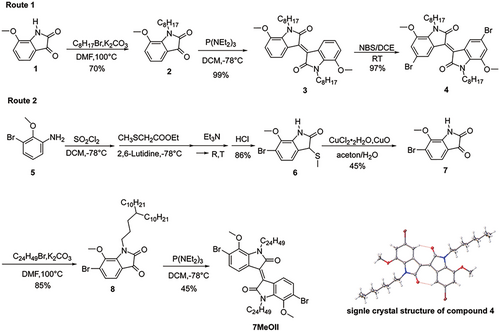
To solve this problem, a different strategy (Route 2, Scheme 1) was chosen, using the pre-brominated compound 5 as the starting material. Following Gaussian protocol, compound 6 was obtained in 86% yield. Oxidation of Compound 6 provided isatin 7 in 45% yield, which was then alkylated with 11-(3-bromopropyl)henicosane to give compound 8 in 85% yield. Further treatment of isatin 8 with P(NEt2)3 led to 7MeOII with only 45% yield, considerably lower than the one without bromide, which might be due to the electron-withdrawing effect of Br substitution.
For the synthesis of 5MeOII, we first chose commercially available 5-methoxyisatin 9 as the starting material, which was alkylated with 1-bromo-n-octane in 79% yield, as shown in Route 1, Scheme 2. Compound 10 reacted with P(NEt2)3 to provide 5,5′-dimethoxyisoindigo 11 in 50% yield, which is also lower than its 7,7′-dimethoxy analogs, showing that the position of the methoxy group has a large influence on the yield of the reaction. Unfortunately, the bromination reaction of compound 11 with NBS is rather slow and tedious, and switching to other bromination reagents and other reaction conditions did not help. Furthermore, the product has a very similar Rf value with the starting material, which makes it rather difficult to isolate. We also tried to brominate the corresponding isatin 10, however, the yield was also low (20%), and the product was difficult to purify.
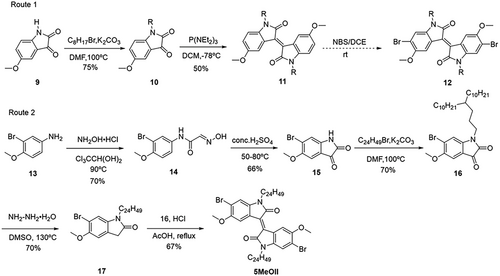
An alternative synthetic route was then adopted, as shown in Route 2, Scheme 2. 3-Bromo-4-methoxyaniline (compound 13) was chosen as the starting material, which was treated with chloral hydrate and hydroxylamine hydrochloride to afford compound 14 in 70% yield. In presence of conc. sulfuric acid, crude 14 underwent cyclization to provide 6-bromo-5-methoxyisatin 15, which was then alkylated with 11-(3-bromopropyl)henicosane to afford compound 16 in 70% yield. A Wolff–Kishner–Huang reduction was used to reduce the carbonyl group to methylene, giving 17 in 70% yield. Direct condensation of 16 and 17 in acetic acid afforded 5MeOII in 67% yield.
2.2 Methoxy Group Influence on the UV–vis Absorption of the Monomers
With both monomers in hand, we investigated their optical properties using UV–vis spectroscopy. For a better understanding of the methoxy substitution effect, the UV–vis absorption spectrum of 6,6′-dibromoisoindigo (diBrII)[48] is also compared. The results are shown in Figure 3a. The molar extinction coefficient is 4.80 × 104 M−1 cm−1 for 5MeOII, obviously higher than that of 7MeOII (2.89 × 104 M−1 cm−1). Both monomers exhibit three major absorption bands, with the more intense absorption peak at a shorter wavelength and two weaker absorption peaks at a longer wavelength. Interestingly, distinct substitution-position effects on the UV–vis absorption were observed. Compared to diBrII, both the longest wavelength absorption peak and shortest wavelength absorption peak of 5MeOII are obviously red-shifted (from 500 to 560 nm and from 280 to 294 nm, respectively), while the peak in between is less influenced (from 396 to 398 nm). On the contrary, both the longest wavelength absorption peak and shortest wavelength absorption peak of 7MeOII show little position change compared with diBrII (from 500 to 502 nm and from 280 to 279 nm, respectively), while the peak in between is obviously red-shifted (from 396 to 426 nm). As a result, 5-methoxy substitution has a more obvious optical bandgap () narrowing effect, and the determined in the solution state is 1.74 eV for 5MeOII and 1.92 eV for 7MeOII, respectively. It can be concluded that the methoxylation position has a non-neglectable influence on the electronic structure of isoindigo.
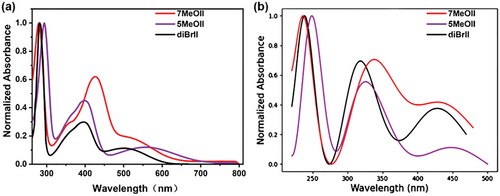
To better understand the substitution position effect, density functional theory (DFT) calculations were performed at the ωB97XD/6-31g(d) level to simulate the UV–vis absorption changes of methoxyisoindigos, and the calculated spectra are shown in Figure 3b. Although all the peaks appear at a shorter wavelength with respect to the experimental ones, the calculated spectral evolution trends are in good agreement with the experimental results: compared with diBrII, 5MeOII exhibits a more pronounced redshift on the two bands at the longest and shortest wavelengths, while 7MeOII shows a more obvious redshift on the band in the middle absorption region. The electronic transition contributions to these three major absorption peaks were calculated and their assignments are listed in Table 1, and the corresponding electronic states are listed in Figures S1–S5, Supporting Information.
| Compounds | λpeaka)[nm] | Contribution |
|---|---|---|
| 7MeOII | 236 | H → L+1 39.6%, H-1 → L+2 22.2%, H-12 → L 20.3% |
| 341 | H-2 → L 78.7%, H-4 → L 15.7% | |
| H-4 → L 70.7%, H-2 → L 14.4% | ||
| 430 | H → L 93.8% | |
| 5MeOII | 244 | H → L+1 47.1%, H-1→ L+2 27.9%, H-12 → L 11.1% |
| 324 | H-2 → L 61.8%, H-4 → L 27.7% | |
| H-4→ L 48.4%, H-2→ L 32.5%, H-6 → L 6.3% | ||
| 438 | H → L 95.2% | |
| diBrII | 239 | H → L+1 47.5%, H-1→ L+2 24.3%, H-10 → L 14.4% |
| 319 | H-2→ L 57.1%, H-4→ L 37.1% | |
| H-4→ L 52.1%, H-2 → L 36.2% | ||
| 431 | H→ L 94.7% |
- a) calculated absorption peak wavelength, H: HOMO; L: LUMO.
The longest wavelength absorption band was assigned to the HOMO→LUMO transition, and the shortest wavelength absorption band was mainly assigned to the HOMO→LUMO+1 and HOMO-1→LUMO+2 transition, while the absorption band in between is composed of two bands, which were both majorly assigned to the mixed HOMO-2→LUMO and HOMO-4→LUMO transitions but with different mixing ratios. If we assume that the redshift of the absorption band is mainly contributed by the transition with a narrower bandgap, then whether the lone pair on the methoxy group could effectively participate in HOMO→LUMO, HOMO→LUMO+1, and HOMO-2→LUMO transitions will determine whether redshift could be observed or not. From Figures S1 and S4, Supporting Information, it can be clearly seen that for 5MeOII, the lone pair on the methoxy group is more effectively delocalized on the HOMO orbital, while for 7MeOII, the contribution of the lone pair to the HOMO orbital is almost neglectable. Thus, the methoxy group of 5MeOII has a stronger influence on HOMO→LUMO and HOMO→LUMO+1 transitions than that of 7MeOII, which well-explains the redshift of both the longest and shortest absorption bands of 5MeOII with respect to diBrII. On the other hand, the lone pair on the methoxy group on 7MeOII is more effectively participated in the HOMO-2 orbital than that on 5MeOII, as shown in Figure S2, Supporting Information, which contributes more to HOMO-2→LUMO transition. As the result, the medial absorption band 7MeOII shows a more obvious redshift.
2.3 Methoxy Group Influence on the Electrochemical Properties of the Monomers
The influence of methoxy substitution position on the ionization potential (IP) and electron affinity (EA) of II was evaluated by cyclic voltammetry, and the results are shown in Figure 4 and the data are listed in Table 2. DiBrII exhibits an irreversible oxidation peak with an onset at 1.6 V and two poorly semi-reversible reduction peaks with an onset at −0.62 V versus Ag/AgCl. Methoxylation at the 7-position (for 7MeOII) causes the oxidation peak to shift to lower potential with the onset located at around 1 V and the reversibility of the oxidation peak is slightly improved. Two distinct reduction peaks can be clearly observed for 7MeOII with the onset at around −0.63 V, and the first reduction peak is reversible. Methoxylation at the 5-position (for 5MeOII) causes the oxidation peak to shift to lower potential with the onset located at around 1.2 V and the oxidation peak becomes fully reversible. However, only one distinct and fully-reversible reduction peak can be clearly observed for 5MeOII with the onset at around −0.65 V. It seems that methoxylation improves the reversibility of both the oxidation and reduction process, which could be correlated to the improved stability of the radical cations and radical anions generated in the process. The IP and EA data are summarized in Figure 4d. It can be seen explicitly that methoxylation causes little change to EA but a dramatic change to IP. Surprisingly, 7-methoxylation leads to lower IP (5.40 eV) than 5-methoxylation (5.70 eV), corresponding to a narrower EA/IP gap, which is opposite to the HOMO/LUMO energy gap change determined by UV–vis spectroscopy. We could not explain this discrepancy at this stage, however, it should be pointed out that EA/IP could not be directly interpreted as HOMO/LUMO as suggested by Brédas.[49]
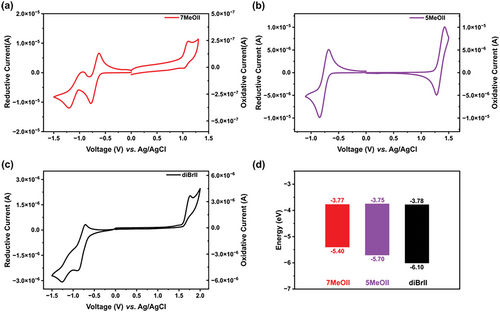
| Compounds | λpeak [nm] | λcutoff [nm] | [eV]a) | Ε (× 104M−1 cm−1]b) | IP [eV]c) | EA [eV]c) |
|---|---|---|---|---|---|---|
| 7MeOII | 279, 426, 502 | 643 | 1.92 | 2.89, 1.77, 0.53 | 5.40 | 3.77 |
| 5MeOII | 294, 398, 560 | 712 | 1.74 | 4.80, 2.80, 0.71 | 5.70 | 3.75 |
| diBrIIdd) | 280, 396, 500 | 611 | 2.03 | 3.62, 1.08, 0.41 | 6.01 | 3.78 |
- a) In chloroform;
- b) = 1240/λcutoff;
- c) Solution CV measurements were conducted in anhydrous dichloromethane at a scan rate of 50 mV s−1 with Bu4NPF6 (0.1 mol L−1) as the electrolyte, and IP and EA were calculated from the redox onset potentials versus Ag/AgCl (EHOMO = −4.4 eV); d)Data from ref. [48].
2.4 Polymer Synthesis and Characterization
With the two brominated compounds (7MeOII or 5MeOII) in hand, we carried out the corresponding copolymerization with two comonomers, namely 5,5′-bis(trimethylstannyl)-2,2′bithiophene (2T) and 5,5′-bis(trimethylstannyl)-2,2′-bithiazole (2Tz), using Pd2(dba)3 as the catalyst in the presence of P(o-tolyl)3 as the ligand in toluene at 115 °C for 72 h, which resulted in four target copolymers, namely P(7MeOII-2T), P(7MeOII-2Tz), P(5MeOII-2T), and P(5MeOII-2Tz) in high yield, as shown in Scheme 3. All of the polymers were subjected to Soxhlet extraction to remove oligomers and other impurities. The polymers have number-average molecular weights (Mn) ranging from 16.2 to 190.2 kDa, as shown in Table 2, with the polymer dispersibility index (PDI) in the range of 1.88–2.60 in comparison to polystyrene standard as determined by high-temperature gel permeation chromatography (GPC). The copolymerization of 7MeOII with both 2T and 2Tz resulted in two polymers with similar molecular weights, while a large discrepancy in molecular weights was observed for the copolymerization of 5MeOII with 2T and 2Tz, probably due to the reactivity difference of 5MeOII toward the two comonomers. All four polymers show excellent solubility in chlorinated solvents, such as chloroform, chlorobenzene, and dichlorobenzene at room temperature, and are soluble in toluene when heated. Differential scanning calorimetry (DSC) analysis (Figure S7) shows that all these polymers exhibit an endothermic transition at ≈95 °C in the second heating scans, which could probably be ascribed to side-chain melting. Thermal gravity analysis (TGA) shows that all four polymers exhibit high thermal stability with a 5% weight-loss temperature above 380 °C (Figure S6, Supporting Information).
2.5 Photophysical and Electrochemical Properties of the Polymers
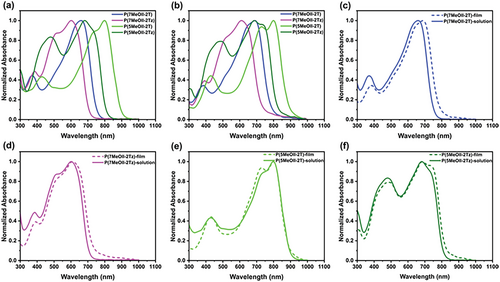
To study the effect of methoxy substitution on the optical properties of the polymers, the UV–vis absorption spectra of P(7MeOII-2T), P(7MeOII-2Tz), P(5MeOII-2T), and P(5MeOII-2Tz) copolymers were measured in o-dichlorobenzene (o-DCB), which are shown in Figure 5a and the data are summarized Table 3. Two trends could be observed: 5MeOII-based copolymers exhibit more red-shifted absorption onset compared to their 7-MeOII counterparts, which is corresponding to its narrower optical bandgap (). For example, is found to be 1.38 eV for P(5MeOII-2T), obviously smaller than that (1.65 eV) of P(7MeOII-2T). The other trend is that 2Tz-based copolymers show blue-shifted absorption onset compared to their 2T counterparts, corresponding to a broader optical bandgap, which can be ascribed to the smaller donor strength of 2Tz with respect to 2T. For example, is 1.76 eV for P(7MeOII-2Tz), wider than that (1.65 eV) of P(7MeOII-2T). It should be pointed out that the optical bandgap of P(7MeOII-2T) is similar to that (1.60 eV) of the parent P(II-2T),[18] showing that methoxylation at the 7-position of II does not alter of the corresponding polymer that much. On the contrary, methoxylation at the 5-position of II leads to around 100 nm redshift of the UV–vis absorption onset of the corresponding polymer with respect to the parent P(II-2T), which infers that the methoxylation position plays a vital role in adjusting the optical bandgap of the polymers. All polymers show typically dual absorption bands with the stronger absorption band located in the long wavelength region, which could be ascribed to the intramolecular charge transfer (ICT) coupled with 0–0 and 0–1 vibration modes of typical D–A polymer structures. Temperature-dependent UV–vis spectroscopic measurement up to 95 °C shows that when the temperature increases, the optical band gaps of the four polymer solutions show little changes (as shown in Figure S9, Supporting Information), indicating that the conformations of the four polymers were relatively stable in solution. Compared with those in solution, absorption spectra of four polymers in thin film state only display a slight red-shift, as shown in Figure 5b–f. For P(7MeOII-2T) and P(7MeOII-2Tz), absorption spectra of both polymers in thin film state show relatively increased 0–0 vibrational absorptions, which indicates that the polymer backbones become more planar in solid state; On the other hand, the spectrum ofP(5MeOII-2T) shows a relatively increase 0-1 vibrational band, while P(5MeOII-2Tz) shows a relatively increase 0-0 vibrational band, indicating that 5'-methoxylation on II has a more sophasticated influence on the final outcome of the planarity of the polymers, which might be correlated to the influence of the comonomers.
| Polymer | Mn [kDa] | PDI | λmax [nm]a) | λcutoff [nm]a) | [eV]b) | IP [eV]c) | EA [eV]c) | [eV]d) |
|---|---|---|---|---|---|---|---|---|
| P(7MeOII-2T) | 67.4 | 1.89 | 660 | 753 | 1.65 | 5.23 | 3.79 | 1.44 |
| P(7MeOII-2Tz) | 51.5 | 1.88 | 620 | 704 | 1.76 | 5.56 | 3.88 | 1.68 |
| P(5MeOII-2T) | 190.2 | 2.20 | 798 | 898 | 1.38 | 5.08 | 3.72 | 1.36 |
| P(5MeOII-2Tz) | 16.2 | 2.60 | 680 | 808 | 1.53 | 5.37 | 3.73 | 1.64 |
| P(II-2T)e) | 37.3 | 2.3 | 719 | / | 1.58 | 5.52 | 3.74 | 1.78 |
- a) In o-DCB;
- b) Eopt g = 1240/λcutoff;
- c) IP and EA energy levels were estimated from redox onset potentials versus Ag/AgCl (EHOMO = −4.4 eV);
- d) ECV g = EEA − EIP; e)Data from ref. [18].
Cyclic voltammetry was performed on all polymers in their film states to estimate their frontier orbital energy levels, and the CV diagrams are shown in Figure 6 and the data are listed in Table 3. The IP values (which are closely related to HOMO energy level) of the polymers were estimated from their oxidation potential onset. P(5MeOII-2T) exhibits the lowest IP of 5.08 eV (corresponding to the highest HOMO energy level), which is close to the work function of Au, while the IP value (5.23 eV) of P(7MeOII-2T) is higher. It should be pointed out that the HOMO energy level of 5MeOII determined by CV is lower than that of 7MeOII, which implies that 5-methoxylation on II has a more drastic influence on the HOMO energy level of the resulting polymer than 7-methoxylation. It is worth mentioning that among the four polymers, the oxidation peak of P(5MeOII-2T) shows the best reversibility, which infers that its radical cations may possess the best stability. The copolymers containing 2Tz exhibit higher IPs than their 2T counterparts, inferring that they have a lower-lying HOMO energy level. For example, P(7MeOII-2Tz) exhibits the highest IP of 5.56 eV, which is correlated to the lowest HOMO energy level among the four polymers. We also determined the IP values from ultraviolet photoelectron spectroscopy (UPS) in films (Figure S10 and Table S1, Supporting Information). The IP values determined by UPS show a very similar trend, in which P(5MeOII-2T) exhibits the lowest IP value of 4.95 eV (IPCV 5.08 eV), while P(7MeOII-2Tz) exhibits the highest IP value of 5.97 eV (IPCV 5.56 eV). It can be seen clearly that the IP value of P(5MeOII-2T) matches best with the work function of Au.
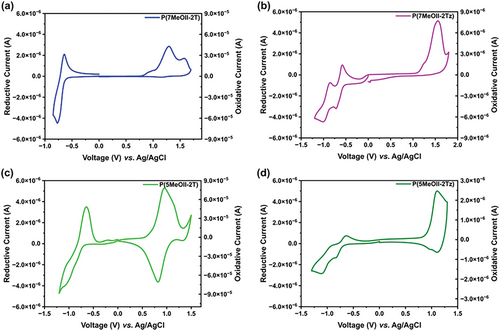
The EA values (which are closely related to LUMO energy level) of the polymers were estimated from their reduction potential onset. Among the four polymers, P(7MeOII-2Tz) exhibits the highest EA of 3.88 eV, which is correlated to its lowest-lying LUMO energy level. Moreover, two semi-reversible reduction peaks can be clearly observed, inferring that the radical anion of P(7MeOII-2Tz) shows the best stability. The EA values decrease in the order of P(7MeOII-2Tz) > P(7MeOII-2T) > P(5MeOII-2Tz) > P(5MeOII-2T), and the reduction peak of P(5MeOII-2T) shows the worst reversibility, indicating the radical anion of it might be the least stable. Compared with the previously reported the parent P(II-2T),18 the HOMO energy levels of P(7MeOII-2T) and P(5MeOII-2T) show a certain increase while the LUMO energy levels remain almost unchanged, and we anticipate that they would exhibit p-type charge transport behavior. On the other hand, P(7MeOII-2Tz) possesses the lowest-lying LUMO energy level, and we anticipate it would show ambipolar charge transport behavior.
2.6 OFET Properties
To evaluate the OFET performance of these four polymers, top-gate/bottom-contact (TG/BC) OFET devices were fabricated. The fabrication details are provided in the Supporting Information. Transfer and output curves are shown in Figure 7 and Figure S10, Supporting Information, and the corresponding data are summarized in Table 4. P(7MeOII-2T), P(5MeOII-2T), and P(5MeOII-2Tz) show unipolar p-type character after thermal annealing treatment at 150 °C for 10 min (optimized conditions). Among the three polymers, P(5MeOII-2T) exhibits the highest hole mobility (µh) of 1.9 × 10−1 cm2 V−1 s−1 with an on/off current ratio (Ion/Ioff) of 101–102. The lower Ion/Ioff may be correlated to its relatively narrower band gap. P(5MeOII-2Tz) shows a µh of 6.6 × 10−2 cm2 V−1 s−1 and a high Ion/Ioff ratio of 105–106, while P(7MeOII-2T) shows a µh of 1.1 × 10−2 cm2 V−1 s−1 and an Ion/Ioff ratio of 104–105. All unipolar p-type devices were tested for 20 cycles, and exhibited little change in the transfer curves, showing that they are quite stable under operation conditions, and a typical example is shown in Figure S11, Supporting Information. Furthermore, forward/back scan curves overlap quite well, which indicates that they have a small hysteresis close to idealized OFETs. Only P(7MeOII-2Tz)-based devices exhibit bipolar transport properties with a µh of 1.3 × 10−4 cm2 V−1 s−1 and an electron mobility (µe) of 3.9 × 10−3 cm2 V−1 s−1 with an Ion/Ioff ratio of 103–104due to its relatively lower LUMO energy level (−3.88 eV). Among the four polymers, P(5MeOII-2T) has better mobility probably due to its better matched HOMO energy level (−5.08 eV) with the work function of the Au electrode. As will be discussion in the following sections, for P(5MeOII-2T), the crystallinity of its films is not the highest among the four polymers, demonstrating that crystallinity is not the only factor that governs charge carrie mobility; however, we could not rule out the contribution of its higher Mn (190 kDa) to its higher mobility. Mn. Compared with the reported mobility of isoindigo polymers without methoxy group substitution, the mobility of these four polymers was reduced, which is unexpected, since we anticipate that the introduction of methoxy group on II may form O···S interaction with the adjacent thiophene ring and make the backbone more planar, which should facilitate the charge transport. There must be other reasons that are unfavorable for charge transport, which will be discussed in the following sections.
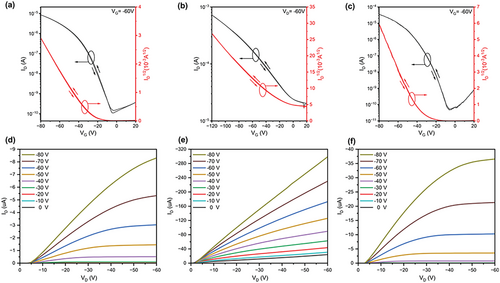
| Polymers | Dielectric layer | μhmax (μhave) [cm2V-1s-1] | µemax(µeave) [cm2V-1s-1] | Vth,h[V] | Vth,e[V] | Ion/Ioff |
|---|---|---|---|---|---|---|
| P(5MeOII-2Tz) | CYTOP | 6.6(6) × 10−2 | – | −35.9 | – | 105–106 |
| P(5MeOII-2T) | CYTOP | 1.9(1.4) × 10−1 | – | −2.7 | – | 101–102 |
| P(7MeOII-2Tz) | CYTOP | 1.3(0.7) × 10−4 | 3.9(3.4) × 10−3 | −71 | 121 | 103–104 |
| P(7MeOII-2T) | CYTOP | 1.1(0.6) × 10−2 | – | −26.6 | – | 104–105 |
2.7 Theoretical and Experimental Evidences on the Planarity of the Polymers
In order to further study the relationship between molecular conformation and properties, we calculated the most stable conformation of the repeating units of the four polymers and the rotation barrier around the MeOII-thiophene/thiazole linkage. As shown in Figure 8, in all cases, the most stable conformations are reached when the methoxy group and the sulfur atom on the thiophene/thiazole ring are on the same side, and the dihedral angle between the phenyl ring and thiophene/thiazole ring is nearly 0°. The rotation barriers of 7MeOII-2T/2Tz are larger than those of 5MeOII-2T/2Tz, which can be ascribed to the higher steric hindrance of the methyl group on 7MeOII pointing closer to the thiophene/thiazole ring due to the repulsion of the alkyl sidechain on the lactam moiety. However, the energy differences between 0° and 180° conformation is not large, ranging from around 1.0 (for 7MeOII-2T linkage) to 3.8 kJ mol−1 (for 5MeOII-2Tz linkage), which infers that the existence of most and 2nd most stable conformation is possible.

To further clarify the existence of O···S interactions, single crystals of thiophene-flanked 7MeOII (using liner C12 sidechain instead of branched sidechain to facilitate crystal growth) were grown and subjected to X-ray diffraction analysis, and the results are shown in Figure 9. It can be seen very clearly that in this case, there are two stable conformations in the single crystal structures, with the sulfur atom either parallel to or antiparallel to the methoxy group. When O and S are on the same side, the distance between the two atoms is 2.906 Å, which is smaller than the sum of the van der Waals radii of O and S atoms (3.25 Å), indicating that there is non-covalent interaction between the O and S atoms. However, the more important information from single crystal analysis is that the existence of O···S interactions do not rule out the existence of the other stable conformation, so the stable conformations presented in the polymeric system should be more complicated than what we wish to control by simply adding O···S interaction to the system.
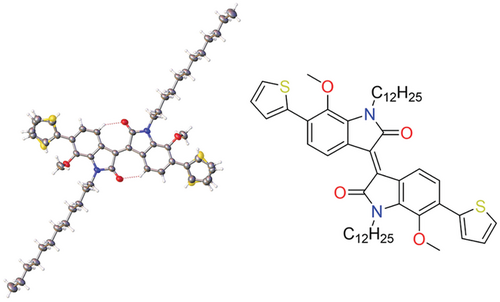
2.8 Microstructure Characterization of the Polymeric Films
To further understand the effect of film morphology and microstructure on the mobility of OFET devices, the thin films of all polymers were investigated by 2D grazing-incidence wide-angle X-ray scattering (2D-GIWAXS) and tapping-mode atomic force microscopy (AFM). Figure 10 displays the 2D-GIWAXS images of four polymers after annealing at 150 °C. All the polymers exhibit clear Bragg diffraction arcs (h00) higher than the 2nd order, which implies the coexistence of both edge-on and face-on orientations. Compared with the polymers based on isoindigo without methoxy substitution, which show a preferable edge-on orientation,18 our results clearly show that the introduction of the methoxy group alters the packing mode of the corresponding polymers. Although whether pure edge-on orientation or mixed orientation should be preferred for charge transport in OFETs is still under debate, at least in our case, switching from edge-on to mixed orientation sounds detrimental to charge transport, which partially explains that the introduction of methoxy group does not lead to improved charge mobility. It can also be seen from Figure 10c that among all the polymers, P(5MeOII-2T) exhibits the most distinct out-of-plane (010) peak, which indicates that it has the most well-established out-of-plane π–π stacking. The in-plane and out-of-plane GIWAXS plots of the four polymers are given in Figure S13, Supporting Information, and the corresponding data were calculated via the Scherrer equation and are shown in Table 5. P(7MeOII-2T) and P(7MeOII-2Tz) are stacked in a similar manner, with the (010) diffraction peak of qz = 1.74 Å−1 observed in the out-of-plane direction, which represents the π–π stacking distance of the polymer backbone in the out-of-plane direction (dπ–π = 3.61 Å); in-plane direction (010) diffraction peak with qz = 1.75 Å−1 is observed in the in-plane direction, which represents the π–π stacking distance of the polymer skeleton in the in-plane direction (dπ–π = 3.59 Å). A strong (010) diffraction peak with qz = 1.75 Å−1 is observed in the out-of-plane direction for P(5MeOII-2T), which represents the strong and relatively ordered π–π stacking of the polymer skeleton in the out-of-plane direction, with a π–π stacking distance (dπ–π = 3.59 Å), while a weaker (010) diffraction peak with qz = 1.73 Å−1 is observed in the in-plane direction, which represents the weaker π–π stacking of the polymer skeleton in the in-plane direction and a longer π–π stacking distance (dπ–π = 3.61 Å). Among the polymers, P(5MeOII-2Tz) exhibits the tightest π–π stacking in both the out-of-plane direction (qz = 1.81 Å−1, dπ–π = 3.47 Å) and in-plane direction (qz = 1.82 Å−1, dπ–π = 3.45 Å) stacking, which is correlated to the preferable antiparallel conformation of 2,2′-bisthiazole units and stronger OS interactions compared to its 7MeOII counterpart.
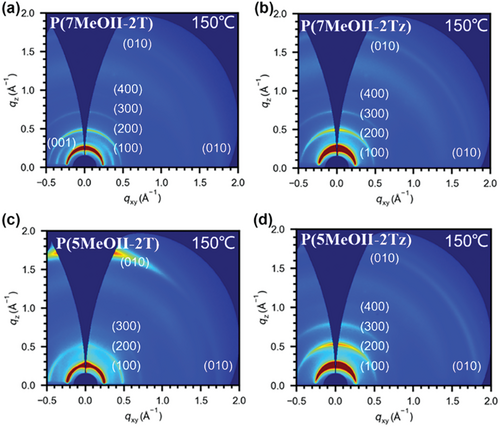
| Polymers | Out-of-plane lamellar(Å−1) |
dL [Å] |
Out-of-plane π–π (Å−1) |
dπ–π [Å] |
In-plane lamellar (Å−1) |
dL [Å] |
In-plane π–π (Å−1) |
dπ–π [Å] |
|---|---|---|---|---|---|---|---|---|
| P(7MeOII-2T) | 0.25 | 25.13 | 1.74 | 3.61 | 0.24 | 26.18 | 1.75 | 3.59 |
| P(7MeOII-2Tz) | 0.25 | 25.13 | 1.74 | 3.61 | 0.24 | 26.18 | 1.75 | 3.59 |
| P(5MeOII-2T) | 0.26 | 24.17 | 1.75 | 3.59 | 0.24 | 26.18 | 1.73 | 3.63 |
| P(5MeOII-2Tz) | 0.27 | 23.27 | 1.81 | 3.47 | 0.26 | 24.17 | 1.82 | 3.45 |
The film quality and surface morphology of thin films were characterized by AFM in tapping mode. After thermal annealing at 150 °C for 10 min, the thin films of P(7MeOII-2T), P(7MeOII-2Tz), P(5MeOII-2T), and P(5MeOII-2Tz) are relatively smooth, showing root-mean-square roughness (RMS) values of 1.06, 1.08, 1, and 2.66 nm, respectively. It is notable that the P(5MeOII-2T) and P(5MeOII-2Tz) films exhibit rod-like nanoscale morphology, which is also preferred for charge transport and is consistent with the device's performance (Figure 11).
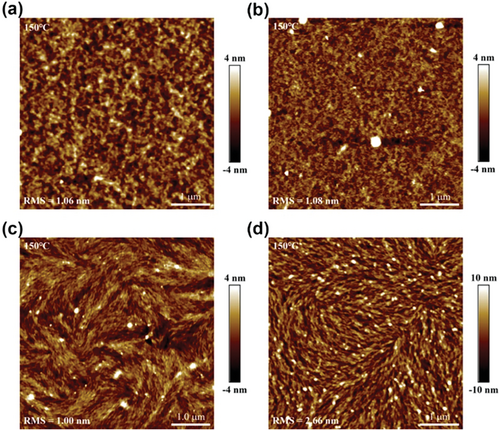
Combining all these evidences together, we can conclude that there are at least two major reasons accounting for the decreased mobility, although methoxylation on the II core leads to polymers with high crystallinity and smooth film morphology. One is that the introduction of O···S interaction does not guarantee the “locked” conformation (although it may be preferred), so the conformation that the polymer adopted in the film state is more complicated than what we expected; the other is that the introduction of methoxy group altered the orientation of the polymers from edge-on to mixed edge-on/face-on, which might be detrimental to charge carrier transport.
3 Conclusion
In conclusion, by synthesizing 7MeOII and 5MeOII, the influence of methoxy group introduction onto isoindigo on the physical and charge transport properties of the corresponding polymers are systematically studied. We found that the substitution positions of the methoxy group have a drastic influence on the optical ad electrochemical properties of both monomers and polymers: methoxylation at 5-position of isoindigo has a more profound influence on the HOMO→LUMO transition, which leads to a more drastic redshift of the absorption band for both monomer and polymers. Theoretical calculations show that 5-position substitution leads to better delocalization of the lone pair on the methoxy group into the HOMO, which accounts for the more obvious redshift. Electrochemical studies reveal that the introduction of the methoxy group elevates the HOMO energy level, but has a neglectable influence on the LUMO energy level of the monomer. However, it does improve the stability of the radical anion generated during the electrochemical process. Both the theoretical calculation and single crystal analysis confirm the existence of O···S secondary orbital interactions, which should improve the planarity of the corresponding polymers. However, single crystal analysis also confirms that two stable conformations (parallel and antiparallel) still exist, implying that the introduction of O···S secondary optical interactions could not render all the repeating units in the polymer to adopt the preferable conformation. Four polymers were synthesized by polymerizing 7MeOII or 5MeOII with either 2T or 2Tz, which were subjected to OFET device fabrication and characterization. GIWAXS study shows that compared to the unsubstituted isoindigo, methoxylation on isoindigo causes the orientation change of the corresponding polymers from edge-on orientation to mixed edge-on/face-on orientation. Among the four polymers, P(5MeOII-2T), P(5MeOII-2Tz), and P(7MeOII-2T) exhibit near ideal p-type OFET characteristics with little hysteresis, among which P(5MeOII-2T) shows the highest hole mobility of 1.9 × 10−1 cm2 V−1 s−1 and a low VT of −3.7 V, which is correlated to its higher molecular weight, suitable ionization potential (5.08 eV) and good crystallinity with the best out-of-plane π–π stacking. P(7MeOII-2Tz) exhibits bipolar characteristics due to its higher electron affinity (3.88 eV). This study cast light on the methoxy-substitution influence on the properties of isoindigo-based polymers, which is instructive to the study of the modification of other electron-acceptors with electron-donating groups. These results also indicate that if we consider introducing the “conformational-lock” effect to boost charge carrier mobility, we should also keep in mind that the introduction of such effects may also change the orientation of the polymers, which may alter the charge transport channel in the films and have adverse effects.
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC 22075105) and start-up funding from Jianghan University.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




