Comprehensive nodal breast VMAT: solving the low-dose wash dilemma using an iterative knowledge-based radiotherapy planning solution
Abstract
Introduction
Aimed to develop a simple and robust volumetric modulated arc radiotherapy (VMAT) solution for comprehensive lymph node (CLN) breast cancer without increase in low-dose wash.
Methods
Forty CLN-breast patient data sets were utilised to develop a knowledge-based planning (KBP) VMAT model, which limits low-dose wash using iterative learning and base-tangential methods as benchmark. Another twenty data sets were employed to validate the model comparing KBP-generated ipsilateral VMAT (ipsi-VMAT) plans against the benchmarked hybrid (h)-VMAT (departmental standard) and bowtie-VMAT (published best practice) methods. Planning target volume (PTV), conformity/homogeneity index (CI/HI), organ-at-risk (OAR), remaining-volume-at-risk (RVR) and blinded radiation oncologist (RO) plan preference were evaluated.
Results
Ipsi- and bowtie-VMAT plans were dosimetrically equivalent, achieving greater nodal target coverage (P < 0.05) compared to h-VMAT with minor reduction in breast coverage. CI was enhanced for a small reduction in breast HI with improved dose sparing to ipsilateral-lung and humeral head (P < 0.05) at immaterial expense to spinal cord. Significantly, low-dose wash to OARs and RVR were comparable between all plan types demonstrating a simple VMAT class solution robust to patient-specific anatomic variation can be applied to CLN breast without need for complex beam modification (hybrid plans, avoidance sectors or other). This result was supported by blinded RO review.
Conclusions
A simple and robust ipsilateral VMAT class solution for CLN breast generated using iterative KBP modelling can achieve clinically acceptable target coverage and OAR sparing without unwanted increase in low-dose wash associated with increased second malignancy risk.
Introduction
Breast cancer is one of the most commonly diagnosed cancers, estimated to account for up to 30% of all new cancer cases in women and contributes significantly to the workload of most radiotherapy departments.1 Radiotherapy treatment is an integral component in local and regional management of breast cancer reducing local recurrence and, in higher risk patients, improving survival in the adjuvant setting.2, 3
The difficulty of modern breast radiotherapy with inclusion of comprehensive lymph nodes (CLN) including supraclavicular, axillary and internal mammary nodes for at-risk patients has created challenges for planning based on three-dimensional conformal radiotherapy (3DCRT).4 This difficulty is exacerbated for patients which have an implant/expander or require simultaneous integrated boost (SIB) to the tumour bed.5 Intensity modulated radiotherapy (IMRT) and volumetric arc therapy (VMAT) improve the balance between target coverage and organ-at-risk (OAR) sparing, but their traditional application tends to be limited to the most demanding CLN-breast cases due to concerns of increased low-dose wash and potential secondary malignancy risk.6 As such, recent CLN-breast planning studies have focussed on modified IMRT or VMAT applications, which deliver a base portion of the dose tangentially using opposed fields or partial arcs (portals) to limit the secondary cancer risk profile similar to that of 3DCRT.7But whilst VMAT plans have the added advantage of fewer monitor units (MU) and shorter delivery times compared to their IMRT counterparts,8 these contemporary base-tangential VMAT techniques are disadvantaged by their increased planning complexity.5, 9-16 Indeed, such modified VMAT approaches require at least one of either base-hybrid plans (3DCRT or IMRT), pre-defined avoidance sectors, junctions, split arcs or non-coplanar beams in order to limit low-dose wash and improve plan quality.
To reduce complexity and improve planning consistency, standardised beam arrangements and application of machine learning via knowledge-based planning (KBP) is appropriate. In particular, KBP software such as RapidPlan™ (Varian Medical Systems, Palo Alto, CA, USA), which leverages curated patient anatomy and high quality plans has been shown to improve quality, consistency and efficiency of breast radiotherapy treatment.17 By applying KBP modelling using iterative learning and complex modified base-tangential techniques as benchmark,18 we hypothesise that a simple ipsilateral VMAT solution for CLN-breast cancer can achieve clinically acceptable target coverage and OAR sparing without an otherwise increase in low-dose wash and potential secondary malignancy risk. This works presents the first clinical implementation of an iterative KBP model in CLN-breast radiotherapy.
Methods
Ethics approval for this retrospective study of low and negligible risk was granted by the Northern Sydney Local Health District Human Research Ethics Committee (LNR/2020/ETH01286).
Preparation
Planning data sets from 60 (30 left-sided and 30 right-sided) de-identified conserved breast cancer patients who previously received CLN radiotherapy treatment were selected at random in this study. Non-contrast CT helical acquisition was with 2 mm slice thickness and 600 mm field of view (5122 pixel matrix) at 120 kVp on a Brilliance Big Bore scanner (Philips Medical Systems, Cleveland, OH, USA). All patients were immobilised in the supine position at 5–10° incline with arms raised above their head on the AccessTM Supine Breast board (Qfix, Avondale, PA, USA). Scans for left-sided patients were acquired in deep inspiration breath-hold (DIBH) using the Real-Time Position Management (RPM) System (Varian Medical Systems).
Two subspecialised breast radiation oncologists (RO) delineated clinical target volumes (CTV) and OARs on each of the 60 patient data sets according to ESTRO consensus guidelines.19 This included conserved breast, tumour bed, axillary nodes, supraclavicular nodes and internal mammary nodes. Planning target volumes (PTV) with a uniform 0.5 cm CTV expansion (cropped 0.5 cm from skin) were added, with heart and left anterior descending coronary artery (LADCA) contoured per Feng et al.20 A remaining volume at risk (RVR), defined as the Body minus all target and OAR structures,21 was included to supplement the assessment of low-dose wash.
- Hybrid (h)-VMAT (in-house low-dose wash benchmark): Extending on work originally published by Jöst et al,22 plan consists of two tangentially opposed IMRT fields to deliver a 30 Gy base to the conserved breast and inferior IMC/Axilla, junctioned with an angled anterior IMRT field (15 or 345 degrees depending on laterality) to deliver a 20 Gy base to the SCF and superior IMC/Axilla. Two ipsilateral VMAT fields spanning an extra 20° beyond the tangential gantry angles are then used to deliver the remaining dose to the target volumes (Figure 1a), with an additional arc in cases where dose coverage was suboptimal due to difficult anatomic configuration.
- Bowtie-VMAT (published benchmark): Includes three ipsilateral VMAT fields spanning 220° but with two of the fields including an appositional avoidance sector (dose-rate drops to zero) to largely limit fluence delivery to tangential sub arcs of approximately 70° range without compromising coverage of the supraclavicular fossa (Figure 1b).
- Ipsi-VMAT (proposed class solution): Three ipsilateral VMAT fields spanning 220° (Figure 1c). For a right-sided treatment the arcs move between 200° and 60° gantry angles. For left-sided treatments the treatment arcs move between 160° and 300°. The start and stop gantry angles were chosen to shape the dose distribution to the patient’s chest-wall off the heart and lung, independent of patient size and geometry.
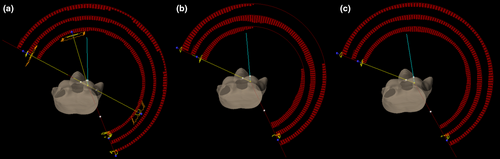
Development of the iterative breast nodal knowledge-based plan model
Benchmarked h-VMAT plans were manually generated by the department’s expert breast planners and repeatedly optimised in order to achieve the clinical objectives detailed in Tables 1 and 2 per the SKAGEN order of priority.23 Planning data sets from 40 of the 60 CLN-breast cancer patients (20 left- and 20 right-sided) were then included in development of the iterative VMAT breast nodal KBP model, using low-dose wash of the h-VMAT plans as benchmark. The model was re-trained initially using outputted bowtie-VMAT plans as input for the next iteration of the model.18 When low-dose wash of the bowtie-VMAT plans met the h-VMAT benchmark through refinement of the models optimisation parameters, ipsi-VMAT plans were then used as input for the final iterations of the model.18 In total five iterations were performed to produce the final refined model, beyond that negligible improvements (change in V5 Gy < 1%) were seen.
| Target name |
Volume (cc) Median (IQR) |
DVH Goal |
h-VMAT Median (IQR) |
Bowtie-VMAT Median (IQR) |
Ipsi-VMAT Median (IQR) |
P-Value (Welch’s t-test) Ipsi-VMAT vs h-VMAT |
P-Value (Welch’s t-test) Ipsi-VMAT vs Bowtie-VMAT |
Reported range from published literature 5, 9, 14-16, 30 |
|---|---|---|---|---|---|---|---|---|
| PTVp_TB |
32.1 cc (29.7–56.4 cc) |
D95% ≥ 95% (54.15 Gy) |
56.1 Gy (55.8–56.3 Gy) |
56.2 Gy (55.9–56.4 Gy) |
56.3 Gy (56.1–56.6 Gy) |
0.14 | 0.16 | |
| D98% |
55.7 Gy (55.4–55.9 Gy) |
55.8 Gy (55.5–55.9 Gy) |
55.9 Gy (55.7–56.2 Gy) |
0.38 | 0.20 | |||
| D50% |
57.8 Gy (57.6–58.0 Gy) |
57.8 Gy (57.6–58.1 Gy) |
57.9 Gy (57.6–58.4 Gy) |
0.13 | 0.38 | |||
| D2% (max) |
59.2 Gy (59.0–59.7 Gy) |
59.6 Gy (59.2–60.1 Gy) |
59.6 Gy (59.2–60.2 Gy) |
0.03* | 0.68 | |||
| HI |
0.057 (0.053–0.064) |
0.059 (0.056–0.065) |
0.057 (0.052–0.061) |
0.89 | 0.06 | |||
| PTVp_Br |
639.4 cc (487.5–961.0 cc) |
D95% ≥ 95% (47.50 Gy) |
48.7 (48.6–49.0) |
47.5 (47.5–47.7) |
47.5 (47.5–47.5) |
<0.01* | 0.12 | 45.6–49.5 Gy |
| D98% |
48.1 Gy (47.7–48.3 Gy) |
46.4 Gy (46.3–46.6 Gy) |
46.3 Gy (46.0–46.5 Gy) |
<0.01* | 0.06 | |||
| D50% |
50.6 Gy (50.2–50.9 Gy) |
50.6 Gy (50.4–51.3 Gy) |
50.7 Gy (50.4–51.3 Gy) |
0.08 | 0.70 | |||
| D2% (max) |
58.1 Gy (57.8–58.5 Gy) |
58.1 Gy (57.9–58.5 Gy) |
58.3 Gy (57.8–58.8 Gy) |
0.25 | 0.68 | |||
| HI |
0.183 (0.175–0.196) |
0.208 (0.204–0.214) |
0.212 (0.204–0.218) |
<0.01* | 0.39 | |||
| PTVn_SCF |
91.6 cc (77.3–110.9 cc) |
D95% ≥ 95% (47.50 Gy) |
47.9 Gy (47.8–48.1 Gy) |
48.4 Gy (48.0–48.6 Gy) |
48.6 Gy (48.3–48.8 Gy) |
<0.01* | 0.18 | 42.5–48.7 Gy |
| D98% |
47.3 Gy (47.1–47.6) Gy |
47.4 Gy (46.8–47.7 Gy) |
47.7 Gy (47.1–48.0 Gy) |
0.23 | 0.29 | |||
| D50% |
49.6 Gy (49.5–49.8 Gy) |
50.5 Gy (50.3–50.9 Gy) |
50.6 Gy (50.3–51.0 Gy) |
<0.01* | 0.55 | |||
| D2% (max) |
51.1 Gy (50.9–51.2 Gy) |
52.2 Gy (51.9–52.8 Gy) |
52.2 Gy (51.8 - 52.6 Gy) |
<0.01* | 0.84 | 51.5–55.0 Gy | ||
| HI |
0.063 (0.062–0.069) |
0.076 (0.071–0.084) |
0.072 (0.066–0.086) |
<0.01* | 0.22 | |||
| PTVn_Ax |
158.8 cc (123.2–204.9 cc) |
D95% ≥ 95% (47.50 Gy) |
48.0 Gy (47.9–48.2 Gy) |
48.6 Gy (48.5–48.9 Gy) |
48.7 Gy (48.5–48.8 Gy) |
<0.01* | 0.80 | 48.9–49.2 Gy |
| D98% |
47.6 Gy (47.3–47.7 Gy) |
47.8 Gy (47.6–48.1 Gy) |
48.0 Gy (47.6–48.2 Gy) |
<0.01* | 0.86 | |||
| D50% |
49.5 Gy (49.5–49.7 Gy) |
50.5 Gy (50.3–50.8 Gy) |
50.6 Gy (50.2–50.9 Gy) |
<0.01* | 0.51 | |||
| D2% (max) |
51.2 Gy (50.9–51.6 Gy) |
52.3 Gy (51.8–52.9 Gy) |
52.4 Gy (51.9–53.3 Gy) |
<0.01* | 0.97 | 55.0 Gy | ||
| HI |
0.065 (0.057–0.074) |
0.069 (0.064–0.085) |
0.072 (0.064–0.085) |
0.33 | 0.95 | |||
| PTVn_IMN |
30.1 cc (26.3–36.7 cc) |
D95% ≥ 95% (42.75 Gy) |
43.6 Gy (43.2–44.3 Gy) |
44.1 Gy (42.8–44.8 Gy) |
44.5 Gy (43.5–45.0 Gy) |
0.05* | 0.37 | 44.6–51.1 Gy |
| D98% |
42.8 Gy (42.3–43.6 Gy) |
43.0 Gy (41.5–43.8 Gy) |
43.4 Gy (42.1–44.1 Gy) |
0.37 | 0.20 | |||
| D50% |
47.4 Gy (46.8–48.0 Gy) |
48.8 Gy (48.2–49.0 Gy) |
48.8 Gy (48.5–49.2 Gy) |
<0.01* | 0.18 | |||
| D2% (max) |
50.8 Gy (50.6–50.97 Gy) |
51.9 Gy (51.5–52.3 Gy) |
51.8 Gy (51.4–52.7 Gy) |
<0.01* | 0.92 | 55.0 Gy | ||
| HI |
0.126 (0.113–0.137) |
0.126 (0.113–0.142) |
0.125 (0.109–0.154) |
0.46 | 0.72 | |||
| Conformity Index | CI (Combined PTV, 45 Gy isodose) |
0.673 (0.663–0.694) |
0.694 (0.686–0.711) |
0.712 (0.693–0.736) |
<0.01* | 0.09 | ||
|
CI (PTVp_TB, 54.15 Gy isodose) |
0.597 (0.536–0.675) |
0.586 (0.523–0.608) |
0.593 (0.531–0.640) |
0.17 | 0.74 | |||
| Monitor Units | Total MU |
839 MU (785–880 MU) |
595 MU (568–628 MU) |
652 MU (616–69 MU) |
<0.01* | <0.01* | 973–1568 MU |
- PTV, planning target volume; TB, tumour bed; Br, conserved breast; SCF, supraclavicular fossa; Ax, axilla; IMN, internal mammary nodes; D, dose; V, volume; Max, maximum; cc, cubic centimetres; HI, homogeneity index; CI, conformity index; DVH, dose-volume histogram; MU, monitor unit; IQR, interquartile range; DNE, does not exist.
- *Statistically significant with P < 0.05.
| Organ Name | DVH Goal | h-VMAT | Bowtie-VMAT | Ipsi-VMAT | P-Value (Welch’s t-test) | P-Value (Welch’s t-test) | Reported range from published literature 5, 9, 14-16, 30 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Number (percentage) of patients who met goal | Median (IQR) | Number (percentage) of patients who met goal | Median (IQR) | Number (percentage) of patients who met goal | Ipsi-VMAT vs h-VMAT | Ipsi-VMAT vs bowtie-VMAT | |||
| Heart Left Lesion | Mean ≤ 3 Gy |
2.0 Gy (1.5–2.5 Gy) |
10 (100%) |
2.0 Gy (1.4–2.4 Gy) |
10 (100%) |
2.0 Gy (1.4–2.5 Gy) |
10 (100%) | 0.87 | 0.98 | 3.0–11.7 Gy |
| V5 Gy ≤ 10% |
2.0% (1.0–4.6%) |
10 (100%) |
2.4% (1.4–4.9%) |
9 (90%) |
1.8% (0.5–5.2%) |
10 (100%) | 0.96 | 0.76 | 11.4–75.7% | |
| V10 Gy ≤ 5% |
0.2% (0.0–1.1%) |
9 (90%) |
0.2% (0.0–1.1%) |
10 (100%) |
0.2% (0.0–1.3%) |
10 (100%) | 0.76 | 0.97 | 4.2–41.7% | |
| D2cc ≤ 20 Gy |
8.1 Gy (5.8–12.5 Gy) |
8 (80%) |
8.8 Gy (7.0–13.9 Gy) |
9 (90%) |
8.0 Gy (5.1–14.4 Gy) |
9 (90%) | 0.89 | 0.93 | 22.5–42.8 Gy | |
| Heart Right Lesion | Mean ≤ 2 Gy |
1.6 Gy (1.4–2.2 Gy) |
7 (70%) |
1.5 Gy (1.4–1.9 Gy) |
9 (90%) |
1.6 Gy (1.4–2.2 Gy) |
7 (70%) | 0.91 | 0.57 | 3.0–11.7 Gy |
| V5 Gy v 6% |
1.6% (0.3–2.4%) |
10 (100%) |
0.8% (0.4–1.8%) |
10 (100%) |
1.7% (0.7–3.1%) |
9 (90%) | 0.65 | 0.35 | 11.4–75.7% | |
| LADCA | D0.04cc ≤ 20 Gy |
10.9 Gy (6.1–20.3 Gy) |
8 (80%) |
13.8 Gy (8.9–18.2 Gy) |
9 (90%) |
14.7 Gy (8.6–19.1 Gy) |
9 (90%) | 0.68 | 0.97 | 31.4 Gy |
| Lung Ipsilateral | Mean ≤ 12 Gy |
12.3 Gy (11.8–13.1 Gy) |
8 (40%) |
11.5 Gy (10.9–12.5 Gy) |
13 (65%) |
11.7 Gy (10.8–12.3 Gy) |
13 (65%) | 0.05* | 0.99 | 13.6–16.4 Gy |
| V5 Gy ≤ 50% |
48.2% (45.3–49.8%) |
16 (80%) |
46.8% (42.9–49.4%) |
16 (80%) |
48.0% (43.7–50.1%) |
15 (75%) | 0.70 | 0.53 | 59.8–84.9% | |
| V10 Gy ≤ 35% |
36.1% (33.5–38.8%) |
8 (40%) |
33.3% (30.8–34.5%) |
16 (80%) |
33.6% (31.4–350%) |
16 (80%) | 0.04* | 0.51 | 45.7–53.5% | |
| V20 Gy ≤ 20% |
25.4% (23.8–28.2%) |
2 (10%) |
22.8% (21.1–23.8%) |
3 (15%) |
23.1% (21.1–23.9%) |
3 (15%) | < 0.01* | 0.71 | 20.8–27.8% | |
| Lung Contralateral | Mean ≤ 2 Gy |
1.9 Gy (1.8–2.2 Gy) |
12 (60%) |
1.9 Gy (1.7–2.0 Gy) |
15 (75%) |
2.0 Gy (1.8–2.1 Gy) |
10 (50%) | 0.71 | 0.06 | 2.0–3.6 Gy |
| V5 Gy (%) |
4.4% (2.5–5.9%) |
4.9% (4.3–5.7%) |
4.7% (3.1–6.3%) |
0.79 | 0.87 | 9.1–23.9% | ||||
| Contralateral Breast | Mean ≤ 3 Gy |
2.2 Gy (1.8–2.5 Gy) |
17 (85%) |
2.0 Gy (1.7–2.4 Gy) |
19 (95%) |
2.1 Gy (1.8–2.5 Gy) |
17 (85%) | 0.57 | 0.62 | 3.4–4.5 Gy |
| V5 Gy (%) |
2.7% (0.4–5.2%) |
6.6% (3.6–9.1%) |
6.6% (4.1–10.8%) |
0.20 | 0.87 | 21.8–30.2% | ||||
| D2cc ≤ 12 Gy |
6.7 Gy (5.2–21.2 Gy) |
13 (65%) |
9.0 Gy (7.5–12.6 Gy) |
15 (75%) |
8.6 Gy (6.9–10.6 Gy) |
17 (85%) | 0.16 | 0.33 | 12.9–22.4 Gy | |
| Oesophagus | D0.1cc ≤ 30 Gy |
16.1 Gy (13.8–22.9 Gy) |
19 (95%) |
15.9 Gy (13.9–20.8 Gy) |
18 (90%) |
16.6 (12.9–19.2 Gy) |
19 (95%) | 0.53 | 0.88 | 27.2–30.7 Gy |
| Spinal Cord | D0.1cc ≤ 20 Gy |
7.3 Gy (6.5–7.9 Gy) |
20 (100%) |
10.9 Gy (8.9–12.2 Gy) |
20 (100%) |
13.3 Gy (11.7–14.6 Gy) |
20 (100%) | < 0.01* | < 0.01* | 12.5–17.2 Gy |
| Humeral Head | D0.1cc ≤ 30 Gy |
29.4 Gy (25.8–31.9 Gy) |
12 (60%) |
26.7 Gy (20.3–32.1 Gy) |
12 (60%) |
24.1 Gy (14.8–28.4 Gy) |
18 (90%) | < 0.01* | 0.24 | 26.0 Gy |
| Thyroid | V30 Gy ≤ 62.5% |
20.8% (2.1–35.9%) |
20 (100%) |
17.7% (0.8–34.3%) |
20 (100%) |
15.6% (0.5–29.4%) |
20 (100%) | 0.32 | 0.55 | DNE |
| Trachea | D0.04cc ≤ 47.5 Gy |
30.3 Gy (25.5–37.3 Gy) |
20 (100%) |
34.2 Gy (27.8–37.9 Gy) |
20 (100%) |
31.6 Gy (25.9–36.3 Gy) |
20 (100%) | 0.96 | 0.34 | DNE |
| Liver | D2cc ≤ 45 Gy |
37.1 Gy (16.9–44.5 Gy) |
8 (80%) |
27.2 Gy (17.8–34.3 Gy) |
10 (100%) |
28.3 Gy (19.0–35.0 Gy) |
10 (100%) | 0.35 | 0.80 | DNE |
- LADCA, left anterior descending left coronary artery; D, dose; V, volume; Max, maximum; cc, cubic centimetres; DVH, dose-volume histogram; MU, monitor unit; IQR, interquartile range; DNE, does not exist.
- *Statistically significant with P < 0.05.
The breast nodal KBP model was used to validate bowtie- and ipsi-VMAT plans on each of the 20 remaining CLN-breast patient data sets (10 left- and 10 right-sided). These plans were generated with a single pass of the KBP model and normalised up to ± 5% to achieve target coverage. The manually generated h-VMAT calculated on the same 20 data sets were used for comparison. For the purpose of this work, a minimum target coverage of D95% > 95% was mandated per departmental protocol for all three plan types to allow direct comparison of OAR dose-volume histograms (DVHs).
Quantitative analysis of the dose to each structure was performed by calculating the population DVH median and interquartile range (IQR) for each plan type. Clinical objectives along with ICRU 83 target dose reporting values (D98%, D50%, and D2%),21 the number of patients meeting each objective and statistical significance of the difference between the ipsi-VMAT and benchmarked plans determined using Welch’s t test for unequal variances were also assessed. In addition, total MUs were assessed to evaluate treatment efficiency and the homogeneity index, HI (= (D2%–D95%)/D50%) calculated to determine the level of dose variation across the PTVs for each plan type. D95% was chosen in the numerator for HI (instead of D98% per ICRU 83) since it defines the minimum target dose coverage goal, with a HI value of zero indicating that the dose distribution is almost homogeneous. Dose conformity was evaluated per the conformity index, CI defined by van’t Riet et al.24 Conformity indexes were calculated for the 54.15 Gy (95% of 57 Gy) isodose coverage of the tumour bed volume and the 45 Gy (90% of 50 Gy) isodose coverage of the remaining target volumes combined, noting a CI value of one indicates the dose distribution is perfectly conformal to the target volume.
Blinded clinician plan comparison
The dose distribution and DVH curves of the three plan types generated on the 20-patient validation cohort were assessed by three subspecialist breast ROs to determine that plan type was preferred. The ROs were blinded to plan type to help prevent any subjective bias based on pre-conceived notions of increased second malignancy risk founded on current published consensus for breast VMAT.8 The ROs completed this review together and came to a consensus of their order of preference for the plan types for each patient. A tally of their preferences was taken with 1 point being given to the preferred plan and no points to the other plans. If two plans were considered equal best, then 0.5 points were given to each of those two plan types; and if all three plans were assessed to be equivalent 0.3 points were given to each.
Results
Validation of the iterative breast nodal knowledge-based plan model
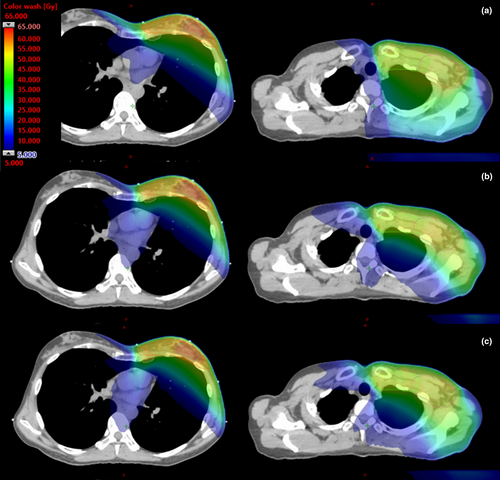
Figure 2 shows target coverage for all plan types was similar due to the optimisation and normalisation process used, with no statistically significant difference between bowtie- and ipsi-VMAT methods. However, as detailed in Table 1, h-VMAT plans did generate improved minimum D95% isodose coverage in the conserved breast (P < 0.01) at the expense of reduced coverage to all nodal target volumes (P < 0.05). When using either of the bowtie or ipsi-VMAT techniques, dose homogeneity was slightly reduced but conformity improved when compared to the hybrid approach. An example of the dose distribution generated by each plan type for the same patient is displayed in Figure 3.
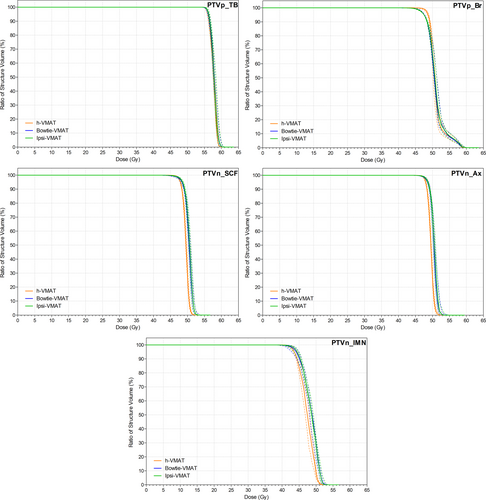
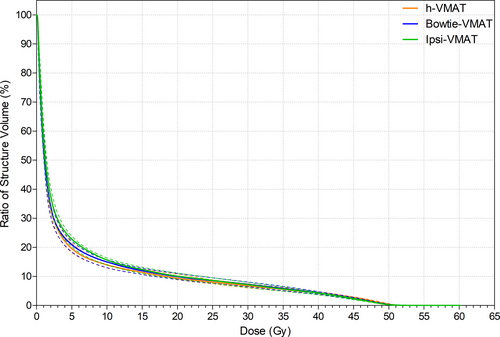
With respect to OAR sparing, Figure 4 shows negligible difference in dose between bowtie- and ipsi-VMAT methods except in spinal cord D0.1cc maximum (P < 0.01). Even so, Table 2 details that the maximum spinal cord dose values remained below the 20 Gy goal for all patient plans. In comparison, the h-VMAT technique generated a statistically significant higher dose to the ipsilateral lung and humeral head in terms of reported mean, V10 Gy, V20 Gy and D0.1cc DVH metrics compared to ipsi-VMAT with reduced planning goal compliance (P = 0.05, P = 0.04, P < 0.01 and P < 0.01, respectively). However, h-VMAT did best reduce spinal cord D0.1cc dose maximum when compared to the alternate VMAT options (P < 0.01) with no statistically significant difference in dose to heart, LADCA, contralateral lung or contralateral breast.
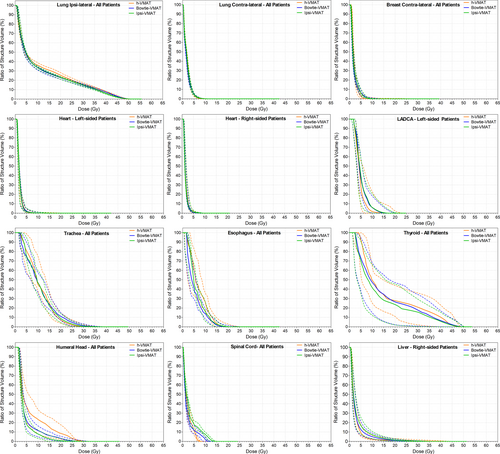
Figure 5 displays the variation in spill dose to the RVR, with no statistically significant difference in low-dose wash between h-VMAT (V5 Gy = 19.6%), bowtie-VMAT (V5 Gy = 20.9%) and ipsi-VMAT (V5 Gy = 22.8%) techniques. However, as detailed in Table 1, h-VMAT generated upwards of 30–40% more MU than both bowtie-VMAT and ipsi-VMAT methods (839 MU, 595 MU and 652 MU, respectively).
Blinded clinician plan comparison
Both ipsi-VMAT and bowtie-VMAT techniques performed well during the blinded RO-planning consensus review. When preferencing all patient plans bowtie-VMAT tallied the highest at 9.8 points, followed by the ipsi-VMAT technique (8.8 points) and lastly the h-VMAT technique (1.3 points). Ratio of scores were similar when filtered for laterality.
Discussion
CLN irradiation can improve outcomes for advanced breast cancer patients, but it can be challenging to achieve target coverage and OAR sparing.8 Recent literature suggests that the balance is changing between the clinical importance of increased low-dose wash of IMRT or VMAT compared to improvements in local cancer control and reduced OAR toxicity.25, 26 Even so, further clinical outcome data are needed to allay concerns of increased potential second malignancy risk.27, 28 Indeed, recent breast nodal planning studies show increasingly complex beam modification methods (hybrid plans, avoidance sectors, other) in order to improve plan quality and limit low-dose wash.5, 8-16, 29, 30 However, by applying iterative KBP modelling, this study demonstrates a simple ipsi-VMAT solution for CLN-breast patients can limit low-dose wash to the heart, lungs, contralateral breast and RVR without compromise in plan quality when compared to tangentially constrained h-VMAT (departmental standard) and bowtie-VMAT (published best practice) benchmark methods. As such, use of a simple VMAT technique should not be discounted in the nodal breast clinical setting so long as optimisation is sufficiently refined to limit low-dose wash and produce the plans intended.
Both ipsi-VMAT and bowtie-VMAT plans in this study were practically equivalent in terms of target coverage, OAR sparing and MU. Compared to the h-VMAT plans, the ipsi-VMAT plans gave improved nodal target coverage, conformity and dose to ipsilateral lung and humeral head. The trade-off was a marginal reduction in breast target coverage, homogeneity and increased dose to spinal cord. This dose distribution was preferred by our subspecialist breast ROs in the blinded plan review in-line with the SKAGEN order of priority (noting the lower dose coverage in conserved breast was in regions of low risk away from the tumour bed),23 with the quality of the ipsi-VMAT plans adjudged to be equivalent to bowtie-VMAT and superior to h-VMAT. In many Institutions, there may be embedded belief that ipsi-VMAT contributes increased risk of second malignancy with its clinical application. Our quantitative and qualitative analysis of this cohort should support an evolution in such beliefs that simple ipsi-VMAT performs equivocally with much more complex techniques in dosimetry. There are clear advantages to workflow, with the highly refined ipsi-VMAT KBP model with preset beam geometry providing an automated class solution that significantly simplifies the CLN-breast planning process by being robust to patient-specific anatomical variation in addition to removing need for any complex beam modification. This has anecdotally helped our department improve planning quality, consistency and efficiency (reduced planning time) compared to our previous in-house h-VMAT solution; whilst the reduced MU load is also expected to improve patient compliance in DIBH to better spare the heart.8, 17
Compared to the majority of DVH objectives commonly reported by published literature in the CLN-breast setting (with standard 50 Gy in 25 fraction prescription), we have demonstrated that an iterative KBP ipsi-VMAT solution achieves superior OAR doses (Table 2) even with addition of a SIB of 57 Gy to the tumour bed. It should be noted, however, that the overlapping SIB volume in the breast target prevents direct comparison of HI to plans with single-dose levels, and that the comparative review is limited . Results could also be influenced by planning left-sided patients in DIBH, using an Acuros XB dose-to-medium calculation algorithm,31 or that the Varian Halcyon treatment unit is installed with a dual layer MLC (reducing MLC leakage). But given the inherent limitations of planning comparison studies, we are prospectively collecting outcome and toxicity data on all patients to compliment this work for future reporting purposes.
Since clinically implementing the ipsi-VMAT technique on our Varian Halcyon treatment unit in mid-2020, we have found need to produce separate KBP models in both breast alone and breast/SCF settings to better spare heart, lungs and contralateral breast. Meanwhile we have found no issue in adapting the models to chest-wall (including expander) and bilateral patients, although all models have had to be reconfigured for hypo-fractionated regimes where prescribed. Similarly, we have found no issue in applying the models for treatment on Varian TrueBeam treatment units (Millennium MLC) so long as jaw tracking is applied to reduce MLC leakage; that is, maintain low-dose wash equivalence between plans. To ensure plan robustness to inter- and intra-fraction motion, skin flash is added per the recommendations of Rossi et al.32 Specifically, an 8mm virtual bolus (−350HU) and 5mm planning PTV extension outside the Body was introduced for optimisation with the breast KBP model before virtual bolus was removed for dose calculation and re-normalisation.33 For any systematic deformations in body structure greater than 1.0cm observed on daily cone beam computed tomography (CBCT), re-planning is requested by treatment staff.32
Conclusions
A simple ipsilateral VMAT solution for CLN-breast cancer generated using iterative KBP modelling can achieve clinically acceptable target coverage and OAR sparing without increase in low-dose wash compared to benchmark base-tangential techniques. This automated class solution significantly simplifies the CLN-breast planning process by being robust to patient-specific anatomical variation in addition to removing need for any complex beam modification.
Acknowledgement
The authors gratefully acknowledge all the staff at Northern Sydney Cancer Centre for making this work possible.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Available from the corresponding author on reasonable request.




