One-year outcomes following physical therapist-led intervention for chronic hip-related groin pain: Ancillary analysis of a pilot multicenter randomized clinical trial
Abstract
Evidence related to physical therapist-led intervention for patients with chronic hip-related groin pain (HRGP) is limited. The purpose was to assess sustainability of treatment effects for people with HRGP undergoing two physical therapist-led interventions. We completed an ancillary analysis of a pilot multicenter, randomized clinical trial. Forty-six patients with chronic HRGP, 15–40 years, were enrolled. Patients were randomized to participate in 10 sessions over 12 weeks of either movement pattern training (MoveTrain) or traditional strength/flexibility (Standard). Participants completed self-report questionnaires before treatment and 6 and 12 months after treatment completion. Outcome measures included Hip disability and Osteoarthritis and Outcome Score (HOOS), Patient Specific Functional Scale and Numeric Pain Rating Scale for average and worst pain. Continuous data were analyzed with mixed model repeated measures analysis of variance (RM-ANOVA) within each group. Numeric pain rating scale (NPRS) was analyzed using multinomial generalized estimating equations (GEE) with a cumulative logit. Reported p values are from statistical contrasts within the RM-ANOVAs and GEEs testing a priori hypotheses regarding change from pretest to month 6, and pretest to month 12. A total of 43/46 (93.5%) participants completed treatment, 40 (87.0%) completed 6 and 38 (82.6%) completed 12 month questionnaires. At 6 and 12 months, both groups demonstrated clinically significant improvements, compared to pretest, in all subscales of HOOS (p < 0.01), Patient Specific Functional Scale (p < 0.001), and NPRS (p < 0.0001). Among patients with chronic HRGP, both MoveTrain and Standard resulted in improved outcomes that were sustained 12 months after treatment. Further investigation in a larger sample is needed to confirm our findings.
1 INTRODUCTION
Chronic hip-related groin pain (HRGP) is a common symptom that affects young to middle-aged adults. Patients with HRGP experience significant limitations in activity participation1-3 thus restricting their ability to complete everyday tasks or participate in school, work, and physical activity including sports.4 Pathoanatomical diagnoses associated with HRGP include femoroacetabular impingement syndrome (FAIS), developmental dysplasia of the hip and other injuries such as labral tears or chondral lesions.5 Physical therapist (PT)-led intervention may be appropriate for patients with HRGP,6-14 yet we know little about the sustainability of treatment effects associated with PT-led intervention strategies.
A growing body of evidence suggests that PT-led intervention can decrease pain and improve function among patients with HRGP.6-14 Most studies report short-term outcomes only and do not report if effects are sustained past the active treatment phase. Only two studies report outcomes 1 year or greater after treatment completion among patients with HRGP who participated in rehabiltation.6, 15 Both studies were randomized clinical trials (RCT) comparing PT-led intervention to surgical intervention for patients with HRGP, specifically FAIS. Patients who received PT-led intervention in these previous RCTs reported improvements in patient-reported function.6, 15 Although these findings related to PT-led intervention are promising, the comparisons to surgical intervention were mixed and differences between treatment groups were small. Griffin et al.6 reported statistically significant, greater improvements among those receiving surgery compared to PT-led intervention, 19% versus 14% improvement in the International Hip Outcome Tool (iHOT), respectively. Similarly, in their intention-to-treat analysis, Mansell et al.15 reported improvements, in both the surgical group and PT-led intervention group, 20% versus 15% improvement in the iHOT, respectively, however the differences were not statistically significant. It is important to note that 70% of patients randomized into PT-led intervention received surgery before final outcome assessment, limiting our ability to draw conclusions about the specific effects of PT-led intervention. In a recent RCT comparing surgical correction of bony morphology associated with FAIS with or without labral repair, to arthroscopic lavage with or without labral repair, Ayeni et al.16 found no differences in patient-reported function 1 year after surgery, except in the Hip Outcome Score activity of daily living domain, in which there was greater improvement in the lavage group, thus questioning the role of surgical treatments (labral repair and osteochondroplasty). Nevertheless, their data also demonstrated fewer reoperations in patients treated with osteochondroplasty. Clearly there is a need to further investigate the role of both operative and nonoperative treatments for HRGP.
While a thorough comparison of the previous RCTs17 goes beyond the scope of this paper, one key limitation of the RCTs6, 15 is the heterogeneity of the PT-led intervention protocols used. For each trial, general treatment guidelines were developed and implemented, however treatment provided to each patient was not standardized. At the discretion of the treating PTs in each study, treatment for a patient may have included any combination of components including strength and flexibility exercises, activity modification, mobilization, and use of orthotics or taping. This heterogeneity suggests that optimal PT-led intervention for patients with HRGP has yet to be established. We completed a pilot, multicenter RCT (ClinicalTrials.gov: NCT02913222) comparing two PT-led interventions for HRGP, movement pattern training (MoveTrain) and traditional strength and flexibility training (Standard) to establish feasibility of completing a definitive trial.8 We established feasibility and reported that both strategies resulted in clinically significant improvements in patient-reported function and hip muscle strength immediately after treatment.8 The MoveTrain group also demonstrated an improvement in lower extremity movement patterns.8
Investigation of the long-term effects of different PT-led interventions are needed to determine how best to optimize treatment for patients with HRGP. This project presents ancillary analysis from our pilot, multicenter RCT. The purpose of this analysis was to determine if the observed posttreatment improvements in patient-reported function were sustained at 6 and 12 months following treatment completion and to provide preliminary data to plan a definitive trial.
2 METHODS
2.1 Study design overview
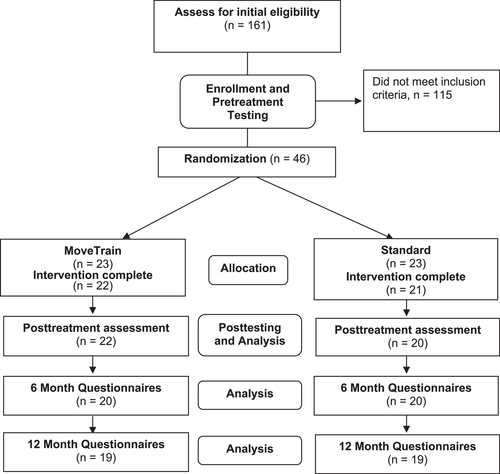
This study is an ancillary analysis using data from a pilot multicenter, assessor-blinded RCT (level of evidence 2). The trial was prospectively registered at ClinicalTrials. gov (NCT02913222). The study design is provided in Figure 1. Methods and results of the pilot study reporting feasibility and patient outcomes immediately following treatment have been published.8 To assess maintenance of treatment effects, questionnaires were sent electronically to patients 6 and 12 months after treatment completion to capture patient-reported outcome measures. This study was approved by the institutional review board of Washington University School of Medicine in St. Louis and University of Pittsburgh and patients signed an informed-consent statement before participating. Signed patient assent and parental permission were obtained for those under the age of 18.
2.2 Participants
Participant recruitment occurred at Washington University School of Medicine in St. Louis and University of Pittsburgh between January 2017 and February 2018. Potential patients were recruited using research volunteer databases, flyers, social media, and from local healthcare clinics. To be included, patients had to be 15–40 years; report anterior groin or deep hip joint pain that was reproduced using the Flexion, ADduction, Internal Rotation (FADIR) impingement test;18 report presence of pain more than 3 months that rated greater than 3/10; and reported functional limitation represented by a modified Harris Hip Score < 90. Patients were excluded if they reported an inflammatory disease; previous hip surgery, fracture, or infection; hip pain due to impact trauma; slipped capital femoral epiphysis or Legg-Calve-Perthes disease; neurological involvement causing balance impairment; radiating numbness, pain or tingling into the thigh; pregnancy; or positive screening tests indicating hip pain was referred from the spine.
2.3 Pretest assessment
After consent was obtained, patients completed self-report questionnaires electronically and participated in a screening examination to determine final eligibility. Once final eligibility was determined, patients participated in a clinical examination that included movement pattern assessment using two-dimensional video capture and hip muscle strength testing using a hand dynamometer.8 Patients were then randomized into MoveTrain or Standard in a 1:1 ratio stratified by site, sex, and Hip disability and Osteoarthritis and Outcome Score (HOOS) Symptoms (determined from median split or our preliminary data).8 Patients were allocated using a variable block size within each stratum. Randomization sequences were generated a priori using a formal probability model and were elicited from the data capture system. It was not possible to blind the patients or the treatment providers, however examiners responsible for collecting outcome measures during the examination were blinded to group. Specific detail for clinical examination and patient randomization have been reported.8
2.4 Intervention
Five experienced PTs, trained in standardized procedures and demonstrated at least 95% on a standardized assessment of treatment fidelity,8 provided treatment. Treatment in both groups consisted of 10 supervised sessions over 12 weeks and included patient goal assessment, education, and instruction in daily performance of a home exercise program (HEP). The HEP included either functional task practice (MoveTrain) or exercise (Standard). Patient-specific tasks, identified by each patient as activities limited by their hip symptoms, were also addressed in both groups. Common patient-specific tasks included daily activities, such as sitting, and activities related work or fitness. The HEP provided in each group was progressed, based on patient performance, by increasing difficulty of the functional task (MoveTrain) or exercise (Standard). Difficulty of the task was progressed by increasing load, increasing repetitions, varying the speed or changing the supporting surface. Group-specific treatment is described briefly below, however specific detail has been reported.8
The goal of MoveTrain was to reduce stresses on hip structures by optimizing biomechanics of daily and patient-specific tasks. The key element of MoveTrain is task-specific instruction to correct abnormal movement patterns demonstrated during daily tasks and patient-specific tasks. For example, if excessive hip adduction was noted during stair descent, instruction was provided to minimize hip adduction. Verbal and visual cues were provided to demonstrate the movement modification. Once the patient was able to complete the motion correctly, they were instructed to perform multiple repetitions of the task as their HEP. Additionally, patients were encouraged to use the trained movement throughout the day, when performing their daily tasks. Instructions to correct abnormal movement patterns during patient-specific tasks such as work or fitness activities were also provided and practiced by the patient.
The goal of Standard was to improve lower extremity and trunk muscle strength and lower extremity flexibility. Published evidence14, 19 and clinical practice guidelines20 were used to select exercises. Exercises included strengthening of muscle groups in all six directions of motion, trunk stabilization using plank-style activities and flexibility for hamstrings, gastrocnemius/soleus, piriformis, and hip flexors. For patient-specific tasks, patients were instructed in modification of intensity, frequency, and duration of tasks that provoked their symptoms.
After treatment completion, patients were encouraged to continue their HEP. The research coordinator contacted patients 3 and 9 months after treatment completion to confirm contact information and monitor for adverse events.
2.5 Posttreatment assessment
Immediately after treatment completion, patients returned for follow up assessment (results reported previously8). Ancillary to the primary pilot study,8 questionnaires were administered electronically 6 and 12 months after treatment completion to assess treatment sustainability.
2.6 Variables measured
The primary outcomes in the pilot RCT were related to feasibility of completing a definitive trial.8 Secondary outcomes to assess patient response to treatment were the HOOS. The HOOS has five subscales representing five domains including pain, symptoms, activities of daily living (ADL), sport and recreation and quality of life (QOL). Scoring of each subscale ranges from 0 to 100, with lower scores indicating greater impairment or activity limitation. The HOOS has high test–retest reliability21, 22 and acceptable content validity.22, 23 The minimum important change for each subscale, among patients with HRGP receiving nonoperative management, has not been established.11, 24
Additional outcomes included the Patient Specific Functional Scale (PSFS),25, 26 a patient-reported outcome measure of patient-specific activity limitations, and pain intensity quantified by a numeric pain rating scale (NPRS). For the PSFS, patients were asked to identify “3–5 activities you are unable to do or having difficulties performing due to the pain or symptoms in your hip.” Patients then rated level of difficulty from 0 to 10, 0 indicating they are unable to perform the activity and 10 indicating they are able to perform the activity at their preinjury level. The final score is an average of all scores provided. The PSFS has high test–retest reliability and construct validity.25, 27 Minimum important differences (MID), identified among patients with various musculoskeletal disorders, for small, moderate, and large patient-perceived change are 1.3, 2.3, and 2.7, respectively.28 For NPRS, patients were asked to rate their average and worst pain in the previous week, 0–10, 0 indicating no pain and 10 indicating worst pain imaginable. Minimum important differences for small, moderate and large patient-perceived change are 1.5, 3.0, and 3.5, respectively.28 Patients were also asked if they elected to have surgery for hip pain after treatment completion.
2.7 Sample-size estimation
The primary pilot study was designed with 23/group to achieve 80% statistical power to detect effect sizes at least as large as 0.9 immediately after treatment.8
2.8 Data analysis
Continuous data were analyzed using mixed model repeated measures analysis of variance (RM-ANOVA) with a random effect for participant and a fixed effect for time point within each treatment group. Unlike traditional RM-ANOVA, a mixed model permits the inclusion of participants who provide data for at least one visit after pretest. For all models, the Kenward-Roger degrees of freedom approximation method was used and a compound covariance structure was found to be the best fitting. When regression residuals were not normally distributed, data were rank transformed before analysis. Reported p values are from statistical contrasts within the RM-ANOVA testing a priori hypotheses regarding change from pretest to month 6, and pretest to month 12. Multinomial generalized estimating equations (GEE) with a cumulative logit was used to analyze the ordinal NPRS. Outcomes are reported in tables as mean ± SD and in figures as mean change from pretest with 95% confidence intervals.
3 RESULTS
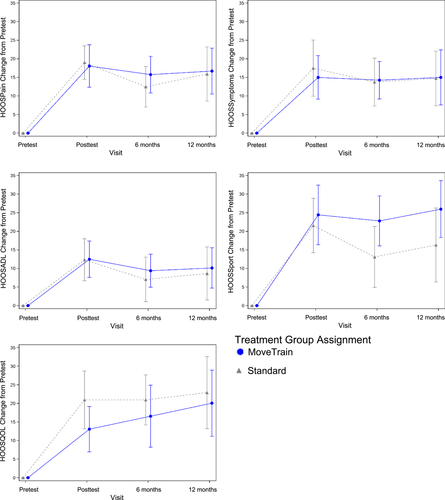
Figure 1 provides participant flow. A complete reporting of participant flow has been reported previously.8 Demographics for the overall sample are provided in Table 1. At 6 and 12 months, patients in both groups demonstrated statistically significant improvements, compared to pretest, in all HOOS subscales, PSFS and NPRS (Table 2). Compared to pretest, improvements for MoveTrain and Standard at 12 months, respectively, were as follows: HOOSPain, 16.7 ± 12.8 (p < 0.0001) and 15.9 ± 15.2 (p < 0.0001); HOOSSymptoms, 15.0 ± 15.4 (p < 0.0001) and 14.7 ± 15.2 (p < 0.0001); HOOSADL, 10.1 ± 11.3 (p < 0.0001) and 8.7 ± 14.8 (p < 0.001); HOOSSport, 26.0 ± 15.9 (p < 0.0001) and 16.3 ± 20.0 (p < 0.001); HOOS QOL, 20.1 ± 18.5 (p < 0.0001) and 22.9 ± 19.5 (p < 0.0001); PSFS, 2.0 ± 2.0 (p < 0.0001) and 1.6 ± 3.2 (p < 0.001); NPRS average, −2.3 ± 1.6 (p < 0.0001) and −2.2 ± 1.9 (p < 0.0001); and NPRS worst, −3.3 ± 1.9 (p < 0.0001) and −3.4 ± 3.4 (p < 0.0001). Figure 2 demonstrate that improvements in the HOOS subscales were similar for both treatment groups at posttest (reported previously8) and at 6 and 12 months. No patients reported undergoing or scheduling surgery for their hip pain.
| Variable | Overall sample N = 43 | By treatment group | |
|---|---|---|---|
| MoveTrain n = 22 | Standard n = 21 | ||
| Age (year), mean ± SD (range) | 29.1 ± 5.3 (range 17–39) | 27.6 ± 5.0 (range 17–37) | 30.6 ± 5.3 (range 22–39) |
| Gender, n (%) | |||
| Male | 17 (40%) | 9 (41%) | 8 (38%) |
| Female | 26 (60%) | 13 (59%) | 13 (62%) |
| Measured BMI (kg/m2), mean ± SD (Range) | 25.5 ± 6.2 (range 17.9–46.1) | 24.9 ± 6.1 (range 17.9–41.8) | 26.1 ± 6.4 (range 20.2–46.1) |
| Study limb, n (%) | |||
| Left | 20 (47%) | 10 (45%) | 10 (48%) |
| Right | 23 (53%) | 12 (55%) | 11 (52%) |
| UCLAa, median (range) | 10 (3–10) | 9.5 (3–10) | 10 (4–10) |
| Pain duration (year), median (range) | 2.0 (0.3–17.0) | 1.8 (0.3–12.0) | 3.0 (0.3–17.0) |
- a Patients are asked to rate their activity level over the previous 6 months. 10 = regularly participates in impact sports; 1 = wholly inactive, dependent on others.
- Abbreviations: MoveTrain, movement pattern training group; UCLA, University of California Los Angeles Activity Score.
| Pretest (mean ± SD) | Posttest (mean ± SD) | 6 months (mean ± SD) | 12 months (mean ± SD) | Within-group changea 6 months (mean ± SD) | Within-group changeb 12 months (mean ± SD) | |
|---|---|---|---|---|---|---|
| MoveTrain (n = 22) | MoveTrain (n = 22) | MoveTrain (n = 20) | MoveTrain (n = 19) | MoveTrain (n = 20) | MoveTrain (n = 19) | |
| Variable | Standard (n = 21) | Standard (n = 20) | Standard (n = 20) | Standard (n = 19) | Standard (n = 20) | Standard (n = 19) |
| HOOS scores | ||||||
| HOOSPainc | ||||||
| MoveTrain | 66.6 ± 14.9 | 84.7 ± 13.5 | 83.4 ± 11.3 | 85.8 ± 17.3 | 15.8 ± 10.6d | 16.7 ± 12.8d |
| Standard | 70.7 ± 11.9 | 89.3 ± 8.7 | 84.4 ± 12.2 | 86.1 ± 15.0 | 12.5 ± 11.7d | 15.9 ± 15.2d |
| HOOSSymptomsc | ||||||
| MoveTrain | 63.2 ± 16.6 | 78.2 ± 14.1 | 79.8 ± 14.5 | 79.7 ± 20.1 | 14.3 ± 10.8d | 15.0 ± 15.4d |
| Standard | 69.8 ± 15.8 | 86.3 ± 13.5 | 84.0 ± 12.3 | 84.7 ± 12.1 | 13.8 ± 13.8d | 14.7 ± 15.2d |
| HOOSADLc | ||||||
| MoveTrain | 79.5 ± 15.0 | 92.0 ± 11.2 | 90.4 ± 10.3 | 91.7 ± 15.5 | 9.4 ± 9.5d | 10.1 ± 11.3d |
| Standard | 84.2 ± 14.8 | 95.7 ± 6.2 | 92.9 ± 8.7 | 92.2 ± 10.5 | 7.1 ± 12.8e | 8.7 ± 14.8f |
| HOOSSportc | ||||||
| MoveTrain | 58.5 ± 20.0 | 83.0 ± 16.4 | 83.1 ± 15.7 | 88.5 ± 17.0 | 22.8 ± 14.4d | 26.0 ± 15.9d |
| Standard | 69.3 ± 17.2 | 90.6 ± 11.2 | 84.1 ± 15.2 | 83.3 ± 18.7g | 13.1 ± 17.5f | 16.3 ± 20.0e, g |
| HOOSQOLc | ||||||
| MoveTrain | 55.1 ± 14.8 | 68.2 ± 16.8 | 73.8 ± 18.8 | 76.6 ± 19.3 | 16.6 ± 17.8d | 20.1 ± 18.5d |
| Standard | 53.0 ± 15.3 | 73.1 ± 15.3 | 75.0 ± 15.6 | 74.3 ± 22.5g | 20.9 ± 14.4d | 22.9 ± 19.5d, g |
| PSFS, mean of up to 5 activities | ||||||
| PSFSh | ||||||
| MoveTrain | 5.8 ± 1.9 | 7.5 ± 2.2 | 7.2 ± 2.3 | 8.0 ± 2.5 | 1.6 ± 2.1d | 2.0 ± 2.0d |
| Standard | 5.3 ± 1.4 | 7.2 ± 2.2 | 7.1 ± 2.1 | 7.0 ± 2.8g | 1.7 ± 1.7f | 1.6 ± 3.2e, g |
| Pain numeric rating scales | ||||||
| Pain (Avg)i | ||||||
| MoveTrain | 4.1 ± 2.0 | 1.3 ± 1.5 | 1.6 ± 1.7 | 1.4 ± 1.9 | −2.6 ± 2.0d | −2.3 ± 1.6d |
| Standard | 3.4 ± 1.5 | 1.0 ± 1.4 | 1.6 ± 2.0 | 1.3 ± 1.6 | −1.6 ± 1.4d | −2.2 ± 1.9d |
| Pain (Worst)i | ||||||
| MoveTrain | 5.6 ± 2.0 | 2.0 ± 1.7 | 2.8 ± 2.0 | 2.1 ± 2.1 | −2.8 ± 1.8d | −3.3 ± 1.9d |
| Standard | 5.7 ± 1.9 | 1.9 ± 2.2 | 2.6 ± 2.5 | 2.5 ± 2.9 | −3.0 ± 2.8d | −3.4 ± 3.4d |
- Abbreviations: HOOS, Hip Disability and Osteoarthritis Outcome Score; HOOSADL, function in activities of daily living; HOOSQOL, quality of life; HOOSSport, function in sports and recreation; MoveTrain, movement pattern training group; PSFS, Patient Specific Functional Scale.
- a Change is calculated by subtracting the pretest value from the 6 month value.
- b Change is calculated by subtracting the pretest value from the 12 month value.
- c Patient-reported outcome measures with 100 = no disability.
- d Significant difference compared with baseline score within the same group (p < 0.0001).
- e Significant difference compared with baseline score within the same group (p < 0.01).
- f Significant difference compared with baseline score within the same group (p < 0.001).
- g n = 18, one patient did not complete all HOOS subscales.
- h Patient Specific Functional Scale assesses the level of difficulty performing up to five activities due to hip pain or symptoms. 0 = unable to perform activity; 10 = able to perform activity at preinjury level.
- i Pain rated by patients using a verbal numerical pain rating scale. 0 = no pain; 10 = worst pain imaginable.
4 DISCUSSION
To investigate the sustained effects of two PT-led interventions for patients with HRGP, we completed an ancillary analysis of data collected during a pilot RCT. Participation in MoveTrain or Standard resulted in clinically significant, yet similar improvements in pain and function that were sustained for 12 months. These improvements were similar to improvements we noted immediately following treatment.8 This suggests that either PT-led intervention may be effective for patients with HRGP, however a more definitive RCT is needed. Consensus among PT-experts recommend a minimum of 3 months of PT-led intervention be provided for patients with HRGP.11, 20 Despite these recommendations, recent studies report that fewer than half of patients with HRGP participate in PT-led intervention before undergoing surgery.29, 30 Our results, provide preliminary support for the recommendation to participate in PT-led intervention before making decisions related to surgical intervention and that participation in PT-led intervention may result is sustained benefits. Nevertheless, future studies with larger sample sizes are needed to definitively determine the optimal treatment strategy.
Two previous studies have reported outcomes 1 year or longer among patients with FAIS, a subtype of HRGP.6, 15 Although study methods differed, each PT-led intervention protocol used in the previous studies6, 15 and in ours resulted in improvements in patient function that were sustained a minimum of 12 months after treatment completion. Comparing treatment protocols across studies may provide insight into treatment components that likely contributed to the observed improvements. Key elements provided in all protocols in the previous and current studies included (1) PT-led supervised sessions and HEP instruction, (2) exercises or task practice that used basic overloading principles to improve hip muscle performance that were progressed based on each patient's performance, and (3) instruction in modification of symptom-provoking, patient-specific activities. These three components are consistent with current consensus recommendations11, 20 and systematic reviews reporting short term PT-led intervention effects,10, 14 and may represent key treatment components to be provided to patients with HRGP.
Differences among treatment protocols may represent areas requiring further investigation to optimize future treatment strategies. Key differences among treatment protocols were the inclusion of mobilization techniques and flexibility exercises. Neither treatment arm in our study used joint or soft tissue mobilization. Mansell et al.15 included joint and soft tissue mobilization as a core component. Griffin et al.6 allowed joint mobilization as an optional component, used at the discretion of the treating PT, however forceful manual techniques into restricted range of motion were not allowed. All treatment protocols, except our MoveTrain, included flexibility exercises, however exercise instruction differed. In our Standard protocol and the protocol by Griffin et al.,6 patients were not allowed to do painful, hard end stretches into restricted range of motion and were discouraged from performing exercises that placed the hip into a position associated with impingement, combination of hip flexion, adduction, and internal rotation. Based on exercise images provided by Mansell et al.,15 it appears patients were encouraged to move in an impingement position for some exercises. Activity modifications provided also differed. Both treatment protocols of the current study and the protocol in Griffin et al.6 included instruction to modify activity performance to limit placing the hip in the impingement position. Only patients in our MoveTrain were given instructions to modify abnormal movement patterns observed by the treating PT, such as pelvic drop during ambulation or excessive hip adduction motion during stair descent.8 Activity modifications provided by Mansell et al.15 were not described. Among the studies, other differences included the frequency and duration of the supervised sessions and inclusion of additional treatments such as a corticosteroid injection and orthotics/taping.
No patients enrolled in our study reported receiving surgery during the 12 months following treatment completion. Mansell et al.,15 reported that 70% of patients randomized to PT-led intervention, received surgery before final outcome assessment compared to 8% reported by Griffin et al.6 It is unknown the specific factors that may have contributed to the relatively high cross-over rate reported in Mansell et al.15 Differences among PT-led intervention protocols, described above, should be further explored. The length of follow up period also differed. Mansell et al.15 included a longer follow up period of 2 years compared to 1 year in our study and that of Griffin et al.6 It is important to note the outcomes of the intention-to-treat analysis reported by Mansell et al.,15 represent treatment associated with PT-led intervention and surgery and may not best reflect outcomes specific to PT-led intervention. Nevertheless, comparison of treatment protocols across studies reveal that further research is needed to assess the effect of PT-led intervention including hip muscle strengthening, joint and soft tissue mobilization, flexibility exercises and activity modification.
A major strength of our primary pilot study is standardization of each treatment protocol that may allow us to ultimately assess the specific effects of each treatment strategy. Treatment provided in each group was based on a specific theoretical mechanism. The primary goal of MoveTrain, was to optimize hip biomechanics during daily, work and fitness activities. The primary goal of Standard was to improve muscle strength and flexibility. As we reported previously, the MoveTrain group demonstrated improved biomechanics by reducing hip adduction motion during a single leg squat after treatment, however those in the Standard group did not, despite improved muscle strength.8 Both groups resulted in improved patient outcomes at 12 months, however we do not know the sustainability of treatment effects greater than 12 months, nor do we know if integrity of the hip joint structures were directly affected by either treatment. Future treatment studies incorporating advanced imaging to assess articular cartilage and other joint structures over longer time periods may provide additional insight into each treatment mechanism.31-33 Although the confidence intervals around the mean change within each group suggest that between-group differences are not significantly different, it is interesting to note that the MoveTrain group reported improvement in HOOSSport that was almost 10 points greater than the Standard group. It is possible that instruction and practice in patient-specific tasks, including sporting activities, may allow patients to return to their sport more effectively. This speculation would need to be confirmed in a larger trial.
4.1 Limitations
Statistical comparisons between groups are not presented because the study was not powered to detect between-group differences at 6 and 12 months. Confidence intervals, provided in Figure 2, for within-group changes from pretest reflect precision of these estimates. We did not have a control group to assess effect of time. Pain duration in our sample was a median of 2 years, therefore it is unlikely the improvements observed were due to time alone. In our proof-of-concept study,7 we compared treatment outcomes between patients who received MoveTrain and patients placed on a waitlist. Those patients who received no treatment reported no change or a small decline (HOOSADL) in all HOOS subscales compared to pretest. We did not measure hip muscle strength or movement patterns 12 months after treatment completion, therefore we do not know if improvements in the mechanism targeted by the treatment received were sustained over time. We used the HOOS as a measure of functional ability. Recently, patient-reported outcome measures, such as the iHOT34 and the Copehhagen Hip and Groin Outcome Score (HAGOS) have been recommended for use in patients with HRGP, however these recommendations are based primarily on surgical studies.24 Further research is needed to determine the best patient-reported outcome measure for nonoperative management.24 Responsiveness of outcome measures are specific to patient population and the intervention provided, therefore MIDs for PSFS and NPRS from previous studies may not be generalizable to our patient sample. Future work is needed to establish minimally important differences for nonoperative management of patients with HRGP.
Our findings suggest that patients with chronic HRGP may benefit from PT-led intervention incorporating either MoveTrain or strength and flexibility training and treatment benefits may persist 12 months after treatment completion. We do not know if treatment effects persist longer than 12 months. A future, larger trial to definitively assess the efficacy of MoveTrain and Standard treatment and to determine factors associated with long-term outcomes will improve our ability to develop treatment strategies for people with HRGP.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Megan Burgess, Visnja King, Suzanne Kuebler, Joseph Mancino, Dave Wortman for providing treatment during the trial; Kathy Brown, Martha Hessler, and Darrah Snozek their assistance with trial procedures and data collection. This study was supported by the following grants: R21HD086644 and NIH T32HD007434 from the National Center for Medical Rehabilitation Research, National Institute of Child Health and Human Development; the Orthopaedic Research Grant from the Foundation for Physical Therapy Research; Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). Additional support was provided by Program in Physical Therapy at Washington University School of Medicine, Clinical and Translational Science Award (CTSA).
AUTHOR CONTRIBUTIONS
Marcie Harris-Hayes, G. Kelley Fitzgerald, Karen Steger-May, Allyn M. Bove contributed to the study design, data collection, data analysis, data interpretation, and manuscript preparation. Michael J. Mueller and John C. Clohisy contributed to the study design, data interpretation, and manuscript preparation. All authors have read and approved the final submitted version of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.