Current approaches to facial nerve schwannoma surgery
Abstract
Background
Facial nerve schwannomas (FNSs) are exceedingly rare benign tumors. This study aims to report on a series of excised FNSs, providing clinical information and details on their surgical management, including novel approaches.
Methods
We retrospectively reviewed patients who underwent surgical excision of FNSs in a private otology clinic and public tertiary referral center. The main outcome measures were facial nerve function, complete tumor removal, postoperative complications, tumor recurrence, and hearing.
Results
Seventeen patients (10 men and 7 women) with a mean age of 44.23 years (SD, 12.21) underwent surgery during the study period. The most common symptom was facial nerve dysfunction (58.8%). Facial and otoneurologic symptoms (hearing loss, tinnitus, and vertigo) were observed in 88.8% and 77.7% of patients, respectively. The middle cranial fossa (MCF) was the most common approach (six patients, 35.2%), followed by translabyrinthine (TL), transmastoid (TM), and combined TM-MCF (three patients, 17.6% each). Exclusive endoscopic transcanal suprageniculate (ETS) and mastoid combined with cervical approaches were applied once in two patients, 5.8% each. Total tumor removal was achieved in all cases. No significant postoperative complications were observed. The mean follow-up period was 193.2 months (SD, 119.5) and no tumor recurrence was observed.
Conclusion
This study provides further evidence for the safety and efficacy of various surgical approaches for FNS, and incorporates the endoscopic transcanal approach.
Level of evidence
4.
1 INTRODUCTION
Facial nerve schwannomas (FNSs) are exceedingly rare, benign tumors comprising 1% of all intrapetrous lesions and 0.15%–0.8% of intratemporal tumors.1, 2 Compared to vestibular schwannomas, FNSs are even rarer, they are diagnosed in only 1%–3% of all cerebellopontine angle (CPA) and internal auditory canal (IAC) tumors.3, 4 Schwannomas, followed by epidermoid and hemangiomas, are the most common tumors involving the facial nerve. They are usually slow-growing, multi-segment tumors originating from any segment of the nerve.5-7 In cases of Bell's palsy with unfavorable evolution (no recovery within 6 months), facial weakness, or recurrent facial paralysis, suspicion of a facial nerve neoplasm is warranted, even though only 5%–10% of these palsies are caused by tumors.2
The management of FNSs remains controversial, lacking consensus.8 Available options include watchful waiting (observation and scanning), radiation therapy, and surgery. Owing to the negative impact of possible postoperative facial paralysis, complete surgical excision is only indicated when there is temporal lobe or brainstem compression or in cases with poor facial nerve function.9
Depending on the tumor location, classical surgical approaches include the translabyrinthine (TL), retrosigmoid (RS), transmastoid (TM), and middle cranial fossa (MCF) vias. Each method has its own indications, limitations, and intrinsic sequelae. Recently, endoscopic ear surgery has gained traction as a minimally invasive technique, with the exclusive endoscopic transcanal suprageniculate (ETS) approach emerging as a viable option for treating geniculate ganglion (GG) tumors in selected cases.10-12
This study aims to report on a series of FNSs cases undergoing surgical intervention and introduce the authors' updated strategy for approach.
2 METHODS
This retrospective case series involved chart reviews of patients who underwent complete tumor excision for FNSs in both a private practice otology clinic and a public third-level adult university hospital from January 1993 to December 2022. The study protocol was approved by the Institutional Review Board of the participating institutions (HCB/2023/0351).
Inclusion criteria comprised the intratemporal location of the tumor with or without extratemporal extension, brainstem or temporal lobe compression, or facial nerve function equal to or worse than IV on the House–Brackmann scale. All the cases were histopathologically confirmed as schwannomas. Exclusion criteria included exclusive extratemporal location, poor general condition, and syndromic disease.
The tumor size and location were determined based on imaging studies (CT and MRI), with the main affected segments being the CPA, IAC, labyrinthine, GG, tympanic, mastoid, and extratemporal portions. Facial nerve dysfunction was assessed using the House–Brackmann scale.13 Hearing results were evaluated according to the guidelines of the American Academy of Otolaryngology—Head and Neck Surgery.14 Class A (PTA hearing level < 30 dB and WRS ≥70%), class B (PTA >30 and ≤50 dB, and WRS ≥50%), class C (>50 dB and WRS ≥50%), and class D (any PTA and WRS <50%).
- Tumors <1.5 cm affecting the CPA and IAC with A and B hearing classes were proposed for the MCF approach.
- Tumors affecting the CPA, IAC, labyrinthine, or GG segments of class C or D (or tumors affecting the CPA and/or IAC >1.5 cm) were proposed for the TL approach.
- Combined approaches (MCF–TM) were indicated for large tumors involving multiple segments in which intracranial–intratemporal anastomosis was required.
- Small tumors (<1 cm) affecting the GG were treated using the ETS approach, regardless of the patient's hearing level. Incus and malleus head removal were required to access the suprageniculate fossa; thus, ossiculoplasty was performed if bone conduction was preserved in the preoperative hearing test.
- Tumors affecting the tympanic or mastoid segments were approached via the TM with tympano–ossicular reconstruction.
| Size | Hearing | Segment affected | Approach |
|---|---|---|---|
| <1.5 cm | A,B | IAC | MCF |
| C,D | IAC | TL | |
| A,B,C,D | GG | ETS | |
| >1.5 cm | C,D | IAC | TL |
| C,D | LAB | TL | |
| A,B | LAB+GG | MCF | |
| A,B | T | TM | |
| A,B | M | TM | |
| C,D | IAC + LAB+T | MCF + TM | |
| C,D | IAC + M | MCF + TM | |
| C,D | GG + P | C + TM |
- Abbreviations: C, cervical; ETS, endoscopic transcanal suprageniculate; GG, Geniculate Ganglion; IAC, Internal Auditory Canal; LAB, Labyrinthic; M, mastoid; MCF, Middle Cranial Fossa; T, tympanic; TL, translabyrinthine; TM, transmastoid.
Facial repair techniques included the greater auricular nerve (GAN) graft, hypoglossal–facial anastomosis (XII–VII), and hypoglossal–facial anastomosis with the GAN as a cable jump graft.
Primary outcome measures were postoperative facial nerve function and disease clearance, while secondary measures included complications, tumor recurrence, and hearing results.
2.1 Statistical methods
All continuous data are expressed as mean (±SD), and noncontinuous variables as percentages. Analyses were conducted using SPSS (version 22.0; SPSS, Chicago).
3 RESULTS
Table 2 summarizes the patient demographics, tumor location, side, size, symptoms, facial nerve reconstruction, pre- and postoperative hearing, and pre- and postoperative House–Brackmann grade.
| Pt-N° | Age | Sex | Side | Size (cm) | Symptoms | Segments | Surgery approach | Pre H-B | Post H-B | Pre-hearing | Post-hearing |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | L | 0.6 | FP + HL | Ty + Ms | TM | IV | III | A | B |
| 2 | 35 | F | R | 0.5 | FP | Ms + P | M + C | V | IV | A | B |
| 3 | 40 | M | L | 1 | FP + HL + V | CPA + IAC | MCF | IV | IV | B | B |
| 4 | 37 | F | L | 1.2 | FP | IAC + Lab | MCF | IV | IV | B | B |
| 5 | 28 | M | R | 0.25 | FP + HL | GG + Lab+Ty | MCF | V | IV | C | D |
| 6 | 33 | F | L | 1.2 | FP | Lab+GG | MCF | V | III | C | D |
| 7 | 61 | M | R | 1.5 | FP | Lab+GG | MCF | IV | IV | C | C |
| 8 | 59 | F | R | 0.7 | FP + V | GG + Lab+Ty | ETS | V | V | D | D |
| 9 | 46 | M | R | 1.7 | FP + HL | IAC + Lab+GG + Ty | MCF + TM | IV | IV | B | D |
| 10 | 20 | M | R | 3 | FP + HL + T | IAC + Lab+GG + Ty | TL | I | IV | C | DE |
| 11 | 42 | M | R | 0.4 | HL + V | IAC | MCF | IV | III | B | D |
| 12 | 53 | M | R | 0.5 | FP + HL | T + Ms | TM | V | III | B | B |
| 13 | 55 | F | R | 1.5 | FP + HL | GG + Ty + Ms | TM | V | IV | B | B |
| 14 | 46 | M | L | 2.5 | FP + HL + V + T | CPA + IAC + Lab+GG + Ty | MCF + TM | V | IV | B | C |
| 15 | 43 | F | L | 2.3 | FP + HL + V | IAC + Lab+GG + Ty | MCF + TM | IV | IV | B | B |
| 16 | 35 | M | R | 2 | FP + HL + V | IAC + Lab +GG | TL | V | IV | C | DE |
| 17 | 59 | F | R | 2.8 | FP + HL + V + T | CPA + IAC + Lab+GG + Ty | TL | IV | IV | C | DE |
- Abbreviations: C, cervical; CPA, cerebellopontine angle; DE, dead ear; ETS, endoscopic transcanal supracochlear; F, female; FP, facial palsy; GG, Geniculate Ganglion; HL, hearing loss; IAC, Internal Auditory Canal; L, labyrinthine; M, male; MCF, middle cranial fossa; Ms., mastoid; P, parotid; T, tinnitus; Ty, tympanic; TM, transmastoid; V, vertigo.
There were 10 men (58.5%) and 7 women (41.1%), with a mean age of 44.23 years (SD, 12.21). Six cases (35.2%) had left-sided lesions. The most common presenting symptom was facial nerve dysfunction in 10 cases (58.8%), followed by otoneurological symptoms such as hearing loss, vertigo, and tinnitus in four cases (23.5%). Three patients (17.6%) presented with mixed symptoms. Sixteen patients (94.1%) presented with facial nerve dysfunction during the disease course: 5 patients (29.4%) had recurrent facial palsy, 7 patients (41.1%) had progressive facial palsy, and 4 patients had acute facial palsy (23.5%). One patient (5.8%) had a normal preoperative facial nerve function. In our series, only 4 patients (23.5%) presented with otoneurological symptoms, and 13 patients (76.4%) developed otoneurological symptoms over the course of the study period: 12 patients (70.5%) had hearing loss, 3 (17.6%) had tinnitus, and 7 (41.1%) had vertigo or unsteadiness. Analysis of the affected segments revealed that the GG and labyrinthine portions were the most frequently involved segments in 11 cases (64.7%), followed by the tympanic segment in 10 cases (58.8%), IAC segment in 9 cases (52.9%), mastoid segment in 4 cases (23.5%), CPA in 3 cases (17.6%), and extratemporal segment in 1 case (5.5%) (Figures 1 and 2). Only one case (5.8%) affected one segment (IAC). The tumors affected more than one segment in 16 cases (94.1%). The mean number of affected segments was 2.8.
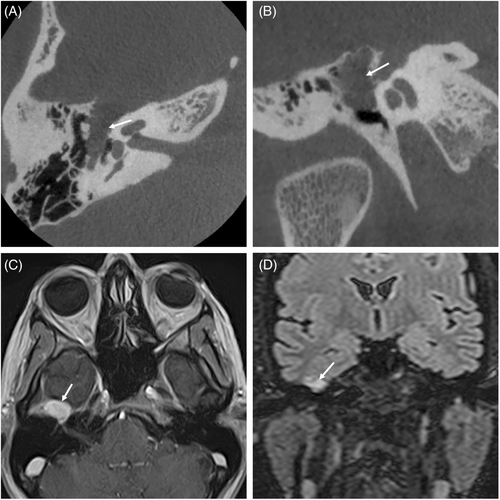
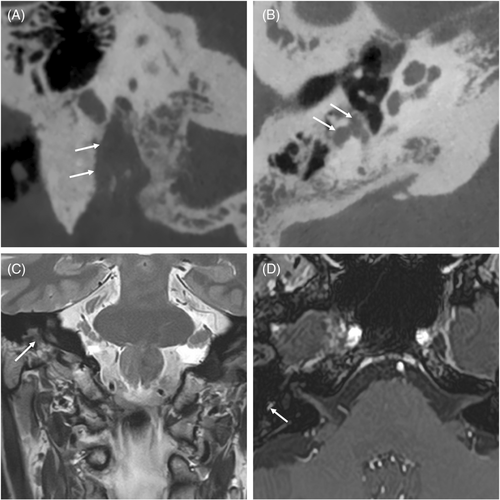
Preoperatively, House–Brackmann grade ranged from I to V; one large tumor that compressed the temporal lobe was grade I (5.8%), and grades IV and V were observed in eight patients each (47.05%).
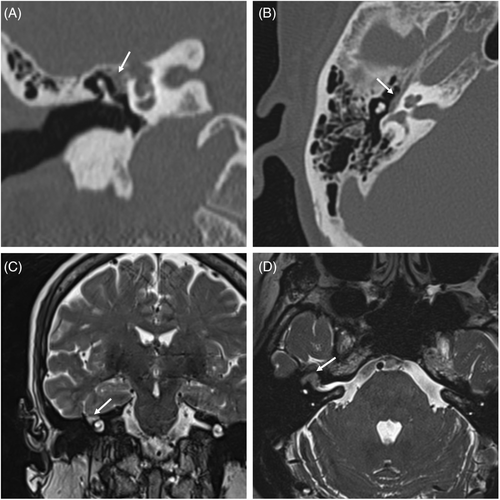
Macroscopically, total tumor removal was achieved in all 17 patients. The most common approach was MCF in 6 cases (35.2%), followed by TL, TM, and combined MCF–TM approaches in three cases each (17.6%); the ETS approach was performed in 1 case (5.8%) (patient 8, shown in Figure 3; see Video S1 illustrating technique); and the combined mastoid and cervical approach was performed in 1 case (5.8%).
Facial nerve reconstruction was performed through an “end to side” hypoglossal–facial anastomosis in 11 cases (64.7%), reaching a mean House–Brackmann of 3.8; through GAN graft in three cases (17.6%) resulting in House–Brackmann grade 3.3; through hypoglossal–facial anastomosis with GAN as a cable jump graft in two cases (11.7%) achieving House–Brackman grade IV; one case was not reconstructed because the patient accepted only static procedures. Collagen tubules to approximate, isolate, and stabilize the anastomosis were used in three cases in which the stumps could not achieve complete contact. At 1-year postoperatively, 16 patients achieved House–Brackmann grades III and IV.
No significant postoperative complications were observed. There was one case (5.8%) of postoperative headache after the MCF approach, and one case (5.8%) of CSF leak after the TL approach. Both conditions resolved with conservative management. The patient undergoing the endoscopic transcanal procedure was discharged without incident a few hours after the intervention. The mean hospitalization time was 6.4 days (SD, 2.0).
The mean follow-up period was 193.2 months (SD, 119.5), with neither tumor recurrence nor residual disease detected. Complete audiometric data were obtained in all cases. The preoperative hearing level was class A or B in 10 patients and class C or D in seven patients. Postoperatively, the hearing level was class B in 7 patients and class C or D or Dead Ear in 10 patients. Hearing was preserved in 50% of the MCF cases.
4 DISCUSSION
This study outlines the authors' experience with the surgery treatment of 17 FNSs. To summarize the diverse scenarios and surgical options, the authors introduced their own algorithm based on tumor size, location, hearing level, and the patient's general condition. This new algorithm incorporates classic approaches to the FNSs and an exclusive transcanal endoscopic approach recently described by Marchioni et al.11 Some studies have reported algorithms based solely on tumor location,15, 16 while others have considered both tumor location and hearing.17, 18 Table 3 summarizes the main studies focusing on surgical approaches.
| No Pts | Surgical Approach | Segments involved | Facial repair | Facial postop (HB) | |
|---|---|---|---|---|---|
| McMonagle et al.19 | 33 | 17 TL | 1 pt: 2 | 2 pt: End to end | 9 pt: I |
| 6 RS | 1 pt: 5 | 5 pt: GAN | 2 pt: II | ||
| 3 MCF + TM | 1 pt: 5,6 | 6 pt: HF | 13 pt: III | ||
| 3 TM + C | 1 pt: 0,1,2 | 7 pt: SN | 5 pt: IV | ||
| 2 TM | 1 pt: 2,3,4 | 13 pt: None | 4 pt: VI | ||
| 1 MCF | 1 pt: 0,1,2,3,4 | ||||
| 1 TO | 1 pt: 1,2,4,5,6 | ||||
| 1 pt: 0,1,2,3,4,5 | |||||
| 1 pt: 0,1,2,3,4,5,6 | |||||
| 2 pt: 3 | |||||
| 2 pt: 0,1,2,3 | |||||
| 2 pt: 3,4,5,6 | |||||
| 3 pt: 4,5,6 | |||||
| 3 pt: 1,2,3,4 | |||||
| 4 pt: 0 | |||||
| 8 pt: 0,1 | |||||
| Bacciu et al.20 | 23 | 10 RS | 2 pt: 1 | 2 GAN | 10 pt: I |
| 10 TL | 8 pt: 0 | 3 FPS | 2 pt: II | ||
| 13 pt: 0,1 | 3 HF | 9 pt: III | |||
| 4 D | 1 pt: V | ||||
| 5 None | 1 pt: IV | ||||
| 5 SN | |||||
| Kim et al.15 | 18 | 2 TM + MCF | 1 pt: 2,3,4,5,6 | 4 GAN | 1 pt: III |
| 2 MCF | 1 pt: 2,3,4 | 6 SN | 1 pt: V | ||
| 6 ITF | 1 pt: 2,3 | 7 HF | 15 pt: IV | ||
| 8 TM | 1 pt: 1,2,3,4 | ||||
| 1 pt: 2,3,4,5 | |||||
| 1 pt: 0,1,2,3 | |||||
| 2 pt: 3,4,5 | |||||
| 3 pt: 4,5 | |||||
| 3 pt: 3,4,5,6 | |||||
| 4 pt: 4,5,6 | |||||
| Shirazi et al.18 | 16 | 1C | 1 pt: All | 1 FF | 1 pt: I |
| 1MCF + ITF | 1 pt: 2,3,4 | 1 HF | 1 pt: Static | ||
| 3 C + TM | 1 pt: 4,5,6 | 10 GAN | 2 pt: IV | ||
| 3TM + MCF | 1 pt: 3,4,5 | 3 SN | 12 pt: III | ||
| 4TM | 1 pt: 6 | 1 None | |||
| 4TL | 1 pt: 0,1,2 | ||||
| 2 pt: 0,1,2,3,4 | |||||
| 2 pt: 0,1,2,3 | |||||
| 2 pt: 2,3 | |||||
| 2 pt: 3,4 | |||||
| 2 pt: 5,6 | |||||
| Liu et al.21 | 12 | 10 TL | 1 pt: 1,2,3,4 | 7 GAN | 2 pt: VI |
| 2 TM | 1 pt: 1,2,3,4,5,6 | 3 SN | 2 pt: Early | ||
| 1 pt: 0,1,2,3,4,5,6 | 2 HF | 3 pt: III | |||
| 1 pt: 1,2,3 | 2 pt: VI | ||||
| 1 pt: 0 | |||||
| 1 pt: 0,1,2,3,4 | |||||
| 2 pt: 0,1,2,3,4,5 | |||||
| 2 pt: 4,5,6 | |||||
| 2 pt: 0,1 | |||||
| Minovi et al.17 | 11 | 1 C + TL | 1 pt: 2,3 | 2 pt: HF | 6 pt: III |
| 1MCF + TM | 1 pt: 2,3,4,5 | 4 pt: SN | 2 pt: IV | ||
| 2 TL | 1 pt: 0,1,2 | 5 pt: GAN | 3 pt: LFU | ||
| 2 C | 2 pt: 6 | ||||
| 2 MCF | 2 pt : 0,1 | ||||
| 3 TM | 2 pt: 2,3,4 | ||||
| 2 pt: 3,4,5 | |||||
| Chung et al.16 | 8 | 1 ITF | 1 pt: 3,4,5,6 | 1 SN | 1 pt: VI |
| 1 MCF | 1 pt: 2,3,4,5 | 2 HF | 3 pt: IV | ||
| 1 TM | 1 pt: 3,4,5 | 2 GAN | 4 pt: III | ||
| 1 MCF + TM | 1 pt: 0,1,2,3,4,5,6 | 3 None | |||
| 2 TL | 2 pt: 1 | ||||
| 2 C | 2 pt: 6 |
- Note: Pt, patients; 0 = CPA; 1 = IAC; 2 = Labyrinthine; 3 = Geniculate Ganglion; 4 = Tympanic; 5 = Mastoid; 6 = Parotid.
- Abbreviations: C, cervical; D, decompression; FF, free flap; FPS, facial preservation surgery; GAN, greater auricular nerve; HF, hypoglossal to facial; ITF, infratemporal fossa; LFU, loss of follow up; MCF, middle cranial fossa; RS, retrosigmoid; SN, sural nerve; TL, translabyrinthine; TM, transmastoid; To, transotic.
The demographic data in this study were comparable to those reported in previous publications.2, 19-21 The clinical presentation of FNSs varies depending on tumor location.5 Tumors affecting the CPA, IAC, or labyrinthine regions typically present with sensorineural hearing loss, vertigo, or tinnitus. Tumors affecting the GG or extratemporal facial nerve usually cause facial palsy. Patients with tumors affecting the tympanic and mastoid portions may present with conductive hearing loss or facial dysfunction. There was no correlation between tumor size and clinical symptoms, although intracranial and intratemporal FNSs usually exhibit more severe clinical manifestations than extracranial FNSs.7, 16 According to most studies, facial palsy is the most common presenting symptom, ranging from 41% to 82% of cases, which aligns with the results of the present study.18, 22, 23 However, other authors have referred to otoneurological symptoms as the most common clinical presentation.19 FNS has a predilection for GG, likely related to the major structural reorganization found in this region.1, 24 According to Falcioni et al.,6 the GG and tympanic segments were the most commonly affected (75%). Kertesz et al.25 reported that the GG, labyrinthine, and tympanic segments were involved in 68%, 52%, and 43% of the cases, respectively. According to McMonagle,19 the GG and intracanalicular facial nerves are affected in 45% and 43% of the cases, respectively. The findings of our study are in agreement with these results. Multi-segment tumors occurs almost twice as often as single-segment tumors (64% and 36%, respectively), with a reported average of 2.57 segments affected,25 which is comparable to the present series.
High-resolution bone-algorithm CT and advanced MRI techniques indicate the precise location and extension of the FNS, facilitating surgical planning and minimizing misdiagnosis between vestibular schwannomas and FNSs affecting the CPA and IAC segments. Due to the complex course of the facial nerve in the temporal bone, its radiological appearance varies. However, MRI typically indicates a mildly hypointense or isointense lesion relative to the brain on a non-contrast T1-weighted series and enhancement after gadolinium contrast.25
Consensus on the management of FNSs is lacking, although there is a trend towards less aggressive approaches. In cases of normal or mild facial dysfunction without temporal lobe or brainstem compression, various options are available: watchful waiting with periodic ENOG control, serial imaging, and photographic documentation, considering that observation before surgery does not result in worse facial outcomes,21 bony decompression surgery, tumor debulking, and stereotactic radiation therapy.9, 26, 27 The authors advocated for complete tumor excision and nerve grafting when the House–Brackmann evaluation reached grade IV, considering that the best possible functional outcome after reconstruction was grade III. This valid option has also been reported by other authors.19, 21, 28-30 Tumor debulking may be an alternative to preserve facial nerve continuity: some authors reported facial nerve sparing (≥50% of the fibers) in 25%–42% of their cases.4, 24, 31 However, this technique may compromise future nerve reconstruction if access to the proximal stump is difficult because of tumor growth towards the brainstem.
Considering the indications, limitations, and sequelae, we observed that the MCF approach allowed hearing preservation in half of the cases. The pitfalls of this approach include technical difficulty, temporal lobe compression, and the impossibility of restoring facial nerve continuity during the same surgical procedure after total tumor removal.17, 19 The TL approach is a well-known surgical approach for otosurgeons that enables the excision of tumors of any size and direct facial nerve reconstruction. The only exceptions were cases in which the proximal stump was too short in the brainstem. The pitfalls related to labyrinthectomy include dead ear and unsteadiness.17, 19, 20 Large tumors involving multiple segments require combined approaches (MCF–TM), enabling intracranial–intratemporal facial nerve anastomosis if needed15, 18, 19 (case 9). The TM approach is suitable for tumors affecting the tympanic and mastoid segments. Tympanoplasty reconstruction can be performed, and facial nerve function can be repaired using a GAN graft.15, 18 The authors recently incorporated the ETS approach for small tumors (<1 cm) affecting the GG regardless of the hearing level. In this technique, chain removal is mandatory; however, hearing can be restored using ossiculoplasty. This approach results in less morbidity, brief hospital admission, and faster recovery than MCF; thus, it could be an alternative in selected cases (i.e., contraindications to the MCF approach). In such cases, facial nerve reconstruction can be achieved through hypoglossal–facial anastomosis.11, 12 In the authors' opinion, the RS approach does not offer good control of the intracanalicular portion of the facial nerve.20 However, surgeon's expertise and patient's preferences should be finally considered.
It is generally accepted that a brief time interval is related to an optimal outcome.30 All facial nerve reconstructions in the present study were performed between 3 and 6 months after tumor removal. Regardless of the reconstruction technique, these results are comparable to those previously reported.16, 17, 19, 21, 24 The authors preferred to use the GAN because of its easier access and lower morbidity compared to the sural nerve.
The main limitations of the present study are its retrospective design and small sample size. Surgical time, hospitalization time, and cost of each technique were not evaluated. Additionally, it would have been interesting to assess patients' quality of life. Nevertheless, we believe that the information provided herein may be helpful to specialists dealing with these rare tumors.
In conclusion, the surgical treatment of FNSs remains controversial, although the tendency is to minimize aesthetic and functional sequelae using more conservative approaches. Tumor size, location, hearing status, and the patient's general condition determine the most suitable approach. Finally, the present study provides further evidence of the safety and efficacy of various surgical approaches to FNSs, including the novel ETS, as a minimally invasive technique for selected cases.
FUNDING INFORMATION
This study was not supported by any sponsor or funder.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants, but are available from the corresponding author (F.L.).




