Charge Regulation Stabilizes the Formation of Ionic Liquid-Based Amphiphilic Oligomer Droplet Interface Bilayers
[Correction added on April 10, 2025, after first online publication: the eighth author's affiliation has been corrected in this version].
Abstract
Amphiphilic charged oligomers (oligodimethylsiloxane – methylimidazolium cation, ODMS-MIM (+)), assemble into bilayers using the droplet interface bilayer (DIB) platform, possess similar size and functionality as phospholipid bilayers, but exhibit increased stability. The oligomer ionic headgroups (MIM(+)) are covalently bound to monodisperse, short-chain (n = 13) hydrophobic tails (ODMS). These self-assemble as monolayer brushes at the oil–aqueous interface of water droplets that are influenced by both the charged cationic headgroups, and the nature of the covalently attached tails in the organic phase. Charge regulation (CR) stabilizes the formation of ordered, molecularly close-packed brush phases, which results in highly insulating, stable DIB membranes, with contributions from specific ion-pairing effects, Debye screening, and voltage-dependent electrocompressive stresses. In the oil phase, interactions between hexadecane, a good solvent for ODMS, and the hydrophobic tails result in extended waiting times for bilayer formation compared to phospholipid DIBs, for which hexadecane is a poor solvent. Close agreement between experimental values and predictions for two key parameters, the critical membrane thickness, hc, and maximal grafted headgroup density, Γ0, validate an electrostatic CR model consisting of adsorption and partial neutralization of counterions at a charged interface.
1 Introduction
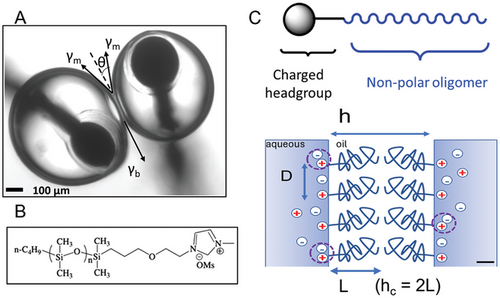
Neuromorphic devices assembled from nanoscale stimuli-responsive biomolecules and lipid bilayer membranes offer unique advantages for low-power, reconfigurable circuitry capable of sensing, signal processing, learning, and memorization involving many types of physical and chemical phenomena not possible with solid-state systems.[1] Droplet interface bilayers (DIBs), which form between two or more aqueous droplets in oil take advantage of the tendency of lipid bilayers to form spontaneously at the liquid/liquid interface between two immiscible phases,[2] as shown in Figure 1A. Recently, we have shown the potential for using planar lipid bilayers in DIBs with peptide ion channels as synapse-inspired memristors,[1] and without peptides as memcapacitors.[3] Both of these memelements can be integrated into neuromorphic computing applications.
Amphiphilic polymers can self-assemble into synthetic nanoscale membranes with tunable behaviors reminiscent of lipid-based membranes, but with more robust interfaces,[4, 5] ensuring the fabrication of extended neuromorphic networks large enough to elicit brain-like computation. For both lipids and amphiphilic polymers, membrane assembly can involve a delicate balance of enthalpic and entropic interactions, however, this balancing can be different for lipid bilayers in long-chain alkanes like hexadecane from that of polymers. Hexadecane interactions with the hydrophobic acyl chains in diphytanoylphosphatidylcholine (DPhPC), a common lipid used in DIBs, are not as important as chain–chain interactions, thus hexadecane is a poor-quality solvent for DPhPC. In this case, directional attractive interactions resulting from shape anisotropy and enthalpic forces (e.g., hydrogen bonds) have important roles in the spontaneous self-assembly of lipid bilayers at hydrophobic–hydrophilic interfaces, which are just a few nanometers thick. Entropic depletion forces involving solvent exclusion also contribute to lipid bilayer formation.[6] For amphiphilic polymers for which hexadecane is a good solvent, meaning solvent–chain interactions are more important, membrane formation may involve a more subtle interplay between enthalpic and entropic forces.
Most polymer-based membranes are formed using block copolymers, which self-assemble into well-characterized morphological phases.[5, 7-9] However, these self-assembled phases can be large and complex while exhibiting long-range repulsive forces – based on osmotic stress and other excluded volume interactions – that hinder membrane formation. Moreover, block copolymer membranes are compressible and up to an order of magnitude thicker than lipid bilayers, features which can complicate the search for design rules affecting assembly and the emergence of neuromorphic behaviors.
Voltage-dependent formation of nonionic triblock copolymer membranes using DIBs made from hydrophilic polyethylene oxide (PEO) blocks flanking a core of hydrophobic polydimethylsiloxane (PDMS) has been described.[10] However, the aqueous droplets functionalized with these polymers did not spontaneously adhere to each other below a minimum voltage threshold because the hydrophobic oils commonly used for the formation of DIBs were thermodynamically good solvents for the hydrophobic PDMS core. Entrapped oil between the two droplets in the hydrophobic block of the copolymer resulted in an energy barrier that had to be overcome before membrane self-assembly could proceed.
Our strategy has been to develop simpler, short-chain, amphiphilic oligomers that structurally resemble lipids. We synthesized oligomers that are highly amphiphilic due to their charged, ionic headgroups (methylimidazolium cation MIM(+)), which were covalently bound to short, hydrophobic oligo-dimethylsiloxane (ODMS) chains (number of monomer segments N = 13) that were monodisperse (i.e., all molecules had identical molar mass,[11] Figure 1B). The choice of a small, monodisperse oligomeric molecule allowed us to avoid the large polydispersity of molecular weights and other unwanted molecular properties typically seen with larger and more complex polymers. Equilibrium membrane thicknesses of ODMS-MIM(+) bilayers were determined to be ≈5 nm, similar to those of lipid bilayers.
Figure 1C illustrates the geometry of the interactions between oligomers in apposed leaflets in the bilayer. Adjacent MIM(+) headgroups are represented by positive charges at the liquid–liquid interface, separated by the distance D (≈1 nm). This distance is dependent on electrostatic repulsion, which is affected by the activity of the Cl(-) counterions in solution and the degree of neutralization of the cationic charges, via the ionic strength and specific ion–ion interactions, shown schematically by the dashed circles in the figure. The length of the ODMS tails for each leaflet is given by L and the separation distance between apposed leaflets by h.
A novel feature of this approach was the inclusion of positively charged MIM(+) headgroups, which were free to move in two dimensions at the aqueous/oil interface of each droplet in the DIB, but which were also covalently bonded to the hydrophobic ODMS tails residing in the oil phase. We found that the fluid liquid–liquid interfaces in a DIB allowed for charge regulation (CR) effects, involving the modulation of the interfacial positive charge of the cationic headgroups by counterions in the aqueous environments of the droplets, to influence the formation and stability of ODMS-MIM(+) bilayers, a unique capability lacking in more commonly studied solid supported lipid or polymer bilayer membranes.[12]
In this paper, we used the DIB platform to electrically probe the formation and stability of polymer bilayer membranes made with ODMS-MIM(+) oligomers immersed in hexadecane. The next section has three subsections: 1) the importance of electrostatic effects for ODMS-MIM(+) monolayers; 2) the sensitivity of bilayer conformation to charge regulation; 3) how charge regulation affects bilayer disjoining pressure; 4) comparison with experimental data, namely, the critical membrane thickness, hc, and the maximal headgroup grafting density in the plane of the oil–water interface, Γ0, which was estimated from pendant drop interfacial tension measurements. There was a close, quantitative agreement between the experimental values of these parameters with a generic charge-regulated model consisting of the adsorption and partial neutralization of Cl(-) counterions at grafted MIM(+) surface sites at an aqueous–oil interface.
2 Results and Discussion
Voltage-dependent ODMS-MIM(+) bilayer formation at two values of the ionic strength is shown schematically in Figure 2. DIB formation was monitored with capacitive ionic currents that were induced in the electrically insulating bilayers with a small, triangular (±25 mV, 10 Hz) voltage waveform. Bilayer formation coincided with an abrupt increase in the capacitive current as the membrane thickness shrunk due to the adhesion of the two initially separate monolayers (bottom panels Figure 2A,B). It is important to note that the ionic currents displayed in Figure 2A,B were almost completely capacitive (given by dV/dt), due to the highly insulating bilayer (R ≅ 0.5 GΩ). Bilayers formed at low ionic strength however (such as in Figure 2A) were unstable, leading to DIB droplet coalescence.
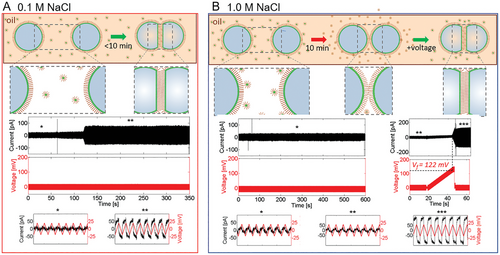
We found that bilayer formation between pairs of droplets was spontaneous for droplets at low ionic strength (I < 0.5 m, Figure 2A), or, at higher ionic strength, after a sufficiently long waiting period (≥30 min), or above a voltage threshold (120 ± 20 mV (n = 20 trials)) (Figure 2B). However, bilayers formed at high ionic strength were very stable (rupture potential 360 ± 130 mV (n = 20), bilayer lifetimes hours), while those at low ionic strength resulted in weaker bilayers (rupture potential 160 ± 50 mV (n = 3)), with only seconds to minutes lifetimes. Additionally, stable bilayer formation at high ionic strength was irreversible, with the bilayer remaining intact even when the transmembrane potential returned to 0 mV. Control experiments performed to rule out colligative and multivalency effects are described in the Supporting Information.
The backbone tails of the ODMS-MIM(+) oligomer were too hydrophobic to be miscible in aqueous droplets. Hence, ODMS was dissolved in the oil phase, where it is believed to form inverse micelles.[11] Due to the charged headgroups, the oligomers were highly amphiphilic, and monolayers spontaneously formed at the oil–aqueous liquid/liquid interface, with the ionic liquid headgroup located on the aqueous side of the interface and the hydrophobic ODMS tails residing in the oil.
2.1 Electrostatic Effects in ODMS-MIM(±) Monolayers
Prior pendant drop tensiometry (PDT) and sum frequency generation spectroscopy (SFG) measurements of monolayer formation showed that the ionic strength-dependent kinetics of self-assembly were comprised of two distinct regimes, namely, an initial one where oligomers first adsorbed and reoriented on relatively fast time scales, followed by conformational sampling and frustrated packing at longer times.[11] The rates of these events were found to scale with the ionic strength (I → 1 m NaCl) and the species of counterions. This involved a time-dependent, structural transition of the ODMS tails, from being mainly parallel to the interface with low packing density at low ionic strength to extending out more into the oil phase to increase oligomer packing density at the interface with elevated ionic strength. This is consistent with lower monolayer tensions at elevated strength measured with PDT. This is significant since it indicated that the hydrophobic tail backbone configurations in hexadecane were controlled by electrostatic interactions occurring in the aqueous phase, even though the electrolytes were not in direct contact with the tails. These changes also coincided with the disruption of the ordered hydrogen-bonding network of water at the interface with increasing ionic strength, as indicated by SFG spectral changes in the ─OH stretching region of water.[11]
Additional SFG/MD studies of the monolayer showed that ion pairing and specific ion effects could result in dramatic changes in monolayer assembly, including enhanced ODMS adsorption, morphological changes of the monolayer, and disruption of hydrogen bonding with water at the interface.[13-16] Importantly, they reduced the electrostatic repulsion of the MIM(+) headgroups in the aqueous phase, forcing the ODMS tails to extend into the oil phase. At all ionic strengths, “softer”, more polarizable, and surface-active anions (like SCN−, compared to Cl−) resulted in denser, more ordered brushes, with extended tails and smaller tilt angles normal to the interface, the result of enhanced charge neutralization as a function of changes in the ionic strength.
Because of their strong amphiphilicity, the ODMS-MIM(+) oligomers were effectively “terminally grafted” at the fluid liquid–liquid interface. This means that the cationic MIM(+) headgroup grafting points were restricted to diffuse in two dimensions parallel to the interface. Further, since the ODMS tails were covalently bonded to the headgroups, they were effectively grafted at the liquid–liquid interface as well. Our previous PDT and SFG results on monolayers at the oil–aqueous interface indicated that the amphiphilic oligomers had first adsorbed at the interface as dilute “mushrooms”, characterized by the hydrophobic tails adopting coiled conformations with segments adopting a random walk, tracing out non-overlapping hemispheres in the oil phase.[11] This was followed by increased overlap between neighboring tails at higher surface coverage, forcing them to stretch out from the interface into an extended “brush” configuration.
2.2 Sensitivity of Bilayer Conformation to Charge Regulation
In this subsection, changes in ODMS-MIM(+) geometry as functions of surface charge density, specifically, oligomer packing density and chain length, will be explored. Our starting point for this analysis is the equilibrium separation distance between the charged methylimidazolium headgroups at the oil–aqueous interface for each droplet before the droplets are brought together to form the droplet interface bilayer, based on our previously reported atomistic molecular dynamics (MD) calculations of the ODMS-MIM(+) bilayer membrane.[13]
In Figure 1C, there is a critical thickness, h = hc = 2L, which corresponds to the point where the hydrophobic tails from the two apposed monolayers begin to sterically interact with each other. For leaflet separation distances above hc, corresponding to monolayers that are in proximity but are not touching, the interaction forces are weakly attractive, consisting of van der Waals and electrocompression forces that increase with decreasing distance. Below this value, the forces are strongly repulsive due to osmotic stress. These forces are also dependent on the in-plane separation distance between adjacent MIM(+) headgroups, given by D.
We used the minimum of the radial distribution function of the in-plane MIM(+)-MIM(+) headgroup pair from atomistic molecular dynamics (MD) simulations we had performed on the monolayer[13] to determine D0 (0.90 nm), which we assigned as a minimum distance of closest approach between nearest neighbor imidazolium groups. The corresponding values for the maximum possible grafting and charge densities for the brush phase from the simulations were Γ0 = 1.57 oligomers nm−2 and σ0 = 0.25 C m−2 respectively.
In Equation (4), σ1 is the modification of the maximum charge density, given by σ0, when multiplied by the fraction of dissociated MIM(+) cations at the liquid–liquid interface, α. The resulting charge density σ1 is decreased from σ0 because of the formation of specific MIM(+)-Cl(-) ion pairs, which neutralized a fraction (1 − α) of the headgroups. In Equation (5), σ2 is the ionic strength-dependent charge density at the interface. It is what the charge density would have been if there were no ion pair formation, and it was only dependent on the ionic strength of the solution (and pH; however, in this study, we assume a constant pH = 7.4).
Ion pair formation, characterized in terms of the association constant 1/Kd (Equations (1)–(3)), resulted in charge neutralization at the interface, not just an increase in the effective activity coefficients of nearby ions in the solution. This is an example of a charge-regulated system resulting from the adsorption and partial neutralization of Cl(-) counterions at grafted MIM(+) surface sites, giving rise to a Langmuir-type adsorption profile as a function of the ionic strength.
For a mixed system consisting of inert (nonbinding, Na(+)) and surface-binding (MIM(+)) cations, Equations (4) and (5) can be solved simultaneously, from which both σ and the surface potential, ψ0, can be determined numerically from the maximum charge density σ0 and dissociation constant Kd. This can be accomplished by finding the value of ψ0 that minimizes the difference σ1 − σ2 = 0, with the constraint Δσ ≤ 10−7 C m−2 using a nonlinear reduced gradient optimization script,[21] and by assuming a value for the dissociation constant in Equation (1) from a series of structurally similar alkyl-methylimidazolium chlorides: Kd ≅ 0.2 m.[22] When this condition is realized, σ1 = σ2 = σ.
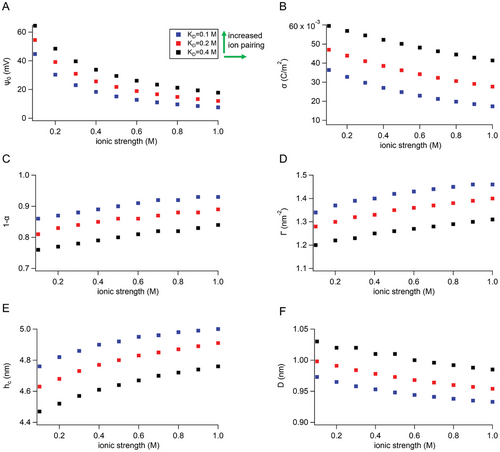
Figure 3 shows, (A) the membrane surface potential, ψ0, (B) the effective membrane surface charge density, and (C) the fraction of associated (neutralized) MIM(+) sites by chloride ions (1 − α), and (D) the corresponding headgroup grafting density at the liquid–liquid interface: Γ0 = σ0(1 − α)/e , all as functions of the ionic strength in the droplet aqueous solutions (e is the elementary charge, 1.6 × 10−19 C). (E) and (F) show the critical leaflet separation distance (membrane thickness) hc where the apposed ODMS tails begin to overlap, and the in-plane headgroup separation distance, D. In Figure 3F, the value of D is dependent on ionic strength and Kd and varies from 0.95 to 1.05 nm. The corresponding hc values, determined from Equation (6), were in the range of 4.4–5 nm and were dependent on ionic strength and Kd as well. These values were consistent with experimental-specific capacitance measurements of ODMS-MIM(+) bilayers as a function of ionic strength, from 0.1 to 1 m. The specific capacitance was used to experimentally determine bilayer thickness from CM (µF cm−2) = εε0/h, where εε0 is the relative electric permittivity and h is membrane thickness (Figure 5B, Table S1, Supporting Information).
Since the ODMS tails were covalently bound to the charged headgroups, the grafting number density of the MIM(+) cations, Γ, also represents the grafted ODMS density in the hydrophobic tail region. This assumes that the interactions between the tails, governed by steric, osmotic, and dispersive interactions in the oil phase, did not influence the effective charge density of the ionic liquid MIM(+) cations in the aqueous phase.
The green arrows in Figure 3 indicate the dependence of ion pairing with the value of Kd; lower values of Kd correspond to increased ion pairing, and hence, increased charge neutralization at the interface. Kd controlled the degree of association for MIM(+)-Cl(-) ion pairs while increasing ionic strength increased the chemical activities of all the ions in the electric double layer (including chloride). The trends from these two contributions are clearly shown in the plots of surface charge, σ, in Figure 4B. Both result in increased molecular packing with time.
The values of the charge density and grafted oligomer number density at the interface with increasing ionic strength were not able to reach their maximum values, Γ0 and σ0, values since not all of the positively charged headgroups were completely neutralized by the counterions (determined by 1/Kd), even at the highest applied ionic strength (1 m). Similarly, the in-plane separation distances of the charged headgroups, D (proportional to ), did not reach the corresponding minimum value D0.
2.3 Charge Regulation Affects Bilayer Disjoining Pressure
In this subsection, the total disjoining pressure between the two ODMS-MIM(+) monolayer leaflets of the two aqueous droplets as a function of separation interval when forming the DIB will be derived. Disjoining pressures are sensitive to the electrochemical variables of charge regulation, parametrized by Kd, and the ionic strength. This is because they affect the oligomer packing density at the interface and the bilayer thickness.
At higher compression (lower h), the osmotic component (π = c × RT) from residual hexadecane concentration in the brush (c) dominates the elastic term. These forces balance at a critical distance h = hc, which is the distance where the ODMS chains from the two apposing monolayers begin to touch. This value increased somewhat at higher ionic strength and ionic pairing because of chain elongation due to charge neutralization, which led to enhanced excluded volume interactions, stretching the chains. At h ≥ hc, Pbrush = 0.
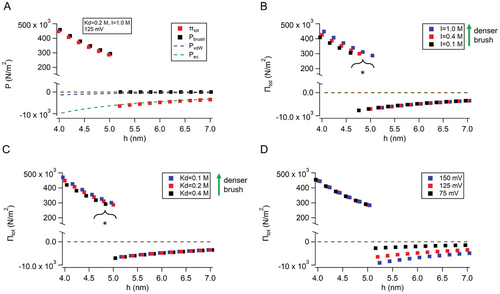
Figure 4B–D shows plots of the total disjoining pressure, Πtot, as functions of the three parameters associated with charge regulation at the ODMS-MIM(+) interface in this study: that is, the ionic strength (B), the dissociation constant, Kd, of the MIM(+)-Cl(-) specific ion pair (C), and the electrocompressive potential (D). Varying Kd or ionic strength changed the repulsive components of the total disjoining pressure (Pbrush) but did not affect the attractive components (PvdW or Pec). However, varying the applied voltage not only changed the attractive component Pec, but also PvdW, because electrocompression thinned the bilayer, which increased the van der Waals interactions according to Equation (9).
Either decreasing Kd or increasing ionic strength increased repulsive component pressures, as shown in Figure 4B,C, but in different ways. In Figure 4B, Kd was kept constant at 0.2 m, while in Figure 4C, the ionic strength remained the same at I = 1 m. The differences at h ≤ hc between these two panels highlight the dependencies existing between I and Kd. The ionic strength is one of the experimentally accessible independent variables (voltage is the other). It parametrizes Kd via Equations (1)–(3), and the charge density at the interface via Equations (4) and (5). The asterisks in Figure 4B,C indicate changes to the threshold condition h ≤ hc, corresponding to the onset of the repulsive pressure contribution Pbrush to the total disjoining pressure. The threshold condition changes because Pbrush is proportional to both the grafting density Γ (nm−2) and h−2 from Equation (8), which in turn depend on the extent of electrostatic shielding of the charges and charge neutralization of the headgroups by chloride ions.
At early times, the ODMS-MIM(+) monolayers at the oil–water interface of each DIB droplet likely had many heterogeneous, interstitial void spaces in the plane of the membrane (corresponding to the mushroom structures from SFG/MD experiments that formed at early times), resulting in poor electrical resistivity and instability against droplet coalescence, due to an increased probability of nucleation and runaway conductive pore growth in the bilayer. This same behavior has been observed in MD simulations of polymer chains in a theta-solvent versus a good-quality solvent when confined in two dimensions.[34] The higher repulsive pressures due to charge regulation in Figure 4B,C correspond to denser brushes, with increased molecular packing in the plane, resulting in a stable, highly insulating membrane. The CR model (Equations (1)–(6)) is a combination of Gouy–Chapman electrostatic theory and the Grahame equation of the surface charge density. It presupposes a continuous charged surface or interface without holes or leaks, and so pinholes, voids, or any other defects at earlier times or at lower salt are not treated by the model. This point is somewhat academic since these conditions typically resulted in abrupt DIB droplet coalescence.
2.4 Comparison of CR Model with Experimental Data
The voltage-dependent capacitive currents in Figure 2 chronicle the formation and life cycles of droplet interface bilayers in real time, given by the step-like increases in membrane capacitive currents seen in the middle current/voltage plots in Figure 2A,B. The onset of conductive currents, tended to be closely followed in time by abrupt loss of the bilayer due to droplet coalescence in the DIB. Therefore, they were not considered in this study.
The data in Figure 2 give qualitative information (e.g., rupture potentials, DIB lifetimes) about the formation and stability of ODMS-MIM(+) bilayers under changes in ionic strength. This data is not quantitative, but we are still able to validate our model quantitatively, from a) experimental measurements of the specific capacitance of the DIBs, which enable membrane thickness determination, and b) estimates of the grafting densities of the charged headgroups, deduced from interfacial tension measurements of pendant droplets (Figure 5D). Both predicted values of the model were very close to their corresponding experimental counterparts (Table 1).
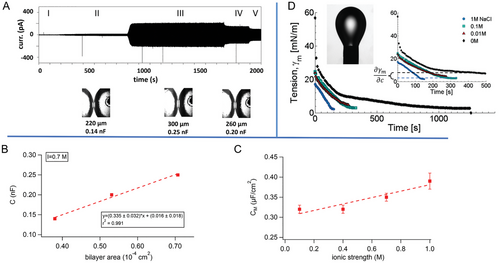
Figure 5A includes bright-field images of the DIB interface at different points in time corresponding to the regions II, III, and IV in the plot of capacitive current. (This current is like those in Figure 2.) This sequence of images enabled the determination of the membrane areas at specific values of the membrane capacitance. This gives the specific capacitance (CM = C/A), which is related to the bilayer thickness, h, by CM (µF cm−2) = εε0/h. It is estimated from the slope of membrane capacitance versus bilayer area in Figure 5B. It is also dependent on ionic strength, as seen in Figure 5C, which shows that the specific capacitance increases by ≈15% over the range I = 0.1 to 1 m. These data are also listed in Table S1 (Supporting Information). The value at 1 m (0.39 µF cm−2) corresponds to a membrane thickness (5.2 nm) that is very close to that predicted for the critical membrane thickness, hc at I = 1 m in Figure 3E (4.9 nm).
The ionic strength-dependent tension data in Figure 5D shows that increased ionic strength sharply decreases the interfacial tension of ODMS-MIM(+) monolayers, suggesting more stable bilayers at high salt (I = 1 m). This is consistent with the large variations observed in ionic strength-dependent rupture potentials and membrane lifetimes, and previous reports correlating surface tension of bilayers with membrane leakiness.[36]
The two parameters, hc and Γ1 m are in fact the most important parameters in the CR model and the disjoining pressure plots in Figure 4. They are also experimentally accessible. Table 1 is a comparison of experimentally determined values for hc and Γ1 m from the data in Figure 5 with those predicted from the CR model. The two experimental parameters are close in value to the predictions, corroborating the model.
Table S2 (Supporting Information) contains interfacial energy parameters of ODMS-MIM(+) bilayers from the monolayer interfacial tension measurements on pendant drops, and contact angle measurements (θ) from bright-field optical images of DIBs (Figure 1A). Bilayer tensions can be estimated from monolayer tensions using γb = 2γm × cos(θ). From these data, the Young–Dupré relation gives for the specific adhesion energy density of the bilayer, −∆F = 2γm [1 − cos(θ)]: 0.27 mJ m−2 at 0.1 m and 0.19 mJ m−2 at 1 m ionic strength. These values are close to those experimentally measured for DPhPC DIBs.[33] The higher adhesion energy at lower salt [NaCl] is likely the result of the interfacial tension almost doubling that at high salt, due to incomplete Debye shielding of the MIM(+) cations. The fact that the low salt, 0.1 m ionic strength bilayer formation was spontaneous but short-lived is consistent with an initial tension-dependent, higher adhesion energy during nucleation of the bilayer, but also points to the greater importance of the final brush density in forming stable, robust, highly insulating bilayers.
3 Conclusion
In this paper, we determined that ODMS-MIM(+) DIB bilayer formation and stability could be modified by charge regulation of the positively charged MIM(+) headgroups in the fluid, 2D, liquid–liquid interface on each individual aqueous droplet in the DIB. Charge regulation in this context refers to the manipulation of the electrostatic repulsion between the charged imidazolium cations at the liquid–liquid interface by Cl(-) counterions, either by changes to the ionic strength, which changed the Debye screening of the headgroup cations or by the formation of specific MIM(+)-Cl(-) ion pairs, which neutralized the surface charge.
Reducing the electrostatic repulsion between headgroups at the interface (decreasing D) resulted in the formation of dense brush layers, consisting of packed ODMS tails in the oil phase. This resulted in a long-range, steric, repulsive disjoining pressure as the chains from the two droplets in a DIB began to overlap. Attractive van der Waals forces and electro-compression, which is caused by an attractive, voltage-dependent dielectric stress, reduced the repulsive disjoining pressure of the brushes somewhat, by thinning the membrane and expelling solvent, resulting in the formation of a shallow, attractive energy well, located just to the right of the critical distance where the monolayer leaflets from the apposed droplets first make contact (h = hc).
This behavior was consistent with those from previous reports where we used SFG spectroscopy and MD simulations to determine the structure and dynamics of ODMS-MIM(+) monolayers at the oil–aqueous interface.[11, 13-16] There we showed that at low ionic strength, the siloxane linkages appeared to initially orient themselves parallel to the aqueous–oil interface, but extended out into the oil phase at elevated ionic strength to form dense brushes to accommodate more oligomers at the interface.
The longer required waiting time for forming a well-packed, highly insulative DIB bilayer with ODMS-MIM(+) compared with diphytanoylphosphatidylcholine (DPhPC), a common lipid used in DIBs, was the result of hexadecane being a better solvent for ODMS-MIM(+) than it is for DPhPC. In a good solvent, solvent–monomer interactions are more favorable than monomer–monomer interactions. Additional time was needed by the ODMS tails to shed interstitial alkane molecules, before enabling the formation of dense brush layers. Given enough time, entropy-driven solvent exclusion decreased the osmotic pressure caused by solvent retention that had hindered DIB formation. An applied voltage also induced bilayer formation via electrocompression, which reduced the membrane thickness, driving out residual solvent in the process.
The structural simplicity of the charged ODMS-MIM(+) molecules and their monodisperse uniformity in size gave them many of the properties of small-molecule ionic surfactants, which allowed for more thorough and quantitative analyses of their structure and ordering at the interface than possible with block copolymers. This analysis will aid the search for the design rules necessary for the assembly of novel, bio-inspired neuromorphic devices. For example, the magnitude of the ionic strength in the droplets effectively acted as a “switch” between two very different types of behavior: the spontaneous formation of a “volatile” bilayer membrane of lower stability at low to moderate values of the ionic strength (≤400 mm), or a “nonvolatile” bilayer at higher ionic strength that, although requiring a voltage threshold or waiting time to be exceeded for assembly, was irreversible.
Specific ion-pairing effects are likely important regulators of charge at the interface. Although not measured directly here, ion-pair interactions, parametrized by an association constant, 1/Kd, were very sensitive to, and could be systematically modified by, the ionic strength. In this case, the driving force for ion-pairing was not Coulombic, but instead, originated from structural disturbances of neighboring water molecules by MIM(+), which was hydrophobic due to a delocalized π-bonded network along the imidazole ring. The water molecules in the vicinity of the headgroup become significantly more ordered than those found at a neat oil–aqueous interface, resulting in a net loss of entropy.[18-20] Ion-pairing alleviates these structural disruptions of water.[18] This screens the interfacial potential seen by bulk water molecules analogously to Debye screening but with a different charge screening mechanism.
This is supported by SFG spectra that were captured from ODMS-MIM(+) monolayers with anions with different polarizabilities in the Hofmeister series (F−, Cl−, NO3−, and SCN−) as the counterions in the aqueous phase.[13, 15] The more surface active and polarizable anions resulted in more compact ODMS tails in the oil phase, indicative of stronger anion interactions with the MIM(+) headgroups and the hydrogen-bonded network with water at the interface (Figure S1, Supporting Information). One would expect then that for a bilayer formed using the DIB platform, the shift in membrane assembly behavior from spontaneous to voltage-activated would occur at lower values of the ionic strength for the more polarizable anions compared to Cl(-).
In this paper, we did not vary the extent of ion pairing between the MIM(+) cations at the interface and counterions in solution directly (i.e., we did not experimentally vary Kd or the type of counterion other than chloride). However, by simultaneously solving Equations (4) and (5) for different values of the ionic strength, we were able to determine how varying the degree of association affected the grafting density in the brush. In general, ionizable surface sites are rarely completely dissociated but are partially neutralized by specific ion binding from the solution.[18] If Kd was large than the counterions would be inert (not able to bind to the interface), the surface charge would be constant at σ0, and the brush density and hence, bilayer stability would be independent of ionic strength. This was not the case here.
Synaptic plasticity in soft-matter neuromorphic systems like DIBs-based memristors and memcapacitors is sensitive to ambient electrochemical environments, including temperature[1] and pH.[37] The small, monodisperse ODMS oligomers studied here can form dense brush layers, over time, regulated by ionic charges. This is a different structural motif compared to lipid bilayers, which are lamellar liquid crystals governed by relatively weak dispersive intermolecular interactions. They also represent a different approach than the more conventional use of block copolymers, which can phase separate to form membranes with nanoscopic features, but which are typically much larger and bulkier, making them difficult to characterize structurally, and complicating efforts to correlate electrical behaviors with specific physical states of the membrane. These siloxane oligomers, on the other hand, are on the same length scale as lipid molecules, and are all compositionally and structurally identical, making such correlations accessible. We are currently working on adapting the DIB platform to X-ray and neutron reflectometry, to correlate the dynamic behavior described here to time-dependent structural changes in the bilayer.
The requirement of a threshold, like the one here for ODMS-MIM(+) bilayer formation at high salt concentration, is a defining characteristic of both solid-state and soft-matter memristors,[1] and is reminiscent of the nonlinear electrical behavior inherent in how integrate-and-fire post-synaptic neurons store and transmit information in both biological and artificial neural networks.[38] Additionally, the difference between volatile and nonvolatile bilayer formation in ODMS bilayers is suggestive of the differentiation of short-term plasticity from long-term synaptic plasticity, including learning and memory, in neuroscience.[39] With these bilayers, ionic strength in effect functions like a switch between volatile and nonvolatile behaviors, governed by stability.
Here, we show that a parameter as easily adjustable as the concentration of a simple salt [NaCl] in the aqueous droplets of a DIB could lead to facile chemical tunability of the interface and new avenues for controlling synaptic plasticity in memelements composed of soft materials that go beyond phospholipid bilayers.
4 Experimental Section
ODMS-MIM (+) Synthesis
Detailed procedures for the synthesis of the ODMS-MIM (+), mesylate co-anion, OMs (-), ionic oligomers, including NMR characterization, can be found in the Supporting Information of Ref. [11]. The ODMS-MIM(+)− OMs(-) ionic liquid oligomer was introduced in the oil phase as inverse micelles, with the OMs(-) sequestered in the center of the micelle presumably. It was unlikely that much of it was transferred into the aqueous phase of the droplet from the oil to impact the total ionic strength. The chloride counterion was included in the aqueous phase of the droplet at much higher concentrations than was possible for the mesylate co-anion, which effectively exchanges the more surface active OMs(-) with Cl(-).
Ionic Oligomer DIB Formation and Characterization
Droplet interface bilayers were formed as described previously in studies of lipid-based DIBs.[2] The aqueous droplets consisted of deionized water (18.2 MΩ × cm) containing 10 mm 3-(N-morpholino propanesulfonic acid (MOPS, Sigma), with a pH of 7.4, and varying amounts of sodium chloride (NaCl, 0.1 to 1 m) for electrical conductivity and to control the ionic strength, which is given by I , where ci is the molar concentration of the ion i, zi is its valence, and the sum is taken over all the ions in the solution. Here it was assumed that the solution concentration of the initial mesylate counterion to the MIM (+) head group was negligible compared to the chloride anion at all NaCl concentrations. The droplets were anchored to silver/silver chloride (Ag/AgCl) wires (Goodfellow) by coating their ball-ended tips with a 1% agarose gel solution. The droplets, anchored on the electrodes, were submerged in hexadecane (≥99%, Sigma) which contained 2 mg mL−1 ionic oligomer dissolved in it (unless otherwise indicated in dilution experiments). Droplets were placed into contact after allowing enough time for monolayers to assemble and attain equilibrium packing.
Electrical Measurements and Imaging
Ionic oligomer membrane formation was detected as an increase in membrane capacitance by supplying a 10 Hz, 10 mV triangular waveform from a function generator clamped at zero bias voltage (Agilent). Due to the highly resistive and capacitive nature of the bilayer membrane, the resulting current response was square-like. To determine voltage thresholds for DIB formation, a custom LabView code was used to overlay the triangular waveform on a slowly increasing bias ramp. These input signals were routed through an Axopatch 200B or an Axopatch 1D patch-clamp amplifier (Molecular Devices). For convention, positive current refered to current flowing from the positive terminal of the Axopatch headstage. The capacitance of the polymer interface was extracted from sections of the square-wave current response. In parallel, changes in the minor axis of the membrane, R, were acquired from bright field images of the droplets viewed from below through a 4× objective lens on a Nikon TE-300 inverted optical microscope. The images were post-processed to extract bilayer area values. The bilayer areas from these measurements were then used to calculate the specific capacitance, CM, from which the membrane thickness hc was obtained. Simultaneously, images of contact angles of the DIB as functions of applied voltages were captured and used to help determine changes to both the monolayer and bilayer interfacial tensions (Supporting Information, Ref. [33]). All current recordings were made using the patch clamp amplifiers listed above and a Digidata 1440 data acquisition system (Molecular Devices). For all measurements, droplets and measurement probes were placed under a custom-made Faraday cage to minimize ambient electrical noise.
Acknowledgements
This manuscript was authored by UT-Battelle, LLC, under Contract No. DE-AC0500OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript or allow others to do so, for the United States Government purposes. B.D. was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. R.L.S. was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Science and Engineering Division. J.K. was supported through the Scientific User Facilities Division of the Department of Energy, Office of Science, sponsored by the Basic Energy Science Program, DOE Office of Science, under Contract No. DEAC05-00OR22725. K.H., J.-M.C., Z.L., G.T., and C.P.C. performed work at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility and sponsored at Oak Ridge National Laboratory by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Z.L. and G.J.T. carried out electrical measurements on DIBs. R.L.S., J.M.C., B.D., J.K., and C.P.C. carried out data analysis. K.H. and Y.L. prepared the ODMS-MIM (+) sample. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.