Patina formation and diffuse dispersion of alloying metals from weathering steels at urban atmospheric conditions—A combined laboratory and field investigation
Abstract
The increasing use of weathering steels (WS) has raised concerns regarding the diffuse emission of alloying elements. This research paper investigates the release of iron (Fe) and the alloying elements (Cr), copper (Cu), manganese (Mn) and nickel (Ni) from two commercially available WS at urban field conditions in Stockholm, Sweden, during 1 year and in the laboratory at simulated urban conditions. The amount of released metals is evaluated and compared with recommended levels of metals in drinking water and the formation and evolution of the protective patina is studied in parallel to assess the influence on the metal release process. Only minor amounts of the alloying elements are released and is only linked to the outermost surface composition of the patina during the very beginning of the exposure. The released amounts are found to be lower compared with the corresponding levels recommended by the World Health Organisation for drinking water.
1 INTRODUCTION
Weathering steel (WS) is a construction material widely used in applications ranging from heavy-duty constructions like bridges and shipping containers to cladding material for facades to works of art. The low carbon (<0.2 wt%) high strength low alloyed (<5 wt%) steel has an improved corrosion resistance at atmospheric conditions compared with traditional carbon steels and has hence a long-term durability with low maintenance requirements.[1, 2] Its resistance to atmospheric corrosion derives from the formation of a rust layer, a patina, consisting of iron (Fe) oxides/hydroxides and the involvement of small content of the alloying elements, typically Cr, copper (Cu), manganese (Mn), nickel (Ni) and silicon (Si) in appropriate ratios. This patina is denser and has improved barrier properties (corrosion resistance) compared to the rust layer formed on carbon steel, which is much more voluminous and poorly attached to the steel substrate. Some WS grades are alloyed with phosphorus (P) to improve the corrosion resistance further, however, with the drawback of reduced impact toughness and welding properties. WS are, depending on the P content, divided into two types. Phosphorus-alloyed WS are used for aesthetic applications, for example, facades and works of art, whereas non-P WS are suitable as structural steels in load-bearing structures.[3, 4]
The formation of a dense patina on WS slows down the corrosion rate of the steel substrate, which makes the use of any protective coating, as commonly used on carbon steel at atmospheric conditions, redundant. The use of WS compared to carbon steel also has sustainability advantages such as reduced maintenance, long-term durability, recyclability and no environmental impact (carbon footprint) associated with coatings.
The formation of the dense patina of improved barrier properties (corrosion resistance) compared to carbon steel is favoured by regular daily cycles of wet and dry conditions, low deposition rates of chlorides and moderate atmospheric pollutant levels, for example, of SO2 and NOx. This is, depending on the prevailing environmental conditions, typically achieved within a time period of 2–8 years.[1, 2, 5] Periods with non-regular wet and dry cycles, long periods of wetness or high deposition rates of chlorides and pollutant levels, for example, SO2, promote the formation of corrosion products that have lower adherence to the steel and are more porous, such as the case for carbon steel and iron. The general criterion for the use of WS is mostly restricted to low corrosivity environments (C2–C3) but can be used in environments with higher corrosivity (C4) by compensating for the increased corrosion loss by using a thicker material, under which conditions an aesthetically attractive well-adherent patina evolves and stabilises with time.[1, 6, 7] The primarily formed corrosion product is γ-FeOOH (lepidocrocite) that, with time, evolves into amorphous ferric oxyhydroxide, and after several years, the final corrosion product consists of a layered structure with an inner layer of α-FeOOH (goethite) and an outer layer of γ-FeOOH.[1, 4-6] The α-FeOOH layer is enriched in Cr, Cu and P[8] with a denser structure compared with γ-FeOOH providing improved barrier properties compared with corrosion products formed on the traditional carbon steels.[5, 9] However, if WS are exposed to chloride-rich environments, the formation of β-FeOOH (akagenite) is promoted, thus providing a less dense structure with decreased barrier properties and corrosion rates similar to traditional carbon steels.[10]
Environmental interactions inevitably result in the possibility that metals can be dissolved from the patina and, by the action of rainwater, be released from the surface to the adjacent environment. The extent and characteristics of this release of metals from WS are of interest as it is important to ensure that the extent and chemical form of released metals do not induce any adverse effects on the environment. Some metal release studies have been made on WS at laboratory conditions using accelerated methods simulating chloride-containing environments[6, 11] and at marine field conditions.[9] The field exposure showed no significant release of either Cr or Cu, whereas Fe, Mn and Ni were continuously released with the highest extent during the first year of the 3-year exposure.[9] All investigations disclosed the main alloying elements to be released to varying extents.[6, 9, 11] The laboratory study further clearly showed that metal release testing at stagnant conditions is not representative of real-life exposure scenarios.[11]
Investigations on the release of metals from WS in urban environmental conditions are scarce or non-existing. Obtaining such data is crucial for informed decision-making by manufacturers, architects, contractors and end-users. A solid scientific understanding of diffuse metal dispersions serves as the basis for potential regulations and restrictions set by regulatory bodies.
The objective of this study was to address the following research questions related to the diffuse emissions of alloying metals from commercially available WS when used, for instance, in building façades under atmospheric conditions: (i) Are alloying elements of WS released into the environment under atmospheric conditions, and can their dispersion be linked to the composition of the outermost surface oxide? (ii) Can the composition, morphology and evolution of the patina on WS at outdoor conditions be mimicked at simulated rain events at laboratory conditions and where is the alloy constituents located? (iii) Do the release of the alloying constituents exhibit any time-dependent patterns? and (iv) Are the levels of dispersed alloying elements from WS of environmental concern?
2 MATERIALS AND METHODS
2.1 Materials
Two commercial WS, WS-1 and WS-2, supplied by SSAB Europe, and pure iron, Fe (99.62 wt% Fe), supplied by Stena Stål AB, Västerås, were investigated. Their nominal bulk compositions, based on supplier information, are given in Table 1. The surface conditions of WS-1 and WS-2 were kept as-received from the suppliers to ensure that the investigation was performed on end-user relevant surfaces. The supplied surfaces of both WS surfaces were shot blasted (WS-1 by steel grit and WS-2 by glass grit) to remove the mill scale.
| Material | C [max wt%] | Mn [wt%] | Cu [wt%] | Cr [wt%] | Ni [max %] | Al [wt%] | Si [wt%] | P [wt%] | S [max wt%] | V [wt%] | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WS-1 | 0.12 | 0.20–0.50 | 0.25–0.55 | 0.50−1.25 | 0.65 | 0.015–0.060 | 0.25–0.75 | 0.07–0.15 | 0.03 | – | bal |
| WS-2 | 0.19 | 0.80–1.25 | 0.25–0.40 | 0.40−0.6 | 0.40 | 0.020–0.06 | 0.30–0.65 | 0.035 | 0.030 | 0.40 | bal |
| Fe | 0.060 | 0.23 | – | – | – | 0.051 | 0.010 | 0.011 | 0.014 | – | bal |
Two different surface configurations of pure Fe were investigated, one duplicate of aged and corroded surfaces previously exposed to two consecutive 8 h rain events, then kept in a desiccator for several years before exposure in the present study (Fe-aged) and one duplicate abraded to 1200 SiP then aged for 24 h in a desiccator (Fe-fresh).
2.2 Surface/patina characterisation
Elemental composition and surface morphology were determined using a combination of techniques including scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS) (Philips XL-30 with an Oxford X-max 20 mm2 SSD EDS detector) and X-ray photoelectron spectroscopy (XPS) (Al X-ray monochromatic source operating at 150 W (10 mA, 15 kV), Kratos UltraDLD spectrometer, Kratos Analytical, Manchester, UK). Wide and high-resolution spectra were acquired at two separate areas (each sized ≈ 0.35 mm2) for C 1s, O 1s, Cr 2p, Cu 2p, Fe 2p, Mn 2p, Si 2p and P 2p. Energy correction was made against the C 1s peak of adventitious carbon set l at 285.0 eV.
The potential presence of crystalline corrosion products in the patina was investigated by means of grazing incidence X-ray diffraction (GIXRD, X'pert PRO ANALYTICAL system with a CuKα radiation source and an incidence angle of 2° or 8°).
Functional groups and compositional surface information were acquired by means of infrared spectroscopy using a Lumos II IR microscope (FTIR, Bruker instrument, 4000–600 cm−1 with a minimum lateral resolution of 5 μm).
The visual appearance of the patina was documented using a Canon Powershot G16 camera (at settings f/2.8, ISO 400 and 1/200).
2.3 Total metal release analysis
Flame (F-AAS) or graphite furnace (GF-AAS) atomic absorption spectroscopy (PerkinElmer PinAAcle900T) was used to determine the total extent of release of Fe (F-AAS) and Cr, Cu, Mn and Ni (GF-AAS) into rainwater (either natural or artificial, see below). Limits of detection (LOD) and quantification (LOQ) and calibration standards for the main alloy metals are presented in Table 2.
| GF-AAS | LOD [µg L−1] | LOQ [µg L−1] | LOQ [g m−2 100 mm−1] | Calibration standards [µg L−1] |
|---|---|---|---|---|
| Cr | 0.2 | 1.0 | 0.076 | 0, 10, 30, 100 |
| Cu | 0.2 | 1.0 | 0.076 | 0, 10, 30, 100 |
| Mn | 0.3 | 1.1 | 0.084 | 0, 10, 30, 100 |
| Ni | 0.5 | 1.5 | 0.12 | 0, 10, 30, 100 |
| F-AAS | LOD [mg L−1] | LOQ [mg L−1] | LOQ [g m−2 100 mm−1] | Calibration standards [mg L−1] |
|---|---|---|---|---|
| Fe | 0.012 | 0.020 | 1.5 | 0, 3, 10, 30 |
2.4 Field and laboratory exposure
The field exposure was conducted at the KTH test station in Stockholm, Sweden, according to the ISO DIS 17752:2011 standard using one panel (0.25 × 0.15 m) of each WS mounted on individual Plexiglas fixtures (inclined 45° from the horizontal, facing south).[12] This orientation is the worst-case scenario for metal runoff in outdoor conditions. Generated release rates must be recalculated to reflect, for example, vertical surfaces as the case for facades, by considering effects of inclination, degree of sheltering, wind direction, and so forth.[13-15] Runoff water was continuously collected in HDPE bottles and sampled 14 times (each containing runoff water from several rain events of different durations) during the 1-year exposure (June 2020–August 2021) with collection times ranging from 1 to 11 weeks (Figure 1).
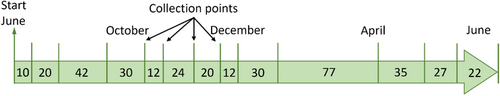
All runoff water was collected directly after interaction with the WS surfaces to, as far as possible, avoid contact with any complexing agent/surface immobilising the released metals.
Due to a long period of subzero temperatures during winter and early spring in 2021, minor volumes of runoff water were collected resulting in an 11-week long period. To determine background concentrations and enable background corrections of the metals of interest, runoff water was collected from a separate Plexiglas fixture without any WS.
A laboratory setup with well-documented reliability to simulate field exposures was used in parallel to investigate short-term metal release kinetics, details given by Herting et al.[16] Duplicate panels were exposed to five consecutive 4 h rain events (pH 4.8 and 5 mm/h) separated by dry periods of varying duration when the panels were kept in a desiccator (Figure 2).
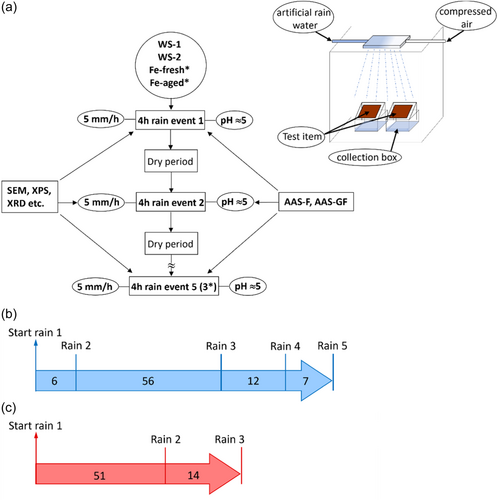
Duplicate samples of the two Fe surfaces were exposed to three consecutive 4 h rain events during a previous pilot study (data not yet published) using the same setup and conditions as for the WS.
An important difference between the rain periods in the field exposure and the rain events in the laboratory exposure is that the laboratory rain events only include one rain event giving high kinetic resolution, whereas one collection period usually contains the pool of several rain events.
3 RESULTS AND DISCUSSION
In the following, patina formation and metal runoff characteristics of alloying elements from the two different WS, WS-1 and WS-2, are presented and linked to the formation of corrosion products with the intention of answering set research questions.
3.1 Are alloying elements of WS released into the environment under atmospheric conditions, and can their dispersion be linked to the composition of the outermost surface oxide?
Time-resolved metal release rates (bar diagrams) of Fe, Cr, Mn, Ni and Cu, and corresponding changes in the relative mass composition of the same elements (also including Si and P) in the outermost surface oxide (top 5–10 nm, pie diagrams), determined by means of XPS, are presented in Figure 3 for WS-1 under both laboratory and field conditions.
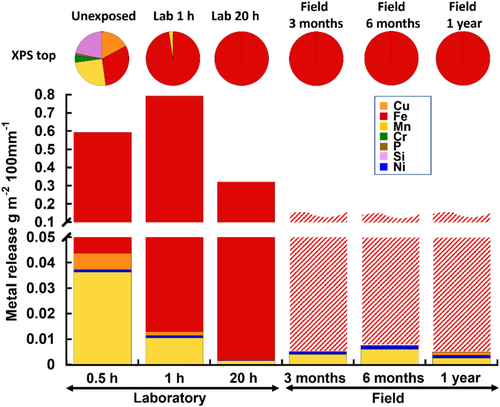
The relative proportions of oxidised metals in the outermost surface oxide reflect the surface conditions at the onset of each exposure period. The unexposed surface oxide (top 5–10 nm) comprised oxidised Fe, along with oxidised Mn, Cu and Cr—the alloy constituents. Ni was not identified in the outermost surface oxide, while Si and P were present in their oxidised states. The relative amount of oxidised metals and elements diminished following the sequence: Fe > Mn > Si > Cu ≫ Cr ≫ P (oxygen was excluded from this comparison). Consistent with findings in the literature, extended exposure under both laboratory and field conditions resulted in the formation of an outermost surface oxide primarily consisting of oxidised Fe (Figure 3).[6, 9, 17] No evidence of any alloy constituents in the outermost surface oxide of the material was discernible at prolonged laboratory exposure conditions (up to 20 h) or when exposed to the 1-year field conditions.
The amounts of released metals from WS-1 determined after the initial rain event of the laboratory exposure (Fe ≫ Mn ≫ Cu ≫ Ni, Si and P not measured) correlated qualitatively with the relative compositional ranking of the same elements in the outermost surface oxide (Fe > Mn > Si > Cu ≫ Cr ≫ P). Neither this ranking nor the metal bulk content (Fe ≫ Cr ≈ Ni > Cu ≈ Mn, see Table 1) could be used to predict long-term released metal fractions.
The results clearly show the highest extent of released alloy constituents and changes in outermost surface composition to take place during the first hour of the laboratory exposure (Figure 3), leaving a surface oxide predominantly composed of oxidised Fe and small amounts of Mn. Even though oxidised Fe was the only element observed in the outermost surface oxide after 20 h of exposure (5 laboratory rain events), some release of the alloying elements could be observed. Cr and Cu were released during the first rain event of the 20 h laboratory exposure, whereas Mn and Ni were continuously released at low concentrations throughout the total exposure period. The release rate of Fe was slightly reduced with time (1–20 h).
The release of metals from the patina of WS-1 by the action of rainwater at field conditions, that is, 14 collection periods reflecting 3, 6 and 12 months of exposure, showed similar results as the laboratory investigation with the predominant release of Fe followed by the release of small amounts of Mn, Ni and Cu as also found during marine field exposures.[9] No Cr was detected in the runoff water. Due to rapid complexation of released Fe with organic matter and other ligands, already at the Plexiglas fixtures, especially in the gutter transporting the runoff water to the runoff water collecting vessel, the total amount of released Fe per collection period could not be determined. The total amount of released Fe was instead recalculated (possibly underestimated) based on the extent of immobilised Fe in the Plexiglas gutter and downpipe and the pooled amount of Fe in the collected runoff water. The results are therefore presented as striped unfinished bars in Figure 3.
Oxidised Fe was the predominant constituent of the outermost surface of the patina after both 3 months of field exposure conditions and 20 h of simulated rain exposure at laboratory conditions (5 rain events). Field and laboratory conditions revealed the released amount of Mn to be of similar magnitude, whereas the release of Ni was lower after 20 h of laboratory exposure compared with field conditions. The results elucidate that the 20 h laboratory exposure, despite differences in environmental conditions, reasonably well can simulate long-term field runoff scenarios of the alloying constituents of WS, as previously shown for, for example, Zn.[16]
Surface oxide composition and metal release results from the parallel investigation of the WS (WS-2) with higher Mn but lower Cr content compared with WS-1 (c.f. Table 1) are presented in Figure 4. Despite a higher bulk content of Mn compared with WS-1, no Mn could be observed in the outermost surface oxide of the unexposed surface, which predominantly was composed of oxidised Fe and Si (Si > Fe). Concordantly, Fe was the main element released (Si and P not measured). Low levels of released Mn, Ni, Cu and Cr were observed despite their absence in the outermost surface layer (Fe ≫ Mn > Ni > Cu > Cr). After 20 h of laboratory exposure was the relative Si content of the outermost surface considerably reduced, and some Mn (1.6 wt%) could be observed. This corresponded in time with a measurable increase in the released amount of Mn (from 0.0020 to 0.0035 g m−2 100 mm−1). Even though the composition of the outermost surface of the patina after 3 months of field exposure was similar as observed after 20 h of laboratory exposure, the release of Mn was almost four times higher. No Mn could be observed in the top patina layer either after 6 months or 1 year of exposure. Nevertheless, the extent of released Mn remained at relatively similar levels (0.0053–0.0158 g m−2 100 mm−1).
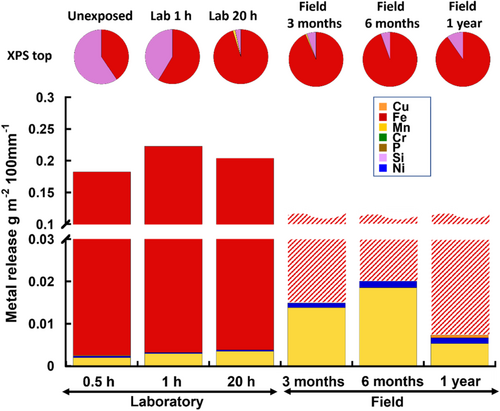
Small amounts of Ni were continuously released during both the laboratory and field exposures at levels <0.002 g m−2 100 mm−1 despite its absence on the outermost surface.
In all, minimal amounts of the alloying elements in WS are released by the action of rainwater during atmospheric exposure and in proportions only initially linked to the composition of the outermost surface oxide.
3.2 Can the composition, morphology and evolution of the patina on WS at outdoor conditions be mimicked at simulated rain events at laboratory conditions and where are the alloy constituents located?
Changes in the visual appearance of unexposed WS-1 and the laboratory and field-exposed surfaces with time are presented in Figure 5a–f. Yellow–brown features were initially observed giving the surface an orange hue (Lab 1 h), observed with SEM as patches of corrosion products inhomogeneously distributed over the surface (Figure 5g–l). These patches evolved with time (up to 20 h) into a fully covering layer. The visual appearance of the field-exposed surfaces was more reddish brown compared with the 20 h laboratory samples. Variations in visual appearance are probably linked to disparities in the thickness of the patina and the conditions of exposure. The laboratory-exposed samples were stored in a desiccator with low relative humidity (<10%) during varying intervals (see Figure 1) between rain events, while the field samples were continuously exposed outdoors under unsheltered conditions. The SEM images of the top surfaces of the laboratory-exposed samples (up to 20 h), Figure 5g–l, revealed rapid formation of corrosion products exhibiting the typical morphological characteristics of Lepidocrocite (γ-FeOOH), that is, 'sandy, globular and laminar' features, already after 1 h of exposure.[17-19]
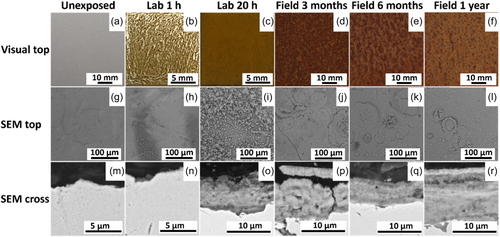
The cross-sectional SEM images of the patina after 20 h of laboratory exposure showed similar layered structures and thicknesses of corrosion products within the patina as observed for the field-exposed samples (Figure 5m–r). The individual layers are believed to be a result of repeated dry/wet periods gradually building up the corrosion products of different morphology (depending on prevailing environmental characteristics) layer by layer with time and to be mainly composed of Lepidocrocite. As extensively described in the literature, the patina is with prolonged time (≫1 year) anticipated to include the even more stable Fe-oxyhydroxide, goethite (α-FeOOH), which provides the WS with improved corrosion resistance compared with, for example, carbon steel.[1, 4-6, 9] Its formation is promoted by repeated dry and wet cycles and the alloying elements that become incorporated into the goethite structure forming a dense and uniform layer at the substrate interface.[1, 2, 4]
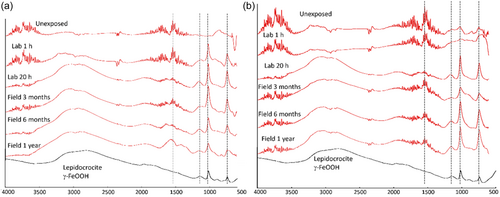
Compared with the laboratory-exposed samples, the 3 months field sample of WS-1 displayed the same characteristic features (larger and more pronounced) of Lepidocrocite, covering the surface.[17-19] This morphology did not significantly change with time up to 1 year of exposure. The formation of a patina predominantly composed of Lepidocrocite on WS-1 at both laboratory and field conditions was confirmed by means of FTIR, revealing bands at 740, 1018 and 1150 cm−1; the bands at 1870–1590 and 1585–1410 cm−1 are assigned to carbonate groups (Figure 6).[19, 20] Based on the observation of weak and broad diffraction peaks (XRD) (Figure S1) and literature findings, Lepidocrocite was mainly present in its amorphous state.[1, 17-21]
Compared with WS-1, the formation of corrosion products on WS-2 evolved more slowly (Figure 7a–f) during the laboratory exposure. A yellow hue was visible after 1 h (Figure 7b) being similar to but less pronounced compared with the patina formed on WS-1 (Figure 5) most probably linked to the formation of non-uniformly located patches of corrosion products over the surface. Compared with WS-1, these patches were after 1 h smaller and thinner (Figures 5b and 7b). After 20 h, the patina was fully covering the surface and revealed an orange-greenish appearance (Figure 7c). Similar findings were observed for the field-exposed surfaces, though with a more homogeneous appearance compared with WS-1. The unexposed WS-2 (Figure 7g) revealed a large number of surface features, marked with red arrows, all over the surface. Based on elemental analysis by means of EDS, these features were shown to be Si rich. These observations agreed with the compositional analysis of the outermost surface by means of XPS (Figure 4), showing a relative Si/Fe mass ratio of 0.6. Since the bulk content of Si in the alloy was less than 0.65 wt% (Table 1), its presence at the surface is believed to be of external origin and most probably to be a consequence of the shot blasting procedure, using glass grits to remove the mill scale upon material manufacture. Since any surface treatment influences both the corrosion and metal release characteristics, the role of Si should in future studies be investigated. It is though plausible that its presence at least initially might explain the slower evolution of the corrosion products and initial differences in surface appearance on the WS-2 surface compared with WS-1 without these Si-rich surface features.
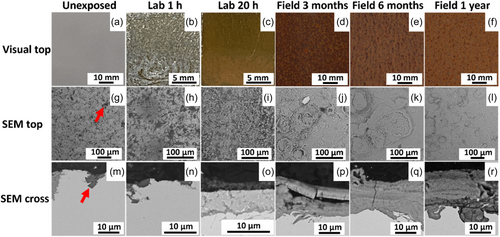
No effect of the Si surface features on the visual appearance was observed on the field-exposed surfaces, being very similar as WS-1 after 1 year. The Si-rich features were no longer observed at the outermost patina surface but were shown to be present both at the bulk/patina interface and incorporated within the patina (Figure S2). The corrosion products formed on WS-2 at both laboratory and field conditions displayed an almost identical morphology, typical for Lepidocrocite, as observed for WS-1 (Figure 5), though forming more slowly compared with WS-1.[17-19]
As discussed above, Mn was the only alloying element observed in the outermost surface of both WS-1 (only unexposed and after 1 h of laboratory exposure) and WS-2 (after 20 h and 3 months of laboratory and field exposure conditions) (Figures 3 and 4). These measurements were complemented with elemental analysis of cross-sections of the patina of both WS-1 and WS-2 after 1 year of field exposure with the aim to identify the location of the alloying elements within the patina (Figure 8).
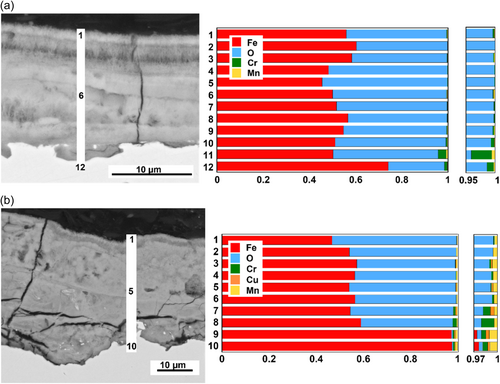
Cr was predominantly observed in the inner layer of the corrosion products next to the substrate for both WS-1 and WS-2, with small amounts intermittently present in most layers of the patina (Figure 8). Cu was observed in low amounts within some layers, preferentially the inner layer, of the patina of WS-2 (Figure 8b). Despite a slightly higher Cu bulk content of WS-1 compared with WS-2 (Table 1), no Cu was observed in the investigated sections of the patina of WS-1 (Figure 8a). However, this could merely be a consequence of its local presence within the patina and/or in concentrations below the limit of detection (≈ 0.1 wt%). The inhomogeneous distribution of both Cu and, to some extent, Cr within the patina may explain their intermittent release from the patina of both WS-1 and WS-2, Figures 9b and 9a, respectively. Low intermittent distribution of Mn was also observed within the patina of WS-1, with significantly higher levels in all layers of WS-2. These findings agree with a higher extent of Mn release, see Figure 3, and are in qualitative agreement with the higher Mn bulk content of WS-2 compared with WS-1. Despite measurable low amounts of released Ni, no Ni was observed (most probably due to too low concentrations) within the patina by means of EDS on either WS-1 or WS-2.
In all, the patina forming on WS at both laboratory and field exposure conditions showed µm-thick layered structures, being predominantly composed of amorphous Lepidocrocite (γ-FeOOH) of different morphology. The alloying elements of Cr and Cu were predominantly present in the patina layer next to the substrate for both WS-1 and WS-2 but also present in low amounts intermittently distributed within the entire patina thickness. Mn followed the same pattern but in higher amounts for WS-2.
3.3 Does the release of the alloying constituents exhibit any time-dependent patterns?
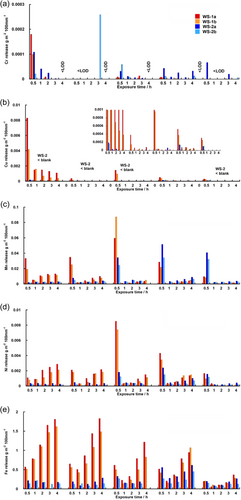
The release kinetics of the alloy constituents from WS-1 and WS-2 was investigated during the five consecutive rain events (pH 4.8, 5 mm h−1) simulated at laboratory conditions. Time-resolved released amounts of Cr, Cu, Mn, Ni and Fe during each rain episode, normalised to the rainfall quantity, are presented for duplicate samples in Figure 9. The total released amounts of each element during the five rain events are presented in Figure S3. It should be noted that these results reflect surfaces inclined 45° from the horizontal, a rain pH of 4.8 and a rain intensity of 5 mm h−1 (average annual rain intensity in Stockholm, Sweden). Except for the effects of surface inclination, the absolute levels of released metals are strongly connected to the rain composition and intensity and hence contact time between the rain. These effects are thoroughly investigated elsewhere.[13-15]
Released amounts of Cr during each rain event (Figure 9 a), were below the system limit of quantification (<0.076 g m−2 100 mm−1) with several results below the limit of detection (Table 2). Most Cr was released from WS-1 during the first 30 min of the first rain event, followed by decidedly intermittent levels during the forthcoming rain events. Similar results, with variations, were observed for WS-2. The low and intermittent release suggests an inhomogeneous presence of Cr within the patina as described above for the field-exposed samples with Cr preferentially located in the inner patina layers adjacent to the substrate (c.f. Figure 8).
Cu was released with clear first-flush behaviour from WS-1 for all rain events, in particular, pronounced during the first rain event (Figure 8b), with decreasing levels for the subsequent rain events.[22, 23] These findings are consistent with the compositional findings by means of XPS showing considerable amounts of oxidised Cu in the outermost surface for the unexposed surface of WS-1 (Figure 3). The extent of released Cu from WS-2 was considerably lower (no Cu observed in the outermost surface, Figure 4) and followed similar trends.
In relation to the release of the other alloying elements, the amounts of released Mn were one order of magnitude higher, showing an evident first flush behaviour during all rain events for both WS-1 and WS-2 (Figure 8c). This behaviour was more evident during the 3rd rain event for WS-2 and most probably connected to the initial barrier effect of Si-rich features hindering the rate of corrosion (and metal release) as described above. The relatively high extent of released Mn was qualitatively linked to its relatively large presence (25 wt%) of oxidised Mn on the unexposed surface and after 1 h for WS-1 (Figure 3) and its minor presence (due to rapid patina formation of Lepidocrocite) observed after 20 h for WS-2 (Figure 4).
The release of Ni (Figure 8d) followed the same general trends as observed for Mn, though at one order of magnitude lower. Its presence in the outermost surface or within the patina could not be confirmed either by XPS or EDS. This suggests Ni to be present in very small amounts inhomogeneously distributed in the patina.
Fe was continuously released from both materials during all five rain events, without showing any first flush effect, though with slightly decreasing total amounts released with increasing number of rain events (Figure 8e). The kinetic results reflect the patina composition consisting of amorphous Lepidocrocite, with an initial barrier effect of the Si features (from the shot blasting process) on WS-2. After four rain events, the extent of released Fe was similar between WS-1 and WS-2.
The long-term kinetic release (based on the 14 sampling periods, each comprising different numbers or rain events) of Cr, Cu, Mn and Ni during the 1-year field exposure is presented in Figure 10. The results show a similar overall trend as observed in the last rain event of the laboratory conditions with intermittently released amounts of both Cr and Cu at levels close to or below background levels from both WS-1 and WS-2 (Figure 10a,b). Mn and Ni were continuously released from both materials during the field exposure, with more Mn released from WS-2 compared with WS-1 and similar amounts of released Ni (one magnitude lower compared with Mn) from WS-1 and WS-2. These findings elucidate that the laboratory setup simulating rain events at laboratory conditions can be used to gain a mechanistic understanding of metal release patterns and that long-term effects at field conditions are simulated following at least five rain events at laboratory conditions.
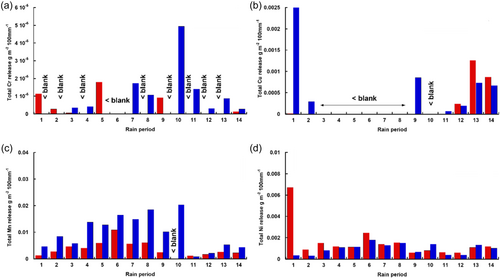
In all, Fe, Mn and Ni followed similar release patterns, albeit at different levels, during all five laboratory rain events and at field conditions. This suggests that the release of these elements is connected and also present in the outer layers of the patina. The intermittent release of Cr and Cu indicates their presence at deeper layers of the patina, possibly at the interface between the substrate and patina and hence not as readily released.
3.4 Are the levels of dispersed alloying elements from WS of environmental concern?
The combined laboratory and field exposure results presented above elucidate that low levels of the alloying elements (Mn ≫ Ni > Cu > Cr) were released from commercial WS at atmospheric unsheltered conditions. These levels correspond to annual released amounts of 0.016–0.036 g Mn m−2, 0.00041–0.0019 g Cu m−2, 0.000015–0.000028 g Cr m−2 and 0.0033–0.0075 g Ni m−2 (representing surfaces inclined 45° from the horizontal, facing south and an annual rainfall quantity of 590 mm year−1).[24]
When translating these rates to a vertical surface like a façade, where runoff is significantly influenced by factors such as prevailing wind direction, degree of sheltering, orientation and the actual contact area with rainwater,[13-15] a conservative calculation would yield: 0.0096–0.022 g Mn m−2, 0.00025–0.0011 g Cu m−2, 0.0000090–0.000017 g Cr m−2 and 0.0020–0.0045 g Ni m−2.
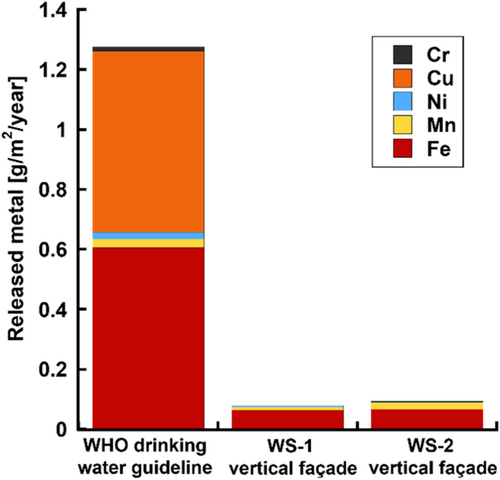
To contextualise the amounts of metals released from WS in terms of environmental and human health, a comparison was made with the World Health Organisation's (WHO) recommended maximum levels for Mn, Ni, Cu and Cr in drinking water (Figure 11).[25] The recommendations, provided in µg L−1, were recalculated to the consistent unit used in this study, g m−2 100 mm−1. Upon comparison, it is evident that the amounts of Ni, Cu and Cr released from both WS-1 and WS-2 were considerably below the drinking water limits. The released levels of Mn were slightly lower from WS-2 and well below the drinking water threshold value from WS-1.
To provide additional context for the released metal amounts from the WS, a comparison was conducted with the corresponding released amounts of Cu, Cr and Ni from pure Fe surfaces (abraded and aged) exposed to two rain events under laboratory conditions (Figure 12).
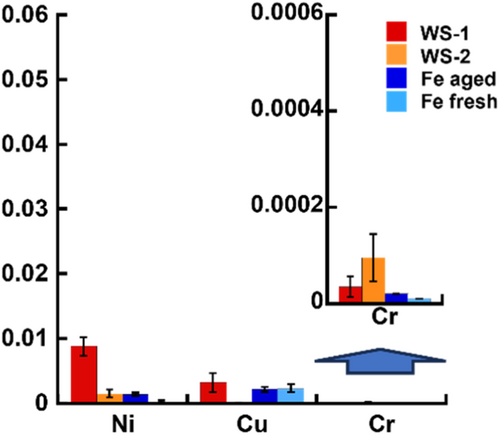
The results clearly show that the extent of released Ni, Cu and Cr from the WS's is of the same order of magnitude as the release from 99.62 wt% pure Fe (low carbon steel). The presence of Cr, Cu and Ni in the carbon steel is not intentionally added but rather small residual traces as impurities from the iron ore used in the manufacturing. These results imply that the intentional alloying of WS with low amounts of Ni, Cu and Cr does not largely influence the extent of released metals.
Additionally, prior studies have demonstrated that released Cu, Zn, Cr and Ni from outdoor constructions can be effectively immobilised by solid surfaces like pavement, concrete, limestone and soil in the immediate vicinity of a building. These surfaces act as sinks, effectively capturing and containing the released metals.[26-29]
4 CONCLUDING REMARKS
Two commercial WS, WS-1 and WS-2, were investigated under laboratory and urban field conditions, and the total metal released in the runoff water was measured and the corrosion products formed on the surface were characterised.
The action of impinging rainwater resulted in the release of the alloying elements, Cr, Cu, Mn and Ni, in the same order of magnitude as the release from 99.62 wt% pure Fe and lower than the WHO corresponding recommended drinking water levels.
AUTHOR CONTRIBUTIONS
Gunilla Herting was involved in conceptualisation, investigation, writing—original draft, revision and editing and project management. Esa Virolainen was involved in conceptualisation and writing—revision and editing. Inger Odnevall was involved in conceptualisation, investigation, writing—original draft, revision and editing.
ACKNOWLEDGEMENTS
The financial support of SSAB Europe is highly appreciated.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




