Effectiveness of Sucralfate comparing to normal saline as an oral rinse in pain reduction and wound healing promotion in oral surgery
Abstract
Objectives
To study the effectiveness of Sucralfate suspension oral rinse compared to normal saline alone for pain reduction and wound healing promotion in open oral surgical wounds. The primary outcome of this study was postoperative pain VAS score reduction. The secondary outcome was wound healing promotion based on wound grade and maximal wound length reduction.
Materials and methods
A total of 30 patients with secondary healing intraoral surgical wounds were enrolled in this study. Sucralfate suspension (1 g/5 mL) was prescribed to a randomized experimental group as an oral rinse every 6 h for 14 days in addition to standard postoperative care. Postoperative pain VAS score, wound grade, and wound length were collected and compared with baseline from initial to final visit during 2 weeks.
Results
The mean change of VAS score was significantly lower from baseline in the Sucralfate group on day 3 (−0.77 in control and −2.15 in Sucralfate, p < .05) and day 7 (−2.15 in control and −3.62 in Sucralfate, p < .05). Wound grade distribution over time was the same in both Sucralfate and control groups. The mean change in wound length was not significantly different between the two groups. No adverse reaction to Sucralfate was reported during the study participation.
Conclusions
Sucralfate suspension oral rinse can be recommended as an effective topical analgesic solution in postoperative secondary healing of intraoral wounds with no significant interference. The benefits of wound healing promotion have yet to be proven.
Level of evidence
1b.
1 INTRODUCTION
Oral surgical wounds have obvious pain characteristics and prolonged healing resulting from multiple factors such as bare mucosa left for secondary healing, rich innervated sensory nerve fibers, and inevitable exposure to mechanical and chemical irritation.
There have been many attempts to invent novel cytoprotective agents and dressings for intraoral open surgical wounds, however, only a few have reached clinical trials and come at very high costs. The use of topical analgesics including NSAIDs, corticosteroid, and lidocaine can relieve postoperative pain however they must be used with caution for systemic adverse effects and unclarified risk of wound complications such as prolonged wound healing and postoperative bleeding.
Sucralfate is an effective cytoprotective agent that has long been used to treat peptic ulcers. It is also proven to be clinically effective in both pain relief and healing promotion in other mucosal ulcers such as post tonsillectomy wound,1 post uvulopalatoplasty wound,2 aphthous ulcer,3 chemoradiation mucositis and proctitis,4 chronic venous ulcer.5 Cytoprotective property of Sucralfate results from direct contact of Sucralfate with exudative matrix protein released from injured cells to form a physical barrier that coats over wound bed epithelium, thereby, preventing it from exposure to the external environment, protecting from physical shearing force while reducing irritation of cut free nerve endings and muscles. Sucralfate also induces PGE-2 and fibroblast GF mucosal concentration by unknown mechanism which also helps in facilitating the reepithelialization process.
In this clinical trial, we introduce the use of Sucralfate as an oral rinse in the postoperative intraoral open wound. This is the first study to assess drug effectiveness in terms of both pain reduction and wound healing promotion.
2 OBJECTIVE
To study the effectiveness of Sucralfate as an oral rinse compared to normal saline alone (as a standard treatment in our hospital routine) for pain reduction and wound healing promotion in open oral surgical wound.
The primary outcome is postoperative pain VAS score reduction.
The secondary outcome is wound healing promotion assessed by wound grade and maximal wound length reduction.
2.1 Study design
Randomized controlled trial.
2.2 Population
Patients with expected postoperative secondary healing intraoral wound were enrolled from June 2018 to November 2020. Enrolled patients were affirmed to meet all inclusion and exclusion criteria. Voluntary consent was obtained.
2.2.1 Inclusion criteria
Patients aged 18–90 scheduled for intraoral surgery with planned secondary healing wound in any oral subunits with wound length of more than 1 cm.
2.2.2 Exclusion criteria
Patients with previous radiotherapy involving head and neck area, previous reconstruction surgery, HIV infection, previous adverse reaction to Sucralfate, chronic renal failure with dialysis, and current use of drugs with Sucralfate interaction.
2.3 Methodology
The enrolled sample population were randomized into experimental and control groups using computer-generated block of four with allocation concealment. Sucralfate was then distributed only to the randomized experimental group on postoperative day 0. All patients were instructed and assigned the same analgesic protocol. Study participants were then scheduled for wound assessment and VAS score collection on day 7 and day 14.
Initially, 43 enrolled patients were assessed for eligibility, 10 patients were excluded due to enrollment criteria and 3 patients declined voluntarily participation, resulting in the final enrollment of 30 patients included in the study and randomized into. Control group (n = 16) and Sucralfate group (n = 14). In the control group, three patients were lost to contact, four patients were lost to wound assessment follow up on day 7 and three patients were lost to wound assessment follow up on day 14. In the Sucralfate group, one patient was lost to contact, five patients were lost to wound assessment follow up on day 7, and one patient lost to wound assessment follow up on day 14. Collected data was then analyzed and reported in reference to CONSORT guideline.
The research manuscript was submitted and approved by Institutional Review Board (IRB no. 356/62).
2.4 Data collection
Demographic data of patients associated with wound healing and pain were collected together with operative details.
Pain VAS score was collected as continuous data using a questionnaire depicting a 10-cm blank line marked from 0 to 10 to represent a continuum of pain with word anchors of “no pain” on the left and “worst pain” on the right. Patients were asked to record their worst pain level on days 0, 3, 7, and 14.
The number of rescue medications used according to the analgesic protocol, which are the number of paracetamol tablets taken and doses of morphine injection requested in the first 3 days postoperation were collected (Table 1).
| Analgesic protocol |
| Reported pain score > 3, oral paracetamol (500 mg q 4 h) was applied |
| Reported pain score > 6, morphine (4 mg IV q 4 h) was applied |
| No other analgesic modality such as local nerve block or PCA applied |
| No other analgesic medication allowed |
On postoperative day 0, the main investigator opened labeled envelopes of the patient groups that were pre-generated by computer. Sucralfate was prescribed only to the randomized experimental group for 14 days. All patients in Sucralfate group were instructed to use one sachet (5 mL) of Sucralfate suspension (1 g/5 mL) to rinse and held within the mouth for 2 min before swallowing every 6 h. Patients were instructed to store Sucralfate at room temperature, without refrigeration for maximal drug efficacy. Same oral hygiene care with normal saline was instructed and standard postoperative treatment was administered to both groups.
Wound assessment was done on day 0, 7, and 14 by measuring the maximal length of the wound in cm and taking a picture for wound grade assessment (Figure 1). Wound maximal length was measured by the main investigator using a plastic ruler. All pictures were taken with the same digital camera, calibrated with a 2 cm round mirror in the monitor screen.
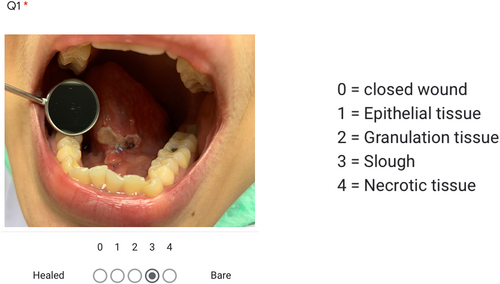
Wound grade adapted from PUSH score6 was collected using a Google Form questionnaire that included wound pictures. Four otolaryngologists, blinded to patient grouping status were asked to assess wounds. In the event of any discordant score, the final score was resolved through consensus discussion among the assessors.
Adverse reactions and comments regarding drug administration were collected at the end of the study.
2.5 Data analysis
Baseline characteristics of the sample population were analyzed by Fisher's exact test and chi square test. Pain VAS score reduction and wound length reduction were analyzed by GEE (Generalized Estimating Equation). Wound grade distribution at each time interval was analyzed by chi square test. All statistical tests were performed using SPSS version 27.
3 RESULTS
A total of 30 patients were enrolled. 4 patients (13%) were lost to contact after the date of their operation. Twenty-six patients followed up until the end of the study on day 14.
Data collected from the remaining 26 patients (n = 13/group) were then used for analysis. Mean age of the patients was 48.7 years (range 22–80 years) with an equal number of males and females in each group, 7 and 6 respectively. Diabetes, smoking, and malnutrition status (using significant weight loss >5% in the last 6 months as an indicator), wound size (small; 1–2 cm, large; >2 cm) in control group and Sucralfate group were not statistically different. Details of wound subsite, final diagnosis, and operation were shown in Table 2.
| Sucralfate | Control | |
|---|---|---|
| Patient characteristics | ||
| Gender | ||
| Male | 7 (53.8%) | 7 (46.2%) |
| Female | 6 (46.2%) | 6 (53.8%) |
| Mean age | 50 (26–75) | 47.3 (22–80) |
| Diabetes mellitus | 1 (7.7%) | 2 (15.4%) |
| Smoking | 4 (30.8%) | 2 (15.4%) |
| Malnutrition | 0 (0%) | 0 (0%) |
| Rescue medication | ||
| Paracetamol (tablets/n) | 5.25 (n = 8) | 6 (n = 11) |
| Morphine | 0 (0%) | 0 (0%) |
| Wound size | ||
| Small (1–2 cm) | 10 | 9 |
| Large (>2 cm) | 3 | 4 |
| Subsite | ||
| Floor of mouth | 2 | 3 |
| Oral tongue | 7 | 5 |
| Buccal | 3 | 3 |
| Palate | 0 | 1 |
| Retromolar | 1 | 1 |
| Operation | ||
| Excision (n = 6) | 3 | 3 |
| Oral tongue | Chronic inflammation 1 | Verrucous hyperplasia 1 Squamous papilloma 1 |
| Palate | 0 | Torus palatinus 1 |
| Buccal | Fibroma 1 | 0 |
| Retromolar | Fibrous scar 1 | 0 |
| Glossectomy (n = 10) | 4 | 6 |
| Squamous cell carcinoma 4 | Squamous cell carcinoma 5 Squamous dysplasia 1 |
|
| Laser excision (n = 7) | 4 | 3 |
| Buccal | Lichen planus 1 Chronic inflammation 1 |
Lichen planus 1 Keratosis 1 |
| Retromolar | 0 | Chronic inflammation 1 |
| Oral tongue | Leukoplakia 1 Chronic inflammation 1 |
0 |
| Marsupialization (n = 2) | Wharton's duct stone 1 | Wharton's duct stone 1 |
| Frenulectomy (n = 1) | Tongue tie 1 | 0 |
| N | 13 | 13 |
Pain VAS score reduction at each time point and interval changes are shown in Figure 2. There was a statistical difference in days 3 and 7 (p < .05). On day 3, mean VAS score reduction in Sucralfate group reduced by almost half from baseline (44.1%) while in the control group, there was a 13.5% reduction
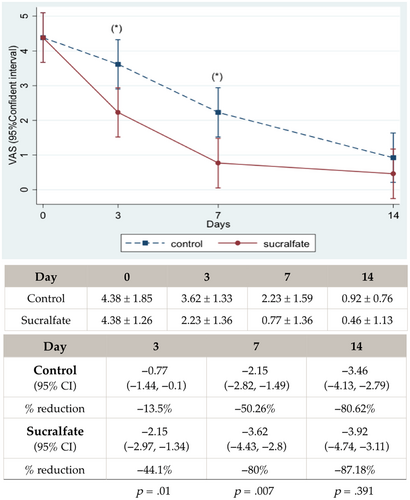
For the primary outcome, overall patient VAS at day 0 had a normal distribution with a mean VAS score of 4.20 and baseline had no statistical difference between the two groups. The number of rescue paracetamol use was 5.25 tablets/patient in Sucralfate group and 6 tablets/patient in control group, which was also not statistically different.
Morphine injection was not requested in any of the patient groups. There was no dropout of pain VAS data but home records of paracetamol use were missing in some OPD patients (n = 7).
For the secondary outcome, wound healing was assessed by wound grade and wound length reduction on day 7 and day 14 compared to baseline. Incomplete wound assessment data in some patients were due to transportation issues during the COVID-19 pandemic lockdown in Thailand. Data analysis by intention to treat and per protocol were carried out and were found to have the same statistical significance. To minimize randomization bias in the small sample size, wound assessment was done through intention to treat analysis.
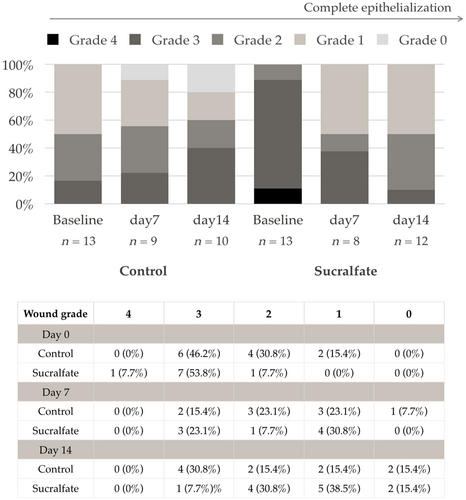
Figure 3 demonstrates wound grade distribution on day 0, 7, and 14 for both control and Sucralfate group. There were no statistical differences in the 2-week interval. The majority of the patients started with grade 3 wound however, only 15.4% achieved grade 0 (complete epithelialization) in the 2-week postoperative follow up.
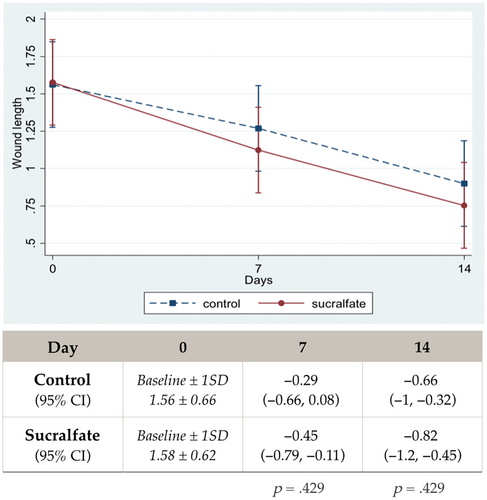
Overall mean wound length at baseline of both groups was 1.57 cm with normal distribution. Figure 4 demonstrates wound length reduction over time. In the Sucralfate group, mean wound length slightly decreased more between groups but was not statistically different on either day 7 or day 14.
No adverse reaction of Sucralfate was reported. Some patients comment on the administration frequency needed to reapply.
4 DISCUSSION
The oral surgical wound is painful and is associated with prolonged healing. There are limited topical analgesics for pain management. Our study shows an initial moderate pain score of 4.20 at day 0 with the same baseline characteristics between groups. After a 14-day period of Sucralfate in the experimental group, the pain score was significantly reduced early on in the postoperative period (p = .007 on day 7). On day 3, the Sucralfate group had a significant reduction in pain score of up to 44.1% from baseline while in the control group, it took more time for pain score to be reduced by 50.26% on day 7. This correlates with the previous study of post tonsillectomy1 and post uvuloplasty wound2 in which Sucralfate has a great effect on improving postoperative pain. This could be explained by the mechanism of Sucralfate and how direct contact with the open wound surface can seal it underneath from the external environment. However, the coating barrier formed by Sucralfate in this suspension form (1 g/5 mL) wears off from the epithelial surface in 5–6 h,7 so it is recommended to reapply Sucralfate every 5–6 h.
Adverse reactions from Sucralfate use have been reported as minor and rare including bloating and constipation in high dosages. Systemic absorption is only 2%–5% through the gastrointestinal tract. Sucralfate use of up to 12 g/day is not a lethal dose.6 In our study, we found no adverse effect from Sucralfate and in terms of wound healing, Sucralfate also shows no interference with the healing process. A previous clinical trial studying Sucralfate use in post tonsillectomy wound1 and major aphthous ulcer3 even found that Sucralfate accelerates reepithelialization time. Theoretically, Sucralfate could facilitate the healing process by recruiting essential cytokines in the inflammatory stage (days 0–3) and by its coating effect, provides an optimum wound healing environment in the proliferative or reepithelialization stage (weeks 1–2) of wound healing. However, in this study Sucralfate group showed a neither superior nor inferior effect in wound healing promotion. Wound grade distribution and wound length reduction were the same at all time points in both groups over the 2-week postoperation period.
Although there are no previous clinical studies on the wound healing process, specifically in the oral surgical wound, a preclinical experimental study7 and some previous clinical studies in oral surgical secondary healing wound1, 2, 8 have reported that it takes about 2 weeks to complete epithelialization similar to other areas of the mucosal wound. Still, only 15.4% of our sample population had complete reepithelialization on day 14.
4.1 Limitations
Due to the artificial pineapple flavor of Sucralfate suspension used in this study, patients in the experimental group could not be blinded. Finally, there were many patient dropouts during the research period and the number of potential participants was limited as a result of the COVID-19 pandemic lockdown restrictions in 2019.
5 CONCLUSION
Sucralfate suspension oral rinse can be recommended as an effective topical analgesia in postoperative secondary healing intraoral wounds with no significant interference in wound healing and rare systemic side effects. Further study in larger sample population or multicenter trial should be conducted to confirm the treatment effect in wound healing promotion. More specific studies on the wound healing process and scoring systems in the surgical oral wound are warranted to advance oral wound care for oral surgical patients.
ACKNOWLDGMENTS
Sample products and financial support for participants during the research period were sponsored by Siam Pharmaceutical Company Limited.




