Quantitative analysis of cysteine-34 on the anitioxidative properties of human serum albumin in hemodialysis patients
Abstract
The purpose of this study was to quantitatively evaluate the extent of contribution of cysteine-34 (34Cys) on the antioxidant effect of human serum albumin (HSA) and to elucidate the physiological implication in hemodialysis (HD) patients. The ratio of oxidized albumin correlated directly with the thiol content in plasma of the HD patients who received intravenous iron. Moreover, the degree of oxidation of 34Cys in HSA purified from the HD patients' plasma correlated with the thiol content in plasma. The radical scavenging activity of purified HSA was dependent on the degree of 34Cys oxidation. A recombinant mutant, C34S, was produced to confirm the role of 34Cys. The activity of C34S was about half of that of wild-type HSA (WT-HSA). Consistent with this observation, the protective effects of C34S were significantly lower than those of WT-HSA in suppressing cytotoxicity and vascular endothelial growth factor production induced by fenton reaction in a human vascular endothelial cell model. These results indicate that the 34Cys residue of HSA may account for more than 40% of the antioxidant effect of HSA in vivo, and thus may exert a protective effect on vascular endothelium function via an antioxidative mechanism in chronic renal disease. © 2011 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 100:3968–3976, 2011
Abbreviations used:
HSA, human serum albumin; CKD, chronic kidney disease; CVDs, cardiovascular diseases; ROS, reactive oxygen species; HD, hemodialysis; DPPH, 1,1′-diphenyl-2-picrylhydrazyl; NEM, N-ethylmaleimide; DTNB, 5,5′-dithiobis (2-nitro benzoic acid); HUVECs, human umbilical venous endothelial cells.
INTRODUCTION
It has recently become clear that oxidative stress is an important participant in a number of pathological processes, including the onset and progression of chronic renal disease (CKD) and its complications, such as cardiovascular diseases (CVDs), and that it affects the prognosis for survival.1 It has been proposed that an enhancement in oxidative stress by reactive oxygen species (ROS) represents a common pathomechanism by which cardiovascular risk factors affect the vessel wall to induce and amplify vessel and organ injury. Thus, an appropriate and quantitative evaluation of this process and a reduction in oxidative stress in the circulating blood are important from the viewpoint of protecting the vascular endothelium and vascular smooth muscle cells against oxidative stress.2-4 Because antioxidant enzymes, such as catalase and superoxide dismutase, are present at lower levels in the blood vessels than in other types of cells, protein-derived thiol groups, which have a high reactivity for ROS, could represent an important source of reducing power in relation to the mechanism for protection against oxidative stress in the blood. Actually, a recent study clearly demonstrated that a reduction of blood thiol content accentuates oxidative stress and leads to an increased mortality rate in hemodialysis (HD) patients.5,6
About 80% of the thiol groups in the blood are derived from cysteine-34 (34Cys), which is the only Cys present in the free state in human serum albumin (HSA), the most abundant protein in plasma. As the classical function, HSA involves the regulation of osmotic pressure and serving as a carrier for many endogeneous and exgeneous substances in plasma. In particular, the reversible binding ratio of several drugs to HSA is very important for absorption, distribution, metabolism, excretion, and toxicity considerations, and contributes to the analysis of earlier stages of the overall drug discovery process.7 In addition to these functions, the antioxidant effect of HSA has recently attracted attention as a new function, and it has been proposed that HSA represents a very abundant and potentially important circulating antioxidant.8-10 In fact, the administration of albumin favorably influences plasma thiol-dependent antioxidant status as well as the levels of protein oxidative damage in patients with acute lung injuries.11 This administration also confers robust protection against oxidative stress and has a favorable influence on redox-signaling processes regulating inflammation in patients with acute respiratory distress syndrome.12
In physiological conditions, the 34Cys residue of HSA is present in two different forms. Human mercaptoalbumin (HMA; reduced) is present in the free state of thiol group. Human nonmercaptoalbumin (HNA; oxidized) is present in a disulfide that is formed reversibly with Cys or glutathione, or as an oxide that is formed irreversibly such as in a sulfinic acid, sulfonic acid, or a similar structure.13 Interestingly, in the past 10 years, it has been shown that HMA and HNA occur in equilibrium, depending on the surrounding redox state, and that their ratio varies depending on the age and the diseased state.14-16 In a previous study, using high-performance liquid chromatography (HPLC), we monitored the redox state of the 34Cys residue of HSA and showed that an overdosing of intravenous iron in the treatment of anemia in HD patients results in an intensification of oxidative stress in the blood.17 In another study, we also reported that oxidative stress was significantly less accentuated, despite equivalent antianemic effects, when intravenous iron was given once weekly for 3 months rather than thrice weekly for 1 month.18 On the basis of these results, we reevaluated the conventional regimen for intravenous iron treatments in Japan from the viewpoint of oxidative stress.
In the previous studies, 34Cys was shown to be a good indicator for evaluating oxidative stress in the systemic circulation, which suggests that it has an antioxidant effect in the blood. However, the antioxidant effect of the 34Cys residue in HSA has been reported, to a limited extent, as the result of an in vitro qualitative evaluation.8,19-22 In addition, much remains unknown regarding the physiological implications of the antioxidant effect of 34Cys.
The purpose of this study was to quantitatively evaluate the contribution of the 34Cys on the antioxidant effect of HSA and to elucidate the physiological implications in HD patients being treated with intravenous iron. We first investigated the relationship between the oxidation of albumin and the thiol content in plasma of HD patients. HSA was then isolated and purified from these plasma samples, and the in vivo antioxidant effect of the 34Cys was quantitatively evaluated. Finally, to clarify the physiological implications of the antioxidant effect of 34Cys, this residue was replaced by serine (C34S), and the resulting functional changes were investigated using human umbilical venous endothelial cells (HUVECs) as a vascular endothelial cell model.
MATERIALS AND METHODS
Materials
N-ethylmaleimide (NEM); 5,5′-dithiobis (2-nitro benzoic acid) (DTNB); and 1,1′-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Nacalai Tesque (Kyoto, Japan). CM-H2DCFDA was purchased from Invitrogen (Eugene, OR, USA). HUVECs were purchased from Dainippon Sumitomo Pharma Company Ltd. (Osaka, Japan). All chemicals were of analytical grade.
Patients
The protocol used in this study was approved by the Institutional Review Board of Kumamoto University (Kumamoto, Japan), and informed consent was obtained from all patients. Eleven stable HD patients (five men and six women) aged 47 to 87 years were enrolled in this study. Patients were administered 40 mg of chondroitin sulfate–iron colloid (Blutal®; Chugai Pharmaceutical Company, Ltd., Tokyo, Japan) intravenously after every dialysis session (thrice a week) for 4 weeks (total dosage: 520 mg of iron). Blood samples were drawn from the arterial line at the start of HD treatment at 0, 4, 8, 12, and 20 weeks after a 2-day interval from the last HD treatment. This study was registered at the ClinicaTrials.gov (http://clinicaltrials.gov/; ID: NCT00298441).
Purified Albumin from HD Patients
Human serum albumin samples were isolated as described previously.23 The samples were then dialyzed against deionized water for 48 h at 4°C, followed by lyophilization. The purity of the HSA samples was at least 95% and the percentage of dimers did not exceed 7%, as evidenced by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and native-PAGE, respectively.
Chromatographic Analysis of Albumin in HD Patients
High-performance liquid chromatography was used to analyze serum albumin as described previously.17,18 From the HPLC profiles of HSA, the ratios of oxidized to unoxidized albumin were estimated by dividing the area under the curve for the reduced form (HMA) to the oxidized form (HNA). The level of HNA/HMA ratio represents an appropriate marker of oxidative stress in CKD.24,25
Reactivity of THIOLS with DTNB
Fifteen microliter aliquots of serum samples and an HSA solution (2.0 × 10−4 M) were preincubated at 37°C. The increase in absorbance at 405 nm was monitored against time after the addition of DTNB (final concentration 5.0 × 10−4 M).26
Chemical Modification of Cysteine Residue of Commercial HSA
Commercial HSA (C-HSA) was donated by the Chemo-Sero—Therapeutic Research Institute (Kumamoto, Japan). It was defatted by treatments with an aqueous suspension of activated charcoal at 0°C, after which it was acidified with H2SO4 to pH 3, deionized, freeze-dried, and stored at −20°C until used.23 Modification of thiol groups of C-HSA was accomplished by incubating 10 mg/mL C-HSA with NEM in four different concentrations (2.5, 5, 7.5, and 10 mM). After 30 min of incubation at room temperature, excess NEM was eliminated by repeated dialysis against 0.15 M NaCl.8
Radical Scavenging Potential of HSA
The radical scavenging potential of HSA (20 μM) was determined from the decrease in the absorbance of DPPH radicals at 540 nm due to their scavenging of an unpaired electron of the stable DPPH radical.27
Carbonyl Group Determination
Protein-bound carbonyl groups were quantitated by using the method of Levine et al.28
Synthesis and Purification of Wild-Type HSA and C34S Mutant
The recombinant DNA techniques used to produce recombinant wild-type HSA (WT-HSA) and the single-residue mutant C34S were essentially those described by Watanabe et al.23 Far- and near-ultraviolet circular dichroism measurements and intrinsic fluorescence spectroscopic analysis of the C34S mutant were completely consisted with those of WT-HSA, indicating that secondary and ternary structures were largely preserved in C34S.
Measurements of ROS in HUVECS
The effect of several substances on the generation of ROS in HUVECs was quantitated using a previously described method.24,29,30 To investigate the effect of several substances on the generation of ROS in HUVECs, we measured the fluorescence intensities of CM-H2DCFDA as an ROS probe. HUVECs (104 cell/well) were preincubated in 96-well plates for 24 h at 37°C in a culture medium. Confluent HUVECs were then incubated with 5 μM CM-H2DCFDA for 30 min at 37°C in a serum-free medium. After the removal of the media from wells, several substances at 37°C in serum-free medium with or without additives were added to the cells. The fluorescence intensity was measured at 490 nm excitation and 530 nm emission using a fluorescence microplate reader (CORONA multi microplate reader, Tokyo, Japan). The mean fluorescence intensity ratio is presented as the percentage of the control value, after subtraction of background fluorescence.
Cytotoxicity Assays
Cytotoxicity was determined after 24 h posttreatment growth in 60-mm culture plates with the Cell Counting Kit-8 assays (Dojinkagaku, Kumamoto, Japan).
VEGF Secretion Measurements
Commercially available enzyme-linked immunosorbent assay kits (Biosource International, Camarillo, California) were used to measure VEGF secretion according to the manufacturer's instructions.
Biochemical Analysis
Serum iron, transferrin, ferritin concentrations, standard hematological parameters, and other biochemical parameters were measured at a contract laboratory (SRL, Inc., Tokyo, Japan).
Statistics
Statistical significance was evaluated using the two-tailed, unpaired Student's t-test for comparisons between two means or analysis of variance followed by Newman–Keuls method for more than two means. A p value of less than 0.05 was regarded as statistically significant. Results are reported as mean ± standard error.
RESULTS
Relationship Between Albumin Oxidation and Thiol Content of 34cys in Plasma and Purified Hsas
For up to 12 weeks after the initial administration in the group receiving intravenous iron thrice weekly for 1 month, changes in thiol content over time and the oxidized albumin ratio in plasma were monitored using the DTNB method and HPLC, respectively. As shown in Figure 1a, a statistically significant negative correlation was found between the thiol content and oxidized albumin ratio in plasma (p < 0.01, r = 0.652). To explore this relation in purified HSAs system, we isolated and purified HSA from plasma of those patients. Comparing the changes in the oxidized albumin ratio between plasma and purified HSAs, a good positive correlation was found between them (p < 0.01, r = 0.958, Fig. 1b). These results confirm that the redox state of purified HSA is not only a good indicator of the thiol content but also the oxidized state of HSA in plasma. We further examined the relationship between the oxidized albumin ratio and the thiol content of 34Cys in purified HSA from the patients on HD. A significant negative correlation was also found between these two parameters (p < 0.01, Fig. 1c). To clarify this relation, we prepared HNAs by NEM with different 34Cys thiol contents, and examined the relationship between the thiol content and oxidized albumin ratio in this system as a standard. Similar to the results obtained for the purified HSA, the oxidized albumin ratio decreased significantly depending on the degree of 34Cys modification. As expected, a negative correlation was observed between them (Fig. 1c). Interestingly, this linear relation was fitted to the regression observed for the purified HSA system as shown in Figure 1c, again confirming that the oxidized albumin ratio reflects the thiol content of 34Cys in vivo.
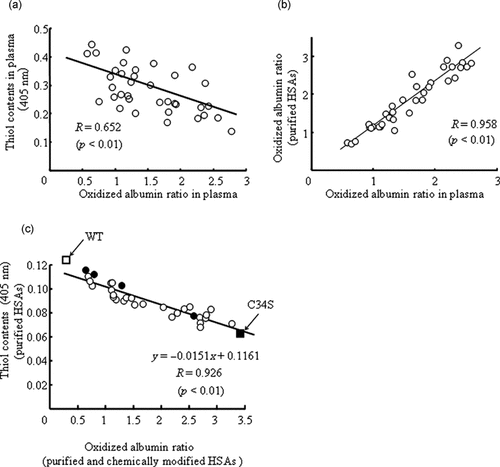
Correlation between oxidized albumin ratio and thiol content in plasma (a); correlation between oxidized albumin ratio in blood and purified HSAs (b); correlation between oxidized albumin ratio and thiol contents of purified HSAs, Cys-dependent chemically modified HSAs, WT, and C34S (c) Values are expressed as mean ± SE; n = 11 patients for each week. ○, Purified HSAs; •, chemically modified HSAs; □, WT; and ▪, C34S.
Relationship Between the Radical Elimination Potential of HSA and the THIOL Content of 34CYS in Purified HSAS
The radical scavenging potential of purified HSA was determined by using DPPH, a synthetic radical species that is commonly used in the determination of the antioxidant effect of various substances. As shown in Figure 2, a significant negative correlation was found between the radical scavenging potential and oxidized albumin ratio in purified HSA (p < 0.01). The radical scavenging potentials of various NEM-modified HSAs were also determined and examined as above (Fig. 1c) as a standard. As shown in Figure 2, the radical scavenging activity of modified HSA decreased significantly and the decrease was proportional to the degree of 34Cys modification. Interestingly, this regression was close to that for purified HSA (Fig. 2). This result also strongly suggests that the 34Cys residue plays a key role in the antioxidant effect of HSA.
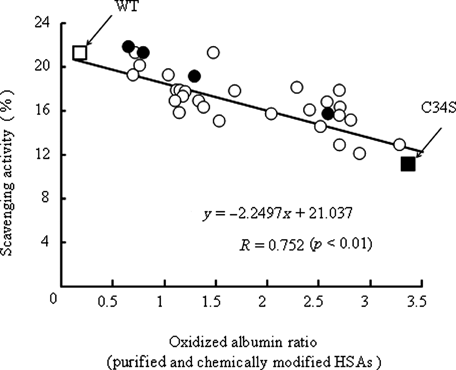
Correlation between oxidized albumin ratio and radical scavenging ability of purified HSAs or Cys-dependent chemically modified HSAs or the data of purified HSAs, WT, and C34S ○, Purified HSAs; •, chemically modified HSAs; □, WT; and ▪, C34S. Values are expressed as mean ± SE; n = 11 patients for each week.
Comparison of the Antioxidant Effects of Recombinant WT-HSA and the C34S Variant
To specifically evaluate the antioxidant effect of 34Cys in HSA, C34S was prepared by replacing 34Cys with serine using site-directed mutagenesis. Here, we selected serine as the substituent residue due to minimize Cys variation. The oxidized albumin ratio of C34S was first determined, and the data compared with that for WT-HSA. As expected, the free thiol group was not detected in C34S by HPLC and the DTNB method (data not shown). Applying the oxidized albumin ratio and the thiol content of these two albumins to the regression line in Figure 1c, the values for WT-HSA and C34S well fitted to the upper and lower ends of the line, respectively (Fig. 1c). Furthermore, an evaluation of radical scavenging ability using DPPH revealed that the antioxidant potential of C34S decreased to about half of that of WT (Fig. 3). Applying this radical scavenging potential to a regression line equation as shown in Figure 2, the values for C34S appeared to the lower end of the line (Fig. 2). This finding also supports the conclusion that 34Cys is a major contributor to the antioxidant potential of HSA.
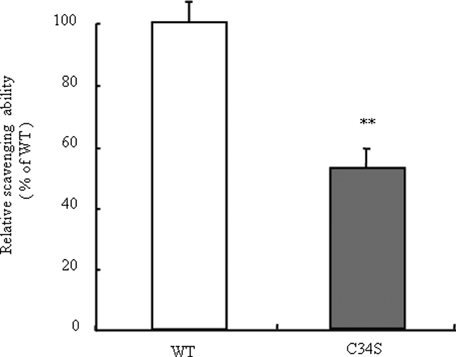
Radical scavenging ability of WT and C34S. Values are expressed as mean ± SE; n = 3. **p < 0.01 as compared with WT.
Relationship Between Antioxidant Effect of 34CYS and Cytotoxicity
Clarifying the effect of increased ROS production with iron preparations on vascular endothelial cell damage is significant from the viewpoint of preventing the onset of CVD in HD patients. Hence, we examined the relationship between the antioxidant effect of 34Cys and vascular endothelial damage using HUVEC. To mimic the oxidative reaction in the blood vessels of HD patients, H2O2 was used as the ROS and an iron preparation as the stimulant for ROS production. As shown in Figure 4a, the production of ROS in HUVEC by the Fenton reaction of the iron preparation and H2O2 was evaluated using CM-H2DCFDA. CM-H2DCFDA has been used as a detector of ROS. This dye is nonfluorescent when chemically reduced, but after cellular oxidation, it becomes fluorescent. In the case of WT-HSA, it was suppressed in a dose-dependent manner. Although C34S also suppressed ROS production, the effect was significantly lower than that of WT. Interestingly, such a dose-dependent effect of WT-HSA was larger than that for C34S. These results demonstrate that the 34Cys residue of HSA serves as an antioxidant against the accentuation of intracellular ROS production in an in vitro cellular system as well as in vivo (Figs. 1 and 2).
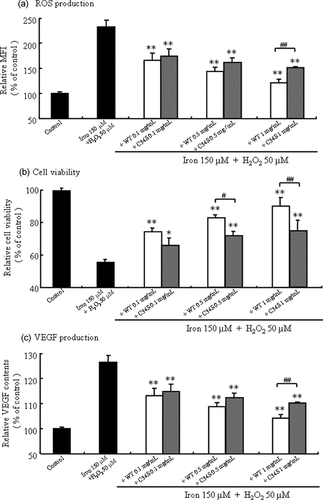
Effects of WT and C34S on intracellular (a) ROS production, (b) cell survival, and (c) VEGF production on HUVEC. Values are expressed as mean ± SE; n = 3. **p < 0.01, *p < 0.05 as compared with iron 150 μM + H2O2 50 μM; ##p < 0.01, #p < 0.05 as compared with WT.
We then determined whether the suppression of ROS production by 34Cys mitigates vascular endothelial damage, as estimated from cytotoxicity as a reduction in cell survival rate and the production of VEGF using HUVEC. WT-HSA and C34S were added 1 h after the addition of the iron preparation and H2O2 to achieve an approximately 50%, and the effect of 34Cys on ROS-derived cytotoxicity and the production of VEGF were examined. Both albumins significantly increased the viability of HUVECs in a dose-dependent manner, although their activities were different (Fig. 4b). In addition, the effect of WT-HSA on cell survival rate was approximately twice as large as that for C34S (Fig. 4b). These albumins also significantly inhibited the production of VEGF in HUVEC. However, C34S was significantly less effective than WT-HSA in VEGF production (Fig. 4c).
DISCUSSION
The findings reported herein serve to demonstrate that the 34Cys residue of albumin may responsible for more than 40% of the antioxidant effect of HSA in vivo, and thus may have a protective effect on vascular endothelium function in CKD patients via the antioxidative defense mechanism. To our knowledge, this is the first report in which the contribution of 34Cys in the function of HSA has been quantitatively evaluated by using the site-directed mutagenesis, and the physiological implications discussed.
A rapidly accumulating body of evidence suggests that oxidative stress could play an important role in the pathogenesis of CKD and its complications. In general, living organisms have developed complex antioxidant systems designed to prevent tissue damage that occurs as a consequence of chemical reactions involving ROS. In blood vessels, the 34Cys residue of HSA constitutes the largest pool of thiols in the circulation. As a result, it has attracted attention as a component of the antioxidant mechanism.8-10 In fact, Bourdon et al.8 reported that more than 70% of the free radical-trapping activity of serum was due to HSA. Carballal et al.31 also concluded that albumin thiol groups may well be an important scavenger of ROS. However, the contribution of 34Cys to the antioxidant effect of HSA has not yet been clarified. In addition, little research has been conducted relative to the antioxidant effect of 34Cys in human pathological conditions.
We initially found a negative correlation between the oxidized albumin ratio and thiol content in plasma from HD patients for up to 12 weeks after initial administration in the case of a group receiving intravenous iron (Fig. 1a). A similar relationship was also observed between oxidized albumin ratio in plasma and in purified HSA from HD patients, and also between the oxidized albumin ratio and the thiol content of 34Cys in purified HSA (Figs. 1b and 1c). These data clearly indicate that the degree of 34Cys oxidation in HSA is a good indicator of the thiol content in plasma. In fact, the thiol content and oxidized albumin ratios of HSA with different degrees of 34Cys modification by NEM were well fitted to the regression obtained for purified HSA (Fig. 1c). Such consistency between in vitro and in vivo data provides additional support for this conclusion. Himmelfarb et al.5 recently reported that a reduction in blood thiol content accentuates oxidative stress, leading to an increased mortality rate in HD patients. In general, thiols are preferential targets of ROS, and they can be oxidized by a wide spectrum of ROS, at rates several orders of magnitude faster than other amino acids. Combined with these findings, our results clearly indicate that 34Cys could play a key role in the antioxidant mechanism in the systemic circulation of HD patients. In fact, the radical scavenging potential of purified HSA from HD patients was significantly dependent on the degree of 34Cys oxidation (Fig. 2). A similar relationship was also observed for HSA that contained different levels of NEM-modified 34Cys (Fig. 2), and these in vitro data were completely consistent with the data for purified HSA (Fig. 2), thus emphasizing the importance of 34Cys in the antioxidant defense system in the circulation in HD. This conclusion was further supported by the fact that the radical scavenging potential of C34S was remarkably lower than that of WT-HSA (Fig. 2).
Surprisingly, the sole 34Cys residue accounts for about half of the total radical scavenging activity of WT-HSA even though it contains 585 amino acid residues (Fig. 4b). It can be concluded that, at least in the evaluation system used, the thiol group of 34Cys may account for more than 40% of the antioxidant effect of HSA because, in this study, we used WT-HSA that contained approximately 80% HMA. Such an extraordinary high potential of 34Cys may have additional physiological implications, for instance, in the protection of vascular endothelial function. To clarify this, we compared WT-HSA and C34S in terms of their ability to protect cells and examined the relationship between the antioxidant effect of 34Cys and the prevention of vascular endothelial cell damage. Our data clearly showed that WT-HSA inhibited the ROS induced by iron preparations, suppressed the production of VEGF, and improved the cell survival rate (Fig. 4). In fact, VEGF is thought to act as a proinflammatory and proarteriosclerotic factor in angiotensine II (AII)-induced hypertension.32 Furthermore, AII, via its type-1 receptor, stimulates nicotinamide adenine dinucleotide phosphate oxidase and enhances the production of ROS, which, in turn, contributes to endothelial dysfunction and vascular inflammation.33,34 Therefore, these findings provide support for the view that WT-HSA may have a protective effect on vascular endothelial cell function by attenuating oxidative stress to suppress VEGF production. Although C34S also decreased ROS levels, resulting in the suppression of cytotoxicity and VEGF production, these effects were significantly less than those of WT-HSA (Fig. 4). Interestingly, the differences in ROS elimination activity between these two albumins are entirely consistent with the difference in the improvement in cell survival rate. Thus, these results suggest that 34Cys plays a significant role in protecting endothelial cell damage via the antioxidant effect in CKD patients.
Taken together with the present data, the favorable functions of albumin for vascular endothelial cells could be largely dependent upon the reactivity of the thiol group in 34Cys. Generally, the thiol reactivity of Cys in proteins against ROS is higher than that for low-molecular-weight thiols such as Cys and glutathione because the microenvironment around Cys in a protein induces a lower pKa value of the thiol group and consequently enhances its reactivity.35 Indeed, the pKa of 34Cys in HSA (pKa = 5.0) is also shifted to lower range due to its unique microenvironment, which is characterized by a hydrophobic region with several localized, charged amino acid residues.36 On the contrary, changes in the carbonyl content of a protein are induced by the oxidation of noncysteine amino acids residues. Thus, it is possible that those noncysteine residues, especially Met residue, another sulfur-containing amino acid that is sensitive to oxidative stress, also contribute to the antioxidant effect of HSA. In this study, however, the role of Met on the antioxidant effect has not been examined. Several studies have shown that surface-exposed Met residues may represent an endogeneous antioxidant defense to protect against extensive protein oxidation.37-39 In fact, Bourdon et al.8 carried out chemical modifications of Cys and Met on HSA using NEM and chloramine T, respectively, and reported that these residues accounted for 40%–80% of the total antioxidant activity of HSA. Because we estimate that the antioxidant effect of C34S in HSA is more than 40%, it can be concluded that the contribution of Met or other residues may be roughly less than 40%. This conclusion is further supported by the finding that, unlike the thiol content of 34Cys, no significant correlation was found between changes in carbonyl content and the radical scavenging potential in purified HSA (data not shown). Additional investigation using mutants that replace Met residues will be necessary to develop a comprehensive understanding of the antioxidant properties of HSA.
CONCLUSION
The findings reported herein indicate that the antioxidant effect of HSA is largely dependent on 34Cys and its possible contribution to the maintenance of intravascular homeostasis, including the protection of vascular endothelium under CKD conditions. In fact, the excess 34Cys oxidation was not only found to have a decreased antioxidant activity but also able to trigger the oxidative burst of human neutrophils.27 This suggests that the treatments wherein the levels of 34Cys oxidation are minimized at low levels in CKD may be beneficial for preventing the onset and progression of serious complications, such as CVD, which affect the prognosis for survival.




