Non-coding RNA and cholesteatoma
Funding information: Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja, Grant/Award Number: 451-03-9/2021-14/200017
Abstract
Objective
Cholesteatoma is a challenging chronic pathology of the middle ear for which pharmacologic therapies have not been developed yet. Cholesteatoma occurrence depends on the interplay between genetic and environmental factors while master regulators orchestrating disease progression are still unknown. Therefore, in this review, we will discuss the diagnostic and therapeutic potential of non-coding RNAs (ncRNA) as a new class of regulatory molecules.
Methods
We have comprehensively reviewed all articles investigating ncRNAs, specifically micro RNAs (miRNAs) and long ncRNAs (lncRNA/circRNA) in cholesteatoma tissue.
Results
Candidate miRNA approaches indicated that miR-21 and let-7a are the major miRNAs involved in cholesteatoma growth, migration, proliferation, bone destruction, and apoptosis. Regulatory potential for the same biological processes was also observed for miR-203a. The NF-kB/miR-802/PTEN regulatory network was in relation to observed miR-21 activity in cholesteatoma as well. High throughput approaches revealed additional ncRNAs implicated in cholesteatoma pathology. Competitive endogenous RNA (ceRNA) analysis highlighted lncRNA/circRNA that could be “endogenous sponge” for miR-21 and let-7a based on the hypothesis that RNA transcripts can communicate with and regulate each other by using shared miRNA response elements.
Conclusion
In this review, we summarize the discoveries and role of ncRNA in major pathways in cholesteatoma and highlight the potential of miRNA-based therapeutics in the treatment of cholesteatoma.
Level of Evidence: NA.
1 MOLECULAR BASIS OF THE CHOLESTEATOMA DEVELOPMENT
Cholesteatoma development may depend on the interplay between genetic and environmental factors,1 however, the molecular mechanisms underlying cholesteatoma pathogenesis remain undefined. Cholesteatoma investigations have progressed from evaluating individual candidate genes to genome-wide studies to elucidate molecular mechanisms of development on a genomic scale.2-5 Despite that numerous processes and pathways have been determined to harbor dysregulated genes identified in cholesteatoma,2-6 no discrete pharmacological targets have been identified yet. To identify master regulators of these pathways and provide pharmacologic targets for medical management of cholesteatoma, we need to look beyond the protein-coding genes and into the universe of noncoding RNA (ncRNA) molecules (Table 1).
| miRNAs associated with cholesteatoma | Regulated direction of expression | miRNA expression validation | Regulatory mechanism in cholesteatoma | Cellular function | Proposed ceRNA interaction in cholesteatoma | References |
|---|---|---|---|---|---|---|
| miR-21 | Upregulated in cholesteatoma versus normal skin | qRT-PCR | Downregulation of PTEN and PDCD4 | Increased proliferation of keratinocytes | lncRNA-uc001kfc.1 circRNA-102747 |
[7-10] |
| let-7a | Upregulated in cholesteatoma versus normal skin | qRT-PCR | Downregulation of HMGA2 | Decreased proliferation of keratinocytes | circRNA-101458 | [8, 10, 11] |
| miR-802 | Upregulated upon NF-kB activation | miRNA transfection qRT-PCR |
Downregulation of PTEN | Increased proliferation of keratinocytes in vitro | — | [12] |
| miR-203a | Downregulated in cholesteatoma versus normal skin | qRT-PCR | Upregulated Bmi1 and subsequent increase of p-Akt level | Increased proliferation, migration, and antiapoptotic abilities | — | [13] |
| miR-16-1-3pa | Upregulated in cholesteatoma versus normal skin | Microarray qRT-PCR |
Regulation of PI3K/Akt signaling pathway | Hyper-proliferation of cholesteatoma | — | [14] |
| miR-10a-5pa | Downregulated in cholesteatoma versus normal skin | Microarray qRT-PCR |
Regulation of PI3K/Akt signaling pathway | Hyper-proliferation of cholesteatoma | — | [14] |
- a Only qRT-PCR validated miRNAs identified in high throughput analysis have been presented.
2 THE EXPANDING UNIVERSE OF ncRNAs
Only 1%–2% of the human genome is transcribed in coding RNAs, able to encode a sequence of amino acids in proteins.15 The RNA that does not code for proteins are called ncRNA. In this narrative review, we will focus on the two ncRNA subgroups, short ncRNAs and long ncRNAs16, 17 because of their diagnostic and therapeutic potential.
2.1 Small ncRNA
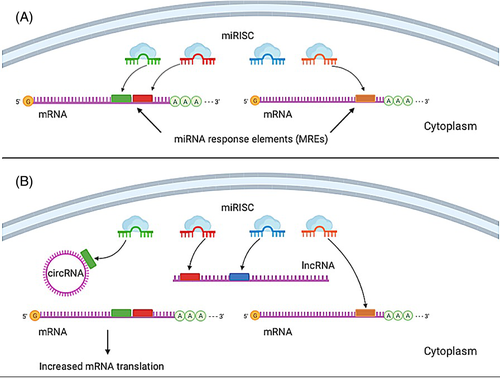
The dominant class of small ncRNAs, microRNAs (miRNAs), are around 20 nucleotides in size. miRNAs orchestrate gene expression of almost every biological process18-20 and consequently are associated with various diseases.21-23 miRNAs are negative regulators of gene expression acting through induction of mRNA degradation or inhibition of its translation (Figure 1A).24 Different miRNAs could share the same target mRNA while single miRNA could target multiple mRNAs.18 These properties of miRNA molecules meet most of the required criteria for an ideal biomarker,25 while miRNA mimics and antagomiRNA are considered as promising therapeutics.26 As miRNA dysregulation has been observed in cholesteatoma, in this review, we will highlight the subsequent effects on cellular mechanisms and possible medical applications.
2.2 Long ncRNA
lncRNAs show great capacity for gene expression regulation at both transcriptional and posttranscriptional levels through the sequence- and structure-specific mechanism.27, 28 Competitive endogenous RNA (ceRNA) hypothesis states that all types of RNA transcripts can communicate with and regulate each other by using shared miRNA response elements (MREs).29 lncRNA can act as miRNA decoy capturing active miRNAs, buffering that way regulatory activity of those miRNA on their target mRNAs, which share the same MREs.29, 30 Subclass of ncRNAs are circular RNAs (circ-RNA),31 covalently closed continuous loops, highly conserved and tissue-specific.32-34 circRNAs have been reported to harbor multiple miRNA binding sites, which seems to be a typical feature of this class of RNA molecules.35, 36 Interaction between the miRNA and lncRNA/circRNA represents a complex interaction system (Figure 1B). This mechanism of gene expression regulation, important in all aspects of physiology and disease,37-39 will be discussed in the context of cholesteatoma molecular pathology research.
3 LITERATURE SEARCH AND INCLUSION CRITERIA
Literature search was carried out on Pubmed.gov database using different combinations of keywords: (cholesteatoma) AND ((miRNA) OR (miR)); (cholesteatoma) AND ((long noncoding RNA) OR (lncRNA) OR (ceRNA)); (cholesteatoma) AND (miRNA) AND (microarray) ending with August 2020. Studies in which ncRNA profiling was performed in cholesteatoma tissue from adult and pediatric patients were included in the review. Studies investigating ncRNA expression in samples other than cholesteatoma tissue from patients with cholesteatoma were excluded. Based on the defined search and inclusion criteria, eight studies represented the basis of this review. Although one additional study has passed the search criteria it was not included in this review due to the incompatibility with inclusion criteria.
4 ARE miR-21 AND LET-7 BALANCING CHOLESTEATOMA BETWEEN BENIGN AND INVASIVE NATURE?
The first two miRNAs assigned to be important in cholesteatoma were miR-21 and let-7 as well as their interplay.7, 8, 11 Both miRNAs have been found upregulated in cholesteatoma compared to normal skin7, 8 with a more pronounced difference in pediatric samples.8 At the time the studies have been performed, miR-21 was known to be an onco-miR due to its role in tumorigenesis through regulation of potent tumor suppressors such as PTEN and PDCD4.40, 41 The downregulation of PTEN in cholesteatoma compared with levels in normal skin inversely correlates with p-Akt levels.42 PI3K-Akt pathway is important for the induction of cell proliferation and terminal differentiation,43 and thus was proposed as a possible mechanism of development and progression of cholesteatoma through downregulation of PTEN.42 In addition to PTEN, PDCD4 was also shown to suppress benign and malignant skin tumor formation and progression.44 This critical regulator of apoptosis, which inhibits the procaspase-3 mRNA translation, is shown to be dependent on miRNA regulation under apoptotic stimuli.45 It was indeed confirmed by western blot that downregulation of PTEN and PDCD4 correlates with upregulation of miR-21, making a significantly greater reduction in PTEN and PDCD4 protein levels in pediatric versus adult cholesteatoma.8
Additionally, a similar profile of expression changes both between cholesteatoma and normal skin and between adult and pediatric cholesteatoma was observed for let-7a.8 By investigating protein levels of its target HMGA2, it was shown that its levels are reduced in cholesteatomas, especially in pediatric cholesteatomas.8 This small, nonhistone chromatin-associated protein has no intrinsic regulatory activity on gene expression. However, its capability to alter chromatin architecture could influence gene transcription through the influence on the assembly of multiprotein complexes of transcriptional factors.46 It was reported that in vitro disruption of HMGA2 suppression by let-7 miRNA enhances oncogenic transformation.47, 48 Eventual inhibition of HMGA2 by let-7a upregulation in cholesteatoma may lead to increased keratinocyte apoptosis and a reduction in the proliferation of cholesteatoma cells.
Based on findings of miR-21 and let-7a expression in cholesteatoma and joint regulation of their targets, a balancing mechanism has been proposed, perpetuating the growth and invasiveness of cholesteatoma by PTEN and PDCD4 downregulation but keeping it in a benign stage through HMGA2 inhibition.8 To explain the interplay of the two miRNAs in cholesteatoma it was shown that let-7a transfected mimics inhibited the growth, migration, and invasion of cholesteatoma keratinocytes in vitro.11 It was suggested that the observed effect in cholesteatoma keratinocytes could be explained by the mechanism through which let-7a downregulates miR-21, causing subsequent regulation of its targets.11 However, knowing that both miR-21 and let-7 were upregulated in cholesteatoma ex vivo, implies that the sole interaction between the induced expression of let-7 and downregulation of miR-21 was not a sufficient mechanism. Additional mechanistic studies are needed to confirm this interesting hypothesis aiming to tackle the paradoxal nature of cholesteatoma.
5 NF-kB/miR-802/PTEN REGULATORY NETWORK BEYOND miR-21 IN CHOLESTEATOMA
As previously mentioned, multiple miRNAs could share the same target mRNA18 making the additive fine-tuning of gene expression. This effect was also found to be important in cholesteatoma, by describing additional PTEN regulation, beyond miR-21, through the first time revealed NF-kB/miR-802/PTEN regulatory network.12 The increased mRNA levels of proinflammatory cytokines TNF-a, IL-1b, and IL-6 and increased phosphorylation of NF-kB subunit P65 in the clinical samples of cholesteatoma tissues compared to retroauricular skin have been found.12 Through comprehensive analysis integrating bioinformatics and in vitro manipulation of miR-802 in primary keratinocytes, it was demonstrated that activated NF-kB increase expression levels of miR-802, which subsequently promotes cell proliferation through regulation of PTEN.12 The observed mechanism was suggested to be important in cholesteatoma development and progression as well.12 However, there are no studies investigating miR-802 expression in cholesteatoma tissue, while the single high-throughput study performing miRNA expression profiling in cholesteatoma compared to normal skin to date has not reported differential expression of miR-802.14 These discrepancies warrant further ex vivo validation in cholesteatoma tissue on a larger number of samples to confirm the in vitro observations especially by taking into account the complex perimatrix-matrix molecular interplay which has been suggested to play a major role in cholesteatoma.6
6 miR-203 IN CHOLESTEATOMA PATHOLOGY
The next miRNA investigated in cholesteatoma pathology, miR-203a13 is specific to epithelial tissue by affecting the growth, differentiation, and physiology of keratinocytes; and is an important contributor to skin development.49-51 Additionally, studies investigating miR-203a in various cancer pathologies have suggested its cancer-suppressive potential.52-55 Also delay of chronic wound healing is linked with over-expression of miR-203 in diabetes mellitus.56 miR-203a is significantly lower in cholesteatoma than in normal retroauricular skin, while its bioinformatically predicted target Bmi1 showed correlated upregulation on the protein level. Using luciferase assay on HaCaT cell model, the miR-203/Bmi1 interaction has been confirmed, while the same cells displayed hyperproliferation, a low rate of apoptosis, and abnormal migration in low levels of miR-203.13 It has been described that Bmi1 increases the level of p-Akt, which is considered as one of the mechanisms of tumor cell proliferation, migration, and antiapoptotic abilities.57-59 p-Akt was also linked with the development of cholesteatoma.60, 61 The upregulation of EGFR/Akt/NF-κB/cyclinD1 survival signaling pathway in cholesteatoma epithelium compared to normal skin was suggested to be involved in cellular hyperplasia of cholesteatoma,60 while its activity in PI3K/Akt/PKB survival signaling may be an additional factor of early keratinocyte differentiation arrest.61 Further research of the upstream mechanisms responsible for miR-203a downregulation in cholesteatoma remains to be elucidated.
7 HIGH-THROUGHPUT miRNA EXPRESSION PROFILING IN CHOLESTEATOMA
miRNA microarray technique for miRNA expression profiling identified 44 upregulated and 175 downregulated miRNAs in acquired middle ear cholesteatoma compared to normal skin.14 The bioinformatic analysis of 19 candidate miRNAs suggested that these miRNAs might be important factors in the etiopathogenesis of middle ear cholesteatoma, by regulating genes involved in cell proliferation, apoptosis, cell cycle, differentiation, bone resorption, and remodeling.14 Subsequent qRT-PCR validation of miRNAs of particular interest has confirmed the upregulation of miR-21-3p and miRNA-16-1-3p while miRNA-10a-5p levels were deceased in cholesteatoma versus normal skin.14 However, the discrepancy in the expression of miRNA-584-5p and miRNA-338-5p between qRT-PCR validation and microarray analysis warrants the need for further studies with a larger number of samples. Although the study has a limitation in sample size, the robustness of the high throughput methodology makes acquired data valuable information and a good starting point for the development of novel research hypotheses involving novel miRNA candidates and mechanisms of their activity in cholesteatoma development.
8 REGULATION OF miR-21 AND LET-7 EFFECTS BY lncRNA IN CHOLESTEATOMA
ceRNA research in cholesteatoma proposed “endogenous sponges” for miR-21-3p and let-7a-3p.9, 10 Using network analysis of lncRNA/miRNA/mRNA interactions, the authors have discovered lncRNA-uc001kfc.1 as a possible regulator of miR-21 effects in cholesteatoma and hypothesized that this lcnRNA molecule could be a potential drug target for the treatment of cholesteatoma.9 The same group aimed to investigate the ceRNA hypothesis in cholesteatoma with circRNAs.10 The most notable observation was that circRNA-102747 and circRNA-101458, downregulated in cholesteatoma compared to normal skin, interact with miR-21-3p and let-7a-3p. However, other interactions with targeting miRNAs, beyond miR-21 and let-7a, detected in both studies9, 10 should not be neglected in upcoming cholesteatoma research.
9 PERSPECTIVES
The important role of ncRNAs in cholesteatoma pathology and the contemporary scarcity of the experimental results in the field could be easily deduced from the thorough analysis of available literature encompassed by the current review. We have integrated and highlighted the progress of the previous research of ncRNAs in cholesteatoma (Table 1, Figure 2). The key ncRNAs and target genes are joined in Figure 2 to depict regulation of ncRNAs in cholesteatoma, their interplay, and putative modulation of target genes associated with pathology.
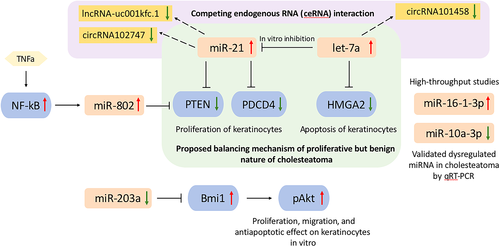
Based on our literature review we can conclude that miR-21 and let-7a are the two most highlighted ncRNAs dysregulated in cholesteatoma pathology (Figure 2). It is widely recognized that ncRNAs exert many biological properties that can make them noteworthy biomarkers for disease follow-up and minimally invasive therapeutics.62 Therapeutics based on blockade of miRNA in different pathologies are reported recently. Beneficial effects on miR-21 expression inhibition have been observed with miR-21 antagomir in hypertrophic scarring of the skin63 and inhibition of acute tissue injury in LPS-treated human pulmonary alveolar epithelial cells (HPAEpiC).64 Even more, a personalized nanomedicine approach with functional nanoparticles was proposed to target both, miR-21 and target genes using the same therapeutic.65 For the let-7, clinical trials are already ongoing for the evaluation of the therapeutic potential of this nc-RNA in various diseases such as obesity, diabetes, and cancer.66 Although the recently developed analytical models indicate time requirement before many miRNA products reach the market67 the progress which has been made in the past few years envisages a promising future.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.




