Investigation of the Physical Properties of Poly(ortho- and para-trifluoromethoxy styrenes)
Abstract
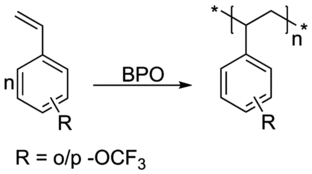
Ortho and para -OCF3 substituted styrenes are prepared by the Grignard cross-coupling reaction between -OCF3 substituted bromobenzene and vinyl bromide and further polymerized in bulk using benzoyl peroxide. The glass-transition temperatures (Tg) are 80 °C and 73 °C for the ortho and para –OCF3 substituted polystyrenes, respectively, which are much lower compared with polystyrene (100 °C). The size of the -OCF3 group is large and the ether bond is flexible; thus, the -OCF3 substituted polystyrenes have larger free volumes, which has more effect on the Tg value compared with that of the steric hindrance effect. The refractive indices are 1.4908 and 1.4809 at 650 nm for ortho and para –OCF3 substituted polystyrenes, respectively, which are much lower than that of polystyrene (1.5861) due to the presence of fluorine atoms in the polymer unit.