Primary site surgical resection in cM1 oral cavity squamous cell carcinoma
Abstract
Objective
To investigate primary site surgical resection and overall survival (OS) in clinically distantly metastatic (cM1) oral cavity squamous cell carcinoma (OCSCC).
Methods
The 2006–2018 National Cancer Database was queried for patients presenting with cM1 OCSCC who underwent chemotherapy. Binary logistic, Kaplan–Meier, and multivariable Cox proportional hazards regression models were implemented.
Results
Of 278 patients satisfying inclusion criteria, 139 (50.0%) underwent chemotherapy alone, 80 (28.8%) underwent chemoradiotherapy, 25 (9.0%) underwent surgical resection + adjuvant chemotherapy, and 34 (12.2%) underwent surgical resection + adjuvant chemoradiotherapy; 5-year OS was 9.4%, 15.2%, 8.3%, and 23.8%, respectively (p < .001). Compared with those not undergoing surgical resection, patients undergoing surgical resection underwent radiotherapy more frequently (57.6% vs. 36.5%) but multiple-agent chemotherapy less frequently (40.7% vs. 74.4%) (p < .005). Twenty-one (36.2%) patients undergoing surgical resection had positive surgical margins. Academic facility (adjusted odds ratio [aOR] 3.19, 95% CI 1.54–6.62) and Charlson-Deyo comorbidity score ≥1 (aOR 2.82, 95% CI 1.25–6.32, p < .025) were associated with increased odds of undergoing surgical resection. Compared with chemotherapy alone, chemoradiotherapy (adjusted hazard ratio [aHR] 0.56, 95% CI 0.38–0.83) and surgical resection + adjuvant chemoradiotherapy (aHR 0.37, 95% CI 0.21–0.66) were associated with higher OS (p < .005). Immunotherapy (aHR 0.48, 95% CI 0.28–0.81, p = .006) was also independently associated with higher OS.
Conclusion
A minority of patients with cM1 OCSCC underwent primary site surgical resection. Despite the high rate of positive surgical margins, surgical resection + adjuvant chemoradiotherapy was associated with higher OS than chemotherapy alone, chemoradiotherapy, or surgical resection + adjuvant chemotherapy. Definitive local therapy may benefit select patients with cM1 OCSCC.
Level of evidence: 4.
1 INTRODUCTION
Surgery and neck dissection is the standard of care for resectable oral cavity squamous cell carcinoma (OCSCC) presenting without clinical evidence of distant metastasis, and advances in adjuvant radiotherapy and chemotherapy have significantly improved survival and locoregional control.1-11 Approximately 2% of OCSCC presents with clinically distant metastasis (cM1) which often contraindicates definitive local therapies such as surgical resection and radiotherapy.12, 13 In cM1 OCSCC, systemic therapies such as chemotherapy and immunotherapy are the standard of care, but response rates are exceedingly poor, with most patients dying of disease progression within months.14-17 cM1 OCSCC, in particular, is suggested to have a worse prognosis than cM1 squamous cell carcinoma of other head and neck primary sites, highlighting a continued need for more effective targeted and systemic therapies.18
Recent studies suggest that surgical resection, curative-dose radiotherapy (≥60 Gy to the primary site), and other local therapies have a survival benefit in cM1 head and neck squamous cell carcinoma (HNSCC).19-29 Surgical resection is included in the management of locoregionally advanced OCSCC, but its utility in cM1 OCSCC remains unclear because of the heterogeneous clinical presentation and lack of high-quality clinical trial data. Considering that the morbidity and mortality of cM1 OCSCC are largely driven by primary tumor progression and poor locoregional control, determining the utility of surgical resection may benefit certain patients. Our study of the National Cancer Database (NCDB) investigates primary site surgical resection and associated survival outcomes in cM1 OCSCC. To our knowledge, our study is also the first to present a cohort of exclusively cM1 OCSCC.
2 METHODS
2.1 Data source
The NCDB is jointly sponsored by the American Cancer Society and the American College of Surgeons Commission on Cancer (CoC).30-32 The NCDB collects data from >1500 CoC-accredited hospitals within the United States, including >34 million patients and capturing >70% of newly diagnosed head and neck cancer each year.30-32 The Rutgers New Jersey Medical School and Perelman School of Medicine at the University of Pennsylvania Institutional Review Boards exempted our study because of the de-identified nature of patient data. The American Cancer Society and CoC are not responsible for the validity of the statistical analyses or conclusions derived herein.
2.2 Inclusion criteria
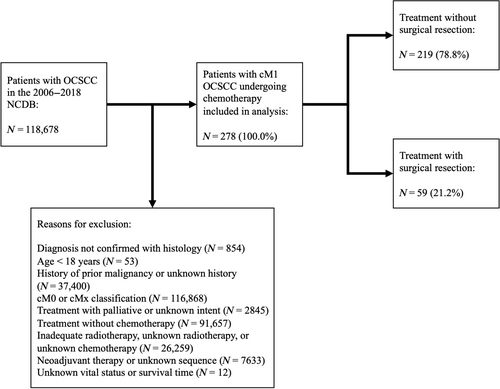
The 2006 to 2018 NCDB was retrospectively reviewed for patients with cM1 OCSCC undergoing chemotherapy (Figure 1). OCSCC was identified using the International Classification of Diseases for Oncology, 3rd Edition histology (“8070–8072”), behavior (“3”), and topography (“C00.0–C00.9,” “C02.0–C02.3,” “C02.8,” “C02.9,” “C03.0–C03.9,” “C04.0–C04.9,” “C05.0,” “C05.8,” “C05.9,” “C06.0–C06.9”) codes. Patients were excluded if they had an age <18 years; history of prior malignancy; unknown surgical resection, radiotherapy, or chemotherapy status; treatment with palliative intent; an unknown number of chemotherapy agents; neoadjuvant radiotherapy or chemotherapy; salvage surgery; or unknown vital status or survival time.
2.3 Variables
Patient data included age at diagnosis, sex, race, facility type, Charlson-Deyo comorbidity score (CDCS), history of prior malignancy, histology, primary site, grade, clinical tumor-nodal-metastasis classification (American Joint Committee on Cancer, 7th and 8th edition), site of distant metastasis, pathologic extranodal extension, lymphovascular invasion, surgical margin status, treatment, length of stay and 30-day readmission following surgical resection, 90-day mortality, vital status, and survival time. Sex was classified as male or female, as provided by NCDB. Cases with a CDCS of 0 had no recorded comorbid conditions. Microscopic, macroscopic, or unspecified residual tumor was considered positive surgical margins (PSM). Tumors were graded low (eg, well or moderately differentiated) or high (eg, poorly differentiated, undifferentiated, or anaplastic). Primary site surgical resection was defined as local tumor destruction, local tumor excision, wide excision, radical excision, and unspecified surgery. Neck dissection was defined as the removal and examination of ≥10 lymph nodes, a previously validated threshold in HNSCC.33-39 Radiotherapy was defined as external beam radiation with volume in the head and neck and a dose between 60 and 80 Gy. The primary outcome of our study was 5-year overall survival (OS). Survival time was calculated as the time from diagnosis to either death or 5 years of follow-up.
2.4 Statistical analysis
Patients undergoing surgical and nonsurgical treatment were compared with the Chi-square and Mann–Whitney U-tests, as appropriate. A multivariable binary logistic regression model handling missing data with listwise elimination and adjusting for all variables in the univariable analysis based on a priori co-author consensus was implemented to identify patient demographics and clinicopathologic features independently associated with undergoing surgical resection. Kaplan–Meier analysis was performed with the log-rank test to estimate 5-year OS. A multivariable Cox proportional hazard regression model handling missing data with listwise elimination and adjusting for all variables in the univariable analysis was implemented to identify patient demographics, clinicopathologic features, and treatment independently associated with OS. The proportionality of hazards was evaluated using time-dependent covariates and was not violated in any regression models. Variables included in multivariable analyses were selected a priori by author consensus. To account for immortal time bias and patients with favorable baseline prognosis having a higher likelihood of undergoing definitive local therapy, sequential landmark survival analysis was performed for patients surviving ≥3, ≥6, and ≥12 months from diagnosis.22 Patients in the NCDB satisfying identical inclusion criteria but undergoing neoadjuvant therapy were included in sensitivity analysis. The two-sided threshold for statistical significance was set at p < .05. SPSS version 25 (IBM) was used for statistical analysis.
3 RESULTS
3.1 Patient demographics, clinicopathologic features, and treatment
Of 278 patients satisfying inclusion criteria, the majority were male (70.9%) and White (81.6%) with low-grade disease (52.9%) of the tongue (33.5%) or floor of the mouth (23.0%) classified as cT4 (55.8%) and cN1-3 (79.1%) (Table 1). Tumors were most frequently classified as cT4N2 (N = 98, 35.3%), cT2N2 (N = 28, 10.1%), cT4N0 (N = 23, 8.3%), cT3N2 (N = 20, 7.2%), cT4N1 (N = 19, 6.8%), and cT4N3 (N = 12, 4.3%). Eight-five (30.6%) patients had metastasis to the lung only, 29 (10.4%) had metastasis to the bone only, eight (2.9%) had metastasis to the liver only, and 156 (56.1%) had metastasis to other sites, multiple sites, or unknown sites. One hundred thirty-nine (50.0%) patients underwent chemotherapy alone, 80 (28.8%) underwent chemoradiotherapy, 25 (9.0%) underwent surgical resection + adjuvant chemotherapy, and 34 (12.2%) underwent surgical resection + adjuvant chemoradiotherapy. Few patients underwent first-course immunotherapy (N = 25, 9.0%).
| No surgical resection | Surgical resection | p | Total | |
|---|---|---|---|---|
| No. | 219 | 59 | – | 278 |
| Age at diagnosis, median years (IQR) | 60 (54–69) | 62 (57–69) | .504 | 61 (54–69) |
| Sex | ||||
| Male | 151 (68.9) | 46 (78.0) | .176 | 197 (70.9) |
| Female | 68 (31.1) | 13 (22.0) | 81 (29.1) | |
| Race | ||||
| White | 174 (79.8) | 52 (88.1) | .267 | 226 (81.6) |
| Black | 32 (14.7) | 4 (6.8) | 36 (13) | |
| Other | 12 (5.5) | 3 (5.1) | 15 (5.4) | |
| Facility type | ||||
| Academic | 90 (41.5) | 32 (56.1) | .047 | 122 (44.5) |
| Nonacademic | 127 (58.5) | 25 (43.9) | 152 (55.5) | |
| Charlson-Deyo comorbidity score | ||||
| 0 | 177 (80.8) | 39 (66.1) | .016 | 216 (77.7) |
| ≥1 | 42 (19.2) | 20 (33.9) | 62 (22.3) | |
| Primary site | ||||
| Tongue (except base) | 73 (33.3) | 20 (33.9) | .060 | 93 (33.5) |
| Gingiva | 16 (7.3) | 7 (11.9) | 23 (8.3) | |
| Floor of mouth | 45 (20.5) | 19 (32.2) | 64 (23.0) | |
| Palate (except soft palate and uvula) | 22 (10.0) | 1 (1.7) | 23 (8.3) | |
| Other, unspecified | 63 (28.8) | 12 (20.3) | 75 (27.0) | |
| Grade | ||||
| Low | 101 (46.1) | 46 (78.0) | <.001 | 147 (52.9) |
| High | 56 (25.6) | 7 (11.9) | 63 (22.7) | |
| Unknown | 62 (28.3) | 6 (10.2) | 68 (24.5) | |
| cT classification | ||||
| 1 | 9 (4.1) | 5 (8.5) | .039 | 14 (5.0) |
| 2 | 28 (12.8) | 15 (25.4) | 43 (15.5) | |
| 3 | 26 (11.9) | 9 (15.3) | 35 (12.6) | |
| 4 | 129 (58.9) | 26 (44.1) | 155 (55.8) | |
| x | 27 (12.3) | 4 (6.8) | 31 (11.2) | |
| cN classification | ||||
| 0 | 25 (11.4) | 12 (20.3) | .185 | 37 (13.3) |
| 1–3 | 178 (81.3) | 44 (74.6) | 222 (79.9) | |
| x | 16 (7.3) | 3 (5.1) | 19 (6.8) | |
| Site of distant metastasis | ||||
| Bone | 24 (11.0) | 5 (8.5) | .906 | 29 (10.4) |
| Liver | 6 (2.7) | 2 (3.4) | 8 (2.9) | |
| Lung | 68 (31.1) | 17 (28.8) | 85 (30.6) | |
| Other, multiple, unknown | 121 (55.3) | 35 (59.3) | 156 (56.1) | |
| Pathologic extranodal extension | ||||
| No | 5 (71.4) | 18 (48.6) | .269 | 23 (52.3) |
| Yes | 2 (28.6) | 19 (51.4) | 21 (47.7) | |
| Lymphovascular invasion | ||||
| No | 16 (76.2) | 15 (36.6) | .003 | 31 (50.0) |
| Yes | 5 (23.8) | 26 (63.4) | 31 (50.0) | |
| Radiotherapy | ||||
| No | 139 (63.5) | 25 (42.4) | .003 | 164 (59.0) |
| Yes | 80 (36.5) | 34 (57.6) | 114 (41.0) | |
| Chemotherapy | ||||
| Single-agent | 56 (25.6) | 35 (59.3) | <.001 | 91 (32.7) |
| Multiple agents | 163 (74.4) | 24 (40.7) | 187 (67.3) | |
| Immunotherapy | ||||
| No | 196 (89.9) | 56 (94.9) | .234 | 252 (91.0) |
| Yes | 22 (10.1) | 3 (5.1) | 25 (9.0) | |
- Note: Bold values are statistically significant (p < 0.05).
- Abbreviations: cN, clinical nodal; cT, clinical tumor-nodal; IQR, interquartile range.
Among 59 patients undergoing surgical resection, median (interquartile range) length of stay was seven (2–10) days, and eight (13.4%) patients had readmission to the surgical facility within 30 days of discharge. Thirty-two patients had known pM classification; 28 (87.5%) had pM1 disease. Of the 42 patients undergoing neck dissection, 34 (87.2%) also had clinical nodal disease and 37 (92.5%) had pathologic nodal disease. Of the 21 (36.2%) patients with PSM, 11 (18.6%) had unspecified residual tumors, nine (15.3%) had microscopic residual tumors, and one (1.7%) had macroscopic residual tumors. Five (8.6%) patients had mortality within 90 days of surgical resection.
Compared with those not undergoing surgical resection, patients undergoing surgical resection were more frequently treated at academic facilities (56.1% vs. 41.5%), had CDCS ≥1 (33.9% vs. 19.2%), and higher incidence of cT1-2 disease (33.9% vs. 16.9%) (p < .05) (Table 1). Patients undergoing surgical resection underwent radiotherapy more frequently (57.6% vs. 36.5%) but multiple-agent chemotherapy less frequently (40.7% vs. 74.4%) than those not undergoing surgical resection (p < .005). Utilization of immunotherapy did not differ between surgical and nonsurgical cohorts (5.1% vs. 10.1%, p = .234).
Low-grade (adjusted odds ratio [aOR] 0.26, 95% confidence interval [CI] 0.10–0.70), and unknown grade (aOR 0.20, 95% CI 0.07–0.60) were associated with decreased odds of undergoing surgical resection on multivariable binary logistic regression (p < .025) (Table 2). Academic facility (aOR 3.19, 95% CI 1.54–6.62), CDCS ≥1 (aOR 2.82, 95% CI 1.25–6.32) and floor of the mouth primary site (aOR 3.05, 95% CI 1.20–7.76) was associated with increased odds of undergoing surgical resection (p < .025).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p | aORa (95% CI) | p | |
| Age at diagnosis (years) | 1.00 (0.98–1.03) | .819 | 1.02 (0.98–1.06) | .312 |
| Sex | ||||
| Male | Ref | .179 | Ref | .278 |
| Female | 0.63 (0.32–1.24) | 0.64 (0.28–1.44) | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 0.42 (0.14–1.24) | .115 | 0.36 (0.10–1.32) | .124 |
| Other | 0.84 (0.23–3.08) | .788 | 0.33 (0.04–2.64) | .293 |
| Facility type | ||||
| Academic | 1.81 (1.00–3.25) | .049 | 3.19 (1.54–6.62) | .002 |
| Nonacademic | Ref | Ref | ||
| Charlson-Deyo comorbidity score | ||||
| 0 | Ref | .017 | Ref | .012 |
| ≥1 | 2.16 (1.15–4.08) | 2.82 (1.25–6.32) | ||
| Primary site | ||||
| Tongue (except base) | Ref | Ref | ||
| Gingiva | 1.60 (0.58–4.41) | .367 | 1.76 (0.45–6.98) | .418 |
| Floor of mouth | 1.54 (0.74–3.20) | .245 | 3.05 (1.20–7.76) | .020 |
| Palate (except soft palate and uvula) | 0.17 (0.02–1.31) | .088 | 0.23 (0.02–2.26) | .208 |
| Other, unspecified | 0.70 (0.32–1.53) | .368 | 1.26 (0.47–3.33) | .646 |
| Grade | ||||
| Low | Ref | Ref | ||
| High | 0.27 (0.12–0.65) | .003 | 0.26 (0.10–0.70) | .007 |
| Unknown | 0.21 (0.09–0.53) | <.001 | 0.20 (0.07–0.60) | .004 |
| cT classification | ||||
| 1 | Ref | Ref | ||
| 2 | 0.96 (0.27–3.40) | .955 | 1.99 (0.37–10.75) | .425 |
| 3 | 0.62 (0.16–2.36) | .486 | 0.88 (0.15–5.01) | .886 |
| 4 | 0.36 (0.11–1.17) | .090 | 0.51 (0.10–2.59) | .420 |
| x | 0.27 (0.06–1.21) | .087 | 0.51 (0.07–3.98) | .520 |
| cN classification | ||||
| 0 | Ref | Ref | ||
| 1–3 | 0.52 (0.24–1.11) | .088 | 0.39 (0.14–1.05) | .062 |
| x | 0.39 (0.10–1.60) | .192 | 0.42 (0.05–3.19) | .398 |
| Site of distant metastasis | ||||
| Bone | Ref | Ref | ||
| Liver | 1.60 (0.25–10.36) | .622 | 2.53 (0.29–22.24) | .403 |
| Lung | 1.20 (0.40–3.61) | .745 | 1.86 (0.52–6.60) | .337 |
| Other, multiple, unknown | 1.39 (0.49–3.91) | .534 | 2.15 (0.63–7.35) | .221 |
- Note: Bold values are statistically significant (p < 0.05).
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; cN, clinical nodal; cT, clinical tumor; OR, odds ratio; Ref, reference.
- a N = 273; number of events: 57.
3.2 Five-year OS
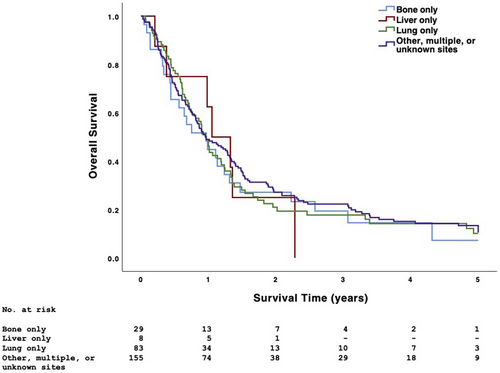
The 5-year OS of patients with primary tumors of the tongue (except base), gingiva, floor of mouth, palate (except soft palate and uvula), and other or unspecified oral cavity sites was 10.2%, 4.3%, 9.1%, 19.0%, and 19.4%, respectively (p = .519). The 5-year OS of patients with metastasis to the bone only, liver only, lung only, and other, multiple, or unknown sites was 9.1%, 0%, 13.2%, and 13.3%, respectively (p = .904) (Figure 2).
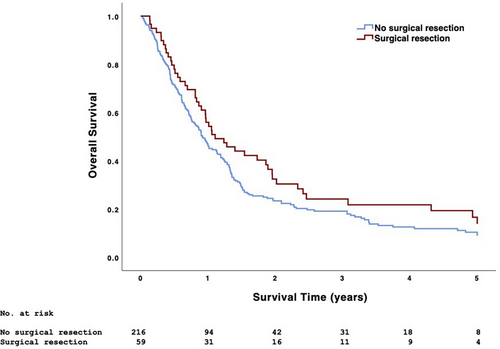
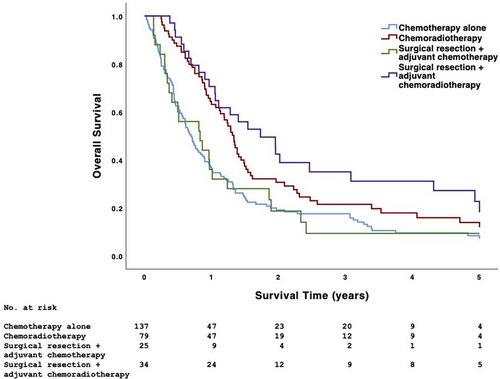
Patients undergoing surgical and nonsurgical treatment had similar 5-year OS (17.1% vs. 11.5%, p = .096) (Figure 3). Surgical treatment was associated with higher 5-year OS than nonsurgical treatment in patients with low-grade disease (20.0% vs. 9.8%) and those undergoing single-agent chemotherapy (23.1% vs. 10.1%) (p < .05) (Table 3). When considering all analyzed treatment combinations, the 5-year OS of patients undergoing chemotherapy alone, chemoradiotherapy, surgical resection + adjuvant chemotherapy, and surgical resection + adjuvant chemoradiotherapy was 9.4%, 15.2%, 8.3%, and 23.8%, respectively (p < .001); median survival (95% CI) was 8.3 (6.9–9.8), 16.0 (14.5–17.5), 10.0 (3.0–16.9), and 20.8 (12.4–29.2) months, respectively (Figure 4). Among patients undergoing surgical resection, those also undergoing neck dissection had similar 5-year OS as those not undergoing neck dissection (20.5% vs. 9.1%, p = .237). Patients with PSM had similar 5-year OS as those with negative surgical margins (12.8% vs. 20.0%, p = .668).
| No surgical resection | Surgical resection | p | |
|---|---|---|---|
| Overall | 11.5 | 17.1 | .096 |
| Primary site | |||
| Tongue (except base) | 9.4 | 13.5 | .244 |
| Gingiva | 6.3 | – | .091 |
| Floor of mouth | 9.5 | 8.1 | .617 |
| Palate (except soft palate and uvula) | 17.1 | – | .328 |
| Other, unspecified | 14.8 | 45.5 | .027 |
| Grade | |||
| Low | 9.8 | 20.0 | .027 |
| High | 9.3 | – | .379 |
| Unknown | 16.2 | 16.7 | .904 |
| cT classification | |||
| 1 | 22.2 | – | .926 |
| 2 | 17.6 | 17.2 | .447 |
| 3 | 14.3 | 17.6 | .981 |
| 4 | 6.5 | 16.7 | .117 |
| x | 23.1 | 25.0 | .691 |
| cN classification | |||
| 0 | – | 27.3 | .048 |
| 1–3 | 11.2 | 15.7 | .132 |
| x | 29.0 | – | .051 |
| Site of distant metastasis | |||
| Bone | 8.7 | – | .740 |
| Liver | – | – | .448 |
| Lung | 12.7 | 15.2 | .228 |
| Other, multiple, unknown | 12.0 | 18.2 | .309 |
| Pathologic extranodal extension | |||
| No | – | 18.8 | .497 |
| Yes | – | 18.9 | .619 |
| Lymphovascular invasion | |||
| No | – | 31.0 | .034 |
| Yes | – | 12.5 | .600 |
| Radiotherapy | |||
| No | 9.4 | 8.3 | .972 |
| Yes | 15.2 | 23.8 | .175 |
| Chemotherapy | |||
| Single-agent | 10.1 | 23.1 | .020 |
| Multiple agents | 12.0 | 8.7 | .505 |
- Note: Bold values are statistically significant (p < 0.05).
- Abbreviations: cN, clinical nodal; cT, clinical tumor.
Age at diagnosis (adjusted hazard ratio [aHR] 1.03, 95% CI 1.01–1.04, p < .001) was associated with decreased OS on multivariable Cox regression (Table 4). Floor of the mouth (aHR 0.61, 95% CI 0.40–0.93), palate (0.42, 0.22–0.80), other or unspecified primary sites (0.64, 0.43–0.95), chemoradiotherapy (0.56, 0.38–0.83), surgical resection + adjuvant chemoradiotherapy (0.37, 0.21–0.66), and immunotherapy (0.48, 0.28–0.81) were associated with improved OS (p < .05).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | aHRa (95% CI) | p | |
| Age at diagnosis | 1.02 (1.01–1.04) | <.001 | 1.03 (1.01–1.04) | <.001 |
| Sex | ||||
| Male | Ref | .230 | Ref | .142 |
| Female | 1.19 (0.90–1.58) | 1.27 (0.92–1.75) | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.23 (0.84–1.79) | .289 | 1.22 (0.81–1.82) | .343 |
| Other | 0.52 (0.26–1.01) | .052 | 0.61 (0.3–1.23) | .170 |
| Facility type | ||||
| Academic | 1.08 (0.83–1.40) | .566 | 1.06 (0.79–1.42) | .694 |
| Nonacademic | Ref | Ref | ||
| Charlson-Deyo comorbidity score | ||||
| 0 | Ref | .496 | Ref | .658 |
| ≥1 | 1.11 (0.82–1.50) | 1.08 (0.77–1.52) | ||
| Primary site | ||||
| Tongue (except base) | Ref | Ref | ||
| Gingiva | 1.00 (0.62–1.60) | .999 | 0.61 (0.33–1.14) | .121 |
| Floor of mouth | 0.87 (0.62–1.22) | .420 | 0.61 (0.40–0.93) | .023 |
| Palate (except soft palate and uvula) | 0.73 (0.44–1.22) | .230 | 0.42 (0.22–0.80) | .008 |
| Other, unspecified | 0.77 (0.55–1.09) | .140 | 0.64 (0.43–0.95) | .027 |
| Grade | ||||
| Low | Ref | Ref | ||
| High | 1.40 (1.02–1.92) | .037 | 1.15 (0.81–1.63) | .440 |
| Unknown | 0.90 (0.66–1.24) | .535 | 0.85 (0.59–1.23) | .397 |
| cT classification | ||||
| 1 | Ref | Ref | ||
| 2 | 1.26 (0.64–2.5) | .504 | 1.48 (0.70–3.12) | .304 |
| 3 | 1.47 (0.74–2.96) | .273 | 1.42 (0.66–3.05) | .367 |
| 4 | 1.75 (0.94–3.24) | .076 | 1.91 (0.95–3.84) | .068 |
| x | 1.19 (0.58–2.44) | .627 | 1.12 (0.51–2.47) | .772 |
| cN classification | ||||
| 0 | Ref | Ref | ||
| 1–3 | 1.08 (0.74–1.57) | .703 | 0.87 (0.57–1.33) | .514 |
| x | 0.74 (0.40–1.40) | .357 | 0.65 (0.30–1.38) | .259 |
| Site of distant metastasis | ||||
| Bone | Ref | Ref | ||
| Liver | 0.97 (0.42–2.25) | .946 | 0.81 (0.33–1.98) | .649 |
| Lung | 0.93 (0.59–1.47) | .763 | 0.79 (0.48–1.30) | .356 |
| Other, multiple, unknown | 0.87 (0.57–1.33) | .519 | 0.66 (0.41–1.08) | .099 |
| Treatment | ||||
| Chemotherapy alone | Ref | Ref | ||
| Chemoradiotherapy | 0.63 (0.46–0.85) | .002 | 0.56 (0.38–0.83) | .004 |
| Surgical resection + adjuvant chemotherapy | 1.01 (0.64–1.59) | .963 | 0.80 (0.45–1.39) | .423 |
| Surgical resection + adjuvant chemoradiotherapy | 0.47 (0.31–0.73) | .001 | 0.37 (0.21–0.66) | <.001 |
| Chemotherapy | ||||
| Single-agent | Ref | Ref | ||
| Multiple agents | 1.27 (0.96–1.67) | .093 | 0.88 (0.61–1.27) | .499 |
| Immunotherapy | ||||
| No | Ref | Ref | ||
| Yes | 0.72 (0.44–1.16) | .175 | 0.48 (0.28–0.81) | .006 |
- Note: Bold values are statistically significant (p < 0.05).
- Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; cN, clinical nodal; HR, hazard ratio; Ref, reference.
- a N = 272; number of uncensored deaths: 229.
3.3 Three-, Six-, and Twelve-month landmark survival analysis
Thirty-four (12.2%) patients had survival time <3 months. After censoring survival time <3 months, surgical resection + adjuvant chemoradiotherapy (aHR 0.48, 95% CI 0.26–0.89, p = .021) remained associated with OS on multivariable Cox regression.
Seventy-eight (28.1%) patients had a survival time <6 months. After censoring survival time <6 months, immunotherapy (aHR 0.49, 95% CI 0.25–0.96, p = .039) remained associated with OS on multivariable Cox regression.
One hundred forty-nine (53.6%) patients had survival time <12 months. After censoring survival time <12 months, neither surgical resection + adjuvant radiotherapy (aHR 0.86, 95% CI 0.31–2.35, p = .765) nor immunotherapy (aHR 0.99, 95% CI 0.43–2.31, p = .986) remained associated with OS on multivariable Cox regression.
3.4 Neoadjuvant therapy sensitivity analysis
Thirteen patients in the 2006–2018 NCDB satisfied identical inclusion criteria but underwent neoadjuvant therapy. The inclusion of these 13 patients in the analysis (N = 291) did not drastically alter our findings. Of these patients, 142 (48.8%) underwent chemotherapy alone, 81 (27.8%) underwent chemoradiotherapy, 29 (10.0%) underwent surgical resection + chemotherapy, and 39 (13.4%) underwent surgical resection + chemoradiotherapy; the 5-year OS was 9.2%, 15.0%, 9.1%, and 26.0%, respectively (p < .001). Age at diagnosis (aHR 1.02, 95% CI 1.01–1.04), palate (0.63, 0.42–0.95), the floor of the mouth (0.45, 0.24–0.84), other or unspecified primary sites (0.65, 0.44–0.95), chemoradiotherapy (0.52, 0.35–0.76), surgical resection + chemoradiotherapy (0.31, 0.18–0.54), and immunotherapy (0.45, 0.27–0.74) were associated with OS on multivariable Cox regression (p < .05).
4 DISCUSSION
For the approximately 2% of OCSCC presenting with distant metastasis, systemic therapies remain the standard of care.12, 22 Despite aggressive treatment, most patients have uncontrolled pain, bleeding, respiratory obstruction, cachexia, and death within months.22, 40 Although local therapies are often withheld in cM1 disease, studies of cM1 HNSCC suggest that definitive local treatment may improve survival in appropriately selected patients.19-29 In addition to the morbidity of locoregional disease, mortality in cM1 OCSCC has strong associations with primary tumor progression and poor locoregional control which raises the possibility that some patients may benefit from surgical resection. Given the lack of high-quality clinical trial data, our study utilizes the NCDB to investigate primary site surgical resection in cM1 OCSCC.
Our study identified 278 patients with cM1 OCSCC undergoing chemotherapy. Most patients had cT3-4 (68.3%) and clinical nodal disease (79.9%), which is often seen with aggressive tumor biology and high metastatic potential. Among patients with 1 known site of distant metastasis, the most frequent site was the lung (69.7%) which accounts for approximately 70% of all distant metastases in HNSCC.41 A minority of patients (21.2%) underwent primary site surgical resection in addition to chemotherapy. Although surgical resection is more often considered in patients with fewer comorbidities, those with CDCS ≥1 had increased odds of undergoing surgical resection. Academic facility was also associated with increased odds of undergoing surgical resection. Academic facilities often have increased access to additional personnel, resources, and multidisciplinary specialists, which assists in coordinating the aggressive, multimodal treatments that patients with cM1 disease may require. Of note, higher cTN classification was not associated with decreased odds of undergoing surgical resection. Although locoregionally advanced tumors are sometimes less amenable to surgical resection because of the difficulty in safely achieving negative margins and extent of surgery required, resecting these tumors often improves quality of life (eg, pain palliation and restoration of critical functions such as breathing and swallowing) which is prioritized in cM1 disease.
The five-year OS in our cohort was 13%; studies report 5-year OS in metastatic OCSCC ranging from 9% to 19% depending on tumor location, size, extent of metastasis, and treatment.42-45 Despite the high rate of PSM (36.2%), patients undergoing surgical resection + adjuvant chemoradiotherapy had the highest OS, followed by those undergoing chemoradiotherapy alone; treatment combinations without radiotherapy were associated with the poorest OS, even if surgical resection was performed. The survival benefit of surgical resection + chemoradiotherapy persisted in sensitivity analysis including patients undergoing neoadjuvant therapy. Our study suggests that surgical resection is not associated with higher OS in cM1 OCSCC unless offered with chemoradiotherapy. A hypothesized abscopal effect in which radiotherapy enhances systemic antitumor immune responses and reduces tumor burden in nonirradiated distant sites may account for this finding.36, 46-49 Our findings are consistent with clinical trials and meta-analyses of cM1 HNSCC which demonstrate the survival benefit of curative-dose radiotherapy in cM1 HNSCC.20, 46, 50-52 Studies of cM1 HNSCC utilizing the NCDB similarly conclude that chemoradiotherapy with a dose ≥60 Gy is associated with higher OS than either chemotherapy alone or chemoradiotherapy with a dose <60 Gy.20, 22 Locoregional radiotherapy, however, is often not utilized in cM1 HNSCC because of poor disease prognosis, the inconvenience of daily radiotherapy sessions, and treatment-related toxicities such as mucositis, dermatitis, xerostomia, dysphagia, and feeding tube dependence.22 Although studies attributing a survival benefit to radiotherapy in cM1 HNSCC excluded patients undergoing surgical resection, our study suggests that the survival benefit of radiotherapy persists in the adjuvant setting.22 The roles of altered fractionation, stereotactic body radiotherapy, radio-sensitizing chemotherapy, and biologic agents in optimizing response to radiotherapy remain areas of future investigation not explored in our study because of limitations in the NCDB.22, 46, 53-55
Patients undergoing surgical resection + adjuvant chemotherapy (without radiotherapy) had similar OS as those undergoing chemotherapy alone. Interruptions or delays in chemotherapy may have mitigated any potential benefit of surgical resection because systemic therapy is typically withheld prior to and immediately following surgery to allow for appropriate wound healing.46 Poor baseline health and the known immunosuppressive effects of surgery may also have mitigated any benefit of locoregional control, contributing to further disease progression.46 Surgical resection may have more benefit in oligometastatic HNSCC, especially if the primary tumor and distant metastases can be removed concomitantly.24, 46, 54-58 Regardless, progression of cM1 disease has significant functional morbidity and despite the risks of surgery, resection may improve quality of life in select patients. Further studies are needed to determine whether the possible survival benefit of definitive local therapy is associated with symptom palliation.
Our study did not identify an association between the number of chemotherapy agents and OS, aligning well with studies of cM1 HNSCC.21, 46 Current chemotherapy agents are often ineffective in slowing the progression of cM1 disease, even when administered in combination, highlighting a continued need for more effective targeted and systemic therapies. Interestingly, surgical resection was associated with higher OS in patients undergoing single-agent chemotherapy suggesting that the possibly improved locoregional control that surgery offers does not benefit patients undergoing multiple-agent chemotherapy. However, there are likely other factors responsible including the specific details surrounding why patients underwent single-agent versus multiple-agent chemotherapy (eg, patients undergoing multiple-agent chemotherapy may not have been candidates for preferred regimens with high-dose cisplatin).
Our study identified an association between immunotherapy and higher OS, aligning with the results of the EXTREME, CheckMate 141, and KEYNOTE-048 clinical trials which demonstrated the survival benefit of cetuximab, nivolumab, and pembrolizumab, respectively, in recurrent or metastatic HNSCC.17, 26, 59-63 Of note, immunotherapy was not routinely utilized in HNSCC during the earlier years of our study period (2006–2018) which may account for a minority of patients (9%) in our cohort undergoing immunotherapy. Interestingly, on landmark survival analysis censoring survival time <3, <6, and <12 months, chemoradiotherapy, surgical resection + chemoradiotherapy, and immunotherapy, respectively, were no longer associated with higher OS, possibly suggesting that the survival benefit of aggressive treatment is specific to advanced cM1 disease with particularly poor prognosis.20, 46 The survival benefit of multiagent chemotherapy and immunotherapy in cM1 HNSCC, specifically in patients undergoing definitive local therapy, warrants further investigation in well-designed clinical trials.64
Limitations inherent in retrospective review of the NCDB include the possibility of inaccurate histologic diagnosis and variable miscoding. The NCDB does not report medical comorbidities, specific site and size of metastases, imaging studies, quality of life, tumor board recommendations, surgeries besides resection (eg, tracheostomy and endovascular procedures), types of chemotherapy and immunotherapy agents, postoperative complications, benefits of surgery besides higher OS (eg, pain palliation and restoration of function), locoregional recurrence, and progression-free survival. The NCDB does not reliably confirm if patients had biopsy-proven pM1 disease. Selection bias is inherent in retrospective analysis comparing primary surgical resection with primary nonsurgical therapy because patients responding to systemic therapy may have been more likely to undergo definitive local therapies or have better performance status. Participation in palliative care, clinical trials, and experimental therapies may have impacted treatment decisions. Our study is unable to comment on the management of cM0 OCSCC that is unresponsive to initial definitive treatment or later presents with distant metastasis. Lastly, the retrospective, hypothesis-generating design of our study prevents us from making firm, evidence-based treatment recommendations.
5 CONCLUSION
A minority of patients with cM1 OCSCC undergoing chemotherapy also underwent primary site surgical resection. Patients undergoing surgical resection more frequently were treated at academic facilities, had CDCS ≥1, and higher incidence of cT1-2 disease. Despite the high rate of PSM, patients undergoing surgical resection + adjuvant chemoradiotherapy had the highest OS, followed by those undergoing chemoradiotherapy alone; treatment combinations without radiotherapy were associated with the poorest OS, even if surgical resection was performed. Our study suggests that surgical resection + adjuvant chemoradiotherapy may have a survival benefit in select patients. cM1 OCSCC is typically considered incurable, and management requires multidisciplinary physicians capable of weighing the potential survival benefit of aggressive treatment with patient-directed goals of care, quality of life, and probability of cure.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.




