Acute toxicity and patient-reported symptom score after conventional versus moderately hypofractionated proton therapy for prostate cancer
Abstract
Introduction
To confirm the feasibility of hypofractionated proton beam therapy (PBT), we compared the acute adverse event rates and International Prostate Symptom Score (IPSS) in prostate cancer patients treated with hypofractionated versus conventionally fractionated (2.0 Gy relative biological effectiveness (RBE)/fraction) PBT.
Methods
We reviewed 289 patients with prostate cancer, of whom 73, 100, and 116 patients were treated with 2.0, 2.5, and 3.0 Gy (RBE)/fraction, respectively. The endpoints were acute genitourinary and gastrointestinal toxicities and the IPSS, evaluated up to 6 months after PBT initiation.
Results
No significant differences were found in acute toxicity rates or the IPSS among the fractionation schedules. Diabetes mellitus, age, and androgen deprivation therapy were not identified as factors associated with the IPSS.
Conclusion
There were no significant differences in adverse events or quality of life among the three fractionation schedules early after PBT.
Video Short
Acute toxicity and patient-reported symptom score after conventional versus moderately hypofractionated proton therapy for prostate cancer
by Iizumi et al.Introduction
The incidence of prostate cancer in Japan is rising due to the ageing population, Westernised lifestyle, and widespread use of prostate-specific antigen screenings.1 Improvements in health consciousness and diagnostic procedures have contributed to early diagnosis of prostate cancer,2 which has led to a high demand for high-quality treatments for localised prostate cancer.
The treatment options for clinically localised prostate cancer include prostatectomy and several forms of radiation therapy (RT). While disease control rates are comparable among these treatments,3, 4 each modality has its own set of complications, side effects, and financial costs, and concerns regarding these factors often critically affect the patient’s choice of treatment modality.5 To determine the best treatment modality from among the numerous options, patient-reported outcome measures (PROMs), such as the International Prostate Symptom Score (IPSS), have been incorporated into clinical practice to assess the patient’s health status and well-being.6, 7 Together with determining adverse event rates, many clinicians are using the IPSS in daily clinical practice for patients with prostate cancer.
Technological advancements in radiation delivery have enabled administration of very high radiation doses to tumours while reducing the margins and irradiated volume of normal tissue. After confirming the benefits of highly conformal RT with safe dose escalation in terms of the biochemical control rate in prostate cancer patients,8 hypofractionated RT was introduced, which has the potential for better efficacy and less toxicity based on the linear quadratic model.9 These characteristics suggest that hypofractionated RT has therapeutic advantages over conventionally fractionated RT. Furthermore, the shorter treatment period associated with hypofractionated RT for prostate cancer leads to possible cost benefits and more comfortable for patients.10, 11 Hypofractionated RT is categorised into moderate and extreme hypofractionation. According to a guideline for hypofractionated RT, moderate hypofractionation is defined as 240–340 cGy/fraction.12 A meta-analysis reported comparable treatment efficacy between moderately hypofractionated and conventional RT.13 However, toxicity and PROM assessments have yielded mixed results.14 In addition, published reports on patient PROM after proton beam therapy (PBT) for localised prostate cancer are still limited. As a result, the optimum hypofractionation schedule that confers less toxicity and better disease-specific PROM in patients with localised prostate cancer is currently unclear.
Proton beam therapy is a type of highly conformal RT. Compared with photon-based external beam RT, PBT results in a significantly different dose distribution, because protons deposit most of their energy in the target area, with a steep decrease in the dose beyond the target. As a result, PBT can reduce the dose received by normal tissues, potentially translating into reduced rates of adverse events.15 To evaluate biological equivalent dose of PBT to photon therapy, a constant relative biological effectiveness (RBE) of 1.1 is used on the assumption that the physical dose of proton beam has a biological effect equivalent to a 10% higher dose of photon. However, several studies have reported that RBE depends on factors, such as dose;16, 17 the biological effect of PBT is likely to be underestimated with a constant RBE of 1.1. Therefore, the variability of RBE derived from the change of fraction size has to be considered as a potential parameter for the difference of the biological effect.
PPS-001 and PPS-002 (UMIN000010510 and UMIN000017679) are open-labelled prospective single-institutional phase II studies designed to confirm the feasibility and efficacy of hypofractionated PBT for localised prostate cancer. After obtaining approval from the institutional review board of our institution and treatment consent forms from the patients, PPS-001 and PPS-002 started in June 2013 and July 2015, and patient accrual was completed in June 2015 and January 2019, respectively. The fraction sizes were 2.5 Gy relative biological effective (RBE) in PPS-001 and 3.0 Gy (RBE) in PPS-002.
To determine the optimum fraction size for moderate hypofractionation for prostate cancer treatment, we reviewed patients registered in PPS-001 and PPS-002 and patients treated with conventionally fractionated RT during the same periods as these two trials. Herein, we report the toxicity and PROM outcomes up to 6 months after the start of PBT in these patients. We hypothesised that the three fractionation schedules would exhibit no significant differences in adverse events or PROM during the observation period.
Patients and Methods
Data collection
This was a single-institutional review of patients registered in two prospective studies of moderately hypofractionated PBT (PPS-001 and PPS-002). Common exclusion criteria were applied in both PPS-001 and PPS-002. Patients with lymph node and/or distant metastasis, severe comorbidities, double primary malignancies, a history of TUR-P within 6 months from the registration of these trials, a history of previous pelvic irradiation, severe dementia, the use of unstoppable anticoagulant drugs or alpha-reductase inhibitor, and miscellaneous inappropriate conditions judged by physicians in charge were not eligible. To compare the therapeutic toxicities and IPSS between these patients and patients treated with conventional fractionation, patients who received PBT at 2.0 Gy (RBE) daily during the same period as those in the PPS-001 and PPS-002 trials were also evaluated, as the conventional treatment group. The present research was approved by the ethical committee of the Institutional Review Board at the University of Tsukuba Hospital (H29-135).
RT planning and prescription
A CT simulation scan was performed for patients who were immobilised in the supine position. The treatment planning CT images were sent to VQA, version 1.7 or 2.0 (Hitachi, Tokyo, Japan). Contouring and planning were performed on VQA with reference to diagnostic MRI imaging to enhance the accuracy of the delineation of a target. The clinical target volume included the entire prostate and base of the seminal vesicles, except in cases of stage T3b disease. For stage T3b cases, the invaded seminal vesicles were also delineated. The planning target volume was formed by anisotropic expansion of the clinical target volume by 10 mm in all directions, except 5 mm in the posterior direction. However, when irradiating organs at risk (OAR) was a concern, modification of the planning target volume was accepted. The conventional group (2.0 Gy (RBE)) received a total dose of 74 Gy (RBE) in 37 fractions for low-risk patients or 78 Gy (RBE) in 39 fractions for intermediate- and high-risk patients. The hypofractionation groups received either a total dose of 70 Gy (RBE) in 28 fractions with a fractional dose of 2.5 Gy (RBE) or a total dose of 63 Gy (RBE) in 21 fractions with a fractional dose of 3.0 Gy (RBE). All groups were treated daily, 5 days per week. All patients were treated with a passive scattering technique with bilateral beam arrangements via daily image guidance with kilovoltage orthogonal imaging using two intraprostatic fiducial markers (Gold Anchor; Naslund Medical AB, Huddinge, Sweden or VISICOIL; RadioMed, Barlett, TN, USA). Full details of the PBT planning have been reported previously.18 Bladder volume was confirmed by daily ultrasound bladder scanning just before each treatment.
Outcomes
Adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0.19 The IPSS was used to evaluate patient-reported functions before starting PBT (pre-PBT), at the end of PBT, and at regular follow-up periods of 3 and 6 months from the start of PBT. To identify patient factors that contribute to worsening of the PROM, the IPSSs according to the presence of diabetes mellitus (DM) (yes or no), age (< median or ≥ median age), and androgen deprivation therapy (ADT) use (yes or no) were also determined for entire group. Analysis of American Urological Association (AUA) symptom index classification, which categorises pre-treatment IPSS of 0–7, 8–9, and 20–35 as mild, moderate, and severe symptoms, was also conducted. Due to the small number of patients in the severe pre-PBT IPSS group, the moderate and severe AUA groups were combined in the IPSS analysis.
Statistical analysis
All statistical analyses were conducted using R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Statistical differences in the patient characteristics and rates of acute toxicities among the three fractionation groups were determined by the Kruskal–Wallis rank sum test and Pearson’s chi-square test. Differences in the IPSS among the different time points in each group or among the three groups at each time point were assessed by the Wilcoxon signed-rank test and Kruskal–Wallis rank sum test. A two-sided P-value less than 0.05 was considered statistically significant.
Results
Patient characteristics
In the present study, 289 patients were included, and Table 1 provides the detailed patient and treatment characteristics. The patient characteristics were similar among the three fractionation (2.0, 2.5, and 3.0 Gy) groups. In the 2.0, 2.5, and 3.0 Gy fractionation groups, there were 7 (9.6%), 15 (15.0%), and 11 (9.5%) patients determined as low risk, 23 (31.5%), 43 (43.0%), and 48 (41.4%) as intermediate risk, and 43 (58.9%), 42 (42.0%), and 57 (49.1%) as high risk, respectively (P = 0.2), and the high-risk group in the study includes very high-risk group according to the NCCN guideline.20 The median ages were 69 (range, 57–86), 67 (53–79), and 68 (53–79) years in the 2.0, 2.5, and 3.0 Gy fractionation groups, respectively (P = 0.06). ADT was conducted in 64 (87.7%), 85 (85%), and 105 (90.5%) patients (P = 0.45), and anticoagulants were used in 15 (20.5%), 11 (11%), and 12 (10.3%) patients (P = 0.07), respectively. There were 22 (30.1%), 12 (12%), and 15 (12.9%) patients with DM in the respective groups (P < 0.01).
| 2.0 Gy (RBE)/fr | 2.5 Gy (RBE)/fr | 3.0 Gy (RBE)/fr | P-value | |
|---|---|---|---|---|
| Number of patients | 73 | 100 | 116 | |
| Start date of PBT | Jan. 2013 to Sep. 2018 | Jun. 2013 to Jun. 2015 | Jul. 2015 to Jan. 2019 | |
| Patient factors | ||||
| Age, median years (range) | 69 (57-86) | 67 (53-79) | 68 (53-79) | 0.06 |
| Comorbidities | ||||
| Diabetes mellitus | 22 (30.1%) | 12 (12.0%) | 15 (12.9%) | <0.01 |
| Use of anticoagulants | 15 (20.5%) | 11 (11.0%) | 12 (10.3%) | 0.07 |
| Tumour factors | ||||
| Clinical tumour classification | ||||
| T1 | 7 (9.6%) | 15 (15.0%) | 23 (19.8%) | 0.21 |
| T2 | 23 (31.5%) | 43 (43.0%) | 67 (57.8%) | |
| T3 | 43 (58.9%) | 42 (42.0%) | 26 (22.4%) | |
| Initial PSA (ng/ml) | ||||
| 4 to <10 | 35 (47.9%) | 68 (68.0%) | 77 (66.4%) | 0.07 |
| 10 to <20 | 22 (30.2%) | 20 (20.0%) | 23 (19.8%) | |
| 20 or over | 16 (21.9%) | 12 (12.0%) | 16 (13.8%) | |
| Gleason score | ||||
| 6 or less | 10 (13.7%) | 21 (21.0%) | 15 (12.9%) | 0.21 |
| 7 | 28 (38.4%) | 45 (45.0%) | 53 (45.7%) | |
| 8 or over | 35 (47.9%) | 34 (34.0%) | 48 (41.4%) | |
| Risk stratification | ||||
| Low | 7 (9.6%) | 15 (15.0%) | 11 (9.5%) | 0.20 |
| Intermediate | 23 (31.5%) | 43 (43.0%) | 48 (41.4%) | |
| High | 43 (58.9%) | 42 (42.0%) | 57 (49.1%) | |
| AUA symptom severity | ||||
| Mild (IPSS ≤ 7) | 33 (45.2%) | 46 (46.0%) | 53 (45.7%) | 0.39 |
| Moderate (8 ≤ IPSS ≤ 19) | 37 (50.7%) | 52 (52.0%) | 58 (50.0%) | |
| Severe (20 ≤ IPSS ≤ 35) | 3 (4.1%) | 2 (2.0%) | 5 (4.3%) | |
| Treatment factors | ||||
| Androgen deprivation therapy | 64 (87.7%) | 85 (85.0%) | 105 (90.5%) | 0.45 |
- AUA, American Urological Association; IPSS, international prostate symptom score; PBT, proton beam therapy; PSA, prostate-specific antigen.Significance of bold value is P<0.002.
Acute genitourinary and gastrointestinal toxicities
Table 2 shows the acute genitourinary (GU) and gastrointestinal (GI) toxicities experienced by the patients. Grade 3 acute GU toxicity was observed in one patient (1.4%) in the conventional fractionation (2.0 Gy) group. Grade 2 acute GU toxicities were observed in 12 (16.4%), 11 (11.0%), and 16 (13.8%) patients in the 2.0, 2.5, and 3.0 Gy fractionation groups, respectively, with no significant difference detected (P = 0.26). No grade 2 acute GI toxicities were observed in any group (P = 0.21). Acute grade 1 GI toxicity was observed in two patients (2.7%) in the conventional fractionation group and in one patient each in the two hypofractionation groups. No significant differences in acute GI toxicities were observed among the three groups.
| Number of patients | 2.0 Gy (RBE)/fr | 2.5 Gy (RBE)/fr | 3.0 Gy (RBE)/fr | P-value |
|---|---|---|---|---|
| 73 | 100 | 116 | ||
| Genitourinary | ||||
| Grade 1 | 35 (47.9%) | 52 (52.0%) | 45 (38.8%) | 0.26 |
| Grade 2 | 12 (16.4%) | 11 (11.0%) | 16 (13.8%) | |
| Grade 3 | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | |
| Grade 4 or worse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Gastrointestinal | ||||
| Grade 1 | 2 (2.7%) | 1 (1.0%) | 1 (0.8%) | 0.21 |
| Grade 2 or worse | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
- fr, fraction; Gy, gray; RBE, relative biological effectiveness.
Patient-reported outcomes according to the IPSS
The IPSSs of the three fractionation groups at each time point are shown in Figure 1. Significant increase in the IPSS, which means worsening of urinary symptoms, was transiently observed at the end of PBT in all groups (P < 0.01). However, at 3 months from the start of PBT, the score was significantly improved in all groups (P < 0.01), but at 6 months, the score had returned to the pre-PBT value (P = 0.92, 0.47, and 0.76 in the 2.0, 2.5, and 3.0 Gy fractionation groups, respectively). There were no significant differences in the IPSS among the three fractionation schedules before PBT (P = 0.57), at the end of PBT (P = 0.31), or at 3 months (P = 0.71) or 6 months (P = 0.70) after PBT initiation.
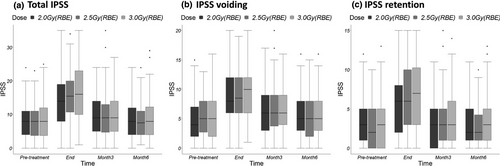
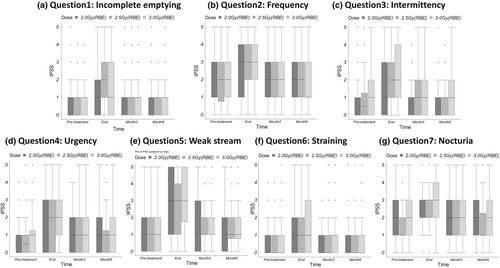
The seven questions of the IPSS pertain to either voiding (questions 3, 5, and 6) or retention symptoms (questions 1, 2, 4, and 7). The scores pertaining specifically to retention or voiding symptoms at each time point are shown in Figure 1. The changes in these scores at 6 months after PBT initiation were similar to the change in the total IPSS. There were no significant differences in the retention or voiding symptom scores among the three fractionation groups before PBT (P = 0.54 for voiding and P = 0.89 for retention symptoms), at the end of PBT (P = 0.62 and P = 0.18), or at 3 months (P = 0.89 and P = 0.64) or 6 months (P = 0.75 and P = 0.61) after PBT initiation. No significant differences among the different time points in each group were observed in the individual scores for any of the seven items of the IPSS questionnaire (Fig. 2).
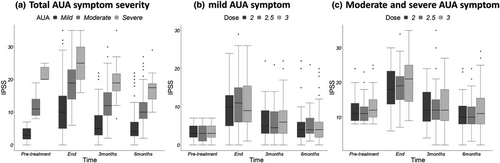
Changes in the IPSS among the time points evaluated according to DM status (yes (n = 49) vs. no (n = 240)), age (< 68 (n = 140) vs. ≥ 68 (n = 149) years), and ADT status (yes (n = 254) vs. no (n = 35)) for all patients are presented in Figure 3. No significant difference in the IPSS was observed according to these patient-related factors. Analysis of AUA symptom index classification is presented in Figure 4. There were no significant differences in the IPSS among the three fractionation groups at each of the four time points in mild AUA symptom group (n = 33, 46, and 53 for 2.0, 2.5, and 3.0 Gy fractionation groups, respectively) and moderate and severe symptom group (n = 40, 54, and 63 for 2.0, 2.5, and 3.0 Gy fractionation groups, respectively). Table 3 shows the results of the three previous studies for the treatment of localised prostate cancer compared to our results.

| Study | No. of patients | Therapy | Total Dose (Gy) | Gy/fr | Toxicity Grading Scale | Toxicity (%) | QoL | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GU | GI | Questionnaire | Results | ||||||||
| G2+ | G3+ | G2+ | G3+ | ||||||||
| Fang et al. 201521 | 94 | IMRT | 79.2 | 1.8 | CTCAE | 28.7 | 0 | 13.8 | 0 | IPSS | No report about early IPSS outcomes. |
| 94 | PBT | 79.2 | 1.8 | 21.3 | 0 | 4.3 | 0 | ||||
| Grewal et al. 201922 | 184 | PBT | 70 | 2.5 | CTCAE | 12.5 | NR | 3.8 | NR | IPSS | No report about early IPSS outcomes. |
| Nakajima et al. 201723 | 254 | PBT | 74–78 | 2 | CTCAE | 15 | NR | NR | NR | IPSS | IPSS was worsened 1 month after PBT, but 6 months after PBT it recovered at the same level as baseline. |
| 272 | 60–63 | 3 | 5.9 | NR | NR | NR | |||||
| Current study | 73 | PBT | 74–78 | 2 | CTCAE | 16.4 | 1.4 | 0 | 0 | IPSS | IPSS was worsened 1 month after PBT, but 6 months after PBT it recovered at the same level as baseline |
| 100 | 70 | 2.5 | 11 | 0 | 0 | 0 | |||||
| 116 | 63 | 3 | 13.8 | 0 | 0 | 0 | |||||
- No., number; Gy, gray; fr, fraction; GU, genitourinary; GI, gastrointestinal; G2, grade 2; G3, grade 3; QoL; quality of life; IMRT, intensity-modulated radiotherapy; PBT, proton beam therapy; CTCAE; Common Terminology Criteria for Adverse Events; NR, not reported; IPSS, international prostate symptom score.
Discussion
Table 3 shows the results of the three published studies on the acute toxicities and IPSS in patients who received hypofractionated PBT or IMRT for localised prostate cancer.21-23 The risk of grade 2 or higher late toxicity was generally low regardless of the treatment. However, wide variations can be seen in the rate of grade 2 or higher acute toxicity and IPSS changes with both IMRT and PBT (Table 3). Multiple factors could explain these discrepant results, but there is a lack of published data regarding different fraction sizes in moderate hypofractionation. In some previous studies,24, 25 acute GU toxicity was identified as a predictive factor for late GU toxicity, and other studies reported a correlation between the PROM score and toxicity26,27. If there are valid associations between acute and late toxicity rates and between toxicity rates and PROM scores, the IPSS early after treatment may be a marker of late toxicity and patient PROM. Given the severe effects of late toxicities like haemorrhage and long-lasting urinary symptoms, it is beneficial for high-risk patients of late toxicity to be found earlier to receive closer follow-up and prophylactic management to reduce the development of the toxicity. In our study, no significant difference in the acute toxicity rate or IPSS among the three groups at 6 months from the start of PBT was observed. In addition, while several reports have assessed hypofractionated RT for localised prostate cancer, most did not include high-risk patients or included smaller sample sizes.28-30 Our results are considered unique because the majority of patients were classified as high risk (58.9%, 42.0%, and 49.1% in the 2.0, 2.5, and 3.0 Gy fractionation groups, respectively).
In our study, we found that rates of grade 2 or higher toxicities were comparable with those of previous reports (Table 3), and there were no significant differences in acute GU or GI toxicity rates among the three fractionation groups. Considering that an alpha/beta ratio of 10 Gy is associated with acute GU and GI toxicities, the biological effective dose (BED) at an alpha/beta ratio of 10 Gy was calculated as 88.8 Gy (for the 74 Gy total dose) or 93.6 Gy (for the 78 Gy total dose) in the conventional (2.0 Gy) fractionation group and as 87.5 Gy and 81.9 Gy in the hypofractionation (2.5 and 3.0 Gy, respectively) groups. These different BEDs suggest that larger fraction sizes reduce toxicity. However, our results did not show any significant differences in acute toxicity rates among the three fractionation groups, in agreement with previous reports on the acute toxicities induced by moderately hypofractionated RT or PBT.23 In addition, we investigated dose–volume histogram (DVH) of bladder to clarify the main cause of GU toxicities, we could not find any correlations between DVH of bladder and the incidence of acute urinary toxicity (Table S1 and S2). Although further research on this issue is required to fill the gap between theoretical expectations and the clinical outcomes observed in our study, our data suggest that hypofractionated PBT using fraction sizes of 2.5 Gy (RBE) and 3.0 Gy (RBE) is as safe as conventional PBT (2.0 Gy (RBE)) in terms of early toxicity. Moreover, highly conformal dose distribution of PBT might contribute to the further reduction of acute and patient-reported toxicity profile of hypofractionated RT.31 Indeed, Vapiwala et al reported significant reduction of acute grade 3 urinary toxicity of patients who received PBT compared with those who received IMRT (2.7% in IMRT vs. 0% in PBT (P = 0.002)),32 and the rate of acute grade 3 urinary toxicity was 0% in the current study. Regarding GI toxicity, all the patients in our cohort did not receive a hydrogel spacer between prostate and rectum. However, the rate of acute GI toxicities observed was so low that the contribution of hydrogel spacer to the improvement of acute GI toxicities might be limited.
The IPSS is a PROM that has been incorporated into clinical practice to assess the urological status and well-being of patients.6, 7 Many urologists and radiation oncologists are already using the IPSS in daily clinical practice for patients with prostate cancer. Because the difference in the BEDs among the three fraction sizes potentially affects the IPSS of prostate cancer patients, we investigated the change in the IPSS at each time point and the difference among the three fractionation groups. Our results confirmed that the pattern of IPSS change was similar among the three fractionation schedules: transient worsening of the IPSS at the end of PBT followed by an improvement by 6 months to pre-PBT values. We also found no significant difference in the IPSS among the four time points, from before PBT to 6 months after PBT initiation. Nakajima et al. previously reported the early IPSSs of patients with localised prostate cancer who received conventionally fractionated (2.0 Gy (RBE)) versus moderately hypofractionated (3.0 Gy (RBE)) PBT.23 In that study, although the IPSSs were increased at the end of treatment, the scores of most patients at 6 months had returned to baseline (before PBT) in both hypofractionation groups, similar to our results. Furthermore, we confirmed that fraction sizes of 2.0, 2.5, and 3.0 Gy (RBE) have similar effects on both the total IPSS and the scores for each item/item category of the IPSS during the early treatment period.
To identify factors likely contributing to a worse IPSS, we also investigated the influence of the following patient factors on the IPSS: DM status, age (< 68 vs. ≥ 68 years), ADT status, and AUA symptom severity. None of these factors showed a significant influence on the IPSS, although a previous study reported that receiving ADT was a predictor of lower QoL, as assessed by the 8-item short form of the Medical Outcomes Study questionnaire (SF-8), after treatment with hypofractionated carbon-ion RT.33 A possible reason for the discrepancy in results between studies is likely due to the use of different questionnaires; IPSS evaluates disease-specific PROM, whereas the SF-8 assesses general PROM with regard to functional, symptomatic, and psychosocial factors related to cancer therapy.34 Therefore, it appears that the influence of the three fractionation schedules on disease-specific PROM is negligible, while the use of PROMs facilitates patient decision-making regarding therapeutic options.
While prospective data collection was a strength of our study, the single institutional design and small patient number potentially introduced patient selection bias. In the present study, the proportion of patients with DM was higher in the conventional fractionation than in the two hypofractionation groups, but having DM did not affect the IPSS. The short observational period was also a limitation of our study, but because early toxicity and PROM changes were the focus of our research, this likely had little influence. Another limitation was that we did not evaluate differences in PROM in terms of bowel function among our fractionation groups, even though several studies revealed that external beam RT for prostate cancer was associated with a negative impact on bowel function.34 However, the negative effect of PBT on PROM regarding bowel function is reportedly limited compared with the effect of photon-base RT in patients with prostate cancer.35 Moreover, although no statistically significant difference was observed among the three fractionation sizes in our study, this might be due to the lack of adequate power. Our cohort did not include patients treated with novel technology of PBT like pencil beam scanning (PBS). Although higher conformal dose distribution of PBS was expected to improve adverse events or quality of life (QoL), Pugh TJ, et al. reported that they could not observe any significant difference about average scores of bowel, urinary, and sexual domain of EPIC between patients treated with PS and with PBS.36 Therefore, the contribution of PBS for the improvement of acute urinary toxicity or IPSS might be limited. However, their study was conducted in a single-institutional setting. A result from future multi-institutional study is aspired. We started a multi-institutional prospective study in October 2020 to investigate changes in patient-reported health-related QoL after PBT with several dose fractionation schedules in prostate cancer patients, using the Expanded Prostate Cancer Index Composite and Short Form Health Survey questionnaires.
Conclusion
We compared acute toxicity rates and IPSS early after PBT prospectively between two moderate hypofractionation schedules and conventional fractionation. No significant difference in acute toxicity or IPSS was observed according to fraction size, suggesting that a shorter treatment course might have greater benefit for patients with localised prostate cancer. We believe that our results will be useful for determining the appropriate fractionation schedule in patients with localised prostate cancer receiving moderately hypofractionated PBT. However, further research is needed to determine optimised fraction size of moderately hypofractionated PBT. To gain more robust data, we will conduct a future study focusing on late toxicity and long-term PROM with a longer observational period in patients participating in the two prospective clinical trials in our institution.
Acknowledgement
The authors would like to acknowledge Masaru Sato, who assisted with establishing the data collection system for this research.
Conflict of Interest
The authors declare no conflict of interest




