Amphiphilic cyclodextrins as nanocarriers of genistein: A spectroscopic investigation pointing out the structural properties of the host/drug complex system
Rosanna Stancanelli
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorMarta Guardo
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorCarmela Cannavà
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorGiovanni Guglielmo
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorPaola Ficarra
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorCorresponding Author
Valentina Villari
CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy
Valentina Villari, CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy. Telephone: +39 090 39762 219, Fax: +39 090 3974130.
Antonino Mazzaglia, CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy. Telephone: 0903974108; Fax: 0903974108.
Search for more papers by this authorNorberto Micali
CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy
Search for more papers by this authorCorresponding Author
Antonino Mazzaglia
CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy
Valentina Villari, CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy. Telephone: +39 090 39762 219, Fax: +39 090 3974130.
Antonino Mazzaglia, CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy. Telephone: 0903974108; Fax: 0903974108.
Search for more papers by this authorRosanna Stancanelli
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorMarta Guardo
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorCarmela Cannavà
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorGiovanni Guglielmo
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorPaola Ficarra
Dipartimento Farmaco-Chimico, Università di Messina, V.le Annunziata, 98168 Messina, Italy
Search for more papers by this authorCorresponding Author
Valentina Villari
CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy
Valentina Villari, CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy. Telephone: +39 090 39762 219, Fax: +39 090 3974130.
Antonino Mazzaglia, CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy. Telephone: 0903974108; Fax: 0903974108.
Search for more papers by this authorNorberto Micali
CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy
Search for more papers by this authorCorresponding Author
Antonino Mazzaglia
CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy
Valentina Villari, CNR-Istituto per i Processi Chimico-Fisici, sede di Messina, Contrada Papardo, Salita Sperone, Faro Superiore, 98158 Messina, Italy. Telephone: +39 090 39762 219, Fax: +39 090 3974130.
Antonino Mazzaglia, CNR-Istituto per lo studio dei Materiali Nanostrutturati, c/o Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Salita Sperone 31, 98166 Messina, Italy. Telephone: 0903974108; Fax: 0903974108.
Search for more papers by this authorAbstract
Nanoggregates of nonionic amphiphilic cyclodextrin (ACyD) modified with hydrophobic chains of intermediate length [(2-oligo-ethyleneoxide-6-hexylthio)-β-CyD, SC6OH] were prepared by emulsification–diffusion method. They are able to entrap an isoflavone, genistein (Gen), and the complexed species are studied at different host/guest molar ratio. The increased isoflavone solubility in the presence of the aggregates of SC6OH is investigated by UV–Vis spectroscopy, whereas size, charge, and structure of aggregates and their complexes with Gen are measured by means of static and quasi-elastic light scattering, and electrophoretic mobility measurements. On the other hand, preparing samples by the conventional method used for liposomes (hydration of an organic film of SC6OH and sonication) gives rise to aggregates with different sizes and lower colloidal stability. It is shown that the improved stability in water of ACyD aggregates both in the absence and in the presence of Gen, obtained by emulsification–diffusion is due to the existence of nanodomains of organic solvent (RH ≅ 120 nm) which cannot be completely removed by evaporation and freeze-drying and in which host/guest complexes are contained. This result shows that residues of organic solvent from preparation step favor the colloidal stability of the aggregate, but their presence must be taken into account in designing systems for drug delivery. © 2010 Wiley-Liss, Inc. and the American Pharmacists Association J Pharm Sci 99:3141–3149, 2010
REFERENCES
- 1 Lukyanov AN, Torchilin VP. 2004. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev 56: 1273–1289.
- 2 Lipinski CA. 2000. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods 44: 235–249.
- 3 Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26.
- 4 Fournier E, Dufresne MH, Smith DC, Ranger M, Leroux JC. 2004. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm Res 21: 962–968.
- 5 Constantinides PP, Lambert KJ, Tustian AK, Schneider B, Lalji S, Ma W, Wentzel B, Kessler D, Worah D, Quay SC. 2000. Formulation development and antitumor activity of a filter sterilizable emulsion of paclitaxel. Pharm Res 17: 175–182.
- 6 Thompson D, Chaubal MV. 2000. Cyclodextrins (CDs)-excipients by definition, drug delivery systems by function (Part I: injectable applications). Drug Deliv Technol 2: 34–38.
- 7 Harada A. 2001. Cyclodextrin-based molecular machines. Acc Chem Res 34: 456–464.
- 8 Caliceti P, Salmaso S, Semenzato A, Carofiglio T, Fornasier R, Fermeglia M, Ferrone M, Pricl S. 2003. Synthesis and physico-chemical characterization of folate-cyclodextrin bioconjugate for active drug delivery. Bioconjug Chem 14: 899–908.
- 9 Dai XH, Dong CM, Fa HB, Yan D, Wei Y. 2006. Supramolecular polypseudorotaxanes composed of star-shaped porphyrin-cored poly(epsilon-caprolactone) and α-cyclodextrin. Biomacromolecules 7: 3527–3533.
- 10 Loftsson T, Jarho P, Másson M, Järvinen T. 2005. Cyclodextrins in drug delivery. Expert Opin Drug Deliv 2: 335–351.
- 11 Memisoglu-Bilensoy E, Doğan AL, Hıncal AA. 2006. Cytotoxic evaluation of injectable cyclodextrin nanoparticles. J Pharm Pharmacol 58: 585–589.
- 12
Ravoo BJ,
Darcy R.
2000.
Cyclodextrin bilayer vesicles.
Angew Chem Int Ed
39:
4324–4326.
10.1002/1521-3773(20001201)39:23<4324::AID-ANIE4324>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 13 Mazzaglia A, Donohue R, Ravoo BJ, Darcy R. 2001. Novel amphiphilic cyclodextrins: Graft-synthesis of heptakis(6-alkylthio-6-deoxy)-β-cyclodextrin 2-oligo(ethylene glycol) conjugates and their ω-halo derivatives. Eur J Org Chem, 1715–1721.
- 14 Donohue R, Mazzaglia A, Ravoo BJ, Darcy R. 2002. Cationic cyclodextrin bilayer vesicles. Chem Commun, 2864–2865.
- 15 Lombardo D, Longo A, Darcy R, Mazzaglia A. 2004. Structural properties of non-ionic cyclodextrin colloids in water. Langmuir 20: 1057–1064.
- 16 Ravoo BJ, Jacquier BJ, Wenz JC. 2003. Molecular recognition of polymers by cyclodextrin vesicles. Angew Chem Int Ed 42: 2066–2070.
- 17 Mazzaglia A, Angelini N, Darcy R, Donohue R, Lombardo D, Micali N, Sciortino MT, Villari V, Monsù Scolaro L. 2003. Novel heterotopic colloids of anionic porphyrins entangled in cationic amphiphilic cyclodextrins: Spectroscopy investigation and intracellular delivery. Chem Eur J 9: 5762–5769.
- 18 Mazzaglia A, Angelini N, Lombardo D, Micali N, Patanè S, Villari V, Monsù Scolaro L. 2005. Amphiphilic cyclodextrin carriers embedding porphyrins: Charge and size modulation of colloidal stability in heterotopic aggregates. J Phys Chem B 109: 7258–7265.
- 19 Sortino S, Mazzaglia A, Monsu Scolaro L, Marino Merlo F, Valveri V, Sciortino MT. 2006. Nanoparticles of cationic amphiphilic cyclodextrins entangling anionic porphyrins as carrier-sensitizer system in photodynamic cancer therapy. Biomaterials 27: 4256–4265.
- 20 Mazzaglia A, Valerio A, Villari V, Rencurosi A, Lay L, Spadaro S, Monsù Scolaro L, Micali N. 2006. Probing specific protein recognition in controlled host nanoassemblies of glycosylated cyclodextrins. New J Chem 30: 1662–1668.
- 21 McNicholas S, Rencurosi A, Lay L, Mazzaglia A, Sturiale L, Perez M, Darcy R. 2007. Amphiphilic N-glycosyl-thiocarbamoyl cyclodextrins: Synthesis, selfassembly and fluorimetry of recognition by Lens culinaris lectin. Biomacromolecules 8: 1851–1857.
- 22 Sortino S, Petralia S, Darcy R, Donohue R, Mazzaglia A. 2003. Photochemical outcome modification of diflunisal by a novel cationic amphiphilic cyclodextrin. New J Chem 27: 602–608.
- 23 Callari FL, Mazzaglia A, Monsù Scolaro L, Valli L, Sortino S. 2008. Biocompatible nanoparticles of amphiphilic cyclodextrins entangling porphyrins as suitable vessels for light-induced energy and electron transfer. J Mater Chem 18: 802–805.
- 24 Rodriguez SG, Allémann E, Hatem Fessi H, Eric Doelker E. 2004. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res 21: 1428–1439.
- 25 Kwon SH, Kang MJ, Huh JS, Ha KW, Lee JR, Lee SK, Lee BS, Han IH, Lee MS, Lee WL, Lee J, Choi YW. 2007. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm 337: 148–154.
- 26 Faqi AS, Johnson WD, Morrissey RL, McCormick DL. 2004. Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol 18: 605–611.
- 27 Stancanelli R, Mazzaglia A, Tommasini S, Calabrò ML, Villari V, Guardo M, Ficarra P, Ficarra R. 2007. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J Pharm Biomed Anal 44: 980–984.
- 28 Quaglia F, Ostacolo L, Mazzaglia A, Villari V, Zaccaria D, Sciortino MT. 2009. The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials 30: 374–382.
- 29 Berne BJ, Pecora R. 1976. Dynamic light scattering: With applications to chemistry, biology and physics. New York: John Wiley and Sons.
- 30 Brown W, Nicolai T. 1996. In: W Brown, editor. Dynamic properties of polymer solutions. Dynamic Light Scattering: The method and some applications. Oxford: Clarendon. pp 272–318.
- 31 Chu B. 1991. Laser light scattering—Basic principle and practice. 2nd edition. San Diego: Academic.
- 32 Villari V, Micali N. 2008. Light scattering as spectroscopic tool for the study of disperse systems useful in pharmaceutical sciences. J Pharm Sci 97: 1703–1730.
- 33 McNeil-Watson F, Tscharnuter W, Miller J. 1998. A new instrument for the measurement of very small electrophoretic mobilities using phase analysis light scattering (PALS). J Colloid Surf A 140: 53–57.
- 34 Hunter RJ. 1986. Foundations of colloid science, Vols. I–II. New York: Oxford University Press.
- 35
Low amount of organic solvent can be tolerated by rats and correspond in animal with average weight of 250 g to LD50 ButOH = 0.08% (w/w) and
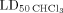 = 0.07% (w/w), respectively. The weight of organic solvent residues, as estimated by scattered intensity, is ∼2.5% (w/w) of ButOH and ∼0.5% (w/w) of CHCl3. By taking in account that the highest concentration of Gen in our formulation is 30 µM (8 µg/mL), it results that the maximum dose of Gen is ∼70 µg for a rat of 250 g. Moreover, this dose is much lower of medium dose of Gen (∼18 mg) per day tolerated by a rat with a average weight of 250 g [26].
= 0.07% (w/w), respectively. The weight of organic solvent residues, as estimated by scattered intensity, is ∼2.5% (w/w) of ButOH and ∼0.5% (w/w) of CHCl3. By taking in account that the highest concentration of Gen in our formulation is 30 µM (8 µg/mL), it results that the maximum dose of Gen is ∼70 µg for a rat of 250 g. Moreover, this dose is much lower of medium dose of Gen (∼18 mg) per day tolerated by a rat with a average weight of 250 g [26].
- 36 Glasstone S. 1948. Textbook of physical chemistry. 2nd edition. New York: Macmillan.
- 37 Johnsson M, Wagenaar A, Engberts JBFN. 2003. Sugar-based gemini surfactant with a vesicle-to-micelle transition at acidic pH and a reversible vesicle flocculation near neutral pH. J Am Chem Soc 125: 757–760.
- 38 Micali N, Villari V, Consoli GML, Consolo F, Geraci C. 2006. Vesicle-to-micelle transition in aqueous solutions of amphiphilic calixarene derivatives. Phys Rev E 73: 051904.
- 39 Falvey P, Lim CW, Darcy R, Revermann T, Karst U, Giesbers M, Marcelis ATM, Lazar A, Coleman AW, Reinhoudt DN, Ravoo BJ. 2005. Bilayer vesicles of amphiphilic cyclodextrins: Host membranes that recognize guest molecules. Chem Eur J 11: 1171–1180.




