Functional characterization of rat plasma membrane monoamine transporter in the blood–brain and blood–cerebrospinal fluid barriers
Abstract
This study investigated the expression and functional roles of rat plasma membrane monoamine transporter (rPMAT) in the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier by using in vitro brain barrier model cells (TR-BBB13 and TR-CSFB3 cells) and multiple in vivo experimental techniques. Quantitative reverse transcription-polymerase chain reaction analysis showed relatively high expression of rPMAT mRNA in TR-BBB13 and TR-CSFB3 cells. 1-Methyl-4-phenylpyridinium (MPP+) was transported into rPMAT-expressing cells in a sodium-independent manner. [3H]MPP+ was taken up concentration dependently by TR-BBB13 and TR-CSFB3 cells with Km values similar to that of rPMAT-expressing cells. [3H]MPP+ transports into these cells were markedly inhibited by serotonin, dopamine, and cationic drugs. rPMAT small interfering RNA (siRNA) significantly suppressed the [3H]MPP+ uptake by TR-BBB13 cells. Intracerebrally injected [3H]MPP+ was eliminated from the brain parenchymal region, whereas brain [3H]MPP+ uptake did not increase with time during in situ brain perfusion, suggesting that the brain-to-blood transport across the BBB predominates over the blood-to-brain transport. Brain microdialysis studies revealed that the elimination across the BBB was significantly decreased by coperfusion of unlabelled MPP+, serotonin, or dopamine. [3H]MPP+ was also eliminated from the CSF. These findings suggest that PMAT in brain barriers functions as the brain-to-blood transporter to regulate brain concentrations of organic cations including monoamines and cationic neurotoxins. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3924–3938, 2011
INTRODUCTION
The blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB) are formed by the brain capillary endothelium and choroid plexus epithelium, respectively, which are cemented by high-resistance tight junctions. Owing to the presence of these brain barriers, solute exchange between blood and brain interstitial fluid and/or cerebrospinal fluid (CSF) is tightly restricted.1,2 However, the presence of multiple transporters gives the BBB and the BCSFB additional functionality; these transporters mediate not only the blood-to-brain transport for supply of nutrients but also the brain-to-blood transport to excrete endogenous and exogenous compounds from the brain.3 For example, glucose transporter (GLUT1/SLC2A1), monocarboxylate transporter (MCT1/SLC16A1), large neutral amino acid transporter (LAT1/SLC7A5) with 4F2hc (SLC3A2), and organic cation/carnitine transporter 2 (OCTN2/SLC22A4) mediate the blood-to-brain influx transport of nutrients.4 Efflux transporters present at the BBB include P-glycoprotein (ABCB1), multidrug resistance protein 4 (MRP4/ABCC4), and breast cancer resistance protein (BCRP/ABCG2).3 In addition, organic anion transporter 3 (OAT3/SLC22A8) and/or organic anion transport polypeptide 1a4 (Oatp1a4/Slco1a4) mediate the brain-to-blood efflux transport of endogenous metabolites across the BBB.3
In relation to the monoamine transporters at the brain barriers, immunohistochemical analysis has revealed that serotonin transporter (SERT/SLC6A4) and norepinephrine transporter (NET/SLC6A2) are expressed at the brain capillary endothelium.5 These monoamine transporters at the brain barriers have been suggested to regulate the levels of monoamines linked with brain edema, endotoxemia, and ischemia. Indeed, biogenic monoamines have been reported to be taken up by the brain capillary endothelium and choroid plexus epithelium.6–8 In addition, it has been reported that serotonin uptake by immortalized brain capillary endothelium includes an Na+-independent component,8 indicating the existence of an additional transporter different from SERT and NET.
Recently, plasma membrane monoamine transporter (PMAT/SLC29A4) has been identified as a novel monoamine transporter, which is expressed in the brain neuronal cells and mediates reuptake of monoamine neurotransmitters and a cationic neurotoxin, 1-methyl-4-phenylpyridinium (MPP+), in an Na+-independent manner with relatively low affinity and high capacity.9–12 PMAT has been suggested to play a role in monoamine reuptake by supplementing the function of high-affinity monoamine transporters such as SERT and NET, especially when interstitial concentrations of monoamines reach high levels. Because SERT and NET are high-affinity and low-capacity transporters with Km values in the nanomolar range (400–600 nM),13,14 they would be easily saturated by the high concentrations of monoamines released from the nerve terminals.
On the basis of the above findings we hypothesized that PMAT would be functionally expressed at the BBB and the BCSFB to regulate monoamine levels in the brain by removing excess amounts of monoamines from brain to the blood circulation. In this study, we characterized the transport function of rat PMAT (rPMAT) cloned from BBB cells and investigated the mRNA expression and function of rPMAT using conditionally immortalized cell lines, TR-BBB13 and TR-CSFB3, as in vitro models for BBB and BCSFB, respectively. For the in vivo functional characterization of rPMAT at the brain barriers, the blood-to-brain influx transport of [3H]MPP+ across the BBB was measured by the in situ brain perfusion method. The brain-to-blood efflux transport of [3H]MPP+ across the BBB was evaluated by the brain efflux index (BEI) and the brain microdialysis methods. Finally, the elimination of [3H]MPP+ from the cerebrospinal fluid was measured after intracerebroventricular (i.c.v.) administration.
MATERIALS AND METHODS
Chemicals
[3H]MPP+ (85.5 Ci/mmol) and [14C]inulin (2.06 mCi/g) were purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, Massachusetts). All other chemicals and reagents were commercial products of reagent grade.
Cell Culture
TR-BBB13 and TR-CSFB3 cells are stable, immortalized rat brain capillary endothelium and choroid plexus epithelium, respectively, derived from transgenic rats harboring temperature-sensitive Simian virus 40 (SV40) large T-antigen, and they express several transporters and retain the in vivo transport rate of several compounds.15–17 These cells were cultured in Dulbecco's modified Eagle's medium (Nissui Pharmaceutical Company, Tokyo, Japan) supplemented with 20 mM sodium bicarbonate, 4.5 g/L d-glucose, 100 U/mL benzylpenicillin potassium, 100 µg/mL streptomycin sulfate, and 10% fetal bovine serum (FBS; Moregate, Bulimba, Australia). They were maintained in 5% CO2 at 33°C, which is the permissive temperature at which the temperature-sensitive SV40 large T-antigen is activated. T-REx Chinese hamster ovary (CHO) cells (Life Technologies, Carlsbad, California) were grown in Nutrient Mixture F-12 HAM (Sigma, St. Louis, Missouri) supplemented with 10% FBS (Biowest, Nuaille, France) and 1% Penicillin–Streptomycin (Life Technologies) in 5% CO2 at 37°C.
Animals
Adult, male Wistar rats weighing approximately 350 g were purchased from Japan SLC (Shizuoka, Japan); they were housed, two or one per cage, with free access to food and water and were maintained on a 12-h dark/12-h light cycle in a room with controlled temperature (24 ± 2°C) and humidity (55 ± 5%). This study was conducted according to the guidelines approved by the Experimental Animal Ethical Committee of Teikyo University.
Isolation of rPMAT Gene and Construction of CHO Cells Expressing rPMAT or Flag-Tagged rPMAT
The rPMAT gene was isolated from TR-BBB13 cells by reverse-transcription polymerase chain reaction (RT-PCR) using Pfx DNA polymerase (Life Technologies). The sequences of primers were as follows: forward primer, 5′-TGC CAT GGG CTC CAT TGG AA-3′; reverse primer, 5′-CTG GCT CAG GGG CCA ACA AG-3′. A 1.58-kb DNA fragment was cloned into pCR2.1-TOPO (Life Technologies). The obtained complementary DNA (cDNA) sequence was 100% identical to the sequence of XM_001072979. The DNA fragment containing the coding region of rPMAT was amplified with the following primers: forward primer, 5′-GGA ATT C CA CCA TGG GCT CCA TTG GAA GCC AG-3′ (underlined letters show the EcoRI site); reverse primer, 5′-TTT TTT CTC GAG TCA GGG GCC AAC AAG GAT GGA G-3′ (underlined letters show the XhoI site). The DNA fragment containing rPMAT with N-terminal FLAG tag was also amplified with a forward primer, 5′-GG AAT TC C ACC ATG GAT TAC AAG GAT GAC GAC GAT AAG ATG GGC TCC ATT GGA GCC AG-3′ (underlined and bold letters show the EcoRI site and the DNA sequence encoding the FLAG tag, respectively) and the above reverse primer containing the XhoI site. The cDNA including rPMAT or FLAG–rPMAT was subcloned into pcDNA4/TO (Life Technologies). The resultant plasmid (rPMAT/pcDNA4/TO or FLAG-rPMAT/pcDNA4/TO) was transfected with Lipofectamine™ 2000 (Life Technologies) into T-REx CHO cells (Life Technologies), which stably express tetracycline repressor protein. The rPMAT/T-Rex CHO cells or FLAG-rPMAT/T-REx CHO cells were isolated by selection with zeocin and blasticidin according to the manufacturer's protocol. rPMAT/T-Rex CHO cells or FLAG-rPMAT/T-Rex CHO cells were induced to express rPMAT or FLAG–rPMAT by 1 µg/mL tetracyclin.
Preparation of Rat Brain Capillary Fraction
The brain capillary fraction was prepared from rat cerebrum by the glass bead column method.18 Cerebrum excised from rats was cut into pieces and homogenized in Hanks' balanced salt saline. The homogenate was added to the same volume of 32% dextran solution and the mixture was centrifuged (4500× g, 10 min, 4°C). The resulting pellets were suspended in buffer A [100 mM NaCl, 4.6 mM KCl, 2.5 mM CaCl2, 0.91 mM KH2PO4, 1.2 mM MgSO4·7H2O, 15 mM 4-(2-hydroxyethyl)- 1-piperazineethanesulfonic acid (HEPES), 10 mM d-glucose, 1.0 mM pyruvate, 25 mM NaHCO3, 1% bovine serum albumin (BSA), pH 7.4]. This fraction was passed through an 85-µm nylon mesh filter. The filtrate was applied to a column of glass beads (350–500 µm). The column was washed with buffer A, and the brain capillaries attached to the glass beads were isolated by gentle shaking to give the brain capillary fraction.
Real-Time PCR Analysis
The mRNA levels of organic cation transporters in TR-BBB13 or TR-CSFB3 cells were measured by quantitative real-time PCR analysis.19,20 Total RNA was isolated from TR-BBB13 or TR-CSFB3 cells with an RNeasy mini kit (QIAGEN, Valencia, California). Single-strand cDNA was prepared from 1.0 µg of total RNA by reverse transcriptase (Superscript III, Life Technologies) using oligo (dT) primer. Quantitative real-time PCR analysis was performed with a 7500 sequence detection system (Life Technologies) with 2× SYBR Green PCR Master Mix (Life Technologies) according to the manufacturer's protocols. Rat genomic DNA was used as a standard in quantitative PCR. The set of primers were 5′-GGC CTA TTG CAC CTA CAG CCT-3′ and 5′-CGG TGG CTG TTT GAA AGC A-3′ for rPMAT; 5′-TGG GCC ATC TTC CTC AAC A-3′ and 5′-TCT GGA TCC TGC CTT AGG AAC A-3′ for rat multidrug and toxin extrusion (rMATE) 2. The primers for rat organic cation transporter (rOCT) 1, rOCT2, rOCT3, rOCTN1, rOCTN2, rMATE1, and rat glyceraldehyde-3-phosphate dehydrogenase (rGAPDH) were used as described in a previous report.19 The thermal protocol was set to 2 min at 50°C, followed by 10 min at 95°C, and then 40 cycles of 15 s at 95°C and 1 min at 60°C. Total RNA was isolated from rat whole brain, brain capillary fraction, and choroid plexus; then, single-strand cDNA was prepared and quantitative real-time PCR was performed as described above. The relative expression levels of rPMAT mRNA in rat tissues were calculated using the comparative Ct (2−ΔΔCt) method for relative quantification based on GAPDH mRNA expression according to the manufacturer's protocols. The control, lacking the reverse transcription (RT) enzyme, was assayed in parallel to monitor genomic contamination. To confirm specificity of amplification, the PCR products were subjected to melting curve analysis.
In Vitro Uptake Studies
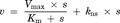 ()
()Immunocytochemical and Western Blot Analyses of CHO Cells Expressing FLAG–rPMAT
The expression of FLAG–rPMAT was measured by immunocytochemical and western blotting analysis.21 Briefly, FLAG–rPMAT/T-Rex CHO cells were seeded on Glass Bottom Culture Dishes (MatTek, Ashland, Massachusetts). After incubation with 1 µg/mL tetracycline for 24 h, the CHO cells were fixed with 4% paraformaldehyde and treated with 1% Triton-X 100. The cells were then incubated with a mixture of 1 µg/mL monoclonal antibody against FLAG (Sigma) and 5 µg/mL anti-calnexin antibody (Stressgen Biotechnologies, Victoria, Canada) at room temperature for 1 h and further incubated with Alexa-488-conjugated anti-mouse immunoglobulin G (IgG) antibody (Life Technologies) and Alexa-546-conjugated anti-rabbit IgG antibody (Life Technologies). They were viewed with a confocal laser microscope, Leica TCS-SP5 (Leica, Wetzlar, Germany). For western blotting analysis, homogenates from FLAG–rPMAT/T-Rex CHO cells after the tetracycline treatment for designated times (3 µg protein) were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently electrotransfered to polyvinylidene difluoride membrane (Atto, Tokyo, Japan). The membrane was blocked with Immuno Block (Dainippon Sumitomo Pharma Company, Ltd., Osaka, Japan) at 4°C overnight. The membrane was then incubated with anti-FLAG monoclonal antibody (1:1000) at 4°C overnight and further incubated with anti-mouse IgG (1:1000) (GE Healthcare UK Ltd., Buckinghamshire, England). Immunoreactivity was visualized with an enhanced chemiluminescence kit (GE Healthcare).
RNA Interference Analysis
The small interfering RNA (siRNA) for the target sequence of rPMAT cDNA (CCT GTC AGA CTT TGT GGG CAA) was purchased from QIAGEN. Nonsilencing siRNA (QIAGEN) was used as a negative control. TR-BBB13 cells were seeded on collagen-coated multiwell dishes and grown for 24 h, then transfected with rPMAT siRNA or negative control siRNA (20 or 50 pmol/well) using Lipofectamine™ 2000 (Life Technologies). The uptake of [3H]MPP+ (25 nM, for 3 min) was measured as described above at 48 h after the transfection.
In Situ Brain Perfusion Study
Brain perfusion was performed by the method reported previously.19,20 In brief, each rat was anesthetized and the right carotid artery was catheterized with polyethylene tubing (SP-10) filled with sodium heparin (100 IU/mL). The perfusate (Krebs–Henseleit buffer: 118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, 10 mM d-glucose, pH 7.4) containing [3H]MPP+ (4.2 nM, 0.37 µCi/mL) and [14C]inulin (0.09 µCi/mL), a brain intravascular marker, was passed through the catheter at the rate of 2.0 mL/min with an infusion pump (Harvard Apparatus, South Natick, Massachusetts). After the infusion pump is started, 10 s is required to fill the external carotid artery cannula.22 Therefore, 10 s was subtracted routinely from the gross perfusion time in each experiment. At the end of the uptake for 1–2 min, rats were decapitated and the right cerebral hemisphere was dissected. The samples were solubilized and the radioactivity was measured as described above.
BEI Study
 ()
()The elimination rate constant of [3H]MPP+ from the brain was obtained by least squares fitting of a plot of the 100 − BEI (%) data versus time.
Brain Microdialysis
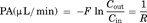 ()
()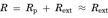 ()
()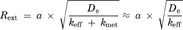 ()
()Intracerebroventricular Administration
The elimination of [3H]MPP+ from the CSF after i.c.v. administration was studied as described previously.27 [3H]MPP+ (640 nM, 0.5 µCi) and [14C]inulin (0.01 µCi), a reference compound, were dissolved in 10 µL of artificial CSF (122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 mM K2HPO4, 10 mM d-glucose, and 10 mM HEPES, pH 7.3). Rats were anesthetized and the tracer solution (10 µL) in the presence or absence of inhibitor was injected into the left ventricle, and aliquots of CSF were withdrawn by cisternal puncture at designated times. The CSF specimens were assayed, as described above, to determine the radioactivity.
Statistical Analysis
Statistical analysis of the data were performed using Student's t-test and one-way analysis of variance, followed by Dunnett's test for single and multiple comparisons, respectively. Differences were considered statistically significant at a P value of less than 0.05.
RESULTS
Isolation of rPMAT cDNA from TR-BBB13 Cells
Rat plasma membrane monoamine transporter cDNA was isolated from TR-BBB13 cells. The open reading frame encodes a protein of 523 amino acid residues with a calculated molecular mass of 58 kDa. A basic local alignment search tool search revealed that the deduced amino acid sequence of rPMAT was highly homologous to those of human PMAT (87%) and mouse PMAT (97%). Hydropathy analysis predicted the presence of 10–12 membrane-spanning domains in rPMAT.
Gene Expression of Organic Cation Transporters
The mRNA level of rPMAT was the highest, followed by rOCTN2, in both TR-BBB13 and TR-CSFB3 cells, whereas the expression of rOCT1–3, rOCTN1, and rMATE1–2 was negligible (Table 1). Another rat brain capillary endothelial cell line, RBEC1, also has a high expression level of rPMAT mRNA [1010 ± 100 copies/ng total RNA, mean ± standard error (SE), n = 3]. rPMAT mRNA expression levels in isolated rat brain capillaries and choroid plexus were 2.4- and 8.3-fold, respectively, greater than that in the whole brain (Fig. 1).
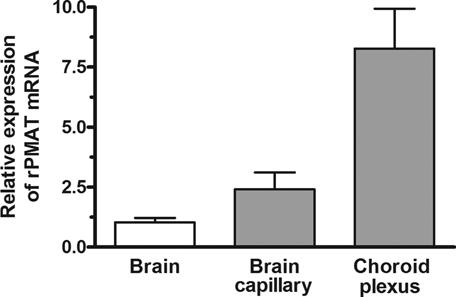
Expression levels of rPMAT mRNA in the whole brain, brain capillary, and choroid plexus of rats. rPMAT mRNA expression was determined by SYBR green real-time PCR analysis in the whole brain, brain capillaries, and choroid plexus of rats. The results were calculated using the comparative Ct (2−ΔΔCt) method for relative quantification based on GAPDH mRNA expression and are shown as a fraction of relative rPMAT expression in whole brain. Each value represents the mean ± standard error (SE) of three determinations.
| mRNA Expression (Copies/ng Total RNA) | ||
|---|---|---|
| TR-BBB13 | TR-CSFB3 | |
| rOCT1 | 0.7 ± 0.4a | 1.5 ± 0.7 |
| rOCT2 | 2.3 ± 0.1a | 19.2 ± 1.9 |
| rOCT3 | 2.3 ± 0.6a | 9.0 ± 0.9 |
| rOCTN1 | 8.9 ± 0.3a | 29.4 ± 5.3 |
| rOCTN2 | 1254 ± 92a | 1621 ± 53 |
| rPMAT | 2543 ± 151 | 3849 ± 484 |
| rMATE1 | 1.3 ± 0.6a | 3.1 ± 0.5 |
| rMATE2 | 1.3 ± 0.4 | 13.8 ± 2.9 |
| rGAPDH | 4124 ± 90a | 3032 ± 174 |
- Each value represents the mean ± SE of three or four determinations.
- aData taken from Okura et al.19
Subcellular Localization and Transport Kinetics in rPMAT-expressing CHO Cells
To determine the cellular localization of rPMAT, we designed an expression construct that has a FLAG tag at the N-terminus of rPMAT. When FLAG–rPMAT was expressed in CHO cells using a tetracycline-inducible system, there was no coincidence of reactivities of FLAG–rPMAT (green) and calnexin (red), a marker protein of endoplasmic reticulum (Fig. 2). These data suggest that FLAG–rPMAT was primarily localized to the plasma membrane of CHO cells. In western blotting analysis, CHO cells expressing FLAG–rPMAT showed a strong band of immunoreactivity with an apparent MW of around 55 kDa (Fig. 3a). The multiple bands may represent different glycosylation states of this transporter because rPMAT has a potential N-linked glycosylation site (Asn515). The expression of FLAG–rPMAT was the highest at 24 h after the tetracycline treatment, and the uptake of [3H]MPP+ reached the maximum level at 24 h after the tetracycline treatment (Fig. 3b). The increase in [3H]MPP+ uptake paralleled the expression of FLAG–rPMAT, indicating that the [3H]MPP+ transport is mediated by FLAG–rPMAT.
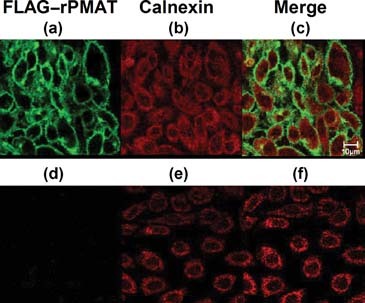
Confocal microscopy imaging of tetracycline-induced FLAG–rPMAT-expressing CHO cells. The cells were immunostained with anti-FLAG antibody (green) or anti-calnexin antibody (red) after treatment (a–c) with or (d–f) without tetracycline. (c and f) Merged pictures of FLAG–rPMAT and calnexin immunoreactivity.
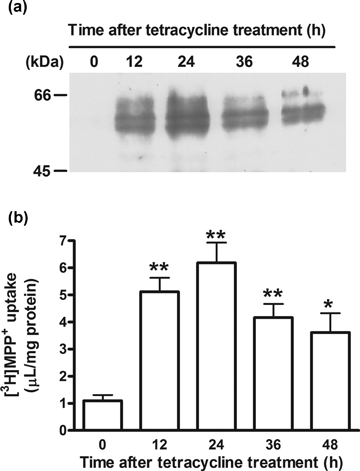
(a) Western blot analysis of FLAG–rPMAT and (b) functional analysis of [3H]MPP+ transport in FLAG–rPMAT/T-Rex CHO cells after treatment with tetracycline (0–48 h). Immunoblotting was carried out with the anti-FLAG antibody at 1:1000 dilution. Uptake of [3H]MPP+ (25 nM) was measured at 37°C for 3 min. Each column represents the mean ± SE of three determinations. Asterisks show a significant difference, *P < 0.05, **P < 0.01 versus 0 h.
The [3H]MPP+ uptake increased with time, and reached 4 µL/mg protein at 15 min in rPMAT- and FLAG–rPMAT-expressing CHO cells (Figs. 4a and 4b). The initial uptake, which was assessed as the uptake at 3 min, by rPMAT and FLAG–rPMAT was concentration dependent, with Km values of 36 and 25 µM, and Vmax values of 40 and 47 pmol/(mg protein min), respectively (Figs. 4c and 4d). The similarity of the kinetic parameters in rPMAT- and FLAG–rPMAT-expressing CHO cells suggests that the N-terminal FLAG tag did not interfere with the membrane localization or transport function of PMAT.
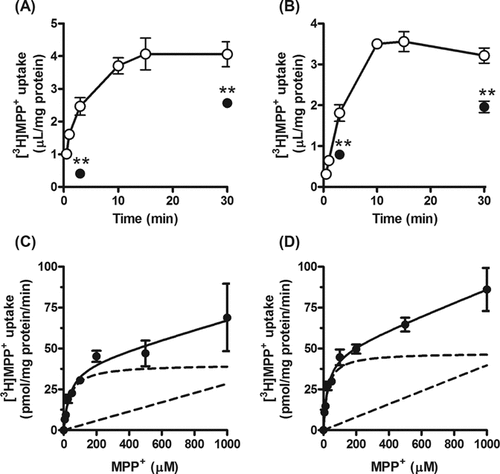
Time course and concentration dependence of uptake of [3H]MPP+ by (a and c) CHO cells expressing rPMAT and (b and d) FLAG–rPMAT. (a and b) [3H]MPP+ (25 nM) uptake was measured after treatment with ◯ or without (•) tetracycline. Each point represents the mean ± SE of four determinations. Asterisks show a significant difference, **P < 0.01 versus tetracycline treatment. (c and d) Uptake of [3H]MPP+ was measured at 37°C for 3 min. The total uptake (•) was analyzed as the sum of saturable (dashed curve) and nonsaturable (dashed line) components. Each point represents the mean ± SE of four determinations.
Inhibition Study in rPMAT-expressing CHO Cells
In the inhibition study, the uptake of [3H]MPP+ was significantly inhibited by unlabeled MPP+ and biogenic amines (serotonin and dopmamine) by more than 70%. Cationic drugs (amantadine, verapamil, quinidine, and procainamide), which have positive charge at physiological pH and relatively high hydrophobicity (i.e., type II cations), greatly inhibited [3H]MPP+ uptake in the PMAT-expressing CHO cells by more than 60% (Table 2). Although norepinephrine and choline moderately inhibited the uptake of [3H]MPP+, histamine, tetraethylammonium, guanidine, L-carnitine, thiamine, and p-aminohippuric acid did not inhibit the [3H]MPP+ uptake.
| Relative Uptake (% of Control) | |||
|---|---|---|---|
| rPMAT/CHO | TR-BBB13 | TR-CSFB3 | |
| Control | 100 | 100 | 100 |
| MPP+ | 12.6 ± 0.7** | 5.77 ± 0.5** | 30.5 ± 2.7** |
| Serotonin | 23.0 ± 1.3** | 25.7 ± 6.9** | 32.0 ± 3.2** |
| Dopamine | 36.6 ± 1.6** | 62.7 ± 7.8* | 38.2 ± 3.3** |
| Norepinephrine | 67.7 ± 6.1** | 58.0 ± 11.7** | 57.7 ± 6.3** |
| Histamine | 93.0 ± 7.6 | 69.1 ± 12.6 | 68.4 ± 7.7** |
| Amantadine | 39.5 ± 2.8** | 28.8 ± 6.0** | 35.9 ± 2.9** |
| Verapamil | 12.7 ± 1.1** | 25.6 ± 3.0** | 25.7 ± 5.3** |
| Quinidine | 14.1 ± 1.5** | 2.02 ± 1.9** | 24.5 ± 3.4** |
| Procainamide | 30.5 ± 1.6** | 15.9 ± 6.6** | 32.5 ± 1.6** |
| Tetraethylammonium | 90.5 ± 2.4 | 56.4 ± 6.3** | 77.6 ± 3.9* |
| Guanidine | 91.3 ± 7.3 | 43.1 ± 12.7** | 76.4 ± 1.8* |
| L-Carnitine | 109 ± 4.5 | 56.5 ± 7.7** | 79.2 ± 3.1* |
| Choline | 77.2 ± 5.0** | 62.8 ± 12.1* | 75.7 ± 6.1** |
| Thiamine | 111 ± 7.7 | 65.0 ± 10.4* | 66.8 ± 4.2** |
| p-Aminohippuric acid | 133 ± 5.0 | 98.4 ± 5.9 | 83.9 ± 14.1 |
- Uptake of [3H]MPP+ (25 nM) was measured for 3 min at 37°C in transport medium (pH 7.4) containing each compound (1 mM).
- Each value represents the mean ± SE of three or six determinations.
- *P < 0.05, **P < 0.01 versus control.
Characterization of rPMAT-Mediated Transport
Replacement of Na+ with N-methylglucamine+ in the uptake medium did not change the [3H]MPP+ uptake (Fig. 5a). In contrast, depolarization of cells with increased extracellular K+ or treatment with valinomycin, a potassium ionophore, caused a significant decrease in the [3H]MPP+ uptake (Fig. 5b). Metabolic inhibitors (rotenone or sodium azide) did not reduce the [3H]MPP+ uptake (data not shown). As shown in Figure 5c, the [3H]MPP+ uptake was increased at lower extracellular pH (6.0), whereas no increased uptake of [3H]MPP+ was observed in the cells without tetracycline treatment.
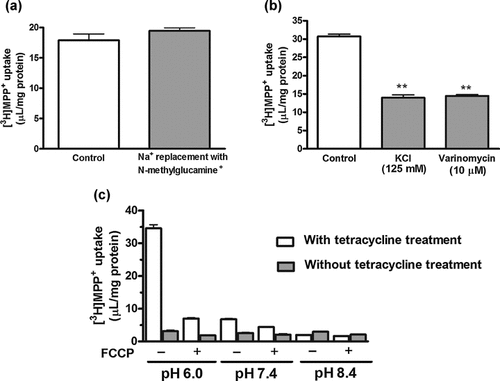
Effect of (a) sodium replacement, (b) membrane potential, and (c) extracellular pH and protonophore on rPMAT-mediated [3H]MPP+ uptake. Uptake of [3H]MPP+ (25 nM) was measured at 37°C for 3 min. (a) The uptake was measured in sodium-containing (control) or sodium-free (Na+ replaced with N-methylglucamine+) buffer. (b) NaCl in the buffer was replaced by KCl. Valinomycin (10 µM) was preincubated for 10 min. (c) The medium pH was changed to 6.0, 7.4, and 8.4 with (+) or without (–) pretreatment with p-trifluoromethoxyphenylhydrazone (FCCP) (10 µM for 10 min). Each column represents the mean ± SE of three determinations.
[3H]MPP+ Transport in TR-BBB13 and TR-CSFB3 Cells
For the functional characterization of rPMAT in brain barrier cells, we examined [3H]MPP+ uptake by TR-BBB13 and TR-CSFB3 cells, which were used as in vitro models of BBB and BCSFB, respectively. The [3H]MPP+ uptakes in TR-BBB13 and TR-CSFB3 cells were time and concentration dependent, with Km values of 108 and 42 µM and Vmax values of 32 and 24 pmol/(mg protein min), respectively (Figs. 6a and 6b). These Km and Vmax values were comparable to the values for [3H]MPP+ transport measured in rPMAT-expressing CHO cells. Replacement of Na+ with N-methylglucamine+ in the uptake medium did not change the [3H]MPP+ uptake into either TR-BBB13 or TR-CSFB3 cells (Figs. 6c and 6d), in agreement with the results in rPMAT-expressing CHO cells. In inhibition studies, most of the organic cations examined showed significant decreases in [3H]MPP+ uptake by TR-BBB13 and/or TR-CSFB3 cells (Table 2). In common with the inhibitory effects on rPMAT-mediated transport, [3H]MPP+ uptakes by both TR-BBB13 and TR-CSFB3 cells were dramatically inhibited by biogenic monoamines (serotonin and dopamine) and cationic drugs (amantadine, verapamil, quinidine, and procainamide), but not an organic anion (p-aminohippuric acid) (Table 2). Norepinephrine, histamine, tetraethylammonium, guanidine, l-carnitine, choline, and thiamine moderately inhibited [3H]MPP+ uptake by TR-BBB13 and/or TR-CSFB3 cells by 21%–57%.
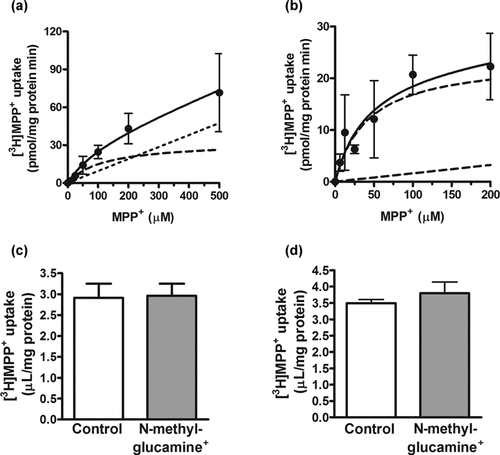
Concentration dependence and extracellular sodium independence of [3H]MPP+ uptake by (a and c) TR-BBB13 and (b and d) TR-CSFB3 cells. (a and b) Uptake of [3H]MPP+ was measured at 37°C for 3 min. The total uptake (•) was analyzed as the sum of saturable (dashed curve) and nonsaturable (dashed line) components. (c and d) Uptake of [3H]MPP+ (25 nM) was measured at 37°C for 3 min in sodium-containing (control) or sodium-free (Na+ replaced with N-methylglucamine+) buffer. Each value represents the mean ± SE of three to five determinations.
Effects of rPMAT siRNA on [3H]MPP+ Uptake by TR-BBB13 Cells
To confirm the involvement of rPMAT in [3H]MPP+ transport in TR-BBB13 cells, we examined the effects of rPMAT siRNA on [3H]MPP+ uptake. The rPMAT siRNA significantly decreased [3H]MPP+ uptake in rPMAT-expressing CHO cells by 72% (data not shown), indicating that rPMAT siRNA effectively suppressed rPMAT function. Similarly, the rPMAT siRNA significantly reduced [3H]MPP+ transport in TR-BBB13 cells by 34% (20 pmol/well) and 59% (50 pmol/well) (Fig. 7). On the contrary, negative control siRNA did not change the uptake. These results suggest that rPMAT mediates [3H]MPP+ transport in TR-BBB13 cells, at least in part.
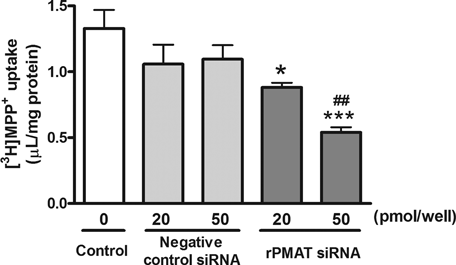
Effect of rPMAT siRNA on [3H]MPP+ uptake into TR-BBB13 cells. TR-BBB13 cells were treated with or without (control) negative control siRNA or rPMAT siRNA (20 and 50 nmol/well) using Lipofectamine 2000™. The [3H]MPP+ uptake (25 nM) was determined 48 h after the siRNA transfection. Each column represents the mean ± SE of four determinations. *P < 0.05, ***P < 0.001 versus control, ##P < 0.05 versus negative control siRNA.
[3H]MPP+ Transport into the Brain Across the BBB
For the in vivo functional characterization of rPMAT at the BBB, we measured the transport of [3H]MPP+ across the BBB. [3H]MPP+ transport into the brain across the BBB was determined by the in situ rat brain perfusion method. The brain/perfusate (B/P) ratios of [3H]MPP+ were 0.031 and 0.029 mL/g of brain at 1 and 2 min perfusion time, respectively (Fig. 8a), indicating that the B/P ratio did not increase with perfusion time up to 2 min. The B/P ratio of [14C]inulin, a vascular space marker, was 0.005–0.006 mL/g of brain. This value is comparable to the previously reported values (0.007–0.017 mL/g of brain).19,28 The B/P values of [3H]MPP+ were six times larger than the vascular space evaluated with [14C]inulin, suggesting that the B/P ratio of [3H]MPP+ may include extremely rapid binding to the capillary surfaces. The B/P ratio of [3H]MPP+ is comparable to the B/P ratios at time zero of choline (0.024 mL/g of brain)28 and oxycodone (0.044 mL/g of brain)19.
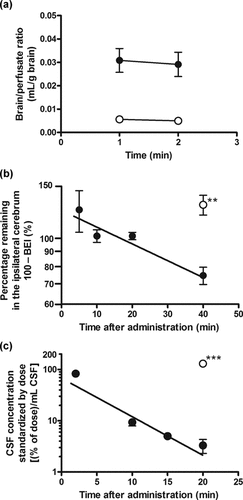
(a) Blood-to-brain, (b) brain-to-blood, and (c) CSF-to-blood transport of [3H]MPP+ across the brain barriers determined by the in situ brain perfusion, BEI, and i.c.v. administration methods, respectively, in rats. (a) Brain uptake of [3H]MPP+ (•) and [14C]inulin ◯ determined by the in situ rat brain perfusion method. The perfusate containing [3H]MPP+ (4.2 nM) and [14C]inulin (0.09 µCi/mL) was perfused into the brain hemisphere through a carotid arterial catheter at a rate of 2.0 mL/min for 1–2 min. Each value represents the mean ± SE of three determinations. (b) Time course of [3H]MPP+ remaining in the ipsilateral cerebrum after intracerebral administration in rats. Rats received intracerebral administration of [3H]MPP+ (4.1 µM) into the Par2 region of the brain in the presence of [14C]inulin as an internal reference. The points were fitted to the first-order elimination rate equation (r2 = 0.874). Open circle represents the case of coadministration of unlabeled MPP+ (100 mM). Each point represents the mean ± SE of four to nine determinations. An asterisk indicates a significant difference, **P < 0.01 versus tracer dose of [3H]MPP+. (c) Time course of [3H]MPP+ remaining in CSF after i.c.v. administration in rats. Rats received i.c.v. administration of [3H]MPP+ (640 nM) into the lateral ventricle. The points were fitted to the first-order elimination rate (r2 = 0.887). Open circle represents the case of coadministration of unlabeled MPP+ (50 mM) into the rat lateral ventricle. Each point represents the mean ± SE of three to six determinations. An asterisk indicates a significant difference, ***P < 0.001 versus tracer dose of [3H]MPP+.
[3H]MPP+ Transport from Brain to Blood Across the BBB
The transport of [3H]MPP+ from the brain to the circulating blood across the BBB was determined by the BEI method. Intracerebrally administered [3H]MPP+ was eliminated from rat brain with an elimination rate constant of 0.013 min−1 (Fig. 8b). The elimination clearance of [3H]MPP+ was calculated to be 0.18 µL/(min cm2) on the assumptions that the distribution volume of MPP+ in the brain and the surface area of the BBB are 1.6 mL/g brain and 120 cm2, respectively.29,30 [3H]MPP+ elimination from the brain was inhibited by unlabelled MPP+ (100 mM) (Fig. 8b). The concentrations of unlabelled MPP+ in the cerebrum was estimated to be 3.3 mM from the injectate concentration (100 mM) divided by the dilution factor, that is, 30.3, which was reported previously.23
Next, we applied the brain microdialysis method, which allows continuous administration of metabolizable compounds such as monoamines through the dialysis probe, to examine [3H]MPP+ transport from the brain across the BBB. After perfusion of [3H]MPP+ via the probe in the absence or presence of unlabelled MPP+, serotonin, dopamine, or p-aminohippuric acid, the PA value in the steady state was calculated as an indicator of the elimination rate constant across the BBB. The PA value was significantly decreased by coperfusion of unlabelled MPP+ (86% decrease), serotonin (50% decrease), or dopamine (67% decrease), but not p-aminohippuric acid (Fig. 9). The replacement of Na+ with K+ in the perfusate reduced the PA value of [3H]MPP+ by 84%. The in vitro probe recovery of [3H]MPP+ was 16.9 ± 1.8% (mean ± SE, n = 7), and it did not significantly change in the absence and presence of inhibitors. The in vivo probe recovery of [3H]MPP+ was 8.99 ± 1.3% (mean ± SE, n = 3), and it remained unchanged during the experimental period.
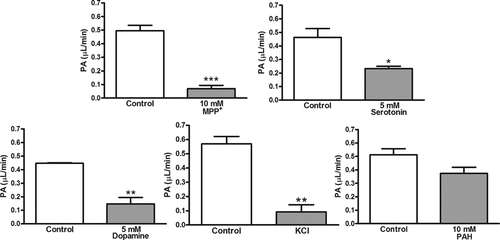
Effect of selected compounds and replacement of Na+ with K+ (KCl) on the efflux of [3H]MPP+ from rat brain determined by the microdialysis method. The perfusate containing [3H]MPP+ (1.2 nM) with or without selected compounds was perfused into the brain hippocampal region through the microdialysis probe at a rate of 5 µL/min for 90 min. Asterisks indicate a significant difference, *P < 0.05, **P < 0.01, ***P < 0.001 versus control. PAH, p-aminohippuric acid.
[3H]MPP+ Transport from CSF to Blood Across the BCSFB
To investigate the transport of [3H]MPP+ from the CSF to the circulating blood across the BCSFB, i.c.v. administration studies were performed in rats. The i.c.v.-administered [3H]MPP+ was cleared from the CSF with an elimination rate constant of 0.175 min−1 (Fig. 8c). The distribution volume (1415 µL) calculated from the ordinate intercept of Figure 8c was 5.7-fold greater than the standard CSF volume (250 µL), suggesting that MPP+ was distributed from the CSF to the surrounding brain parenchyma. The [3H]MPP+ elimination from the CSF was completely blocked by coadministration of unlabeled MPP+ (Figs. 8c and 10). Coadministration of serotonin and dopamine partially inhibited [3H]MPP+ elimination from the CSF, whereas p-aminohippuric acid had no significant effect (Fig. 10). The in vivo elimination clearance of [3H]MPP+ from the CSF was calculated to be 3.3 µL/(min cm2) by multiplying the elimination rate constant by the distribution volume and dividing by previously reported surface area of the BCSFB (75 cm2).31
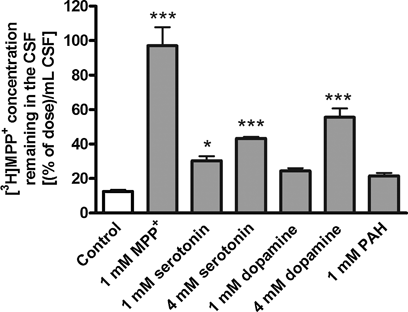
The inhibitory effects of selected compounds on the elimination of [3H]MPP+ from rat CSF after i.c.v administration. Rats received i.c.v. administration of [3H]MPP+ with or without MPP+, serotonin, dopamine, or p-aminohippuric acid (PAH). The concentrations in the CSF were estimated from the injectate amount divided by the standard CSF volume in the rat, that is, 250 µL. Each value, determined 20 min after intracerebroventricular injection, represents the mean ± SE of three to five determinations. Asterisks indicate a significant difference, *P < 0.05, ***P < 0.001 versus tracer dose of [3H]MPP+ (control).
DISCUSSION
The concentrations of monoamines in brain interstitial fluid are regulated through the operation of various influx and efflux transporters located at the brain barriers. Besides the Na+-dependent monoamine transporters, an additional transporter should exist in the brain barriers because serotonin uptake by immortalized brain capillary endothelium includes an Na+-independent uptake component.8 PMAT has recently been identified as an Na+-independent monoamine/OCT expressed in brain neuronal cells.9–12 In the current study, we investigated the mRNA expression and functional properties of rPMAT using in vitro model cells for BBB and BCSFB. Further, blood-to-brain and brain-to-blood transport across the brain barriers were examined by means of multiple in vivo experimental techniques.
Quantitative RT-PCR analysis of transporter mRNAs showed that the level of rPMAT mRNA was the highest, followed by rOCTN2 mRNA, in both TR-BBB13 and TR-CSFB3 cells, whereas the expression of rOCT1–3, rOCTN1, and rMATE1–2 mRNAs was negligible. Of these transporters, rOCTN2 has been reported to be involved in l-carnitine transport across the BBB.32 Further, rPMAT mRNA was detected in isolated brain capillaries and choroid plexus, and the mRNA levels were higher than that in the whole brain. Dahlin et al.11 showed that mouse PMAT mRNA and protein are widely expressed in neuronal cells, but not in astrocytes of mouse brain. Vialou et al.12 showed the anatomical distribution of rPMAT mRNA in various neurons in several brain regions and choroid plexus. Further, expression profiling of the solute carrier gene family in the mouse brain by high-throughput in situ hybridization data generated by the Allen Brain Atlas showed that the mPMAT gene is highly expressed in the brain and the choroid plexus.33 However, previous studies did not examine PMAT mRNA expression in the brain capillary endothelium in detail. Our present finding provides the first evidence that rPMAT mRNA is abundantly expressed not only at the BCSFB but also at the BBB.
For further functional characterization of rPMAT, rPMAT cDNA was cloned from TR-BBB13 cells. The transport of [3H]MPP+ in rPMAT-expressing CHO cells was concentration dependent with a similar Km value to that of hPMAT (33 µM).9 In the inhibition study, biogenic amines (serotonin and dopamine) and type II cations (amantadine, verapamil, quinidine, and procainamide) markedly inhibited the rPMAT-mediated [3H]MPP+ transport. Engel and Wang10 have reported that positive charge and relatively high hydrophobicity of compounds are the principal determinants for interaction with hPMAT. The [3H]MPP+ transport in rPMAT-expressing CHO cells is dependent on membrane potential, but is sodium- or metabolic energy independent and is stimulated in acidic conditions, in line with the previously reported transport properties of hPMAT.9,34 These results suggest that rat and human PMAT have similar functional properties. PMAT can contribute to monoamine transport in particular physiological conditions such as energy depletion and acidosis induced by brain ischemia.35
The transport of [3H]MPP+ in TR-BBB13 and TR-CSFB3 cells was Na+ independent, with comparable Km values to that of rPMAT. The [3H]MPP+ transport was inhibited by most of the tested organic cations. Especially, marked inhibition of the [3H]MPP+ transport by biogenic amines and cationic drugs was observed in TR-BBB13 and TR-CSFB3 cells, as well as in rPMAT-expressing CHO cells. Partial inhibitory effects by tetraethylammonium, guanidine, l-carnitine, and thiamine were observed in TR-BBB13 and TR-CSFB3 cells, but not in PMAT-expressing CHO cells, suggesting that endogenous transporters beside PMAT in these brain barrier cells were related to these inhibitory effects. Indeed, MPP+ has been reported to be a substrate for not only PMAT but also OCT1–3,36–38 DAT,39 SERT,40 and OCTN2.41 Of these transporters, rOCTN2 mRNA was expressed at a relatively high level in both TR-BBB13 and TR-CSFB3 cells. Thus, rOCTN2 may also be involved in [3H]MPP+ transport in these cells.
The treatment of TR-BBB13 cells with rPMAT siRNA resulted in a significant reduction of [3H]MPP+ transport (∼59% decrease), suggesting that rPMAT mediates [3H]MPP+ transport in TR-BBB13 cells, at least in part. The remaining uptake by TR-BBB13 cells treated with rPMAT siRNA may include [3H]MPP+ transport by transporter(s) besides rPMAT, such as rOCTN2, as discussed above. These results strongly suggest that rPMAT is involved in the [3H]MPP+ transport in TR-BBB13 cells.
The immortalized brain capillary endothelial cell lines lack tight junctions, and therefore, further in vivo study is required to clarify the physiological roles of PMAT, including transport direction (brain-to-blood and/or blood-to-brain) at the brain barriers. The blood-to-brain transport of [3H]MPP+ across the BBB was measured by the in situ rat brain perfusion method. The B/P ratio of [3H]MPP+ showed no increase with time, suggesting that little [3H]MPP+ was transported from the blood side into the brain across the BBB. On the contrary, the BEI method revealed that [3H]MPP+ was transported from the brain to blood across the BBB, and that the brain-to-blood transport was saturable. In our preliminaly experiments, serotonin did not inhibit [3H]MPP+ transport from the brain across the BBB, as shown by the BEI method. Therefore, we applied the brain microdialysis method to examine [3H]MPP+ transport from the brain across the BBB because this method enables continuous administration of metabolizable compounds, such as monoamines, through the dialysis probe. The PA value, an indicator of the elimination rate constant across the BBB, was significantly decreased by coperfusion of unlabelled MPP+, serotonin, or dopamine, but not of p-aminohippuric acid. In addition, replacement of Na+ with K+ in the perfusate reduced the PA value. These results suggest that the efflux transport of [3H]MPP+ from the brain across the BBB is inhibited by coperfusion with these inhibitors and changes in membrane potential, in accordance with the in vitro transport properties of [3H]MPP+, that is, the inhibitory effects of monoamines and potassium replacement. Thus, these in vivo transport properties across the BBB suggest that rPMAT expressed at the brain capillary is involved in [3H]MPP+ transport across the BBB in the direction from brain to blood, but not blood to brain.
[3H]MPP+ administered i.c.v. was eliminated from the CSF, and the elimination was partially inhibited by serotonin and dopamine. The in vivo inhibition was less effective than the in vitro inhibition; serotonin and dopamine inhibited [3H]MPP+ uptake as effectively as did the unlabeled MPP+ in the TR-CSFB3 cells. The calculated in vivo elimination clearance of [3H]MPP+ from the CSF [3.3 µL/(min cm2)] was 110 times greater than the in vitro clearance [0.03 µL/(min cm2)] calculated by dividing Vmax/Km values by the surface area parameter (20 cm2/mg protein) in TR-CSFB3 cells. The involvement of rOCT2 and rOCT3 in [3H]MPP+ elimination from the CSF across the BCSFB may be one reason for the difference in the in vivo and in vitro [3H]MPP+ transport because mRNAs of rOCT2 and rOCT3 were expressed in choroid plexus,42 whereas these transporters were downregulated in TR-CSFB3 cells. MPP+ is a substrate for not only rPMAT but also rOCT1–3 and rOCTN2. Specific substrates are important for in vivo analysis of transporter function, although no specific substrate or inhibitor for PMAT has been reported, probably because PMAT has a broad substrate/inhibitor specificity.10 Thus, it is difficult to separate the contributions of other transporters from PMAT-mediated transport. However, the marked rPMAT mRNA expression in the choroid plexus epithelium and the transport properties of [3H]MPP+ across the BCSFB suggest that rPMAT present at the choroid plexus mediates [3H]MPP+ transport from the CSF to the circulating blood, at least in part. The elimination clearance from the CSF [3.3 µL/(min cm2)] was higher than that from the brain parenchymal region across the BBB [0.18 µL/(min cm2)]. However, the estimated surface area of the choroid plexus is manyfold less than that of the brain capillary endothelium. Further, the microvasculature is dense in the brain parenchymal region. The intercapillary distance in the brain is very short (∼50 µm in human) and the intercapillary space is sufficient to accommodate two neurons.43 Therefore, biogenic amines released from the nerve terminals would have better access to the brain capillary endothelium than to the choroid plexus epithelium. This suggests that the PMAT at the brain capillary endothelium makes a larger contribution than the PMAT at the choroid plexus epithelium in maintaining the brain interstitial concentrations of biogenic amines, as well as organic cations.
In this study, we characterized the transport function of rPAMT in the BBB and BCSFB using MPP+, a dopaminergic neurotoxin. Endogenous dopaminergic neurotoxins, including 1,2,3,4-tetrahydroisoquinoline and its derivatives, have similar chemical structures to MPP+ and induce parkinsonism.44 PMAT in the brain barriers may play a role as a transporter molecule for preventing accumulation of cationic neurotoxins in the brain, which is relevant to the pathogenesis of Parkinson's disease. Like monoamines and MPP+, PMAT can interact with relatively highly hydrophobic organic cations. In addition, it has been reported that PMAT can function as a polyspecific transporter that interacts with structurally diverse organic cations as both substrates and inhibitors.10 These findings suggest that PMAT may be involved in efflux transport of a variety of cationic central nervous system (CNS) drugs from the brain and so, may influence the onset of action and duration of pharmacological efficacy of CNS drugs. Thus, we suggest that PMAT is a potential target molecule for new drug discovery programs.
CONCLUSIONS
The present studies indicated that rPMAT mRNA was expressed in brain capillary and choroid plexus of rats, and PMAT in the brain barrier is involved in the brain-to-blood transport of [3H]MPP+, at least in part. These findings should be relevant to the physiological roles of the brain barriers in regulating brain concentrations of organic cations, including monoamines and cationic neurotoxins, in order to maintain the functional brain environment.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research and a Grant-in-Aid for Young Scientists provided by The Ministry of Education, Culture, Sports, Science and Technology.




