New metastasectomy criteria for peritoneal metastasis of hepatocellular carcinoma: A study of the Japanese Society of Hepato-Biliary-Pancreatic Surgery
Abstract
Background/Purpose
Peritoneal metastasis of hepatocellular carcinoma (HCC) is rare. We investigated patients who underwent peritoneal metastasectomy in a multicenter study in Japan.
Methods
The study included 92 patients with HCC who underwent resection for peritoneal metastasis between January 2007 and December 2013. We investigated background and operative factors, as well as overall and recurrence-free survival rates. Patients were classified according to the extent of peritoneal metastasis using the peritoneal cancer index (PCI) and the completeness of cytoreduction (CC) scores to examine whether peritoneal metastasectomy contributed to survival.
Results
The mean maximum tumor size was 4.1 cm. Forty patients (43.5%) had multiple peritoneal metastases. Peritoneal metastasectomy and hepatectomy were performed simultaneously in 48 patients (52.2%). Overall, the 5-year survival rate after resection was 36.0%, and the recurrence-free survival rate was 13.0%. Multivariate analysis revealed that PCI scores of ≤6 + CC scores of 0 were an independent prognostic factor. The 5-year survival rate when these criteria were met was 43%, which was significantly better than that in those who did not meet these criteria (P < .001).
Conclusions
Peritoneal metastasectomy of HCC appears to contribute to improved survival of patients with a PCI scores of ≤6, without remnant tumors.
1 INTRODUCTION
Among extrahepatic metastases of hepatocellular carcinoma (HCC), the incidence rate of peritoneal metastasis (6%–11%) is much lower than the lung, bone, lymph node, or adrenal gland metastasis rates.1, 2 HCC larger than 5 cm, the presence of vascular invasion, a positive resection margin, poorly differentiated HCC, and radiofrequency ablation on HCC near the liver surface are considered to be risk factors for peritoneal metastasis.3, 4
HCC treatment guidelines in Japan recommend systemic chemotherapy, that is, treatment with molecular target drugs for patients with extrahepatic metastases, such as peritoneal metastasis.5 However, because HCC displays expansive growth and has a relatively low tendency to invade the surrounding areas in peritoneal metastasis, metastasectomy may contribute to improving the survival rate. Several studies have reported successful, long-term survival of patients after resection of peritoneal metastasis of HCC.6-9 However, no consensus on the significance of resection has been reached.
It was reported that the median survival of patients with HCC who underwent peritoneal metastasectomy was 33 months, while that in non-resected patients was 14 months, showing a significant prognostic improvement in resected patients.4 However, this study used a relatively small number of patients and did not refer to the tumor sites, or the size or number of peritoneal metastasis. Therefore, the findings need to be confirmed using a much larger sample. We collected data on patients who underwent peritoneal metastasectomy for HCC throughout Japan and classified the collected data according to the extent of peritoneal metastasis and the effect of treatment using the peritoneal cancer index (PCI) and the completeness of cytoreduction scores (CC). Then, we investigated the significance of peritoneal metastasectomy for HCC with the aim of establishing a treatment strategy.10
The current study was funded by the Japanese Society of Hepato-Biliary-Pancreatic Surgery.
2 MATERIALS AND METHODS
A questionnaire survey was completed by patients who underwent peritoneal metastasectomy for HCC between January 2007 and December 2013 in 217 Japanese Society of Hepato-Biliary-Pancreatic Surgery board-certified training institutions. We retrospectively collected the data of 99 patients from 44 institutions who could participate in our study by obtaining approval for the study from the Ethical Review Board. In total, we investigated 92 of the 99 patients, as seven patients were excluded due to insufficient data.
The following background factors of patients were investigated: age, sex, body mass index, the presence or absence of diabetes, hepatitis B surface antigen (HBsAg), and hepatitis C antibody (HCV). Laboratory data based on blood samples were used to investigate the levels of albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, prothrombin activity (PT), platelet count, α-fetoprotein (AFP), and protein induced by vitamin K absence or antagonist-II (PIVKA-II). Histological data regarding a percentage of poorly differentiated primary HCC and the presence or absence of portal invasion were investigated. We investigated treatments for primary HCC considering the presence or absence of a history of HCC rupture, presence or absence of a history of biopsy, hepatectomy, radiofrequency ablation, transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), sorafenib administration, the time between the initial HCC diagnosis to the confirmation of peritoneal metastasis, and the time between the last treatment of HCC and the confirmation of peritoneal metastasis. Moreover, the number of patients who underwent peritoneal metastasectomy and hepatectomy simultaneously, the maximum diameter and number of peritoneal metastases, as well as the PCI and CC scores, was investigated.
The PCI is a system for classifying peritoneal metastasis that was reported by Gilly et al. in 2006. It is based on a maximum of 39 points in which the abdominal region is divided into 13 sections, each of which is given a score of 0–3 according to the size of peritoneal metastasis. The 13 sections are as follows: central, right upper, epigastrium, left upper, left flank, left lower, pelvis, right lower, right flank, upper jejunum, lower jejunum, upper ileum, and lower ileum. If no peritoneal metastasis is found, the score is 0 points. The total diameter of the peritoneal metastasis in each region is calculated with <5 mm as 1 point, between 5 mm and 5 cm as 2 points, and 5 cm or more as 3 points. The points of each of the 13 regions were totaled, and the degree of peritoneal metastasis was evaluated from a minimum of 0 points to a maximum of 39 points. The CC scores score was determined based on the extent of the residual tumor. A score of 0 indicated that no residual nodules were visible, a score of 1 indicated the presence of nodules smaller than 25 mm, a score of 2 indicated the presence of nodules between 25 mm and 50 mm, and a score of 3 indicated the presence of nodules >50 mm.10
This study was conducted following the ethical guidelines set for clinical research involving humans. The study was approved by the Ethical Review Board of each institution, and the approval number of the representative institution is 29–100.
2.1 Statistical analysis
The mean ± standard deviation of age, body mass index, the maximum size of peritoneal metastasis, and the median value and range of the other factors were evaluated. The survival rate was calculated with the Kaplan–Meier method using the log-rank test. Each continuous variable for univariate analysis, including the PCI scores, was divided according to clinically convenient values close to the cutoff value using a receiver operating characteristic (ROC) curve. Multivariate analysis of factors with P-values of < .05 in the univariate analysis was performed to identify independent prognostic factors using the Cox proportional hazards model. All statistical analyses were performed using R version 3.5.3 (The R Foundation for Statistical Computing; https://cran.r-project.org/bin/macosx/).
3 RESULTS
The mean age of the patients under study was 66.8 years, and 70 patients (76.1%) were male. Diabetes was confirmed in 24 patients (26.1%), HBsAg in 27 patients (29.3%), and the HCV antibody in 34 patients (37.0%). Laboratory data based on blood samples before the peritoneal metastasectomy showed that the median albumin was 3.8 g/dL, total bilirubin was 0.73 mg/dL, PT was 86%, and platelet count was 140 000/μL, indicating satisfactory hepatic functional reserve. Tumor marker findings showed that the median value of AFP was 68.6 ng/mL, and PIVKA-II was 260 mAU/mL. Histological findings of primary HCC showed that 28 patients (30.4%) had poorly differentiated HCC, and 23 patients (25.0%) had portal vein invasion. In total, 23 patients (25.0%) had a history of HCC rupture, and 23 patients (25.0%) had undergone a tumor biopsy. Among the patients studied, 55 (59.8%) had a history of hepatectomy, 44 (47.8%) had a history of radiofrequency ablation, and 61 (66.3%) had a history of TACE. Sorafenib was administered to 11 patients (12.0%). The median duration from the initial diagnosis of HCC until confirmation of peritoneal metastasis was 32 months, and the median duration between the last treatment of HCC and confirmation of peritoneal metastasis was 6 months. As for the number of peritoneal metastases, 52 patients (56.5%) had a single tumor, whereas 11 patients (12.0%) had more than 10 tumors. Moreover, the mean maximum size of the peritoneal metastasis was 4.1 cm. Regarding the PCI scores, 57 patients (62.0%) had a PCI scores of 1–3, 23 patients (25.0%) had a scores of 4–6, and 12 patients (13.0%) had a scores of ≥7. Concerning the CC scores, 69 patients (75.0%) had a CC scores of 0, 8 patients (8.7%) had a scores of 1, and 15 patients (16.3%) had a scores of 2. None of the patients had a CC scores of 3 (residual nodules of peritoneal metastasis ≥50 mm; Table 1).
| Factor | n = 92 |
|---|---|
| Age (y) | 66.89 ± 10.65 |
| Gender (%) | |
| Men | 70 (76.1) |
| Women | 22 (23.9) |
| BMI (kg/m2) | 22.94 ± 3.21 |
| Diabetes mellitus (%) | 24 (26.1) |
| Positive of HBs antigen (%) | 27 (29.3) |
| Positive of HCV antibody (%) | 34 (37.0) |
| Albumin (g/dL) | 3.8 [2.5–5.1] |
| AST (IU/L) | 29 [14–170] |
| ALT (IU/L) | 25 [9–161] |
| Total bilirubin (mg/dL) | 0.73 [0.2–1.6] |
| Prothrombin activity (%) | 86 [54–143] |
| Platelet count (×104/μL) | 14.1 [3.7–32.8] |
| α-fetoprotein (ng/mL) | 68.6 [1.7–204014] |
| PIVKA-II (mAU/mL) | 260 [6–33400] |
| Poorly differentiation of primary HCC (%) | 28 (30.4) |
| Portal invasion of primary HCC (%) | 23 (25.0) |
| History of HCC rupture (%) | 23 (25.0) |
| History of HCC biopsy (%) | 23 (25.0) |
| History of hepatectomy (%) | 55 (59.8) |
| History of RFA (%) | 44 (47.8) |
| History of TACE (%) | 61 (66.3) |
| History of HAIC (%) | 10 (10.9) |
| History of sorafenib administration (%) | 11 (12.0) |
| Duration from initial HCC diagnosis | 32 [0–156] |
| Duration from last HCC treatment | 6 [0–145] |
| Simultaneous resection of HCC and peritoneal metastasis | 48 (52.2) |
| Maximum size of peritoneal metastasis (cm) | 4.1 ± 3.1 |
| Number of peritoneal metastasis | |
| 1 | 52 (56.5) |
| 2-5 | 26 (28.2) |
| 6-9 | 3 (3.3) |
| ≥10 | 11 (12) |
| Peritoneal cancer index (%) | |
| 1-3 | 57 (62.0) |
| 4-6 | 23 (25.0) |
| ≥7 | 12 (13.0) |
| Completeness of cytoreduction score (%) | |
| 0 | 69 (75.0) |
| 1 | 8 (8.7) |
| 2 | 15 (16.3) |
Note
- Age, BMI and maximum size of peritoneal metastasis are expressed as mean ± standard deviation. Other data are expressed as median with minimum–maximum values.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HAIC, hepatic arterial infusion chemotherapy; HBs, hepatitis B surface; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PIVKA-II, protein induced by vitamin K absence or antagonist-II; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
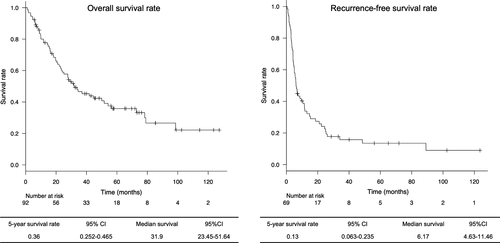
The 5-year survival rate among all resected patients was 36.0%, with a median survival duration of 31.9 months. Moreover, the 5-year recurrence-free survival rate was 13.0%, with median recurrence-free survival duration of 6.1 months (Figure 1).
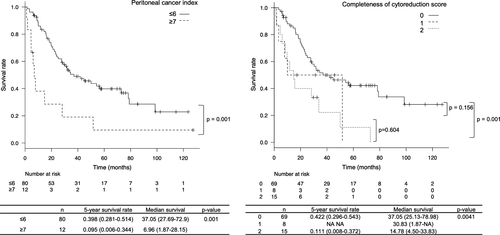
Comparing survival rates using the PCI scores, patients with a PCI scores of ≥7 showed a significantly poorer survival rate than those with lower scores. As for the CC scores, patients with a CC scores of 0 showed a significantly better prognosis than those with a CC scores of 2 (Figure 2).
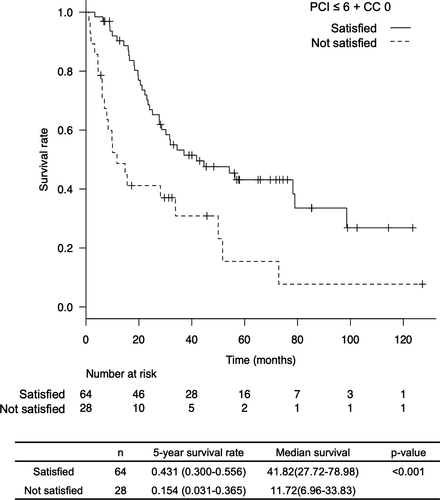
In the univariate analysis to identify the contributing factors of survival, several factors showed a significantly better survival rate: patients with AFP level <500 ng/mL, patients with PIVKA-II level <1000 mAU/mL, patients for whom the duration from the last treatment of HCC until confirmation of peritoneal metastasis was >6 months, patients who underwent peritoneal metastasectomy alone because of intrahepatic HCC absence, patients with a PCI scores of ≤6, and patients with a CC scores of 0. We conducted a multivariate analysis of these factors to identify independent prognostic factors. However, PCI scores of ≤6 and CC scores of 0 were not used in the multivariate analysis because multicollinearity was excluded. Instead, we included a factor of PCI scores of ≤6 + CC scores of 0 in a multivariate analysis. The results showed that a PCI score of ≤6 + CC score of 0 was an independent prognostic factor (Table S1). The 5-year survival rate of patients who had a PCI scores of ≤6 + CC scores of 0 was 43.1%, which was significantly better than that of patients who did not meet this criteria (15.4%, P < .001, Figure 3).
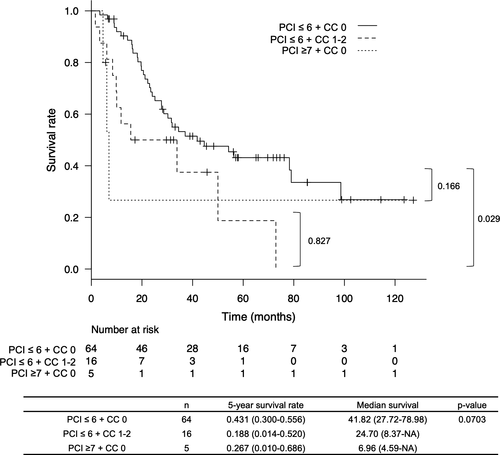
Furthermore, the 5-year survival rate of patients who had a PCI scores of ≤6 but had a CC scores of 1–2 was 18.8%, which was significantly poorer than patients with a PCI scores of ≤6 + CC scores of 0 (P = .029, Figure 4).
4 DISCUSSION
We rarely encounter peritoneal metastasis of HCC. Generally, it occurs in patients who have repeatedly undergone treatment for HCC. However, in some cases, it occurs during the early stage of HCC.11, 12 In general, peritoneal metastasis spreads through tumor seeding.13 The risk of seeding from HCC prevails in cases of direct puncture of the tumor during a biopsy or radiofrequency ablation or in cases of spontaneous rupture of the tumor. In patients with HCC after biopsy, the incidence and yearly occurrence rate of peritoneal metastasis have been reported to be 2.7% and 0.9%, respectively.14 Llovet et al. reported that the incidence of peritoneal metastasis after radiofrequency ablation in HCC was 12.5%.3 Portolani et al. indicated that the incidence of peritoneal metastasis after spontaneous rupture of HCC was 14.3%.15 Additionally, autopsy results revealed the presence of peritoneal metastasis in 9.4% of patients with HCC. A study listed tumor rupture, diaphragm invasion, and lymph node metastasis as risk factors for peritoneal metastasis. However, biopsy or radiofrequency ablation was not included.16
Treatment guidelines for peritoneal metastasis in HCC in Western countries and Japan recommend systemic chemotherapy.5, 17 However, peritoneal metastasis of HCC shows expansive growth, and its resection should be easier than adenocarcinoma. Several case reports have reported the efficaciousness of peritoneal metastasectomy for HCC on long-term prognosis.6-8
To date, there have been four published studies on peritoneal metastasectomy for HCC. Yeh et al. examined 16 patients who underwent peritoneal metastasectomy. In the study, the survival rate of patients who underwent peritoneal metastasectomy was equivalent to those who underwent only hepatectomy.18 Kow et al. investigated 36 patients with peritoneal metastasis, and performed peritoneal metastasectomy on 13 of the 36 patients. Patients who underwent peritoneal metastasectomy showed significantly better survival (33 months) than those who did not undergo surgery (14 months).4 In Japan, a study including nine patients with HCC showed a 42% improvement in the 5-year survival rate of patients who underwent peritoneal metastasectomy, whereas patients who did not undergo the procedure died within a year.9 Moreover, a Japanese group published a research paper on peritoneal metastasis that investigated the largest number of patients so far. Takemura et al. reported that the 5-year survival rate of 32 patients who underwent peritoneal metastasectomy for HCC was 39.0%, with a median survival of 34.5 months. They also concluded that the presence or absence of intrahepatic tumor, as well as its disease control, would contribute to prognosis.19
We have investigated more than 90 patients in this study, the most significant number of patients investigated in a single study on this topic to date. Additionally, the current study includes PCI and CC data.
PCI and CC scores have been used in the severity assessment of diseases such as peritoneal metastasis of digestive cancers or peritoneal cancers since 2006.10 They are currently used to correlate the treatment effects of peritoneal metastasectomy and hyperthermic intraperitoneal chemotherapy, particularly in Europe.20-26
The first report of peritoneal metastasectomy and hyperthermic intraperitoneal chemotherapy combination treatment for HCC was published in 2018. This study investigated 21 patients. The median survival duration was 46.7 months, and the 5-year survival rate was estimated to be 49.4%. Patients showed excellent survival with PCI scores within 15 as well as CC scores of either 0 or 1.27 The results suggest that hyperthermic intraperitoneal chemotherapy treatment for peritoneal metastasis of HCC should be considered.
In this study, the multivariate analysis demonstrated that PCI scores of ≤6 and CC scores of 0 were not independent prognostic factors. However, when we added this combination to the multivariate analysis with PCI scores of ≤6 + CC scores of 0, it was identified as an independent prognostic factor. This result suggests that the combination of two factors focuses more attention on actual patients who can get long survival due to metastasectomy. To support this, the CC scores of 0 and CC scores of 1–2 were compared in Figure 4, considering patients with a PCI scores of ≤6 only, to examine whether metastasectomy should be performed even if a CC scores of 0 is not achieved in such patients. As a result, the 5-year survival rate in patients with CC scores of 1–2 was 18.8%, even if the PCI scores were ≤6, which was a significantly poor prognosis compared to those with CC scores of 0. Therefore, the survival of patients with a PCI scores of ≤6 might be improved by achieving CC scores of 0 metastasectomy.
Furthermore, we examined the prognosis of patients with PCI scores of ≥7, who achieved CC scores of 0 (Figure 4). Since the number of patients was small, 5, there was no significant difference compared to the prognosis of patients with PCI scores of ≤6 + CC scores of 0 (P = .166). However, poor prognosis was demonstrated, with a 5-year survival rate being 26.7% and a median survival duration being 6.9 months. From these results, long-term survival might not be achievable in patients with PCI scores of ≥7, even if CC scores of 0 are achieved.
Recently, the results of a comparative study of lenvatinib and sorafenib (REFLECT study) on unresectable HCC were published.28 The results demonstrated that the median survival duration for lenvatinib was 13.6 months. In our results, the median survival duration following metastasectomy in patients with a PCI scores of ≥7 was 6.96 months. Since the REFLECT study is not limited to patients with peritoneal metastasis, it cannot be unequivocally concluded. However, metastasectomy might not be indicated when a PCI scores of ≥7 is confirmed in preoperative imaging.
According to the results of this study, the 5-year survival rate after metastasectomy was 43.1%, and the median survival duration was 41.8 months in patients with PCI scores of ≤6 + CC scores of 0. Since the therapeutic effect of TACE for peritoneal metastasis is usually insufficient, systemic chemotherapy or metastasectomy is the only treatment option. As mentioned above, patients have different backgrounds, but systematic chemotherapy alone has a median survival of 13.6 months. Therefore, metastasectomy may contribute to the prognosis of selected patients. The PCI scores can be calculated to some extent using preoperative imaging findings. Systematic chemotherapy should be selected if the preoperative imaging findings result in a PCI scores of ≥7. When preoperative imaging findings suggest the possibility of achieving a CC scores of 0 in patients with a PCI score of ≤6, metastasectomy might be considered as a treatment option.
Our research was a retrospective cohort study, which is a limitation of our study. The accuracy of the PCI scores is questionable due to the nature of the questionnaire survey used. In this study, only approximately two patients on average in each institution (99 patients in 44 institutions) underwent metastasectomy over a 7-year period. Therefore, the institution cannot be expected to precisely record the spread of peritoneal metastasis in such rare cases. Furthermore, the PCI scores are likely to be underestimated in postoperative patients because postoperative adhesion and small peritoneal metastasis might be difficult to detect.29 Moreover, comparative investigations were lacking in patients without peritoneal metastasectomy. Various types of systemic chemotherapy will be approved soon, and comparative investigation with these new therapies is required. However, there are no data in the present study that compare metastasectomy with other treatment modalities. Therefore, we cannot conclude from the current results that peritoneal metastasectomy directly contributes to improving the survival of patients.
In conclusion, peritoneal metastasectomy for HCC may contribute to improved survival of patients with a PCI scores of 6 or less, without residual tumors.
ACKNOWLEDGMENTS
We wish to express our sincere gratitude to the Japanese Society of Hepato-Biliary-Pancreatic Surgery for their support and guidance in our research project. We are also genuinely grateful for the kind cooperation of representatives from the following participating institutes: Gunma University, Miyazaki University, Nara Medical University, National Hospital Organization Kyushu Medical Center, Fujita Health University Bantane Hospital, National Hospital Organization Yokohama Medical Center, National Hospital Organization Osaka National Hospital, Nara Prefecture General Medical Center, Saiseikai Maebashi Hospital, Kumamoto University, Hiroshima Prefectural Hospital, Fukushima Medical University, Kinki University, Ehime Prefectural Central Hospital, Mie University, Osaka International Cancer Institute, Saitama Medical University International Medical Center, Hamamatsu University School of Medicine, Osaka Red Cross Hospital, Oita University, Yao Municipal Hospital, Nigata University, Shimane University, Chugoku Rosai Hospital, Hiroshima City Hospital, Teikyo University, Oita Red Cross Hospital, Toyama Prefectural Central Hospital, Jichi Medical University, Kobe University, Osaka City University, Okayama Saiseikai General Hospital, Sapporo-Kosei General Hospital, Yamaguchi University, Iwate Medical University, Fujita Health University, Kanazawa University, Osaka University, Kyoto University, Tokyo Medical and Dental University, Meiwa Hospital, Tokyo University, Kobe City Medical Center General Hospital, and Kumamoto Red Cross Hospital.
AUTHOR CONTRIBUTIONS
HI and MT designed the research and analyzed the patient data. All authors helped with data accumulation. HI drafted the manuscript. All authors have read and approved the final version of the manuscript.




