Staphylococcal infection prevention using antibiotic-loaded mannitol–chitosan paste in a rabbit model of implant-associated osteomyelitis
Abstract
Antibiotic-loaded chitosan pastes have shown advantages in the treatment and coverage of complex musculoskeletal defects. We added mannitol, previously shown to increase antibiotic susceptibility of biofilm, to an injectable chitosan/polyethylene glycol paste for delivery of antibiotics. Ground sponges (0.85% acetic acid solution, 1% chitosan, 0% or 2% mannitol, 1% polyethylene glycol) were hydrated using phosphate-buffered saline with 10 mg/ml amikacin and 10 mg/ml vancomycin added to form pastes. We inoculated rabbit radial defects with 105 colony-forming units of Staphylococcus aureus (UAMS-1) and inserted titanium pins into the cortical bone. Groups compared included mannitol blend pastes, non-mannitol blends, antibiotic-loaded bone cement, vancomycin powder, and no treatment controls. We harvested tissue samples and retrieved the pins retrieved at 3 weeks. All antibiotic-loaded groups lowered bacterial growth and colony-forming unit counts in soft and bone tissue and on titanium pins in in vivo studies. The results indicate this biomaterial is capable of eluting active antibiotics at concentrations that reduce bacterial growth on biomaterials and tissue, which, in turn, may prevent biofilm formation. Blends of chitosan and mannitol may be useful in prevention and treatment of osteomyelitis and implant-associated infections.
1 INTRODUCTION
Musculoskeletal trauma and joint replacement surgeries can leave tissue and implanted materials susceptible to bacterial infections. Periprosthetic joint infections (PJI) throughout the United States are increasing as the demand for joint arthroplasties continues to rise.1 As many as 2% of total joint arthroplasty patients risk developing a PJI after primary surgery.2, 3 Infection recurrence after infection-associated implant failure occurs in as many as 1 of 3 patients.4, 5 The risk of infection increases to as high as 30% after traumatic injuries such as open fracture.4
Both orthopedic implants and devitalized tissue can serve as substrates for the formation of attached bacterial communities, termed biofilms.6, 7 Within biofilms, a subset of bacteria, called persister cells, can enter a semi-dormant metabolic state, which contributes to an inherent tolerance to antimicrobials.8 Although not fully elucidated, mechanisms for persister antimicrobial tolerance could include limited internalization of antimicrobials.9 Studies have demonstrated that some bacterial metabolites and carbon sources stimulate awakening of persisters, allowing antibiotics to eradicate the biofilm at lower concentrations.10, 11 Allison et al.10 showed that mannitol, a sugar polyol and common metabolite of bacteria, increased the susceptibility of biofilms to the aminoglycoside gentamicin, which was demonstrated for both Staphylococcus aureus and Escherichia coli in both in vitro and in vivo infection models. It has been theorized that the addition of mannitol may activate the proton-motive force necessary for antibiotic internalization within persister cells, resulting in enhanced uptake of internally acting aminoglycoside antibiotics (Table 1).
| In vivo study | |
|---|---|
| Treatment group | Amount |
| No treatment | N/A |
| PMMA loaded with antibiotics | 4 mm × 10 mm PMMA cylinder with 37.5 mg vancomycin and 15.6 mg amikacin |
| Sprinkle of powdered antibiotic | 10 mg vancomycin |
| Chitosan–PEG (CP) paste loaded with antibiotics | 0.3 ml of paste with 10 mg/ml vancomycin and 10 mg/ml amikacin |
| Chitosan–PEG–mannitol (CPM) paste loaded with antibiotics | 0.3 ml of paste with 10 mg/ml vancomycin and 10 mg/ml amikacin |
- Abbreviations: PEG, polyethylene glycol; PMMA, poly(methyl methacrylate.
Achieving local antibiotic concentrations necessary for biofilm prevention is not realizable through systemic antibiotic therapy alone. Local antibiotic delivery methods include antibiotic-loaded bone cements (ALBCs), antibiotic-loaded calcium sulfate pellets, and the application of topical antibiotic powder at the time of wound closure.12, 13 However, most poly(methyl methacrylate) (PMMA) based ALBC are nonbiodegradable, requiring secondary surgery for removal.13, 14 Although calcium sulfate pellets are biodegradable and elute high concentrations of antibiotics over the course of several days to weeks,15, 16 limitations include serious wound drainage,17, 18 hypercalcemia,19 and limited wound coverage.20 Biodegradable antibiotic delivery systems that conform to the complex geometries of the joint space increase the likelihood that pathogens are eradicated.21 Injectable drug delivery systems under investigation include temperature-responsive hydrogels,22, 23 fibrin sealants,24, 25 and chitosan pastes.26, 27 Chitosan pastes have the advantages of being injectable, biodegradable, conformable to surgical defects, and can be loaded with clinician-selected antibiotics at the time of surgery.26, 28, 29 Preliminary in vitro studies indicate that the same paste formulations of chitosan and mannitol as tested in this study release mannitol and antibiotics simultaneously for up to 7 days while also degrading 28.2 ± 7.7% over a 14-day period.21 Chitosan–mannitol paste blends released 100% of therapeutics with a first-order burst profile, and release was found to be diffusion driven.21, 30 Interaction between hydroxyl groups of chitosan and hydroxyl groups of mannitol may further promote hydrogen bonding, leading to increased adhesive properties and cohesiveness of the paste.31
In this study, we evaluated the efficacy of a bioinspired supramolecular complexation between mannitol and the carbohydrate polymer chitosan to deliver both mannitol and antibiotics in a controlled manner. Although mannitol has been investigated as a supplement to increase the susceptibility of bacteria to antibiotics, mannitol has not been delivered through a biomaterial drug delivery system for bacterial growth prevention and subsequent biofilm prevention. We evaluated the hypothesis that mannitol–chitosan blends prevent biofilm-associated infection with similar or increased efficacy over other local antibiotic delivery systems using an in vivo osteomyelitis model of S. aureus infection.
2 METHODS
2.1 Fabrication
Chitopharm S chitosan powder (Chitinor AS; 82.46 ± 1.679 degrees of deacetylation; 250.6 kDA average molecular weight) was dissolved at 1% (weight/volume) with 1% (weight/volume) polyethylene glycol (PEG; Sigma-Aldrich; 8000 g/mol average molecular weight) in 0.85% (volume/volume) acetic acid in deionized water solution to form the control chitosan/PEG paste group (CP). Mannitol (Bulksupplements.com) was dissolved at 2% (weight/volume) in the previously described solution to form the chitosan–mannitol paste blend (CPM). The solutions were cast in 25 ml aluminum dishes and frozen overnight at −80°C, then lyophilized in a benchtop freeze dryer (LabConco) to create acidic dehydrated sponges. We then ground the two sponge types separately into fine powders and stored in a desiccator until sterilization with ethylene oxide gas.
To load paste with antibiotics, a 3 ml syringe filled with 1.25 ml of phosphate-buffered saline (PBS) containing 10 mg/ml amikacin (MP Biomedicals) and vancomycin (MP Biomedicals) was coupled via a Luer lock connector (Qosina) to a 10 ml syringe containing 500 mg of ground powder. Mixing of the antibiotic solution with the ground paste occurred by ejecting back and forth between syringes until an evenly mixed paste formed.
To fabricate PMMA cylinders, 2.4 g of amikacin and 1 g of vancomycin were added to 40 g of Orthoset (Microport Orthopedics) powder, before mixing with the liquid component. After mixing for 1 min, we pressed dough into precut 10 mm length cylindrical Tygon tubing with 4 mm inner diameter, using 96-well plates as support. Titanium pins with 0.9 mm diameter were pressed into the center of the cylindrical molds and then removed after setting to create space for the implanted pin within the core.
2.2 Osteomyelitis model in New Zealand white rabbits
This animal model was approved by theIACUCattheUniversityofArkansasMedicalSciences(AUP IACUC at the University of Arkansas Medical Sciences (AUP #3608), taking all appropriate measures to minimize pain and discomfort. In an adaptation of the osteomyelitis model by Smeltzer et al.,32 the current study used titanium pins inserted into bone to create implant-associated infection. Female New Zealand white rabbits weighing 2–3 kg were divided into five groups (n = 6/group) including the following: no treatment control, a hollow PMMA cylinder (Orthoset, Microport Orthopedics) with amikacin (approximately 37.5 mg/implant), and vancomycin (approximately 15.6 mg/implant), vancomycin powder (10 mg) applied into the defect, CP loaded with amikacin and vancomycin, and CPM loaded with amikacin and vancomycin.
Rabbits were anesthetized with 1–2 cc of a xylazine/ketamine mixture intramuscularly for a dose range of 3–7 mg/kg xylazine and 30–40 mg/kg ketamine. Rabbits were maintained on isoflurane administered by nose cone to produce surgical anesthesia and monitored by a veterinary technician. The right forelimb of each rabbit was shaved and prepped using a betadine scrub and rinsed with 70% ethanol. An incision was made on the anterior surface of the right forelimb through the epidermis, musculature, and fascia until the radius was exposed. A 1 cm mid-radial segment was excised from the right radial bone using a miniature saw blade (Exakt, Oklahoma City, OK). Both distal and proximal ends of bone were inoculated with S. aureus (10 μl of 106 colony-forming units [CFUs]/ml; UAMS-1 strain) directly into the intramedullary canal. A titanium pin (0.9 mm diameter, 2 cm length), sterilized by autoclave, was placed into the defect via the medullary canal. Approximately 150 µl of pastes were inserted via syringe to completely fill the space left by the removed segment after insertion of the titanium pin. For vancomycin groups, 10 mg of powder was applied directly to the defect space around the titanium pin. For the PMMA group, the titanium pin was inserted into the center of the cylinder then placed into the defect.
2.3 Bacteriological analysis
Rabbits were euthanized after 3 weeks, with swabs of bone and surrounding soft tissue taken for analysis of present microorganisms. The location of the swab was the same in each case and the procedure was carried out aseptically. The pins were also retrieved and sonicated in PBS for bacteriological analysis. The solutions were serially diluted 10-fold, and 0.5 ml of each suspension was plated in triplicate onto Trypticase Soy Agar (TSA) plates (Becton Dickinson) and incubated at 37°C for 24 h. After incubation, the CFUs developed on the TSA were counted. Plates with one or more colonies were considered a positive culture, with no colonies present indicating a negative culture.
2.4 Radiograph analysis
Radiographs of the right forelimb were obtained immediately after surgery and at Weeks 1, 2, and 3 postoperatively. Methods adapted from Smeltzer et al.33 were used to assess radiographic signs of osteomyelitis and were based on periosteal elevation, widening of the bone shaft, formation of new bone, and deformation of soft tissue. Two investigators who were blinded to the treatment status of each rabbit scored each radiographic sign of osteomyelitis on a five-point scale (0-4), with 0 representing no sign of disease and 4 representing the most severe evidence of disease.
2.5 Histological analysis
Following the sacrifice of animals, the bone and surrounding tissue were harvested and preserved in a 10% neutral formalin solution. Tissue samples were decalcified with formic acid, embedded in paraffin, sectioned into 5 µm slices, and stained with hematoxylin and eosin (H&E) and a modified Gram stain.34 Methods adapted from Smeltzer et al.33 were used to assess histological signs of osteomyelitis and were based on intraosseous acute inflammation, intraosseous chronic inflammation, periosteal inflammation, and bone necrosis. Images were scored on a scale of 0–4, with 0 indicating no inflammation or necrosis, and 4 representing high inflammation and necrosis. Histology scores were determined by two separate evaluators who were blinded with respect to the treatment group.
2.6 Statistical analysis
Statistical analysis was performed using SigmaPlot (Systat Software Inc.) and GraphPad Prism 7.2 software (GraphPad Software Incorporation). In vivo CFU counts, radiographic scores, and histomorphometric scores were analyzed using a non-parametric Kruskal-Wallis one-way analysis of variance with Dunn's post hoc tests to compare groups. The proportion of infected versus cleared culture swabs was assessed using the Fisher Exact test (α = .05).
3 RESULTS
3.1 Bacteriological results
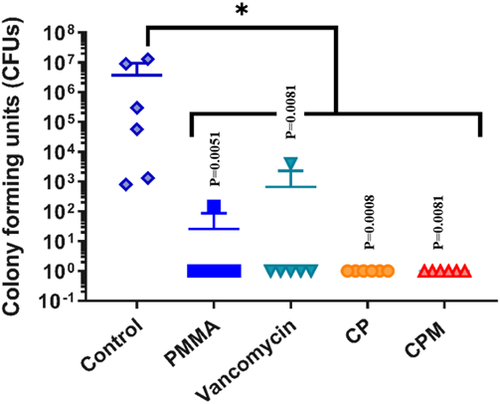
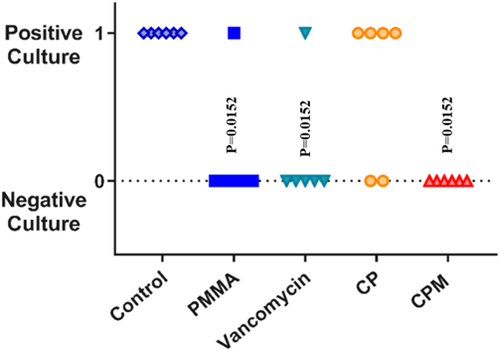
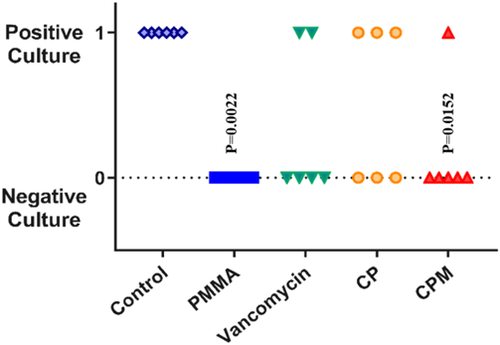
Both antibiotic-loaded paste groups inhibited S. aureus bacterial growth on titanium pins (0 CFUs retrieved) in an implant-associated infection model (Figure 1). Statistical analysis showed all groups to be statistically different from the no treatment group. The CPM paste was the only group with no bacterial growth from harvested soft tissue, compared with positive cultures for non-mannitol blends and for vancomycin powder and PMMA (Figure 2). For the bone culture swabs, PMMA was the only experimental group with no bacterial growth (Figure 3). The CPM paste had a positive culture.
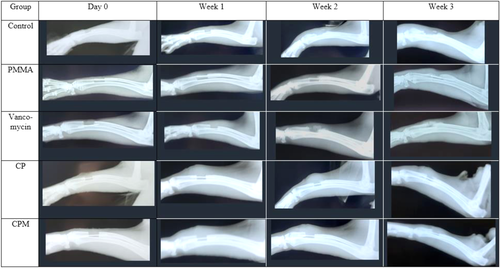
3.2 Radiograph results
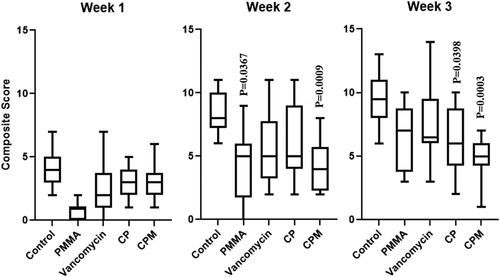
Composite radiograph scores increased at least slightly for each group during each weeklong timepoint. At Week 2, rabbits treated with PMMA and CPM showed significantly lower overall signs of osteomyelitis compared to untreated controls. By Week 3, CPM and CP had significantly lower scores compared to the untreated controls (Figure 5).
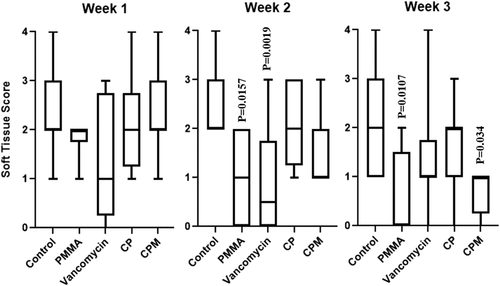
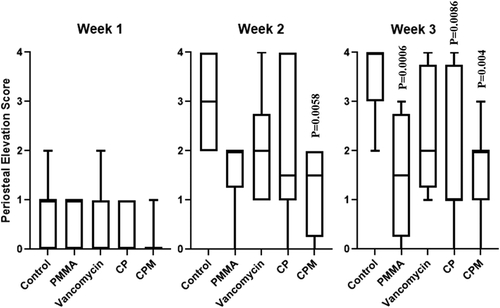
Soft tissue deformation was significantly lower for PMMA and vancomycin groups compared to untreated controls; by Week 3, PMMA continued to have significantly less soft tissue deformation as did CPM treated groups (Figure 5). All groups showed increasing average periosteal elevation scores over time and a broad score distribution; CPM treated groups had significantly lower scores compared to untreated controls at Week 2, whereas PMMA, CP, and CPM all had significantly lower scores in Week 3 (Figure 6).
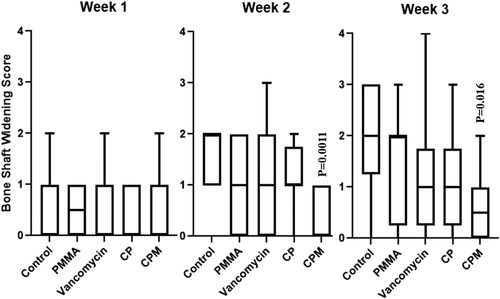
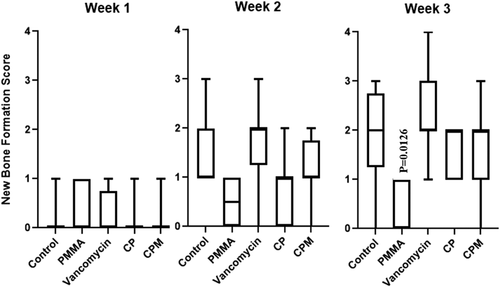
Scores of architectural deformation of the bone, determined by assessing bone shaft widening and new bone formation, showed few differences between groups. CPM had significantly lower scores for bone shaft widening at Weeks 2 and 3 compared to untreated controls (Figure 7). PMMA treated groups had significantly lower scores for new bone formation at Week 3 compared to untreated controls (Figure 8).
3.3 Histomorphometry results
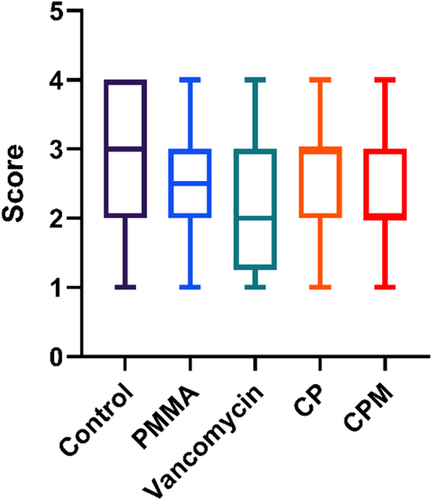
Histological sections of bone in the defect showed areas of severe inflammation for nontreated controls (Figure 10). For PMMA treated groups, there was minimal to moderate inflammation, but little to no tissue growth into the defect area. The vancomycin powder group had tissue fill within the defect, but also areas of high inflammatory cell presence. Both chitosan paste groups had signs of moderate inflammation and soft tissue fill within the defect. No detectable remaining paste was observed in any of the paste groups. There were no significant differences in average histology scores between any of the treatment groups or controls (Figure 11).

4 DISCUSSION
These studies confirmed that the injectable chitosan–mannitol paste delivers antibiotics to complex orthopedic defects and reduces bacterial growth on implants, soft tissue, and bone, which, in turn, could prevent biofilm formation and subsequent infection. This material could serve as a degradable local antibiotic delivery system following musculoskeletal trauma or during periprosthetic joint revision surgeries to prevent osteomyelitis. The added layer of antimicrobial protection provided by these paste blends could lead to a decrease in the number of recurring infections during the wound healing process.
The CPM paste had no bacterial growth from the titanium pin analysis and soft tissue culture, but a positive culture for the bone culture swab. The efficacy of mannitol blends in the in vivo evaluations could be due to several factors. Blending chitosan with mannitol may initiate the formation of a polyelectrolyte complex, forming hydrogen bonding interactions between hydroxyl groups of chitosan and those of mannitol. A previous study demonstrated that mannitol blends had a more extended release of antibiotics, which may have prolonged exposure of contaminated tissue to antibiotics.21 Mannitol may provide carbon sources for stimulating persister cell metabolism or may serve as an osmotic agent that could alter the biofilm-forming ability of S. aureus in vivo.35-37 Recent studies have outlined the antibiofilm properties of erythritol, another sugar alcohol similar in structure to mannitol, suggesting that erythritol is capable of diffusing into the biofilm matrix and weakening the hydrogen bonds between the hydroxyl groups of exopolysaccharides present.38 Lim et al.38 recently attached erythritol to the positive end of the zwitterion, betaine, to show a reduction in Streptococcus mutans biofilm adhesive forces due to an increase in solubility of bacterial exopolysaccharides. A similar effect of weakening the extracellular polymeric substances of biofilm could be occurring with mannitol–chitosan blended pastes. This would lead to the creation of an environment unfavorable for the development of a biofilm, preventing infection before biofilm formation can occur.
Previous studies confirmed that CPM paste degrades 28.2 ± 7.7% over a 14-day period,21 which indicates that it likely stays within the wound site longer than commonly used vancomycin powder. This may further contribute to its extended antimicrobial properties, and can prevent incidence of gram-negative or polymicrobial infections commonly seen with the use of intraoperative vancomycin.39 Though vancomycin has been shown to have toxicity against osteoblasts at concentrations of 5 mg/ml,40 daily release of vancomycin from CP and CPM pastes did not exceed 0.6 mg/ml, indicating that these systems release vancomycin at levels that are not detrimental to bone healing.21 Similarly, amikacin is nontoxic to osteoblasts at concentrations up to at least 2 mg/ml40; both CP and CPM paste did not release more than 0.6 mg/ml/day, further demonstrating the safety of these treatments in bone healing.41 Unfortunately, we were unable to measure chitosan degradation in this model; however, because large areas of chitosan were not seen in histology sections, it was assumed that chitosan had completely degraded within the 3-week timeframe, releasing all antibiotics and mannitol.
Composite radiograph scores indicated that all groups increased at least slightly between weeks, with the highest increase seen from the control group and the lowest by the CPM group. Assessing scores for each assessment category provided a greater insight into the differences amongst treatment groups. Overall, the CPM paste showed significantly lower composite, bone shaft widening, and periosteal elevation scores at Weeks 2 and 3, and significantly lower soft tissue deformation scores at Week 3. In some specimens treated with PMMA, it was difficult to differentiate between the PMMA cylinders and new bone formation, which was a limitation in our analysis. It is important to note the significant variability seen between specimens of all groups for both soft tissue and bone analysis, making it difficult to draw strong conclusions from these data despite statistically significant differences.
Another limitation of this study includes the use of a single strain of microorganism. Although staphylococci are the primary causative species of osteomyelitis,42 infections in a clinical setting may be complex and polymicrobial. Furthermore, not all groups previously evaluated in vitro were included for the in vivo model, with only antibiotic-containing treatment groups examined. In clinical therapy, systemic antibiotic administration is routinely given as prophylaxis,43, 44 though this was not administered in the animal model to avoid confounding effects. Another potential limitation is that to preserve tissues for histological evaluation, we used swab cultures instead of tissue homogenization to detect the presence of bacteria in tissues. The swabbing method has low diagnostic sensitivity in clinical studies; thus, future studies may include a more quantitative metric of bacterial growth in soft tissue and bone. Further studies should be performed to determine local and systemic antibiotic levels as well as systemic inflammatory markers associated with the CPM paste. chitosan–mannitol blends without antibiotics may be examined as adjunctive therapy to systemic prophylaxis in future studies. Furthermore, in vivo studies use a small number of biological replicates, which also limits this study; likewise, only female rabbits were used, so sex differences in response to treatments were not explored. This animal model is representative of preventative therapies and does not use established or mature biofilms; future studies will test CPM paste against established biofilms and its ability to eradicate persister cells.32, 45 Future work evaluating therapy in models of one-stage revision procedures are planned, and further expanded preclinical studies can follow.
5 CONCLUSIONS
In conclusion, the mannitol–chitosan blend exhibited enhanced antimicrobial properties and demonstrated efficacy in reducing bioburden in a model of osteomyelitis. This material could be clinically applied in the context of revision surgeries after a periprosthetic joint infection to improve treatment outcomes and lower the incidence of infection. Subsequent in vitro and in vivo tests will measure the antibiofilm properties of the mannitol–chitosan blend against different species of bacteria relevant to periprosthetic joint infections including methicillin-resistant S. aureus, Staphylococcus epidermidis, and Escherichia coli, as well as Pseudomonas aeruginosa. Future studies will include the evaluation of CPM paste's efficacy at eradicating established implant-associated infection with S. aureus (UAMS-1) models representative of one-stage revision procedures.
ACKNOWLEDGMENTS
Authors acknowledge assistance from Logan Boles, MS, Michael Harris, PhD, and Stasianne Mallin in fabrication of materials and editing. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health (NIH) under Award Number R01AR066050. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICT OF INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
J. Amber Jennings, Warren O. Haggard, Leslie R. Pace, and Zoe L. Harrison conceived the idea. Leslie R. Pace, Madison N. Brown, and Zoe L. Harrison fabricated samples and performed in vitro experiments. Karen E. Beenken and Mark S. Smeltzer performed the in vivo experiments and Joel D. Bumgardner assisted with analysis of in vivo data. Leslie R. Pace and Zoe L. Harrison wrote the manuscript. Madison N. Brown wrote sections of the manuscript and J. Amber Jennings edited sections. J. Amber Jennings, Warren O. Haggard, Karen E. Beenken, and Mark S. Smeltzer were involved in the planning and supervision of the project. All authors discussed the results, provided critical feedback, and contributed to the final manuscript.