MnTE-2-PyP disrupts Staphylococcus aureus biofilms in a novel fracture model
Abstract
Biofilm-associated infections in orthopedic surgery lead to worse clinical outcomes and greater morbidity and mortality. The scope of the problem encompasses infected total joints, internally fixed fractures, and implanted devices. Diagnosis is difficult. Cultures are often negative, and antibiotic treatments are ineffective. The infections resist killing by the immune system and antibiotics. The organized matrix structure of extracellular polymeric substances within the biofilm shields and protects the bacteria from identification and immune cell action. Bacteria in biofilms actively modulate their redox environment and can enhance the matrix structure by creating an oxidizing environment. We postulated that a potent redox-active metalloporphyrin MnTE-2-PyP (chemical name: manganese (II) meso-tetrakis-(N-methylpyridinium-2-yl) porphyrin) that scavenges reactive species and modulates the redox state to a reduced state, would improve the effect of antibiotic treatment for a biofilm-associated infection. An infected fracture model with a midshaft femoral osteotomy was created in C57B6 mice, internally fixed with an intramedullary 23-gauge needle and seeded with a biofilm-forming variant of Staphylococcus aureus. Animals were divided into three treatment groups: control, antibiotic alone, and combined antibioticplus MnTE-2-PyP. The combined treatment group had significantly decreased bacterial counts in harvested bone, compared with antibiotic alone. In vitro crystal violet assay of biofilm structure and corresponding nitroblue tetrazolium assay for reactive oxygen species (ROS) demonstrated that MnTE-2-PyP decreased the biofilm structure and reduced ROS in a correlated and dose-dependent manner. The biofilm structure is redox-sensitive in S. aureus and an ROS scavenger improved the effect of antibiotic therapy in model of biofilm-associated infections.
1 INTRODUCTION
Despite advances in surgical technique and postoperative management, infection remains a major cause of morbidity and mortality within orthopedic surgery.1-3 It is estimated that surgical site infections cost the American health system over 3 billion dollars annually and double the cost of care for an orthopedic trauma patient.4, 5 Infection after fracture fixation portends poor outcomes including impaired bone healing, prolonged recovery, and diminished function. Infection is the most common indication for revision surgery following total knee arthroplasty within the United States and is among the most common indications for revision of total hip arthroplasties, leading to increased length of hospital stay and overall cost to the health system.6 The Centers for Disease Control has estimated that over 65% of infections in the developed world are caused by organisms growing in biofilm structures.7 Materials used in orthopedic surgery including metals and polymers are particularly susceptible to biofilm formation.8, 9 Management of biofilm-associated infections often requires removal of hardware, irrigation, and debridement, with aggressive antibiotic therapy, and a significant number of these infections are not successfully managed. Novel treatment modalities for orthopedic biofilm infections are needed, as are reliable in vivo models to assess candidate therapeutics and to better understand biofilm infections.
Biofilms are cohesive aggregations of bacteria that adhere to materials and are characterized by three-dimensional structures of extrapolymeric substances.10 Past work has demonstrated the generation of reactive oxygen (ROS) and nitrogen species in biofilms are important for the maintenance of biofilm structure.2, 11 Although single-cell bacteria (planktonic) are killed by high concentrations of ROS creating an oxidizing environment, an oxidative environment appears to foster growth in Mycobacteria and some intracellular pathogens.2 There is accumulating evidence that increased ROS and oxidative state may play a complex role in signaling within biofilms influencing the structure of the biofilm matrix.12-14
A variety of compounds have been studied for the capacity to scavenge ROS. Compounds such as urate, ascorbate, glutathione, and others are limited by the stoichiometry of single-electron transfers and the potential to become a pro-oxidant depending on the overall redox state of the system and presence of catalytic metals. Catalytic scavengers such as the superoxide dismutases, catalase, and peroxidases provide essential ROS scavenging around energy generation and protection of cellular and extracellular structures. Metalloporphyrin-containing ring compounds have been synthesized to function as broad scavengers of oxidants and have been used in a variety of clinical situations.15-19 A redox-active metalloporphyrin was effective in modulating intracellular bacterial viability in Mycobacterium abscessus.20
MnTE-2-PyP (chemical name: manganese (II) meso-tetrakis-(N-methylpyridinium-2-yl) porphyrin) is a redox-active compound that scavenges a broad spectrum of oxidants.21 Previous studies have shown it to be effective as a neuroprotectant from oxidative stress in stroke19 and radiation,22 in protecting the lungs during radiation treatment,23 as a radioprotectant in the treatment of head and neck cancer,24 and in improving radiation proctitis.25 Based on the ability of MnTE-2-PyP to scavenge oxidants and the potential role of oxidants in sustaining biofilm structures, we postulated that MnTE-2-PyP would inhibit the formation and/or persistence of bacterial biofilms in vivo and improve the effect of antibiotic treatment. We tested that hypothesis using a known biofilm-forming strain of Staphylococcus aureus in a novel mouse model of infected fracture fixation.
2 MATERIALS AND METHODS
2.1 Animal model
Animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at National Jewish Health. Male C57BL6 mice (Jackson Laboratories) weighing between 25 and 30 g were housed in accordance with NIH guidelines under specific pathogen-free conditions and in 12 h dark and light cycles. The full animal model of induction of an infected fracture and postoperative treatment was repeated four separate times.
2.1.1 Bacteria preparation for animal model
Staphylococcus aureus subsp. aureus (ATCC 29213) was purchased from American Type Culture Collection (ATCC), Manassas, VA and stored at −80°C. A stock plate was prepared by rehydrating the frozen pellet and streaking onto a Trypticase Soy Agar (TSA, Hardy Diagnostics) that was grown overnight at 37°C. Single colonies of S. aureus were removed from a stock plate and were placed in 5 ml of sterile Tryptic Soy Broth (TSB). The inoculation was allowed to grow overnight in an incubator at 37°C shaking at 200 rpm. After 12 h of growth, the culture was removed and read for absorbance at OD600. The culture was diluted to a concentration of 0.001, determined by means of a growth curve, and was allowed to grow for an hour.26 Concentration was again checked to determine growth. If growth was deemed adequate by means of the concentration growth curve, the bacteria was spun down at 25,000 RCF for 1 min and was then resuspended in an equal volume of sterile phosphate-buffered saline (PBS). The prepared culture was then plated to determine exact infection concentration. The culture was plated after surgery to determine whether there was any significant loss of bacteria.
2.1.2 Surgical procedure
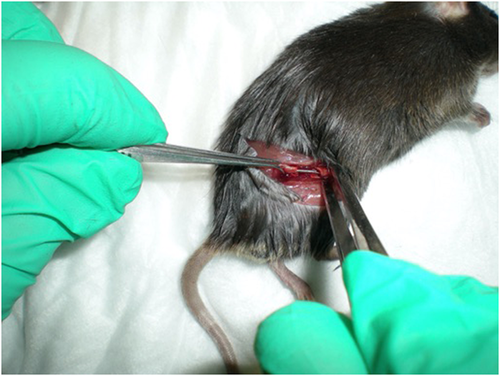
The mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. The right anterolateral thigh was shaved and prepared with chlorhexidine and 70% alcohol. A 6–8 mm incision was made in the skin and the quadriceps muscle identified. The muscle fascia over the femur was incised and stripped to expose the bone. The mid portion of the femur was osteotomized using a bone cutter, and a 5−7 mm portion of a 23-gauge needle was inserted retrograde into the intramedullary canal of the femur to provide fixation of the osteotomy (Figure 1). Ten microliters of prepared S. aureus at a concentration of 1.0 × 104 in PBS was then placed around the fracture site with the muscle allowed to fall back over the bone. The wound was infiltrated with 0.025cc bupivacaine 0.25%. The skin was closed with absorbable suture and sealing adhesive (VetBond, 3 M). Mice were monitored during anesthetic recovery, and when fully recovered, buprenorphine was administered subcutaneously for postoperative pain control. Mice were allowed free activity in standard cages for the duration of the experiment but were provide with gel diet (DietGel 76 A, ClearH2O) for the first 2 postoperative days.
2.1.3 Treatment groups
Mice were randomly assigned to three postoperative treatment conditions after the surgical procedure was completed and the mouse was recovered from anesthesia. Groups were (1) no active treatment (PBS injections and untreated water; 17 mice); (2) oral antibiotics (cephalexin in drinking water) only with PBS injections (18 mice); (3) oral antibiotics (cephalexin in drinking water) plus MnTE-2-PyP (23 mice). These treatments were initiated on postoperative day 1 and were continued through the 14-day treatment period. MnTE-2-PyP was given at a dose of 5 mg/kg (loading dose) on the first treatment day by intraperitoneal (ip) injection, and at a dose of 2.5 mg/kg every other day until the termination of the experiment. Mice not receiving MnTE-2-PyP were injected ip with an equal volume of sterile PBS on all treatment days. Cephalexin was reconstituted in sterile tap water at a concentration of 250 mg/L and mice in treatment Groups 2 and 3 were given unrestricted access to the antibiotic-treated water beginning on the first treatment day and throughout the duration of the experiment. Mice typically resumed weight-bearing 2–3 days postoperatively and were weighed every other day.
2.1.3.1 Toxicity
The animals were monitored for general health and local toxicity. There were no local reactions at the MnTE-2-PyP or PBS injection sites in any animal. General toxicity assessments with organ histology were not done on these animals for MnTE-2-PyP administration because the drug had been used previously in a variety of disease models within our research group and pharmacokinetic assessments of the drug and toxicity had been done.27-29
2.1.4 Tissue collection and processing
On postoperative day 14, animals were euthanized, and tissues were collected. Under sterile conditions, the femur was dissected free from the surrounding muscle and connective tissue and was removed in one piece and weighed. The femur was homogenized in PBS and was then sonicated using a Fisher Sonicator. The intramedullary rod was sonicated in sterile PBS and the supernatant added to the homogenized femoral bone. Residual bacteria in each femur were estimated by determining colony-forming units (CFUs) standardized by the tissue weight. Samples (50 μl) of the supernatant after homogenization and sonication were plated on sterile TSA plates in dilutions of 10−1 to 10−4 (Hardy Diagnostics). The plates were allowed to incubate overnight at 37°C. After incubation, colonies were counted using a manual count of the entire plate to determine CFUs that were in the supernatant. Dilution and colony count were used to calculate the bacteria per gram of tissue.
2.2 In vitro models
2.2.1 Effect of MnTE-2-PyP on biofilm structure
S. aureus (ATCC #29213) was obtained from a frozen stock and was streaked on a TSA (BD) plate and was allowed to grow overnight at 37°C. Single colonies were then removed using a sterile inoculating loop and transferred into a 5 ml of TSB (Bacto Tryptic Soy Broth, BD) culture and were grown overnight at 37°C shaking at 200 rpm.
The overnight culture was removed from the incubator and was diluted 1:1000 with 1% glucose in 0.5× TSB and allowed to grow at 37°C for 1 h. A total of 200 μl of sample was then added to 96-well polyvinyl chloride (PVC) plates. The experimental groups were as follows: no treatment, treatment with 2.5 μM MnTE-2-PyP at t = 0, and treatment with 2.5 μM MnTE at t = 24 h. After 48 h, plates were washed with deionized water, and wells were treated with 50 μl of 0.1% crystal violet in ethanol for 45 min. The wells were washed three times with deionized water, and 200 μl of 95% ethanol were added to each well. Plates were incubated at 4°C for 30 min. Hundred microliters were transferred to a new plate and read for absorbance at OD600. There were 10 replicates for each condition and 4 independent experiments were run on different days.
2.2.2 Dose–response model crystal violet and nitroblue tetrazolium (NBT) assays
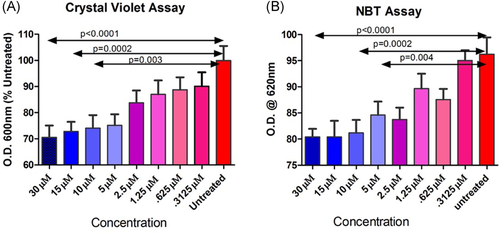
S. aureus biofilms were prepared in two 96-well PVC plates as described. MnTE-2-PyP was prepared in eight different concentrations (30, 15, 10, 5, 2.5, 1.25, 0.625, and 0.1325 μM). After 24 h of growth, MnTE-2-PyP in these varying doses was added to wells in six replicates on each plate. PBS was added to control wells. Biofilms were allowed to grow for another 24 h. NBT was added to the wells of one plate in a concentration of 2 mM.30 Crystal violet was added to the wells of the other plate following the protocol detailed previously. The plates were then allowed to incubate for 20 min before they were read for absorbance at OD620.
2.3 Statistical analysis
Results were analyzed using SAS v9.4 and JMP v.10.0 (SAS). A p < .05 was considered to indicate statistically significant differences. The results of the animal model were analyzed using a generalized linear model and a log transformation of the bacterial counts due to non-normality of the data. Variables for treatment group and the four different temporal runs were included in the model. Data are reported as median and 95% confidence interval for each group. In vitro data were analyzed with an analysis of variance for overall differences between groups and then Student's t test for comparisons between each pair.
3 RESULTS
3.1 Animal model
3.1.1 Animal activity, weights, and postoperative course
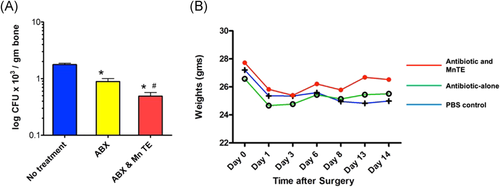
Most animals began full weight-bearing within 24 h postoperatively, and by postoperative day 3, all animals were weight-bearing on the operated leg. All mice lost weight during the first 2–3 days postoperatively. Activity levels and food/water consumption were also diminished during the first few days of recovery. Mean weight by treatment group over the course of the treatment period is shown Figure 2. Mice in all the three groups lost similar amounts of weight by postoperative day 3. Mice in the “No Treatment” group continued to lose weight throughout the duration of the experiment while mice in both the “ABX” group and the “ABX+MnTE-2-PyP” group regained some weight. However, there were no significant differences in weight by treatment group over the course of the project. Findings at the time of tissue harvest varied by the treatment group. Wounds had healed without persistent drainage. The femoral osteotomies showed good callus formation at the time of tissue collection. Soft tissues (quadriceps muscle and periosteum) were healthy and vascularized. Mice treated with PBS only (control group) frequently had purulent material around the osteotomy site consistent with chronic infection. There was no evidence of local drug toxicity from the MnTE-2-PyP injections.
3.1.2 Bacterial counts
Results from the final bacteria counts from the harvested femurs are shown in Figure 2. Quantitative assays for bone bacteria (CFUs/gram of bone) showed that cephalexin treatment was associated with significantly reduced CFU compared with untreated samples. Treatment with both cephalexin and MnTE-2-PyP further and significantly reduced bacteria counts to a mean of 3.1 CFU/g tissue (p < .0001) and yielded several mice without residual bacteria detected as CFUs.
3.2 In vitro models
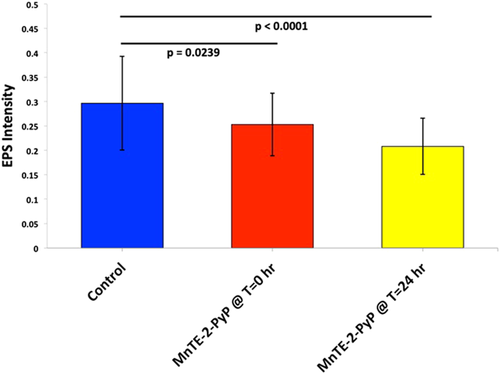
In vitro experiments were conducted to confirm the effect (including dosage-dependency) of MnTE-2-PyP on reducing biofilm concentration and redox state within a highly controlled setting. There were three experimental conditions for the initial in vitro biofilm work: (1) untreated S. aureus biofilm grown over 48 h, (2) S. aureus biofilm treated with MnTE-2-PyP 2.5 µM at time zero for biofilm growth, (3) S. aureus biofilm grown for 24 h and then MnTE-2-PyP 2.5 µM added with growth allowed an additional 24 h to a total of 48 h. The results of four experimental procedures with 10 replicates per group are shown in Figure 3. Biofilm structure is significantly decreased in the samples treated with MnTE-2-PyPat time zero (p = .02) and with treatment started at 24 h after onset (p < .0001), compared with the untreated group. In samples treated with MnTE-2-PyP at 24 h after initial growth was started, the biofilm structure is also significantly reduced compared with the group with treatment at time zero (p = .0361).
The dose–response models depicted in Figure 4 show that treatment with increasing concentrations of MnTE-2-PyP decreased the biofilm structure in a dose-dependent fashion. The parallel NBT assay for ROS showed that the MnTE-2-PyP “reduced” the redox state in a dose-dependent fashion as well. Thus, the lower redox state was linked to the reduced biofilm structure in response to the drug after 24 h of growth.
4 DISCUSSION
The redox-active metalloporphyrin (MnTE-2-PyP) in combination with standard antibiotic treatment was successful in decreasing bacterial counts at 14 days in an biofilm-infected fracture model with hardware. We postulate that the presence of a metal intramedullary fixation device resulted in a biofilm-associated infection and that MnTE-2-PyP acted synergistically with antibiotics to disrupt the biofilm structure leading to enhanced antibiotic penetration and a more effective immune cell response. Modulation of ROS within the biofilm system by acting on the bacterial cellular control of the matrix structure may be a key factor in the disruption of the biofilm structure.
The precise way in which MnTE-2-PyP functions within the biofilm structure is not defined, but the NBT assay demonstrates a parallel reduction in the ROS concentration with a reduction in biomass structure. This suggests that MnTE-2-PyP acts on the bacteria and its biomass through a mechanism in which oxidative state is modified (reduced). MnTE-2-PyP has been previously shown to regulate aerobic glycolysis and redox state which may alter the immune response.15, 31
ROS are byproducts of oxygen metabolism, but may also be actively generated, thus they may naturally accumulate within a biofilm with active metabolic processes or be purposely produced to modulate the local milieu. ROS have a number of important signaling roles within the biofilm.13 There are numerous bacterial polymers within the biofilm matrix that are redox active and may act as either electron donors or electron acceptors within the biofilm.32 Oxidative stress in biofilms and generation of ROS may allow for genetic adaptation and diversification to occur, therefore fostering antibiotic resistance.33 Further, programmed cell death is essential to the survival of biofilms with the death of older cells promoting growth in younger cells, and ROS play a role in this survival cycle.13, 34
The magnitude of the reduction in bacterial growth between the antibiotic alone and the antibiotic with redox modulator is statistically significant in the model, but on initial view does not appear to be a dramatic reduction. This is due in part to the need to create a disease model that establishes an adequate level of local infection without overwhelming sepsis and death to produce a biofilm model. Preliminary model development required testing different concentrations of bacteria to establish a working model with the goal to model a chronic biofilm condition. We successfully demonstrated that the addition of a redox modulating compound improved the effect of antibiotic treatment by reducing the bacterial concentration in the bone.
In developing our animal protocol, we reviewed and modified previously described mouse models. Windolf et al.35 described an infected murine femur fracture model for biofilms utilizing plate and screw fixation. Holstein et al.36, 37 reported on a variety of intramedullary fixation methods in mouse femurs used for studying fracture healing. They noted some instability in single pin fixation, but we found adequate fixation using a retrograde technique and a 23-gauge needle segment. Mice typically resumed weight-bearing between the first and third postoperative day and at the time of surgery, the fixation was assessed for stability. Animals that had inadequate fixation (typically due to some comminution with the osteotomy) were euthanized before randomization. At the time of tissue harvest, we found that the majority of fractures were healing well with exuberant callus formation and that the intramedullary rods remained in place. Since our primary endpoint was infection, having some increased motion at the fracture site may have enhanced the establishment of bone infection. Drug dosages for MnTE-2-PyP in the animal model were selected based on previous animal work with cancer models and radiation protection.38 Lower doses may be effective and can be explored in future work.
MnTe−2-PyP is one of a new class of porphyrin ring compounds developed to modulate redox states and treat oxidative stress or injury. The compounds are significantly more potent than ROS scavengers like ascorbate that are consumed stoichiometrically and they were originally conceived to function like catalytic ROS scavengers such as the superoxide dismutase enzymes. The compounds have been used in a wide range of disease models including stroke, radiation protection, cancer, and diabetes, with human clinical trials currently in progress. They have not had significant toxicity when administered subcutaneously, intravenously, or intraperitoneally.
Our in vitro work corroborated the results of the animal model and supported our hypothesis that MnTE-2-PyP interferes with biofilm structure by modulating redox state. The detailed mechanism by which the drug and redox state affect biofilm persistence has not been defined and may be the subject of further work. These effects are potentially mediated by a wide range of signaling pathways that are redox sensitive.
In summary, we demonstrate that a redox-active metalloporphyrin interfered with the development and persistence of a biofilm in vitro and was synergistic with standard antibiotics to decrease bacterial counts in a biofilm infection mouse model. Thus, MnTE-2-PyP or related compounds may be an effective adjunct to standard antibiotics to improve the killing power in biofilm-associated infections.
ACKNOWLEDGMENT
The authors thank The Schramm Foundation, Elbogen Foundation for the financial support.