Combinatorial Screening for Specific Drug Solubilizers with Switchable Release Profiles
Sebastian Wieczorek
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, D-12489 Berlin, Germany
Search for more papers by this authorSara Vigne
École Polytechnique Fédérale de Lausanne (EPFL), Institute of Materials (IMX), EPFL – STI – IMX – LMOM, MXG 037, Station 12, CH-1015 Lausanne, Switzerland
Search for more papers by this authorTiziana Masini
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
Search for more papers by this authorDaniela Ponader
Max Planck Institute of Colloids and Interfaces, MPI KGF Golm, D-14424 Potsdam, Germany
Search for more papers by this authorLaura Hartmann
Max Planck Institute of Colloids and Interfaces, MPI KGF Golm, D-14424 Potsdam, Germany
Search for more papers by this authorAnna K. H. Hirsch
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Hans G. Börner
- [email protected]
- | Fax: +49 (0)30 2093-7500
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, D-12489 Berlin, Germany
Correspondence to: Prof. H. G. Börner
Search for more papers by this authorSebastian Wieczorek
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, D-12489 Berlin, Germany
Search for more papers by this authorSara Vigne
École Polytechnique Fédérale de Lausanne (EPFL), Institute of Materials (IMX), EPFL – STI – IMX – LMOM, MXG 037, Station 12, CH-1015 Lausanne, Switzerland
Search for more papers by this authorTiziana Masini
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
Search for more papers by this authorDaniela Ponader
Max Planck Institute of Colloids and Interfaces, MPI KGF Golm, D-14424 Potsdam, Germany
Search for more papers by this authorLaura Hartmann
Max Planck Institute of Colloids and Interfaces, MPI KGF Golm, D-14424 Potsdam, Germany
Search for more papers by this authorAnna K. H. Hirsch
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
Search for more papers by this authorCorresponding Author
Hans G. Börner
- [email protected]
- | Fax: +49 (0)30 2093-7500
Laboratory for Organic Synthesis of Functional Systems, Department of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Str. 2, D-12489 Berlin, Germany
Correspondence to: Prof. H. G. Börner
Search for more papers by this authorAbstract
Polymer-block-peptide conjugates are tailored to render hydrophobic small molecule drugs water soluble. The combinatorial strategy selects for bioconjugates that exhibit sequence-specific solubilization and switchable release profiles of the cargo through incorporation of a disulfide linker moiety into the peptide-library design. While the study focused on the photosensitizer m-THPC and reductive carrier cleavage, the approach is generic and might be expanded toward a broad range of poorly soluble small-molecule drugs and other selective cleavage mechanisms to disassemble a peptide binding domain of the bioconjugate-based solubilizer.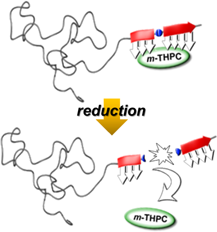
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mabi201400443-sup-0001-SuppData.pdf641 KB | Supporting Data |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 H. D. Williams, N. L. Trevaskis, S. A. Charman, R. M. Shanker, W. N. Charman, C. W. Pouton, C. J. H. Porter, Pharmacol. Rev. 2013, 65, 315.
- 2 T. Kennedy, Drug Discovery Today 1997, 2, 436.
- 3 K. H. Bleicher, H. J. Bohm, K. Muller, A. I. Alanine, Nat. Rev. Drug Discov. 2003, 2, 369.
- 4 W. L. Jorgensen, Science. 2004, 303, 1813.
- 5 J.-H. Zhang, T. D. Y. Chung, K. R. Oldenburg, J. Biomol. Screen. 1999, 4, 67.
- 6 S. Thaisrivongs, M. N. Janakiraman, K.-T. Chong, P. K. Tomich, L. A. Dolak, S. R. Turner, J. W. Strohbach, J. C. Lynn, M.-M. Horng, R. R. Hinshaw, K. D. Watenpaugh, J. Med. Chem. 1996, 39, 2400.
- 7 C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Delivery Rev. 2012, 64, 4.
- 8
S. Dunne,
B. Shannon,
C. Dunne,
W. Cullen,
BMC Pharmacol. Toxicol.
2013,
14, 2050.
10.1186/2050-6511-14-1 Google Scholar
- 9 S. M. Paul, D. S. Mytelka, C. T. Dunwiddie, C. C. Persinger, B. H. Munos, S. R. Lindborg, A. L. Schacht, Nat. Rev. Drug. Discov. 2010, 9, 203.
- 10 H. Ringsdorf, J. Polym. Sci: Polym. Symp. 1975, 51, 135.
- 11 D. V. Santi, E. L. Schneider, R. Reid, L. Robinson, G. W. Ashley, Proc. Natl. Acad. Sci. USA 2012, 109, 6211.
- 12 R. Duncan, Nat. Rev. Drug Discov. 2003, 2, 347.
- 13 S. Gopinathan, A. Nouraldeen, A. G. E. Wilson, Future Med. Chem. 2010, 2, 1391.
- 14 G. Riess, Prog. Polym. Sci. 2003, 28, 1107.
- 15 A. Kuksal, A. Tiwary, N. Jain, S. Jain, AAPS PharmSciTech 2006, 7, E1.
- 16 M. Buonaguidi, V. Carelli, G. Di Colo, E. Nannipieri, M. F. Serafini, Int. J. Pharm. 1997, 147, 1.
- 17 R. J. Amir, S. Zhong, D. J. Pochan, C. J. Hawker, J. Am. Chem. Soc. 2009, 131, 13949.
- 18 M. S. Yavuz, Y. Cheng, J. Chen, C. M. Cobley, Q. Zhang, M. Rycenga, J. Xie, C. Kim, K. H. Song, A. G. Schwartz, L. V. Wang, Y. Xia, Nat. Mater. 2009, 8, 935.
- 19 S. Kim, K. E. Healy, Biomacromolecules 2003, 4, 1214.
- 20 S. K. Choi, M. Verma, J. Silpe, R. E. Moody, K. Tang, J. J. Hanson, J. R. J. Baker, Bioorg. Med. Chem. 2012, 20, 1281.
- 21 G. Leriche, L. Chisholm, A. Wagner, Bio. Med. Chem. 2012, 20, 571.
- 22 Y. Kakizawa, A. Harada, K. Kataoka, J. Am. Chem. Soc. 1999, 121, 11247.
- 23 H. G. Börner, Prog. Polym. Sci. 2009, 34, 811.
- 24 D. Lowik, E. H. P. Leunissen, M. van den Heuvel, M. B. Hansen, J. C. M. van Hest, Chem. Soc. Rev. 2010, 39, 3394.
- 25 P. Wilke, N. Helfricht, A. Mark, G. Papastavrou, D. Faivre, G. H. Börner, J. Am. Chem. Soc. 2014, 136, 12667.
- 26 T. Schwemmer, J. Baumgartner, D. Faivre, H. G. Börner, J. Am. Chem. Soc. 2011, 134, 2385.
- 27 C. M. Kolodziej, S. H. Kim, R. M. Broyer, S. S. Saxer, C. G. Decker, H. D. Maynard, J. Am. Chem. Soc. 2012, 134, 247.
- 28 C. Stutz, I. Bilecka, A. F. Thunemann, M. Niederberger, H. G. Börner, Chem. Commun. 2012, 48, 7176.
- 29 P. Wilke, H. G. Börner, ACS Macro Lett. 2012, 1, 871.
- 30 B. Apostolovic, S. P. E. Deacon, R. Duncan, H. A. Klok, Biomacromolecules 2010, 11, 1187.
- 31 L. A. Canalle, D. Lowik, J. C. M. van Hest, Chem. Soc. Rev. 2010, 39, 329.
- 32 R. Gentsch, F. Pippig, K. Nilles, P. Theato, R. Kikkeri, M. Maglinao, B. Lepenies, P. H. Seeberger, H. G. Börner, Macromolecules 2010, 43, 9239.
- 33 H. G. Börner, Macromol. Rapid Commun. 2011, 32, 115.
- 34 A. K. H. Hirsch, F. Diederich, M. Antonietti, H. G. Borner, Soft Matter 2010, 6, 88.
- 35 S. Wieczorek, E. Krause, S. Hackbarth, B. Röder, A. K. H. Hirsch, H. G. Börner, J. Am. Chem. Soc. 2013, 135, 1711.
- 36 M. O. Senge, J. C. Brandt, Photochem. Photobiol. 2011, 87, 1240.
- 37 C. Hopper, A. Kübler, H. Lewis, I. B. Tan, G. Putnam, G. the Foscan 01 Study, Intern. J. Cancer 2004, 111, 138.
- 38 M. O. Senge, Photodiagnosis and Photodynamic Therapy 2012, 9, 170.
- 39 L. Hartmann, S. Häfele, R. Peschka-Süss, M. Antonietti, H. G. Börner, Macromolecules 2007, 40, 7771.
- 40 D. Ponader, F. Wojcik, F. Beceren-Braun, J. Dernedde, L. Hartmann, Biomacromolecules 2012, 13, 1845.
- 41 K. S. Lam, S. E. Salmon, E. M. Hersh, V. J. Hruby, W. M. Kazmierski, R. J. Knapp, Nature 1991, 354, 82.
- 42 E. Gross, “[27] The cyanogen bromide reaction”, in Methods in Enzymology (Eds: C.H.W. Hirs), Academic Press, 1967, p. 238.
- 43 J. Seidler, N. Zinn, M. E. Boehm, W. D. Lehmann, Proteomics 2010, 10, 634.
- 44 P. Gerber, K. Müller, J. Comput. -Aided Mol. Des. 1995, 9, 251.
- 45 F. Allen, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2002, 58, 380.
- 46V.S. The PyMOL Molecular Graphics System, LLC.
- 47 S. Sasnouski, V. Zorin, I. Khludeyev, M.-A. D'Hallewin, F. Guillemin, L. Bezdetnaya, Biochim. Biophys. Acta 2005, 1725, 394.
- 48 Z. Zmatliková, P. Sedláková, K. Lacinová, A. Eckhardt, S. Pataridis, I. Mikšík, J. Chromatogr. A 2010, 1217, 8009.




