Thermoresponsive Self-Assembly of Nanostructures from a Collagen-Like Peptide-Containing Diblock Copolymer
Tianzhi Luo
Department of Materials Science and Engineering, University of Delaware, Newark, DE, 19716 USA
Search for more papers by this authorLirong He
Institute for Technical and Macromolecular Chemistry, University of Hamburg, Bundesstrasse 45, D-20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Patrick Theato
Institute for Technical and Macromolecular Chemistry, University of Hamburg, Bundesstrasse 45, D-20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Kristi L. Kiick
Department of Materials Science and Engineering, University of Delaware, Newark, DE, 19716 USA
Biomedical Engineering, University of Delaware, Newark, DE, 19716 USA
Delaware Biotechnology Institute, Newark, DE, 19711 USA
Search for more papers by this authorTianzhi Luo
Department of Materials Science and Engineering, University of Delaware, Newark, DE, 19716 USA
Search for more papers by this authorLirong He
Institute for Technical and Macromolecular Chemistry, University of Hamburg, Bundesstrasse 45, D-20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Patrick Theato
Institute for Technical and Macromolecular Chemistry, University of Hamburg, Bundesstrasse 45, D-20146 Hamburg, Germany
Search for more papers by this authorCorresponding Author
Kristi L. Kiick
Department of Materials Science and Engineering, University of Delaware, Newark, DE, 19716 USA
Biomedical Engineering, University of Delaware, Newark, DE, 19716 USA
Delaware Biotechnology Institute, Newark, DE, 19711 USA
Search for more papers by this authorAbstract
Temperature-triggered formation of nanostructures with distinct biological activity offers opportunities in selective modification of matrices and in drug delivery. Toward these ends, diblock polymers comprising poly(diethylene glycol methyl ether methacrylate) (PDEGMEMA) conjugated to a triple helix-forming collagen-like peptide were produced. Triggered by the collapse of the thermoresponsive domain above its LCST, the conjugate undergoes a reversible transition in aqueous solution to form well-defined nanovesicles with diameters of approximately 100 nm, with a transition temperature of 37 °C. The incorporation of CLP domains in these nanostructures may offer opportunities for the selective targeting of collagen-containing matrices.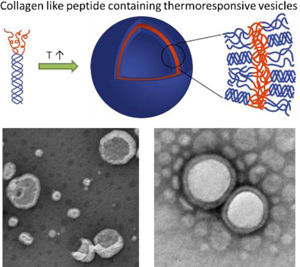
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| mabi201400358-sup-0001-SuppFigs-S1.docx329.6 KB | Figure S1. RP-HPLC trace of collagen like peptide (GPO)7GG, blue line indicates the gradient used. Figure S2. ESI-MS of purified CLP. Figure S3. GPC traces of PEG-CLP diblock and starting materials. a) Normalized refractive index chromatogram of PEG-CLP diblock copolymer and CLP, PEG starting material. b) 214 nm UV absorbance of PEG-CLP diblock and CLP, PEG starting material. Figure S4. 1H NMR spectra (600 MHz) of: a) collagen like peptide; b) PEG5k; c) PEG-CLP diblock in d6-DMSO. Figure S5. DLS data at 50 °C. a) Size distribution by intensity for 1 mg/mL diblock sample; b) Size distribution by intensity for 5 mg/mL diblock sample; c) raw correlation function for 1 mg/mL diblock sample; d) raw correlation function for 5 mg/mL diblock sample; e) Fitting curve using cumulant method for 1 mg/mL sample, empty circles indicate experimental data; f) Fitting curve using cumulant method for 5 mg/mL sample, empty circles indicate experimental data. Figure S6. Size distribution by number at different temperatures. a) PEG-CLP diblock, 1 mg/mL in water; b) PDEGMEMA, 1 mg/mL in water. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 K. E. Kadler, C. Baldock, J. Bella, R. P. Boot-Handford, J. Cell Sci. 2007, 120, 1955.
- 2 G. N. Ramachandran, G. Kartha, Nature 1955, 176, 593.
- 3 J. A. M. Ramshaw, N. K. Shah, B. Brodsky, J. Struct. Biol. 1998, 122, 86.
- 4 K. Watanabe, Y. Nishio, R. Makiura, A. Nakahira, C. Kojima, Int. J. Pharm. 2013, 446, 81.
- 5 C. Kojima, T. Suehiro, K. Watanabe, M. Ogawa, A. Fukuhara, E. Nishisaka, A. Harada, K. Kono, T. Inui, Y. Magata, Acta Biomater. 2013, 9, 5673.
- 6 Z. Ruszczak, W. Friess, Adv. Drug Deliv. Rev. 2003, 55, 1679.
- 7 C. H. Lee, A. Singla, Y. Lee, Int. J. Pharm. 2001, 221, 1.
- 8 T. Z. Luo, K. L. Kiick, Eur. Polym. J. 2013, 49, 2998.
- 9 S. M. Yu, Y. Li, D. Kim, Soft Matter 2011, 7, 7927.
- 10 M. D. Shoulders, R. T. Raines, in Annu. Rev. Biochem., Vol. 78, Annual Reviews. Palo Alto 2009, p. 929.
- 11 J. A. Fallas, J. H. Dong, Y. Z. J. Tao, J. D. Hartgerink, J. Biol. Chem. 2012, 287, 8039.
- 12 A. V. Persikov, J. A. M. Ramshaw, B. Brodsky, J. Biol. Chem. 2005, 280, 19343.
- 13 S. P. Boudko, J. Engel, J. Mol. Biol. 2004, 335, 1289.
- 14 D. Barth, O. Kyrieleis, S. Frank, C. Renner, L. Moroder, Chem. -Eur. J. 2003, 9, 3703.
- 15 W. Yang, V. C. Chan, A. Kirkpatrick, J. A. M. Ramshaw, B. Brodsky, J. Biol. Chem. 1997, 272, 28837.
- 16 L. R. He, P. Theato, Eur. Polym. J. 2013, 49, 2986.
- 17 L. E. R. O'Leary, J. A. Fallas, E. L. Bakota, M. K. Kang, J. D. Hartgerink, Nat. Chem. 2011, 3, 821.
- 18 D. E. Przybyla, J. Chmielewski, J. Am. Chem. Soc. 2008, 130, 12610.
- 19 O. D. Krishna, K. L. Kiick, Biomacromolecules 2009, 10, 2626.
- 20 M. A. Cejas, W. A. Kinnney, C. Chen, J. G. Vinter, H. R. Almond, K. M. Balss, C. A. Maryanoff, U. Schmidt, M. Breslav, A. Mahan, E. Lacy, B. E. Maryanoff, Proc. Natl. Acad. Sci. USA 2008, 105, 8513.
- 21 M. A. Cejas, W. A. Kinney, C. Chen, G. C. Leo, B. A. Tounge, J. G. Vinter, P. P. Joshi, B. E. Maryanoff, J. Am. Chem. Soc. 2007, 129, 2202.
- 22 S. Rele, Y. H. Song, R. P. Apkarian, Z. Qu, V. P. Conticello, E. L. Chaikof, J. Am. Chem. Soc. 2007, 129, 14780.
- 23 M. M. Pires, D. E. Przybyla, C. M. R. Perez, J. Chmielewski, J. Am. Chem. Soc. 2011, 133, 14469.
- 24 M. M. Pires, J. Lee, D. Ernenwein, J. Chmielewski, Langmuir 2012, 28, 1993.
- 25 W. Hsu, Y. L. Chen, J. C. Horng, Langmuir 2012, 28, 3194.
- 26 C. M. R. Perez, L. A. Rank, J. Chmielewski, Chem. Commun. 2014, 50, 8174.
- 27 C. M. R. Perez, A. Panitch, J. Chmielewski, Macromol. Biosci. 2011, 11, 1426.
- 28 V. Hernandez-Gordillo, J. Chmielewski, Biomaterials 2014, 35, 7363.
- 29 A. Y. Wang, X. Mo, C. S. Chen, S. M. Yu, J. Am. Chem. Soc. 2005, 127, 4130.
- 30 Y. Li, D. Ho, H. Meng, T. R. Chan, B. An, H. Yu, B. Brodsky, A. S. Jun, S. M. Yu, Bioconjugate Chem. 2013, 24, 9.
- 31 A. Y. Wang, C. A. Foss, S. Leong, X. Mo, M. G. Pomper, S. M. Yu, Biomacromolecules 2008, 9, 1755.
- 32 Y. Li, C. A. Foss, D. D. Summerfield, J. J. Doyle, C. M. Torok, H. C. Dietz, M. G. Pomper, S. M. Yu, Proc. Natl. Acad. Sci. USA 2012, 109, 14767.
- 33 O. D. Krishna, A. K. Jha, X. O. Jia, K. L. Kiick, Biomaterials 2011, 32, 6412.
- 34 Z. Zhang, Y. X. Lai, L. Yu, J. D. Ding, Biomaterials 2010, 31, 7873.
- 35 J. O. Humtsoe, J. K. Kim, Y. Xu, D. R. Keene, M. Hook, S. Lukomski, K. K. Wary, J. Biol. Chem. 2005, 280, 13848.
- 36 J. M. Harris, R. B. Chess, Nat. Rev. Drug Discov. 2003, 2, 214.
- 37 H. A. Klok, J. Polym. Sci. Pol. Chem. 2005, 43, 1.
- 38 J. X. Ding, C. S. Xiao, Z. H. Tang, X. L. Zhuang, X. S. Chen, Macromol. Biosci. 2011, 11, 192.
- 39 J. Wigenius, P. Bjork, M. Hamedi, D. Aili, Macromol. Biosci. 2010, 10, 836.
- 40 J. Y. Shu, C. Tan, W. F. DeGrado, T. Xu, Biomacromolecules 2008, 9, 2111.
- 41 E. Sahin, K. L. Kiick, Biomacromolecules 2009, 10, 2740.
- 42 J. Kopecek, J. Y. Yang, Abstr. Pap. Am. Chem. Soc. 2011, 241.
- 43 A. N. Elder, N. M. Dangelo, S. C. Kim, N. R. Washburn, Biomacromolecules 2011, 12, 2610.
- 44 O. S. Lee, Y. M. Liu, G. C. Schatz, J. Nanopart. Res. 2012, 14.
- 45 S. Aluri, M. K. Pastuszka, A. S. Moses, J. A. MacKay, Biomacromolecules 2012, 13, 2645.
- 46 S. E. Grieshaber, A. J. E. Farran, S. Lin-Gibson, K. L. Kiick, X. Q. Jia, Macromolecules 2009, 42, 2532.
- 47 S. E. Grieshaber, A. J. E. Farran, S. Bai, K. L. Kiick, X. Q. Jia, Biomacromolecules 2012, 13, 1774.
- 48 Y. Chen, P. Youn, D. Y. Furgeson, J. Control. Release 2011, 155, 175.
- 49 Y. C. Yu, P. Berndt, M. Tirrell, G. B. Fields, J. Am. Chem. Soc. 1996, 118, 12515.
- 50 T. Gore, Y. Dori, Y. Talmon, M. Tirrell, H. Bianco-Peled, Langmuir 2001, 17, 5352.
- 51 J. N. Luo, Y. W. Tong, ACS Nano 2011, 5, 7739.
- 52 O. D. Krishna, K. T. Wiss, T. Z. Luo, D. J. Pochan, P. Theato, K. L. Kiick, Soft Matter 2012, 8, 3832.
- 53 K. T. Wiss, O. D. Krishna, P. J. Roth, K. L. Kiick, P. Theato, Macromolecules 2009, 42, 3860.
- 54 P. J. Roth, K. T. Wiss, R. Zentel, P. Theato, Macromolecules 2008, 41, 8513.
- 55 A. V. Persikov, J. A. M. Ramshaw, A. Kirkpatrick, B. Brodsky, Biochemistry 2005, 44, 1414.
- 56 S. Sakakiba, N. Tanaka, M. Kakudo, K. Okuyama, T. Ashida, Y. Kishida, J. Mol. Biol. 1972, 65, 371.
- 57 A. V. Persikov, J. A. M. Ramshaw, A. Kirkpatrick, B. Brodsky, Biochemistry 2000, 39, 14960.
- 58 K. Kar, P. Amin, M. A. Bryan, A. V. Persikov, A. Mohs, Y. H. Wang, B. Brodsky, J. Biol. Chem. 2006, 281, 33283.
- 59 C. A. Miles, Biopolymers 2007, 87, 51.
- 60 J. B. Cheon, B. C. Kim, Y. H. Park, J. S. Park, J. Y. Moon, J. H. Nahm, C. S. Cho, Macromol. Chem. Phys. 2001, 202, 395.
- 61 S. H. Qin, Y. Geng, D. E. Discher, S. Yang, Adv. Mater. 2006, 18, 2905.
- 62 M. D. C. Topp, P. J. Dijkstra, H. Talsma, J. Feijen, Macromolecules 1997, 30, 8518.
- 63 W. Q. Zhang, L. Q. Shi, K. Wu, Y. G. An, Macromolecules 2005, 38, 5743.
- 64 K. Isoda, N. Kanayama, D. Miyamoto, T. Takarada, M. Maeda, React. Funct. Polym. 2011, 71, 367.
- 65 C. W. Zhao, X. L. Zhuang, C. L. He, X. S. Chen, X. B. Jing, Macromol. Rapid Commun. 2008, 29, 1810.
- 66 H. Nuhn, H. A. Klok, Biomacromolecules 2008, 9, 2755.
- 67 J. R. McDaniel, D. C. Radford, A. Chilkoti, Biomacromolecules 2013, 14, 2866.
- 68 M. G. Cacace, E. M. Landau, J. J. Ramsden, Q. Rev. Biophys. 1997, 30, 241.
- 69 Y. J. Zhang, P. S. Cremer, Curr. Opin. Chem. Biol. 2006, 10, 658.
- 70 B. J. Ravoo, M. C. A. Stuart, A. D. R. Brisson, W. D. Weringa, J. Engberts, Chem. Phys. Lipids 2001, 109, 63.
- 71 H. Wei, S. X. Cheng, X. Z. Zhang, R. X. Zhuo, Prog. Polym. Sci. 2009, 34, 893.
- 72 I. Otsuka, K. Fuchise, S. Halila, S. Fort, K. Aissou, I. Pignot-Paintrand, Y. G. Chen, A. Narumi, T. Kakuchi, R. Borsali, Langmuir 2010, 26, 2325.
- 73 Y. Zhao, H. Y. Gao, G. D. Liang, F. M. Zhu, Q. Wu, Polym. Chem. 2014, 5, 962.
- 74 H. R. Marsden, J. W. Handgraaf, F. Nudelman, N. Sommerdijk, A. Kros, J. Am. Chem. Soc. 2010, 132, 2370.
- 75 T. Koga, S. Kamiwatari, N. Higashi, Langmuir 2013, 29, 15477.
- 76 D. Bacinello, E. Garanger, D. Taton, K. C. Tam, S. Lecommandoux, Biomacromolecules 2014, 15, 1882.
- 77 M. M. Pires, D. Ernenwein, J. Chmielewski, Biomacromolecules 2011, 12, 2429.
- 78 Y. Li, S. M. Yu, Curr. Opin. Chem. Biol. 2013, 17, 968.
- 79 S. Meuer, L. Braun, T. Schilling, R. Zentel, Polymer 2009, 50, 154.
- 80 B. Brodsky, A. V. Persikov, in Fibrous Proteins: Coiled-Coils, Collagen and Elastomers, Vol. 70 ( D. A. D. Parry, J. M. Squire, Elsevier Academic Press, Inc, San Diego 2005, p. 301.
- 81 B. D. Olsen, R. A. Segalman, Mater. Sci. Eng. R-Rep. 2008, 62, 37.
- 82 R. Holmdahl, R. Bockermann, J. Backlund, H. Yamada, Ageing Res. Rev. 2002, 1, 135.




