Divergent effects of distinct perivascular cell subsets for intra-articular cell therapy in posttraumatic osteoarthritis
Masnsen Cherief, Takashi Sono, and Yiyun Wang contributed equally to this work.
Abstract
Intra-articular injection of mesenchymal stem cells has shown benefit for the treatment of osteoarthritis (OA). However, mesenchymal stem/stromal cells at the origin of these clinical results are heterogenous cell populations with limited cellular characterization. Here, two transgenic reporter mice were used to examine the differential effects of two precisely defined perivascular cell populations (Pdgfrα+ and Pdgfrβ+ cells) from white adipose tissue for alleviation of OA. Perivascular mesenchymal cells were isolated from transgenic Pdgfrα-and Pdgfrβ-CreERT2 reporter animals and delivered as a one-time intra-articular dose to C57BL/6J mice after destabilization of the medial meniscus (DMM). Both Pdgfrα+ and Pdgfrβ+ cell preparations improved metrics of cartilage degradation and reduced markers of chondrocyte hypertrophy. While some similarities in cell distribution were identified within the synovial and perivascular spaces, injected Pdgfrα+ cells remained in the superficial layers of articular cartilage, while Pdgfrβ+ cells were more widely dispersed. Pdgfrβ+ cell therapy prevented subchondral sclerosis induced by DMM, while Pdgfrα+ cell therapy had no effect. In summary, while both cell therapies showed beneficial effects in the DMM model, important differences in cell incorporation, persistence, and subchondral sclerosis were identified.
1 INTRODUCTION
Osteoarthritis (OA) is a common chronic joint disease, typifiedby cartilage degeneration, and subchondral bone thickening.1 The current treatmentsare largely limited to pain control with negligible effects on cartilage maintenance. Autologous chondrocyte transplantation has been used to repair cartilage, buttheir tendency to dedifferentiate haslimited therapeutic use.2 Mesenchymal stem/stromal cells (MSCs) reside in bone marrow and many adult tissues, and have been widely explored as a cell based therapeutic for OA.3, 4 Wide variability in outcomes after conventional MSC implantation have also been reported, including fibrocartilage formation and inappropriate ossification.5, 6 Nevertheless, MSCs are known to be of variable cellular composition.7 The cellular heterogeneity resides amid donors, tissue sources and within the MSCs populations.8, 9 Because the most common MSC-based cell therapies are used in bulk, the effects of cell population heterogeneity in terms of therapeutic effects are often masked.10
Mesenchymal progenitor cells have long been known to reside in a perivascular niche,11, 12 and have been well typified within the adipose tissue from mouse,13 human,14, 15 and canine sources.12 Intra-tissue heterogeneity in perivascular cells has been an emerging area of focus, and expression of receptors for platelet derived growth factor (PDGF) has been shown to define functional relevant perivascular cell subsets in human and mouse tissues.16-18 For example, subsets of pericytes expressing different combinations of PDGFRα and PDGFRβ can mediate tissue-specific regeneration.18, 19 Two transgenic perivascular reporter mice have been previously characterized, including Pdgfrα (platelet derived growth factor receptor alpha)13 and Pdgfrβ reporter animals.20, 21 Adipose derived Pdgfrα+ cells are enriched in the tunica adventitiaof large blood vessels, and are less common in microvessels.13 Pdgfrβ+ cells are enriched among microvascular pericytes as well as vascular smooth muscle cells.21, 22 In this study, we compared the precise effects of intra-articular injection of Pdgfrα+ and Pdgfrβ+ perivascular cellson OA after joint destabilization.
2 MATERIALS AND METHODS
2.1 Animals
All animal studies were performed with institutional Animal Care and Use Committee approval within Johns Hopkins University (JHU), complying with all relevant ethical regulations. Pdgfrα-CreERT2 mice were a kind gift from the Bergles laboratory at JHU and are commercially available (stock No.: 018280, Jackson lab). mT/mG mice were purchased from Jackson lab (stock No.: 007576). Pdgfrβ-CreERT2 mice were purchased from Jackson lab (stock No.: 029684). Pdgfrα-CreERT2 or Pdgfrβ-CreERT2 mice were crossed with mT/mG mice to generate Pdgfrα-CreERT2; mT/mGor Pdgfrβ-CreERT2; mT/mG mice which were used for all experiments. Tamoxifen (TM; T5648; Sigma) was provided by intraperitoneal injection as per previously validated protocols to mixed gender, 7 weeks old Pdgfrα-CreERT2; mT/mG mice or Pdgfrβ-CreERT2; mT/mG mice 2 weeks before cell isolation.13, 23 TM was dissolved in sunflower seed oil. Tail genomic DNA was used for genotyping.
2.2 Cell isolation
Pdgfra and orPdgfrβ-CreERT2-mT/mG mice were administered TM 2 weeks in advance of cell isolation. Inguinal fat pads from 10 mice per genotype were dissected, pooled, minced, and digested with collagenase Type 2 (Worthington), 0.5% bovine serum albumin and α-minimum essential medium (Gibco) for 1.5 h at 37°C. mGFP+ cells were isolated with LSR II (BD Biosciences) using FACS Diva software (BD Biosciences) and Flow Jo v10 software (Tree Star, Inc.). Cell debris was excluded based on size characterizationand mGFP+ cells were collected. Cells were cultured for 2–3 passages prior in vivo administration. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) at 37°C in a humidified atmosphere containing 95% air and 5% CO2 in DMEM (Thermo Fisher Scientific, Inc.), with 1% fetal bovine serum (Thermo Fisher Scientific), and 1% penicillin/streptomycin. Medium was changed every 3 days unless otherwise stated. Cells were characterized by flow cytometry after 2 weeks culture expansion. A total of 1 × 106 trypsinized cells were incubated with anti-αSMA-Alexa Fluor 647, anti-Nestin-Cy7, anti-stem cell antigen 1 (Sca-1)-Alexa Fluor 647, CD146-Alexa Fluor 647, and CD34-BV421.
2.3 Destabilization of the medial meniscus
Destabilization of the medial meniscus (DMM) or sham surgery was performed at the age of 10 weeks in mixed gender C57BL/6J mice, in similarity to prior reports.24 Equal numbers of male and female animals were distributed across treatment groups. Briefly, left hindlimbs were disinfected with povidone-iodine and 70% ethanol, and buprenorphine SR (1 mg/kg) was injected subcutaneously. A 1 cm longitudinal incision was made on the medial aspect of the knee joint under general anesthesia using 2% isoflurane. Blunt dissection of the joint capsule along the medial side of the patellar ligament was performed to expose the medial menisco-tibial ligament (MMTL). The infrapatellar fat pad (IFP) was exposed, displaced laterally, and the MMTL was transected using a 11-blade scalpel to destabilize the medial meniscus. The medial joint capsule and the skin were next closed with 5-0 prolene suture (Ethicon). Sham surgery was performed with a similar surgical approach with visualization, but without transection of the MMTL. One week after surgery, either 1 × 106 Pdgfra+ or Pdgfrβ+ cells were injected into the intra-articular space. Equal volume of phosphate-buffered saline (PBS) was injected as a control. In total, 40 mice, 5 males, and 5 females pertreatment group, underwent surgery. Groups included (1) sham operated, (2) DMM operated, PBS treated (control), (3) DMM operated, Pdgfra+ cell treated, and (4) DMM operated, Pdgfrβ+ cell treated. Eight weeks after DMM or sham surgery, animals were sacrificed andhindlimbs were harvested for analysis.
2.4 Histology and histomorphometry
Stifle joints were fixed in 4% paraformaldehyde for 24 h, decalcified with 14% ethylenediaminetetraacetic acid for 14 days, and embedded in OCT compound (Sakura). Sagittal sections of the stifle joint were prepared at 6 µm thickness with a Cryofilm type 3c (SECTION-LAB). Inguinal fat pads from donor Pdgfra orPdgfrβ-CreERT2-mT/mG mice were also dissected, embedded in OCT, and cryosectioned at 30 μm thickness. Routine safranin O/fast greenstaining of stifle joints section was performed with methods adopted from past work.25-27
For immunofluorescent immunohistochemistry, sections were washed in 1× PBS, blocked in 5% normal goat serum (S-1000, Vector Labs) for 30 min, and incubated with primary antibodies specific for ACAN (1:100; ab3778; Abcam), COL10 (1:100; ab58632; Abcam), CD31 (1:100; ab28364; Abcam)and scleraxis (1:100; ab58655; Abcam) at 4°C overnight. Sections were then incubated with Alexa Fluor 647-conjugated secondary antibodies (1:200; ab150083; Abcam) or DyLight 594 (1:100; DI-1594; Vector Labs), and mounted with mounting medium containing DAPI (H-1500; Vector Labs; Table S1). Immunofluorescence images were acquired on a Leica DM 6B or LSM 880 FCS confocal microscope (Carl Zeiss).
2.5 Microcomputed tomography imaging
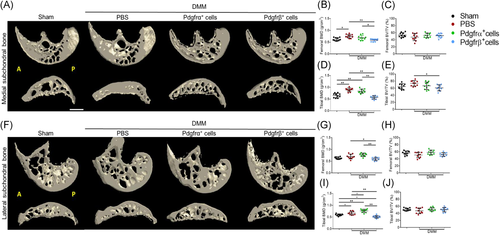
Microcomputed tomography (μCT) analysis of the tibial and femoral subchondral bone was performed using a SkyScan1175 system (Bruker) with the following settings: voltage, 65 kVp; current, 153 μA; resolution, 1.0-mm aluminum filter 10 μm/pixel. The images were reconstructed and analyzed using NRecon v1.7 and CTAn v1.20, respectively. XYZ axes were adjusted by Dataviewer V1.5. 3-Dimensional histomorphometric analysis was performed on cross-sectional images of the tibial and the femoral subchondral bone. We defined the region of interest as the whole subchondral bone medial and lateral compartments and used 15 consecutive images from the medial and lateral tibial plateau for 3-dimensional reconstruction and analysis. Two 3-dimensional structural parameters were analyzed; bone volume density (BV/TV) and bone mineral density (BMD).
2.6 OARSI scoring of the knee joints
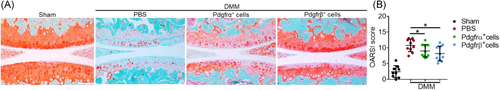
Six frozen semi-serial sagittal sections with 36 µm intervals were prepared from the medial compartment of each knee joint to represent the weight-bearing area of the distal femur and proximal tibia.28 Safranin O/fast green stained sections were analyzed for cartilage injury using the OARSI scoring system.29 A range of 0–24 was determined according to the formula: score = grade (G1 to G6) × stage (S1 to S4). The scoring was done by three different authors (TS, SN, and MC). The average scores of both femur and tibia were summed.
2.7 Statistical analysis
Statistical analyses were performed in IBM SPSS Statistics 16.0. Quantitative data are expressed at mean ± SD. A two tailed the Student t test was used for two-group comparisons, and a one-way Analysis of variance test was used for comparisons of three or more groups, followed by Tukey's post hoc test. *p < .05 and **p < .01 were considered significant.
3 RESULTS
3.1 Isolation of Pdgfrα+ and Pdgfrβ+ cells from mouse subcutaneous adipose tissue
To precisely define the identity of perivascular cell subsets, we used inducible mouse reporter systems for Pdgfrα and Pdgfrβ, and validated cell identity. Each Cre line was crossed with mT/mG reporter animals, which express membranous TdTomato in all cells untilCre recombination occurs when the TdTomato cassette is excised, and membranous mGFP is expressed.30-32 Perivascular cell identity was assessed in the resulting PdgfrαmT/mG and PdgfrβmT/mG reporter animals after exposure to TM.13, 33 Consistent with prior reports, PdgfrαmT/mG reporter activity highlighted perivascular cells within the tunica adventitia, along with a portion of cells within a pericytic location,13 while PdgfrβmT/mG reporter activity highlighted all pericytes and vascular smooth muscle cells.22 After tissue dissociation, by flow cytometry Pdgfrα+ cells comprised 14.5% of total viable cells, while Pdgfrβ+ cells comprised on average 11% of total viable cells (Figure 1). Pericyte and stem cell markers were assessed among Pdgfrα+ and Pdgfrβ+ cell fractions (Figure S1). The expression of Sca-1 expression was high across both cell preparations (90.1% and 89.4%, respectively). In contrast, αSMA and CD146 expression was enriched among the Pdgfrβ+ cell fraction (89.91% and 71.88% among Pdgfrβ+ cells and 12.75% and 4.06% among Pdgfrα+ cells).
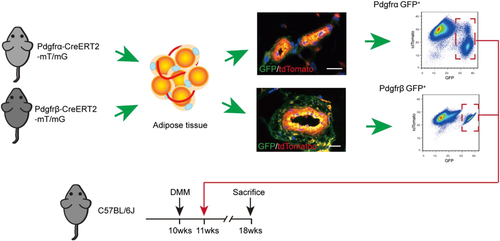
To address the therapeutic potential of either Pdgfrα+ or Pdgfrβ+ expressing perivascular cells, we performed intra-articular injection of either Pdgfrα+ or Pdgfrβ+ perivascular cell subsets (Figures 1-5). Briefly, we injected either FACS purified Pdgfrα+ or Pdgfrβ+ adipose tissue perivascular cells into the stifle joint after DMM (Figure 1). Cells were prepared from inguinal fat pads of either PdgfrαmT/mG or PdgfrβmT/mG reporter animals. Cells were injected as a one-time dose in equal numbers (1M total cells) and compared to PBS control injection. All knee joints were analyzed at 8 weeks after DMM.
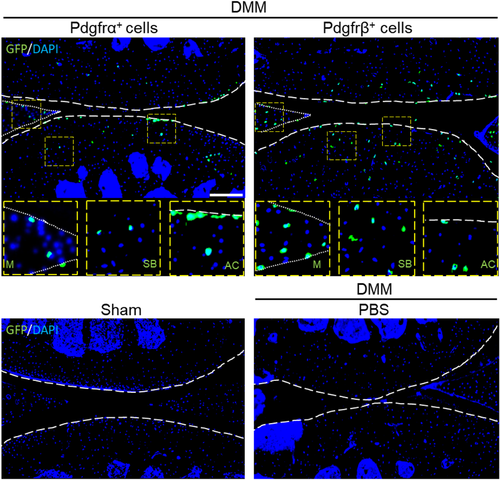
3.2 Pdgfrα+ and Pdgfrβ+ cell engraftment in the stifle joint
Differences in Pdgfrα+ or Pdgfrβ+ cell engraftment and persistence were next evaluated in our model (Figure 2). Pdgfrα+ cells were found superficially within the articular cartilage as well as menisci, while Pdgfrβ+ cells were more widely distributed within all layers of cartilage as well as subchondral bone (Figure 2, upper rows). As expected, no GFP+ cells were identified in control tissues (Figure 2, lower rows).
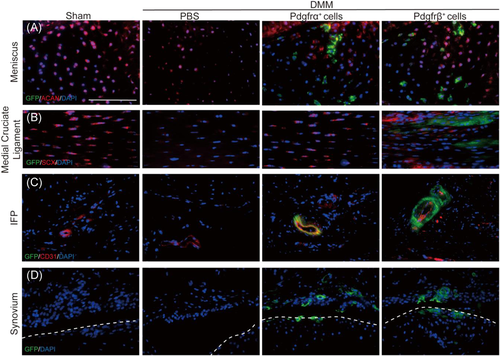
We further examined the distribution of Pdgfrα+ or Pdgfrβ+ cells within the injected joint, examining in detail the meniscus, medial cruciate ligament (MCL), IFP and joint capsule (Figure 3). After DMM surgery, mGFP+ cells were found in the meniscus with relatively similar frequency and distribution across both Pdgfrα+ and Pdgfrβ+ cell treated joints. Interestingly, ACAN immunostaining of the meniscus showed significant changes after cell therapy. Consistent with prior reports,34, 35 DMM operated animals showed a reduction in meniscal ACAN immunostaining in comparison to sham operatd controls (Figure 3A, left). Conversely, both Pdgfrα+ and Pdgfrβ+ cell treated joints showed a relative increase in ACAN immunoreactivity in areas around cell incorporation within the meniscus (Figure 3A, right).
Incorporation of injected cells into the MCL was also observed (Figure 3B). Here, DMM ligamentocytes changed from a consistent spindled shape with elongated nucleus to a more rounded cell shape (Figure 3B, left). This change in morphology was associated with a reduction in scleraxis (SCX) immunoreactivity, consistent with prior reports.36 Significant incorporation of injected Pdgfrβ+ but not Pdgfrα+ cells was found within and around the MCL (Figure 3B, right). As well, SCX expression was partially restored among ligamentocytes upon either Pdgfrα+ or Pdgfrβ+ cell treatment.
Incorporation of injected cells was also found within perivascular areas of the IFP (Figure 3C). This was most conspicuous among larger microvessels of the IFP, and seen among both Pdgfrα+ and Pdgfrβ+ cell injected joints. Finally, incorporation of both Pdgfrα+ and Pdgfrβ+ fluorescent cells was found within the synovial membrane of treated joints (Figure 3D).
3.3 Subchondral bone sclerosis after Pdgfrα+ or Pdgfrβ+ cell therapy
Changes in subchondral bone after cell therapy were next examined by μCT (Figure 4). MicroCT reconstructions of femoral and tibial subchondral bone demonstrated a clear, qualitative increase in bone after DMM, which was most apparent in the medial compartment (Figures 4A,B and 4D). Cell therapies, especially Pdgfrβ+ cell therapy, showed a diminution of this effect. These changes were quantified separately in both the medial (Fiure 4B–E) and lateral aspects of the joint (Figure 4G–J), and separately for both femoral and tibial subchondral bone. As expected, DMM induced a significant increase in BMD within femoral and tibial subchondral bone on both medial and lateral aspects. DMM surgery led to increase in BV/TVs, which was limited to the medial tibial subchondral bone (Figure 4E). Remarkably, Pdgfrβ+ cell therapy completely prevented subchondral bone changes associated with DMM, including a complete reversal of any increase in BMD or BV/TV across all regions of interest. In contrast, no significant change in subchondral bone was found with Pdgfrα+ cell therapy in comparisons to PBS control injection. All analyses were stratified by gender, with similar results (Figure S2). Thus, Pdgfrβ+ but not Pdgfrα+ cell therapy led to improved quantitative metrics of subchondral bone sclerosis after DMM.
3.4 Histologic appearance of articular cartilage after Pdgfrα+ or Pdgfrβ+ cell therapy
Sagittal histologic sections of the knee joint were stained with safranin O/fast Green (Figure 5). Typical degenerative changes to the femoral and tibial articular cartilage were observed among PBS control-treated animals after DMM in comparison to sham surgery (Figure 5A, left hand images). Importantly, a cell dependent effect on arthritis progression was found among either Pdgfrα+ or Pdgfrβ+ perivascular cell therapy (Figure 5A, right hand images). Pdgfrβ+ cell injection led to a significant improvement in the appearance of the femoral and tibial articular cartilage, with less prominent loss of safranin O staining among superficial articular cartilage. In contrast, Pdgfrα+ cell therapy led to a less dramatic improvement in histologic appearance at 8 weeks after intra-articular injection. Using the OARSI histopathology scoring system, cartilage damage and matrix proteoglycan depletionwere measured (Figure 5B). The average OARSI scores (0–24) were determined across treatment groups. As expected, a significant increase in OARSI score was found across DMM-operated mice in comparison to sham-operated animals (10.78 ± 1.97 vs. 2.44 ± 1.89). Pdgfrβ+ cell therapy led to the largest improvement in OARSI score (8.18 ± 2.35), while the Pdgfra+ cell therapy also showed a significant reduction in OARSI score in comparison to PBS control (9.02 ± 1.99). Results were stratified by gender, with similar findings (Figure S3).
3.5 Immunohistochemical staining of articular cartilage after Pdgfrα+ or Pdgfrβ+ cell therapy
Cartilage antigen expression was next assayed after Pdgfrα+ or Pdgfrβ+ cell therapy by immunohistochemistry, including aggrecan (ACAN), and type X collagen (ColX) (Figure 6). As expected, DMM induced a significant loss of ACAN immunoreactivity among superficial articular cartilage in comparison to sham-operated conditions (Figure 6A, upper rows). DMM-operated animals treated with either Pdgfrα+ or Pdgfrβ+ cell therapy showed partial restoration of Acan immunoreactivity (Figure 6A, lower rows).
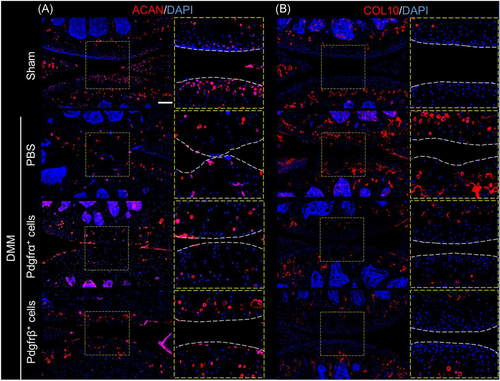
Conversely, the expression of ColX, a marker of chondrocyte hypertrophy and severity of OA, showed the opposite expression patterns (Figure 6B). Here, and as expected, DMM induced a significant increase in ColX immunoreactivity (Figure 6B, upper rows). Both Pdgfrα+ and Pdgfrβ+ cell therapy reversed this increase in ColX expression among DMM-operated stifle joints (Figure 6B, lower rows).
4 DISCUSSION
We compared the effects of intra-articular injection of subcutaneous adipose tissue derived Pdgfrα+ and Pdgfrβ+ perivascular cells subsets on OA after joint destabilization. Eight weeks after, we evaluated the engraftment and persistence on both Pdgfrα+ and Pdgfrβ+ cells in the stifle joint tissues and their therapeutic effects. The surface of articular cartilage was a preferred site of engraftment and persistence for Pdgfrα+ cells. Pdgfrβ+ cells were more widely engrafted in all layers of cartilage and in the subchondral bone. OARSI scoring and cartilage antigen immunohistochemistry showed an improvement from both cell therapies compared to the PBS treated group after DMM. Interestingly, Pdgfrβ+ cells had a remarkable beneficial effect on the cartilage; The proteoglycan content, ACAN, was more marked while the OA marker for hypertrophic chondrocytes, colX, was reduced. Pdgfrβ+ cells are known for their chondrogenic differentiation ability.37 They can also mediate tissue repair via the activation of the PDGF pathway that promotes chondrocyte differentiation, migration, and proteoglycan production.38 Together with their intrinsic homing into the different cartilage layers showed here, they might play an important role in enhancing cartilage regeneration and repair.
The subchondral bone was also preferred tissue of engraftment and persistence for Pdgfrβ+ cells. The subchondral bone sclerosis was remarkably reduced by the Pdgfrβ+ cells. In OA, the sclerosis of the subchondral may be a biomechanical compensational adaptation to the articular cartilage damage.39 Together with their beneficial effect on the articular cartilage and their ability to engraft, persist and stimulate bone repair,40 the Pdgfrβ+ cells may have alleviated the pathologic compensational adaptation response of the osteoarthritic subchondral bone and reduce the sclerosis. Paradoxically, Su et al.41 described that an excessive amount of PDGF-BB, which activates Pdgfrβ signaling after DMM, may result in a pathologic subchondral bone with a higher bone volume fraction.41 The injected and engrafted Pdgfrβ+ cells may play a role in PDGF-BB regulation and hence reduce its effect on the subchondral bone.
Pdgfrβ+ cells not only engrafted and persisted in the articular cartilage and bone, but they were also observed in the MCL, meniscus, IFP, and joint capsule.42 The meniscus is known to host a pool of perivascular stem cells in both the inner avascular region and the vascularized region.43 We saw that the meniscus was remarkably engrafted with both Pdgfra+ and Pdgfrβ+ cells, possibly contributing to meniscal regeneration marked by increasing proteoglycan content after DMM. Significant incorporation of injected Pdgfrβ+ but not Pdgfra+ cells was found within and around the MCL. Scx is a distinct marker for tendon and ligament progenitors and differentiated cells.44 Here, SCX expression was partially restored after DMM among ligamentocytes upon either Pdgfra+ or Pdgfrβ+ cell treatment.
In this study, we injected perivascular cells obtained from the white subcutaneous adipose tissue. Most remarkable effects were observed in the articular cartilage and the subchondral bone and induced by Pdgfrβ+ cells. Once implanted in OA pathologic microenvironment the perivascular cells ameliorated several key radiologic and histologic parameters of OA. Several mechanisms can be involved in the way perivascular progenitor cells mediate their effects after intra-articular injection. In contrast, perivascular cells inhibit osteoclast formation via non-EV derived secondary messengers.45 Future studies must define the extent to which an injectiable Pdgfrβ+ cell therapy plays direct or paracrine roles in OA prevention, and the extent to which anti-inflammatory and/or immunosuppressive actions play a contributory role.11
Several limitations exist toward the broader extrapolation of our results. Firstly, patients with (OA) primarily seek treatment due to pain and disability, yet the primary endpoints we utilized were histological and radiographic measures of joint destruction. Applying behavioral analyses would provide information about gait abnormalities seen in humans and in rodent OA models reflecting similar compensatory behaviors that protect an injured limb from loading.46 A logical next step would be to assess pain levels, motor function and gait after injectable perivascular cell therapies. Second, human and mouse pericytes have analogous antigen expression, and a follow up study should determine if a Pdgfrβ+ cell therapy derived from human fat demonstrates comparable cellular characteristics. Finally, future studies would determine the downstream molecular mediators of a Pdgfrβ+ cell therapy andexpand on whether purified cell therapies with a high pericyte content would be most beneficial for the amelioration or prevention of OA.
ACKNOWLEDGMENTS
Aaron W. James was supported by the NIH/NIAMS (R01 AR070773, K08 AR068316), NIH/NIDCR (R21 DE027922), USAMRAA (W81XWH-180109121, W81XWH-18-1-0336, W81XWH-18-10613), American Cancer Society (Research Scholar Grant: RSG-18-027-01-CSM), the Maryland Stem Cell Research Foundation, and MTF Biologics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health, Department of Defense, or US Army.
CONFLICT OF INTERESTS
Aaron W. James is a paid consultant for Novadip and LifeSprout LLC. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interests policies.